
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 02 June 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.830614
 Floris H. B. M. Schreuder1*
Floris H. B. M. Schreuder1* Mirre Scholte2
Mirre Scholte2 Marike J. Ulehake3
Marike J. Ulehake3 Lotte Sondag1
Lotte Sondag1 Maroeska M. Rovers2,3
Maroeska M. Rovers2,3 Ruben Dammers4
Ruben Dammers4 Catharina J. M. Klijn1†
Catharina J. M. Klijn1† Janneke P. C. Grutters2,3†
Janneke P. C. Grutters2,3†Background: In patients with spontaneous supratentorial intracerebral hemorrhage (ICH), open craniotomy has failed to improve a functional outcome. Innovative minimally invasive neurosurgery (MIS) may improve a health outcome and reduce healthcare costs.
Aims: Before starting phase-III trials, we aim to assess conditions that need to be met to reach the potential cost-effectiveness of MIS compared to usual care in patients with spontaneous supratentorial ICH.
Methods: We used a state-transition model to determine at what effectiveness and cost MIS would become cost-effective compared to usual care in terms of quality-adjusted life-years (QALYs) and direct healthcare costs. Threshold and two-way sensitivity analyses were used to determine the minimal effectiveness and maximal costs of MIS, and the most cost-effective strategy for each combination of cost and effectiveness. Scenario and probabilistic sensitivity analyses addressed model uncertainty.
Results: Given €10,000 of surgical costs, MIS would become cost-effective when at least 0.7–1.3% of patients improve to a modified Rankin Scale (mRS) score of 0–3 compared to usual care. When 11% of patients improve to mRS 0–3, surgical costs may be up to €83,301–€164,382, depending on the population studied. The cost-effectiveness of MIS was mainly determined by its effectiveness. In lower mRS states, MIS needs to be more effective to be cost-effective compared to higher mRS states.
Conclusion: MIS has the potential to be cost-effective in patients with spontaneous supratentorial ICH, even with relatively low effectiveness. These results support phase-III trials to investigate the effectiveness of MIS.
Spontaneous intracerebral hemorrhage (ICH) yearly affects more than 3 million people worldwide (1). ICH has a poor prognosis with 1-month case fatality of 40%, which has not improved in the past decades (2–4). Hematoma volume is one of the most important predictors of an outcome after ICH (5). Therefore, research has been focused on neurosurgical treatment to reduce hematoma volume. Earlier trials comparing neurosurgical open craniotomy to standard care have, however, failed to show improvement in a functional outcome (6, 7). As an alternative, minimally invasive stereotactic aspiration and thrombolytic irrigation of the hematoma were studied in the MISTIE-III trial (8, 9). In 506 patients with a hematoma volume of at least 30 ml, intervention (initiated after a mean of 58 h after the ICH onset) did not seem to be superior to standard medical care (8). In spite of these results, a recent systematic review and meta-analysis of surgical treatments for supratentorial ICH have concluded that surgical treatment for ICH still holds promise, especially with minimally invasive methods and when performed early after the symptom onset (10).
Minimally invasive techniques include stereotactic aspiration and endoscopy-guided approaches. The latter technique uses irrigation-aspiration devices to mobilize the hematoma clot, without the use of thrombolytic agents. Several small case-series of endoscopy-guided minimally invasive surgery have suggested that this technique can safely reduce hematoma volume by 54–94% but lacked a control group or did not report a functional outcome beyond hospital admission (11–13). To properly assess its added value, a phase-III randomized clinical trial is needed to demonstrate the effectiveness of this minimally invasive surgical technique to improve the functional outcome of patients with spontaneous supratentorial ICH, when compared to current usual care.
Given the results of previous trials and the meta-analysis, choosing an optimal trial design and patient population (e.g., distribution of an outcome and age) for such a trial is important as these factors can have a large effect on the (cost-) effectiveness of MIS. One tool that can inform trial design is health economic modeling. Modeling can help to determine the minimal effectiveness and maximum cost from a cost-effectiveness perspective and may determine subgroups in which the intervention is likely to be more or less effective. Before starting a phase-III trial, we aim to assess the conditions that need to be met to reach the potential cost-effectiveness of minimally invasive surgery (MIS) in comparison with usual care in three different populations of patients with spontaneous supratentorial ICH.
We followed the modeling good research practices and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement guidelines (Appendix 1) (14, 15).
To evaluate the conditions that need to be met to reach the potential cost-effectiveness of MIS compared to usual care, we constructed a state transition (Markov) model, representing patient follow-up (Figure 1). Health states represented a functional outcome after the index ICH based on the modified Rankin Scale (mRS) score. The mRS is a widely used functional outcome assessment scale, which ranges from 0 (no symptoms) to 6 (death) (16). To simulate the course of the disease and patient follow-up, we built a model with cycles of 3 months. In each cycle, patients had a probability to move to a different health state.
