
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 18 February 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.837937
This article is part of the Research Topic Insights in Multiple Sclerosis and Neuroimmunology: 2021 View all 20 articles
Acute cerebellitis associated with Homer-3 antibodies is very rare. Here we present a 20-year-old woman who suffered from uncontrollable head shaking quickly from side to side and an unsteady gait for 2 days after the cold. Antibodies were screened by cell-based assays. The indirect immunofluorescence technique results revealed anti-Homer-3 antibody titers of 1:3.2 in the CSF and 1:100 in the serum. The woman was obviously improved after antiviral and immunosuppression (immunoglobin, methylprednisolone and mycophenolate mofetil) treatment. Our report indicated immune-mediated causes should be considered in the acute cerebellitis. Immunotherapy can contribute to the improvement of cerebellar syndrome.
In 2013, Hoftberger et al. reported a 38-year-old man with anti-Homer 3 antibodies who presented with symptoms of acute encephalopathy including headache, nausea, vomiting, and confusion, and cerebellar syndrome (1). Furthermore, seven patients with subacute or insidious “idiopathic cerebellar ataxia,” not acute cerebellitis, were reported (2, 3). Acute cerebellitis associated with anti-Homer 3 antibodies is very rare. Here, we report a female with acute cerebellitis associated with anti-Homer 3 antibodies.
A 20-year-old woman suffered from uncontrollable head shaking twice quickly from side to side (Supplementary Video 1 and Figure 1A) for 2 days after the cold (Table 1). In other words, the head swayed twice quickly and slightly from side to side. The unsteady gait was also observed (Supplementary Video 2). The patient had a history of allergic rhinitis for 2 years. Neurological examination demonstrated bilateral horizontal nystagmus, moderate limb dysmetria, Romberg sign positivity and gait ataxia. The patient was admitted to Nanjing Brain Hospital. On day 1, brain magnetic resonance imaging (MRI) showed an increased signal in the right cerebellar hemisphere without enhancement (Figures 1B,C). On day 3, lumbar puncture was performed, and a pressure of 180 mmH2O, a WBC count of 139 × 10 6 /L (Figure 2), and a protein level of 1.67 g/L were observed. The oligoclonal band was positive. On day 5, the indirect immunofluorescence technique (IIFT) results revealed anti-Homer-3 antibody titers of 1:3.2 in the CSF and 1:100 in the serum (Figures 1D,E). The gynecological sonography was normal. On day 4, mild diffuse waves were observed on electroencephalography. After 66 days of antiviral and immunosuppression (immunoglobin, methylprednisolone and mycophenolate mofetil) treatment, the woman was obviously improved (Supplementary Video 2, Table 1).
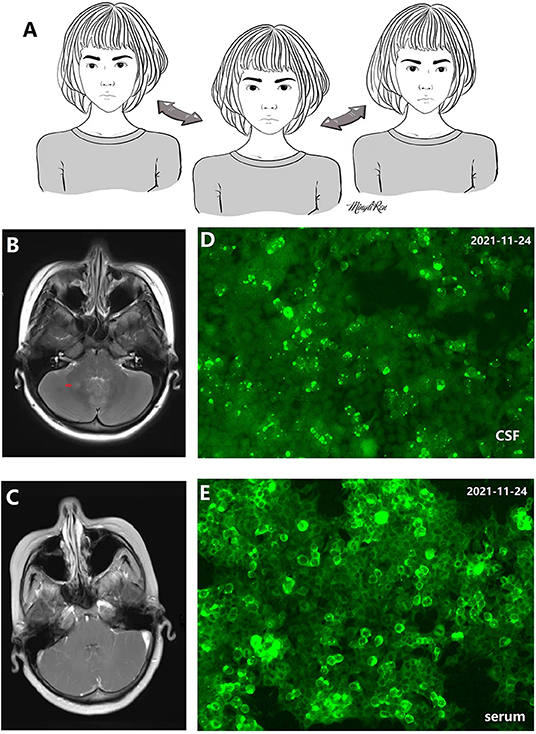
Figure 1. (A) 2D picture associated with Supplementary Video 1 showing uncontrollable and quick head shaking. (B,C) Increased signal in the right cerebellar hemisphere without enhancement. (D) Antibody in cerebrospinal fluid (CSF) recognizes Homer-3 antigens in fifixed HEK293 cells. Anti-Homer-3 antibody titers in CSF: 1:3.2. (E) Antibody in serum recognizes Homer-3 antigens in fifixed HEK293 cells. Anti-Homer-3 antibody titers in serum: 1:100.