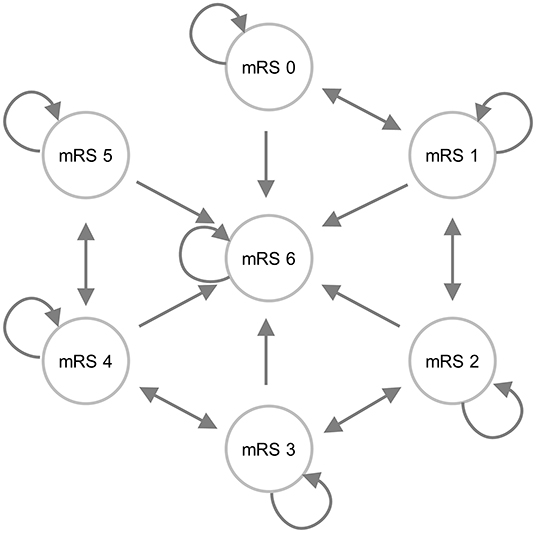
Figure 1. An influence diagram of the state transition model. Patients could enter the model via one of the mRS score health states. The arrows indicate the most likely transitions during follow-up; however, it was also possible to improve from mRS 3 to mRS 1, for example.
The model reflects the change in health states as a result of the two treatment strategies in 3 hypothetical cohorts of patients with spontaneous supratentorial ICH. The cohorts differ in age, case-fatality, and in the distribution of mRS scores. Cohort 1 was based on a report of the 12-month validation of the ICH score in patients with a mean age of 70 years, in which 33% had an mRS 0–3 (Table 1) (17) Cohort 2 was based on the control group of the Tranexamic acid for the primary intracerebral hemorrhage trial (TICH-2) with an average age of 69 years, in which 46% had an mRS of 0–3 (Table 1) (18). Cohort 3 included the control group of the MISTIE-III trial, which had a mean age of 62 years, in which 11% of the patients had an mRS of 0–3 (Table 1) (8). Because decision-making and an outcome after surgical treatment differ between supratentorial and infratentorial ICH, we restricted our model to supratentorial ICH.
The usual care strategy was based on the 2015 American Heart Association/American Stroke Association guideline for the management of ICH and consisted of admission to a stroke unit and pharmacological reduction of blood pressure if indicated (19, 20). As open craniotomy is currently performed in only a small minority of patients, this was not included.
We assumed that only index ICH and age affect a functional outcome and transitions between health states. Because we did not expect MIS to affect the number of ICH recurrences or other cardiovascular diseases beyond the 30-day postoperative period (e.g., ischemic stroke, myocardial infarction), recurrent ICH events were not included in our model.
Transition probabilities determined how the patients could move through different health states in the model. The transition probabilities at 6, 9, and 12 months were derived from the raw data of the 188 conservatively treated patients with supratentorial ICH of Cohort 1 (Table 2) (17). Beyond the 1st year after the index ICH, we assumed that the mRS score was stable for the remaining lifetime. From then on, the patients either stayed in their health state, or died. The probability of dying was based on the mortality rate of the general Dutch population (21), multiplied by the additional risk of dying caused by the ICH. The hazard ratio of dying from ICH-related causes was 0 in mRS 0 and 1, and increased from 1.11 to 2.38 in mRS 2 to 5, respectively (22).
Effectiveness was measured in terms of quality-adjusted life years (QALYs). QALYs were constructed by multiplying the utility weight for each health state (an mRS score) with the remaining life years (Table 3). The utility is a score for health-related quality of life and ranges from 0 (death) to 1 (perfect state of health). We derived utility weights per mRS score from a large individual patient data meta-analysis of 4,479 patients with ICH (23). The average utility weight for an mRS score of 5 is negative, which indicates that this health state is considered to be worse than death. QALYs were discounted with an annual rate of 1.5%, according to the Dutch guidelines for economic evaluation (24).
Costs (in Euros) were calculated from the Dutch health care perspective and were adjusted to the price index of 2019 (23). We included direct healthcare costs in our model, i.e., costs for hospitalization, rehabilitation, nursing home care, visits to general practitioner and outpatient departments, medication, home care, and costs that directly result from MIS (Supplementary Table 1) (20) Resource use of usual care was based on a retrospective cohort of consecutive patients (N = 173) with spontaneous supratentorial ICH treated at our hospital between January 2012 and December 2015, supplemented with data from the literature (Supplementary Tables 2–4) (25, 26) The retrospective cohort provided length of stay at the different hospital wards (i.e., intensive care, medium care, stroke unit, or general ward), length of stay at rehabilitation or nursing home facilities, and the number of outpatient visits. Medication costs and resource use for home care up to the 1st year after the index ICH were derived from the literature (25, 26) and were assumed to remain stable over time thereafter. The frequency of visits to the general practitioner was based on the Dutch multidisciplinary guideline for cardiovascular risk management (27). We calculated the average costs using the mean lengths of stay and standard Dutch unit costs (Table 4) (24). Since costs for MIS are not yet known, we set the total surgical costs (including use of operating facilities, surgical staff, device use, surgical supplies, and postoperative care) at €10,000 by default based on an expert opinion. Costs were discounted with an annual rate of 4%, according to Dutch guidelines for economic evaluation (24).
We validated the model in accordance with the AdViSHE checklist through consulting clinical experts, cross-validation with relevant literature, checks by independent modeling experts, and extreme value, and subunit testing (28).
To assess the conditions that need to be met to reach the potential cost-effectiveness of MIS in comparison with usual care, we evaluated costs and health outcomes per patient over a lifetime horizon for the three patient cohorts. The effectiveness of MIS was assumed to be an absolute improvement toward mRS 0–3 for the patients treated with MIS compared to usual care, using 11% as an example (Figure 2). We assumed that the patients who improved ended up in mRS 0–3 in an increasing extent (i.e., 18% increase in mRS 0, 18% increase in mRS 1, 27% increase in mRS 2, and 37% increase in mRS 3) and that the decrease in mRS 4–6 was relative to the number of the patients in that state in the original cohort. We can illustrate this best using an example population with an equal percentage of the patients in each mRS state, i.e., 14.3% of the patients per health state. The percentage of the patients in mRS 0–3 is then 57.2% and will increase with 11 to 68.2% due to surgery. The percentage of the patients in mRS 0 increases with 18% of this 11 to 16.3%, mRS 1 increases with 18% of 11 to 16.3%, mRS 2 increases with 27% of 11 to 17.3%, and mRS 3 increases with 37% of 11 to 18.3%. The percentages of the patients in mRS 4, 5, and 6 will all decrease with 1/3 of 11 to 10.6%. The transition probabilities for MIS beyond 3 months post-ICH were considered to be equal to the transition probabilities in the usual care strategy.
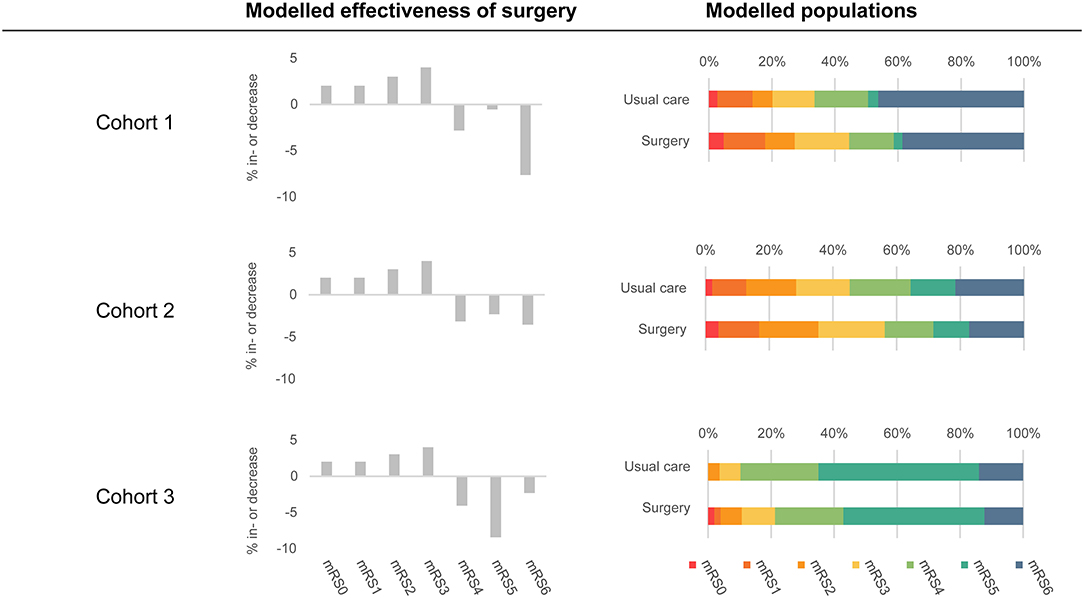
Figure 2. An overview of included populations and modeled effectiveness of MIS. The modeled populations column shows the distributions of the patient populations in both strategies at 3 months post-ICH. The usual care strategy is based on the Hemphill (Cohort 1), TICH-2 (Cohort 2), and MISTIE-III (Cohort 3) populations. The surgery strategy is based on usual care strategy in combination with the modeled effectiveness of surgery (displayed in the middle of the figure). Modeled effectiveness of surgery was based on 11% effectiveness.
We conducted four types of analyses: threshold, two-way sensitivity, scenario, and probabilistic sensitivity analysis. For these analyses, we used the incremental net monetary benefit (iNMB), which was calculated by multiplying the incremental effects (QALYs) of MIS compared to usual care with the willingness-to-pay (WTP) per QALY, subtracting the incremental costs. In the Netherlands, maximum additional costs per QALY range from €20,000 for diseases with a low burden of disease to €80,000 for diseases with a high burden (29). Because of the high disease burden of ICH (1), the highest Dutch cost-effectiveness threshold of €80,000 per QALY was applicable according to the iMTA disease burden calculator (30). An iNMB above 0 means that MIS is cost-effective, whereas an iNMB below 0 implies that it is not. The value of effects or costs where the iNMB is 0 is the threshold for cost-effectiveness. For example, when a new strategy gains 0.1 QALYs extra and costs €1,000 more than the current strategy, the iNMB is 0.1 × 80.000 − 1, 000 = 7, 000. The iNMB is positive, which means that the new strategy is cost-effective. The threshold for cost-effectiveness (iNMB = 0) is found at €8,000 extra costs or 0.0125 gained QALYs for the new strategy compared to the current strategy. All analyses were conducted using R 3.5.3 and the Tidyverse (v1.3.0) package (31).