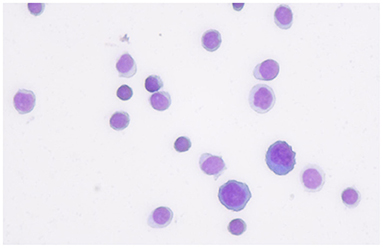
Figure 2. The first cerebrospinal fluid cytology from the patient showed that most of inflammatory cells were lymphocyte.
We described a rare case of cerebellitis associated with Homer-3 antibodies. This patient was positive for the anti-Homer 3 antibody in the CSF and serum, but negative for anti-ATP1A3, ARHGAP26, ITPR1, Hu, Yo, Ri, CV2, Ma2, amphiphysin, Tr(DNER), Zic4, Ma1, GAD65, PKCγ, SOX1, NMDAR, AMPA1, AMPA2, GABAB, LG1, CASPR2, DPPX, lolON5, mGluR5, GlyRα1, GABAARα1, and GABAARβ3.
The Homer family includes Homer-1, Homer-2, and Homer-3, all of which have several isoforms as a result of alternative splicing (4). Homer proteins can be divided into the two structurally distinct groups of short and long Homer proteins. Short Homers include Homer-1A, Homer-2C, Homer-2D, Homer-3C and Homer-3D. Long Homer proteins include Homer-1B, Homer-1C, Homer-2A, Homer-2B, Homer-3Axx and Homer-3Bxx. The short~35 amino acid residue long coiled-coiled domain in the Homer N-terminals may be important for the folding of Homers themselves or involved in interacting with proteins. This short N-terminal coiled-coil domain is present in all Homer-3 proteins except for the Homer-3B. The short domain in Homer-3A is remarkable longer than that in Homer-3C and Homer-3D (5). Homer-3 and mGluR1 (metabotropic glutamate receptor) are expressed predominantly on Purkinje cell dendritic spines (6). Homer-3 is the scaffold protein between mGluR1 and inositol 1,4,5 triphosphate receptors, which regulate the post-synaptic calcium metabolism in Purkinje cells in response to mGLuR1 stimulation (7). Thus, the anti-Homer 3 antibodies might bind Homer-3A, Homer-3C and Homer-3D, especially Homer-3A disturbing the homer 3 function, which could contribute to cerebellar ataxia (1–3, 5). Cerebellar ataxia is also the most common symptom of anti-mGluR1 autoimmunity (8).
In 2007, Zuliani et al. reported a 65-year-old woman with Homer-3 antibodies presenting with subacute cerebellar ataxia. Although the patient received steroids, the cerebellar syndrome had not improved by the last follow-up (2). Guan et al. screened the serum and CSF samples of 750 patients with ‘idiopathic' cerebellar ataxia, and Homer-3 antibodies were detected in 6 patients. Interestingly, 2 patients had RBD, a hot cross bun sign, and dysautonomia, which may be considered diagnostic markers for multiple system atrophy of the cerebellar type (MSA-C) (3). Given that there is no effective treatment for MSA-C, immune-mediated cerebellar syndrome can be improved by immunotherapy (3). Homer-3 antibodies are even more rarer, and screening for antibodies in every patient with acute, subacute and insidious cerebellar syndrome is unrealistic. An interesting symptom, “head socking uncontrollably from side to side (Supplementary Video 1 and Figure 1A),” was observed in this patient with Homer-3 antibodies, which might be a characteristic of cerebellar syndrome with Homer-3 antibodies, and was not reported in the previous studies (1–3). Seasonable immunotherapy can contribute to the improvement of cerebellar syndrome, and delayed treatment might lead to unfavorable outcomes in patients with cerebellar ataxia (3). Figure 1A was depicted by a female patient with anti-N-methyl-d-aspartate receptor encephalitis in our hospital (9). Immunotherapeutic treatment was not delayed, and the patient had no residual problems (9).