Since both costs and effectiveness of MIS are unknown, we explored at which values of these parameters MIS could potentially be cost-effective. We explored this by threshold analysis, in which we fixed the value of one parameter (e.g., costs) and explored at which value of the other parameter (e.g., effectiveness) MIS becomes cost-effective. By default, we set the surgical costs to €10,000 and the effectiveness to an absolute improvement of 11% in mRS 0–3.
To allow the determination of the cost-effective strategy for each combination of cost and effect, we conducted two-way sensitivity analyses. For this purpose, we ranged the effectiveness of MIS (absolute improvement in the mRS score 0–3) between 0 and 20%, and surgical costs of MIS between €0 and €50,000.
In the scenario analysis, we assumed that all the patients receive MIS, but that MIS is effective in a specific health state only, e.g., the patients in mRS 4. It was also assumed that, when the patients improved, they did so by one health state, e.g., mRS 4 patients would transit to mRS 3. The minimal effectiveness of MIS was established by determining at which effect MIS becomes cost-effective, with surgical costs fixed at €10,000.
Uncertainty with respect to the input parameters was addressed in a probabilistic sensitivity analysis by taking 5,000 samples from the distributions of these parameters. MIS costs and effectiveness were incorporated in the probabilistic sensitivity analysis as fixed values. All results were based on probabilistic outcomes with 95% confidence intervals (95%-CI) calculated using the percentile method.
In Cohort 1, the usual care strategy gained, on average, 3.5 (95%-CI: 2.8–4.2) QALYs at an average cost of €79,879 (95%-CI: €49,681–€127,262) per patient. Cohort 2 gained, on average, 4.4 (95%-CI: 3.7–5.1) QALYs at an average cost of €153,961 (95%-CI: €90,591–€246,078) per patient in the usual care strategy. Cohort 3 gained, on average, 0.6 (95%-CI: −0.8–1.8) QALYs at an average cost of €433,191 (95%-CI: €212,123–€712,743). The conditions that needed to be met for MIS to become cost-effective were assessed in comparison with usual care.
For all three populations, we explored the minimal effectiveness and maximum cost at which MIS would be cost-effective. Given €10,000 of surgical costs, MIS became cost-effective when at least 1.3% (95%-CI: 1.2–1.5%) of the patients improved to mRS 0–3 in Cohort 1 (in the usual care strategy, 33.6% had a good outcome, and this increased to 35.1% in the surgical strategy). For Cohort 2, this percentage was 1.1% (95%-CI: 0.9–1.4%), and, for Cohort 3, it was 0.7% (95%-CI: 0.5–0.9%). Given 11% effectiveness of MIS, surgical costs may be up to €83,301 (95%-CI: €71,633–€93,496) for Cohort 1, €164,382 (95%-CI: €120,973–€203,160) for Cohort 2, and €99,581 (95%-CI: €75,749–€118,391) for Cohort 3, for MIS to be cost-effective. Differences between cohorts can be explained by the differences in mRS distributions of poor functional outcomes and the mean age of patients. For example, Cohort 3 had a relatively low threshold, probably due to the high number of the patients in mRS 5 that improve to mRS 0–3 and the relatively young age of this cohort.
Figure 3 shows the two-way sensitivity plot for each cohort. In all cohorts, the cost-effectiveness of MIS was mostly determined by its effectiveness. This indicates that, even when MIS is associated with considerable costs, it would still become cost-effective with low effectiveness. For example, if the effectiveness of MIS is only 1.3%, MIS would be cost-effective with €10,000 treatment costs.
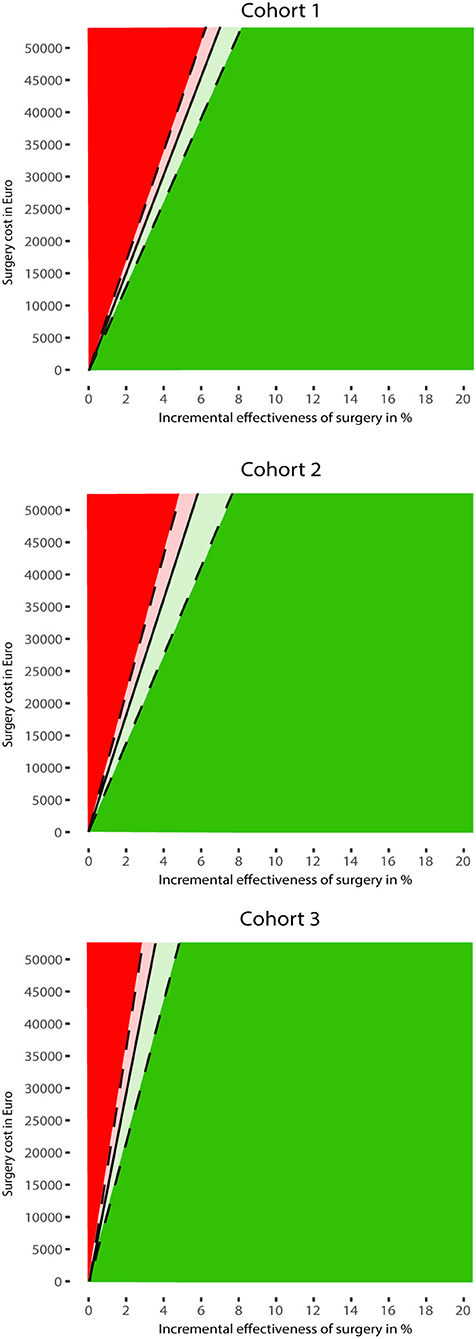
Figure 3. Results of the two-way sensitivity analyses. The MIS strategy is potentially most cost-effective for combinations of surgery cost and effectiveness in the green area. The red area indicates when usual care is most cost-effective. The effectiveness of MIS was assumed to be an absolute improvement toward mRS 0–3 for patients treated with MIS compared to usual care. Both strategies are equally cost-effective at the threshold indicated by the black line. The dashed lines indicate the 95% confidence interval of the threshold line.