In summary, we report a rare patient with cerebellitis with Homer-3 antibodies who improved after immunotherapeutic treatment. The symptom “head shaking” might lead to cerebellar syndrome associated with Homer-3 antibodies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
AM: drafting and revising the manuscript. XW: study concept or design and study supervision. CY, YS, LW, and JG: clinical work. Their contributions helped us to acquire clinical data. All authors contributed to the article and approved the submitted version.
The work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 81501126, http://npd.nsfc.gov.cn/), Science and Development Foundation of Nanjing Medical University (2014NJMU050), Young Medical Key Talents Foundation of Jiangsu Province (Grant No. QNRC2016053), and Training Project for Young Talents of Nanjing Brain Hospital. General project of Nanjing Municipal Health Commission (YKK21110).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.837937/full#supplementary-material
Supplementary Video 1. The patient presented head shaking twice uncontrollably and quickly from side to side, without body shaking.
Supplementary Video 2. The patient was administered antiviral and immunotherapies and improved.
1. Höftberger R, Sabater L, Ortega A, Dalmau J, Graus F. Patient with homer-3 antibodies and cerebellitis. JAMA Neurol. (2013) 70:506–9. doi: 10.1001/jamaneurol.2013.1955
2. Zuliani L, Sabater L, Saiz A, Baiges JJ, Giometto B, Graus F. Homer 3 autoimmunity in subacute idiopathic cerebellar ataxia. Neurology. (2007) 68:239–40. doi: 10.1212/01.wnl.0000251308.79366.f9
3. Liu M, Ren H, Fan S, Zhang W, Xu Y, Zhao W, et al. Neurological autoimmunity associated with homer-3 antibody: a case series from China. Neurol Neuroimmunol Neuroinflamm. (2021) 27:8:e1077. doi: 10.1212/NXI.0000000000001077
4. Shiraishi-Yamaguchi Y, Furuichi T. The homer family proteins. Genome Biol. (2007) 8:206. doi: 10.1186/gb-2007-8-2-206
5. Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Molecular characterisation of two structurally distinct groups of human homers, generated by extensive alternative splicing. J Mol Biol. (2000) 295:1185–200. doi: 10.1006/jmbi.1999.3436
6. Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Difffferential expression of Homer family proteins in the developing mouse brain. J. Comp. Neurol. (2004) 473:582–99. doi: 10.1002/cne.20116
7. Mizutani A, Kuroda Y, Futatsugi A, Furuichi T, Mikoshiba K. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. J Neurosci. (2008) 28:5369–82. doi: 10.1523/JNEUROSCI.4738-07.2008
8. Spatola M, Petit Pedrol M, Maudes E, Maudes E, Simabukuro M, Muñiz-Castrillo S, et al. Clinical features, prognostic factors, and antibody effffects in anti-mGluR1 encephalitis. Neurology. (2020) 95:e3012–25. doi: 10.1212/WNL.0000000000010854
Keywords: cerebellitis, anti-Homer 3 antibody, head shaking, cerebellar syndrome, immunotherapeutic treatment
Citation: Miao A, Yu C, Sun Y, Wang L, Ge J and Wang X (2022) Acute Cerebellitis Associated With Anti-homer 3 Antibodies: A Rare Case Report and Literature Review. Front. Neurol. 13:837937. doi: 10.3389/fneur.2022.837937
Received: 17 December 2021; Accepted: 27 January 2022;
Published: 18 February 2022.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Manish Malviya, Memorial Sloan Kettering Cancer Center, United StatesCopyright © 2022 Miao, Yu, Sun, Wang, Ge and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ailiang Miao, YWlsaWFuZ21pYW8xOTg2QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.