The minimal effectiveness of MIS to be cost-effective was assessed, when only patients in a particular health state improved by only one mRS state (e.g., only a proportion of mRS 4 patients transit to mRS 3 due to surgery). Given €10,000 of surgical costs, MIS would never become cost-effective when only mRS 6 patients transit to mRS 5. This is because patients in mRS 5 had a worse utility score and higher costs compared to mRS 6 patients. MIS is most cost-effective when mRS 5 patients transit to mRS 4. At least 1.5% (95%-CI: 0.8–2.5%; Cohort 1), 1.5% (95%-CI: 0.8–2.5%; Cohort 2) or 1.1% (95%-CI: 0.6–1.7%; Cohort 3) of patients need to improve from mRS 5 to mRS 4 to be cost-effective compared to current care. Thresholds increase when patients with a lower mRS state were treated, with highest thresholds for patients in mRS1 who transit to mRS 0. Then, at least 5.3% (95%-CI: 2.4–10.%; Cohort 1) or 5.3% (95%-CI: 2.4–10.%; Cohort 2) of patients need to improve from mRS 1 to mRS 0 to be cost-effective compared to current care. It was not possible to obtain a threshold for Cohort 3, as there were zero patients in mRS 1 in this cohort.
In patients with spontaneous supratentorial ICH, minimally invasive surgery has the potential to be cost-effective, even with relatively low effectiveness. Assuming €10,000 of surgical costs, MIS would become cost-effective when at least 0.7–1.3% of the patients improve to mRS 0–3 compared to usual care, depending on the population characteristics. When 11% of the patients improve to a favorable functional outcome, surgical costs may be up to €83,301–€164,382. The cost-effectiveness of MIS was mainly driven by its effectiveness. In lower mRS states, MIS needs to be more effective to be cost-effective compared to higher mRS states.
Cross-validation of our model with relevant literature showed that results of the usual care strategy were comparable. We have established the lifetime average number of QALYs of the patients with supratentorial ICH treated with usual care between 0.6 and 4.4, which is most comparable to the published QALYs of 2.8–4.7 after supportive treatment in patients with ICH (32, 33). The QALYs in Cohort 3 were lower compared to the literature, whereas the lifetime costs for the patients in Cohort 3 were higher, which was caused by a larger proportion of the patients with an mRS score of 5 (Figure 2). Lifetime costs of the usual care strategy ranged between €79,879 and €433,191 in our model, which corresponds to €49,081 to €189,744 (using the average 2019 exchange rate of 0.8931)(34) 1, while, in previous studies, these costs ranged from $54,956 to $212,456. (32, 35–37) In general, differences between costs can be explained by different cost perspectives (26, 32, 36, 38), different treatments (38), or different patient populations (26, 35, 37, 39). Our study has a number of strengths. First, we performed our analysis prior to the start of a multicenter phase-III randomized clinical trial. Our modeling study provides valuable insights into patient populations that could benefit most from surgery and minimum effectiveness and maximum costs from a cost-effectiveness perspective. This helps in the design of the phase-III randomized clinical trial. Given the neutral results of recent trials, optimal study design and planning are especially important. Additionally, we adhered to guidelines on (model-based) economic evaluation, and combined evidence from the literature, with data from current clinical practice. To determine the influence of the uncertainty of the input parameters, we performed a probabilistic sensitivity analysis.
Our study also has some limitations. First, transition probabilities and costs were based on relatively small cohorts that were divided in seven categories of mRS scores. In our model, the costs for patients with an mRS of 3 between 3 months and 1 year were based on only 8 patients, and costs were lower than expected based on increasing costs per mRS score. These 8 patients had a relatively good recovery and were discharged to home instead of to a rehabilitation center or nursing home, which resulted in lower costs. Nevertheless, the transition probabilities in our data were comparable to a recently published study of 173 patients who were followed up to 12 months after ICH (40).
Second, the three cohorts were slightly different. Cohort 3 had follow-up data available at 1 month, instead of 3 months for Cohorts 1 and 2. Additionally, Cohort 2 also included infratentorial hemorrhages (7% of total) (18), which may have influenced mRS status. Changes in mRS states at 6, 9, and 12 months were only available for Cohort 1; therefore, we used these probabilities for all three cohorts.
Third, we assumed that hospital length of stay was not influenced by therapeutic strategy other than through differences in a functional outcome. This seemed reasonable to assume, since in MISTIE-III time of ICU admission, was similar between surgically treated patients and patients in usual care (8).
Fourth, we found that the largest impact on cost-effectiveness can be achieved in the higher mRS scores, but it remains difficult to predict the eventual outcome at admission. Several prediction models exist to estimate a functional outcome, however with moderate performance (5, 41). Thus, predicting a functional outcome on an individual basis remains rather inaccurate. Based on our results, surgery should refrain from reducing mortality at the cost of an increase of subjects with mRS of 5, as this results in higher costs and lower QALYs.
Lastly, our model included Dutch healthcare costs, which might limit the applicability to generalize our results to other healthcare systems. However, as we provided extensive details of the input parameters in the model, interested readers can assess the transferability of the results to their specific situation (42) In our model, we only included direct medical costs, e.g., costs resulting from hospital admission, rehabilitation, and medical treatment. We decided not to include indirect costs resulting from the ICH, such as loss of productivity from patients and their caregivers. Because we included only direct healthcare costs, we probably have been conservative and underestimated the cost-effectiveness of MIS.
Given the neutral results of the MISTIE-III trial, one might question whether new trials aimed at reducing hematoma volume are needed. Based on the recent meta-analysis, timing of surgery may modify the effect of surgery (10). In the three large, international, multicenter, randomized clinical trials on neurosurgical treatment of ICH (STICH, STICH-II, and MISTIE-III), average time between the symptom onset and surgery ranged from 26 to 58 h (6–8). Earlier surgery might be more beneficial, as hematoma growth occurs in up to a quarter of patients in the 1st h after the ICH onset (10, 43). Hence, further randomized trials are needed to demonstrate whether minimally invasive surgery performed early after the symptom onset improves a functional outcome after ICH. Several trials using minimally invasive surgery are ongoing or being scheduled (Table 5), of whom some investigate MIS early after the symptom onset. By estimating the potential value of MIS in this modeling study, we have shown that societal investments in phase-III randomized clinical trials are justified to achieve a high level of evidence for the use of neurosurgical treatment of patients with ICH. Once the results of these trials become available, these can be incorporated into the model to estimate the actual cost-effectiveness of MIS.
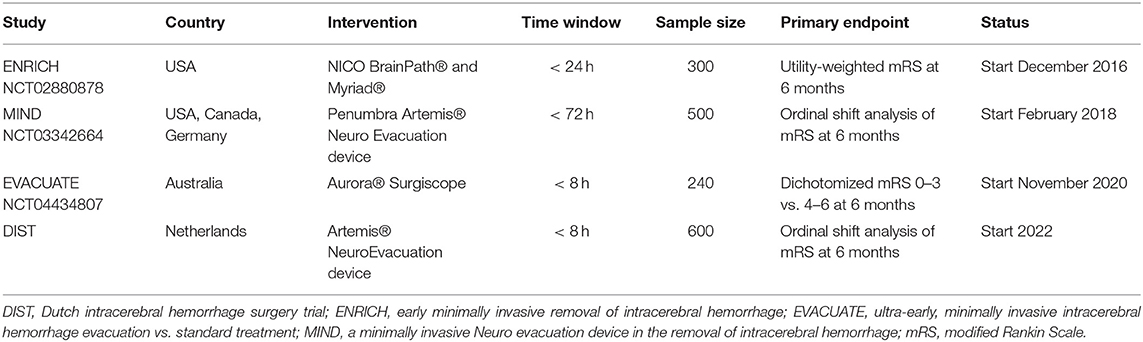
Table 5. An overview of ongoing or scheduled randomized trials using minimally invasive surgery in intracerebral hemorrhage.
Our results have several implications for the design of future phase-III clinical trials investigating the potential effectiveness of MIS in patients with ICH. First, the effectiveness of MIS may be as low as 0.7% to become cost-effective, which indicates that, even a very small improvement in a functional outcome could be deemed worthwhile from a societal perspective. However, this should not be mistaken for a clinically meaningful difference with a corresponding number needed to treat. Second, our study provides insight where the largest impact on cost-effectiveness can be achieved, i.e., in higher mRS states (mRS 4 and 5). Our results illustrate that a transition from mRS 6 to mRS 5 will have a negative impact on cost-effectiveness. As cost-effectiveness of MIS seems mainly driven by its effectiveness, development and application of novel surgical techniques to treat patients with supratentorial spontaneous ICH with a potentially large effect on a clinical outcome is worthwhile also from a cost-effectiveness perspective.
In patients with spontaneous supratentorial ICH, minimally invasive neurosurgery has the potential to be cost-effective, even with relatively low effectiveness. These results underline the need for a multicenter phase-III trial to investigate the effectiveness of MIS to improve an outcome after spontaneous supratentorial ICH. Based on these results, societal costs and patient burden for such a trial seem justified.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
CK, FS, and JG concepted and designed the study. FS, JG, MU, and MS contributed to acquisition and analysis of data. CK, FS, JG, LS, and MS contributed to interpretation of data. FS, MU, and MS drafted the manuscript. CK, JG, LS, MR, and RD contributed to critical revision of the manuscript. CK and MR contributed to funding. CK, JG, and MR contributed to supervision of the study. All authors contributed to the article and approved the submitted version.
This study was sponsored by the Netherlands Cardiovascular Research Initiative, which is supported by the Dutch Heart Foundation, CVON2015-01: CONTRAST and the support of the Brain Foundation Netherlands (HA2015.01.06). FS and CK are supported by the Dutch Heart Foundation (a clinically established investigator grant, 2012T077). CK is supported by ZonMw (ASPASIA grant, 015008048). FS is supported by the Dutch Heart Foundation (a senior clinical scientist grant, T2019T060). MS, MR, and JG are supported by a grant from the Dutch Research Council (No. 91818617).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge Dr. J. C. Hemphill III for his willingness to share the raw data from the ICH score validation study for the functional outcomes of Patients at 3, 6, and 12 months. Special thanks to Dr. Xia Wang for providing additional utility data for our analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.830614/full#supplementary-material
1. ^https://www.exchangerates.org.uk/USD-EUR-spot-exchange-rates-history-2019.html#:~:text=Average%20exchange%20rate%20in%202019%3A%200.8931%20EUR (accessed on April 12, 2022).
1. GBD 2019 Stroke Collaborators. Global, regional and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
3. Samarasekera N, Fonville A, Lerpiniere C, Farrall AJ, Wardlaw JM, White PM, et al. Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke. (2015) 46:361–8. doi: 10.1161/STROKEAHA.114.007953
4. Jolink WM, Klijn CJ, Brouwers PJ, Kappelle LJ, Vaartjes I. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology. (2015) 85:1318–24. doi: 10.1212/WNL.0000000000002015
5. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. (1993) 24:987–93. doi: 10.1161/01.STR.24.7.987
6. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. (2005) 365:387–97. doi: 10.1016/S0140-6736(05)70233-6
7. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. (2013) 382:397–408. doi: 10.1016/S0140-6736(13)60986-1
8. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. (2019) 393:1021–32. doi: 10.1016/S0140-6736(19)30195-3
9. Salman RA, Klijn CJ, Selim M. Minimally invasive surgery plus alteplase for intracerebral haemorrhage. Lancet. (2019) 393:965–7. doi: 10.1016/S0140-6736(19)30309-5
10. Sondag L, Schreuder FH, Boogaarts HD, Rovers MM, Vandertop WP, Dammers R, et al. Neurosurgical intervention for supratentorial intracerebral hemorrhage. Ann Neurol. (2020) 88:239–50. doi: 10.1002/ana.25732
11. Fiorella D, Gutman F, Woo H, Arthur A, Aranguren R, Davis R. Minimally invasive evacuation of parenchymal and ventricular hemorrhage using the Apollo system with simultaneous neuronavigation, neuroendoscopy and active monitoring with cone beam CT. J Neurointerv Surg. (2015) 7:752–7. doi: 10.1136/neurintsurg-2014-011358
12. Tan LA, Lopes DK, Munoz LF, Shah Y, Bhabad S, Jhaveri M, et al. Minimally invasive evacuation of intraventricular hemorrhage with the Apollo vibration/suction device. J Clin Neurosc. (2016) 27:53–8. doi: 10.1016/j.jocn.2015.08.037
13. Kellner CP, Song R, Pan J, Nistal DA, Scaggiante J, Chartrain AG, et al. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. (2020) 12:489–94. doi: 10.1136/neurintsurg-2019-015528
14. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. (2013) 16:231–50. doi: 10.1016/j.jval.2013.02.002
15. Caro JJ, Briggs AH, Siebert U, Kuntz KM, Force I-SMGRPT. Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force−1. Value Health. (2012) 15:796–803. doi: 10.1016/j.jval.2012.06.012
16. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
17. Hemphill JCr, Farrant M, Neill TAJ. Prospective validation of the ICH Score for 12-month functional outcome. Neurology. (2009) 73:1088–94. doi: 10.1212/WNL.0b013e3181b8b332
18. Sprigg N, Flaherty K, Appleton JP, Salman RA, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. (2018) 391:2107–15. doi: 10.1016/S0140-6736(18)31033-X
19. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
20. Neurologie Vereniging voor Neurologie. Richtlijn herseninfarct en hersenbloeding - mei 2019. Available online at: https://richtlijnendatabase.nl/richtlijn/herseninfarct_en_hersenbloeding/startpagina_herseninfarct_-bloeding.html (accessed on January 6, 2021}.
21. Statistics Netherlands. Age and Sex Distribution of Dutch Population 2014 [in Dutch]. Available online at: http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=37530NED&D1=1&D2=101-120&D3=0&D4=l&HDR=T,G1&STB=G2,G3&VW=T (accessed on June 21, 2018).
22. Samsa GP, Reutter RA, Parmigiani G, Ancukiewicz M, Abrahamse P, Lipscomb J, et al. Performing cost-effectiveness analysis by integrating randomized trial data with a comprehensive decision model: application to treatment of acute ischemic stroke. J Clin Epidemiol. (1999) 52:259–71. doi: 10.1016/S0895-4356(98)00151-6
23. Wang X, Moullaali TJ, Berge E, Berge E, Robinson TG, Lindley R, et al. Utility-weighted modified Rankin scale scores for the assessment of stroke outcome: pooled analysis of 20,000+ patients. Stroke. (2020) 51:2411–7. doi: 10.1161/STROKEAHA.119.028523
24. Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, Kanters T, Tan SS. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. [in Dutch]. Diemen: Zorginstituut Nederland (2015).
25. Spieler JF, Lanoe JL, Amarenco P. Costs of stroke care according to handicap levels and stroke subtypes. Cerebrovasc Dis. (2004) 17:134–42. doi: 10.1159/000075782
26. Christensen MC, Morris S. Association between disability measures and short-term health care costs following intracerebral hemorrhage. Neurocrit care. (2008) 9:313–8. doi: 10.1007/s12028-008-9124-5
27. Nederlandse Huisartsen Genootschap. Multidisciplinairy Guideline: Cardiovascular Risk Management 2011 [in Dutch]. Available online at: https://www.nvvc.nl/media/richtlijn/106/2011_MDR_CVRM.pdf (accessed on December 14, 2017).
28. Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. (2016) 34:349–61. doi: 10.1007/s40273-015-0327-2
29. Zwaap J, Knies S, van der Meijden C, Staal P, van der Heiden L. Cost-effectiveness in practice. 2015. The National Health Care Institute (Zorginstituut Nederland). Available online at: https://english.zorginstituutnederland.nl/publications/reports/2015/06/16/cost-effectiveness-in-practice (accessed on April 12, 2022).
30. Versteegh MM, Ramos IC, Buyukkaramikli NC, Ansaripour A, Reckers-Droog VT, Brouwer WBF. Severity-adjusted probability of being cost effective. Pharmacoeconomics. (2019) 37:1155–63. doi: 10.1007/s40273-019-00810-8
31. Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Software. (2019) 4. doi: 10.21105/joss.01686
32. Earnshaw SR, Joshi AV, Wilson MR, Rosand J. Cost-effectiveness of recombinant activated factor VII in the treatment of intracerebral hemorrhage. Stroke. (2006) 37:2751–8. doi: 10.1161/01.STR.0000246611.21999.5d
33. Fletcher JJ, Kotagal V, Mammoser A, Peterson M, Morgenstern LB, Burke JF. Cost effectiveness of transfers to centers with neurological intensive care units after intracerebral hemorrhage. Stroke. (2015) 46:58–64. doi: 10.1161/STROKEAHA.114.006653
34. https://www.exchangerates.org.uk/USD-EUR-spot-exchange-rates-history-2019.html#:~:text=Average%20exchange%20rate%20in%202019%3A%200.8931%20EUR (accessed on April 12, 2022)
35. Gloede TD, Halbach SM, Thrift AG, Dewey HM, Pfaff H, Cadilhac DA. Long-term costs of stroke using 10-year longitudinal data from the North East Melbourne Stroke Incidence Study. Stroke. (2014) 45:3389–94. doi: 10.1161/STROKEAHA.114.006200
36. Navarrete-Navarro P, Hart WM, Lopez-Bastida J, Christensen MC. The societal costs of intracerebral hemorrhage in Spain. Eur J Neurol. (2007) 14:556–62. doi: 10.1111/j.1468-1331.2007.01756.x
37. Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. (2006) 65:547–55. doi: 10.1016/j.surneu.2005.09.032
38. Specogna AV, Patten SB, Turin TC, Hill MD. Cost of spontaneous intracerebral hemorrhage in Canada during 1 decade. Stroke. (2014) 45:284–6. doi: 10.1161/STROKEAHA.113.003276
39. Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on medical costs during the chronic period in patients with spontaneous putaminal hemorrhage: a randomized study. Surg Neurol. (2006) 65:429–35. doi: 10.1016/j.surneu.2005.12.020
40. Sreekrishnan A, Leasure AC, Shi FD, Hwang DY, Schindler JL, Petersen NH, et al. Functional Improvement Among Intracerebral Hemorrhage (ICH) survivors up to 12 months post-injury. Neurocrit care. (2017) 27:326–33. doi: 10.1007/s12028-017-0425-4
41. Mattishent K, Kwok CS, Ashkir L, Pelpola K, Myint PK, Loke YK. Prognostic tools for early mortality in hemorrhagic stroke: systematic review and meta-analysis. J Clin Neurol. (2015) 11:339–48. doi: 10.3988/jcn.2015.11.4.339
42. Welte R, Feenstra T, Jager H, Leidl R. A. decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. (2004) 22:857–76. doi: 10.2165/00019053-200422130-00004
Keywords: intracerebral hemorrhage (ICH), health technology assessment (HTA), cost-effectiveness analysis, neurosurgery, minimally invasive surgery (MIS)
Citation: Schreuder FHBM, Scholte M, Ulehake MJ, Sondag L, Rovers MM, Dammers R, Klijn CJM and Grutters JPC (2022) Identifying the Conditions for Cost-Effective Minimally Invasive Neurosurgery in Spontaneous Supratentorial Intracerebral Hemorrhage. Front. Neurol. 13:830614. doi: 10.3389/fneur.2022.830614
Received: 07 December 2021; Accepted: 19 April 2022;
Published: 02 June 2022.
Edited by:
Andreas Charidimou, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Georgios Tsivgoulis, National and Kapodistrian University of Athens, GreeceCopyright © 2022 Schreuder, Scholte, Ulehake, Sondag, Rovers, Dammers, Klijn and Grutters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Floris H. B. M. Schreuder, ZmxvcmlzLnNjaHJldWRlckByYWRib3VkdW1jLm5s
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.