
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 15 February 2022
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.827544
This article is part of the Research TopicPotential Biomarkers in Neurovascular DisordersView all 50 articles
So far, intense pulsed light (IPL) has been widely used in the treatment of meibomian gland dysfunction (MGD), but there was still a lack of research on its specific mechanism. Determining whether there was a correlation between liposome changes and remission of clinical signs in patients with MGD treated with IPL was of great significance in the clinical evaluation of efficacy in patients with MGD. Our study enrolled the 10 healthy subjects and 26 adult patients, who were diagnosed with MGD and had not received any alternative treatments for at least 3 months. Each patient received a series of three treatments at 3-week intervals. The meibum was collected before the first treatment (T0) and the third treatment (T2). The significant changes in ocular surface parameters before and after IPL treatment were analyzed. The results showed that IPL significantly improved the symptoms of MGD, including ocular surface disease index (OSDI), tear breakup time (TBUT), redness of conjunctival (CR), corneal fluorescein staining (CF), the meibomian gland expressibility (MGE), and meibum quality (all p < 0.05). Lipidomics analysis of the meibum characterized the changes in lipid profiles induced by IPL. A total of 323 lipid species compounds were identified in the spectrum. A total of 41 lipid species were significantly different in patients with MGD (T0) vs. healthy controls. Following IPL treatment (T2), 24 lipid species were significantly different compared with T0: TG (10 lipid species), LPC (6 lipid species), OAHFA (4 lipid species), Cer (2 lipid species), SM (1 lipid species), and PE (1 lipid specie). Among these lipids, 4 of the lipids was a high correlation with TBUT, 5 was TH, 6 was CR, and 11 was meibum quality. In a ward, IPL treatment can achieve the therapeutic effect by changing the alternations of tear film lipids in patients with MGD. The changes in lipid expression profiles are potential indexes to evaluate the therapeutic effectiveness of IPL treatment or other treatments on MGD.
The meibomian gland (MG) is a lipid-rich tissue that is the main source of lipids for the tear film. Meibomian gland dysfunction (MGD) is a chronic, diffuse abnormality of the MGs, commonly characterized by terminal duct obstruction and/or qualitative or quantitative changes in glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease (1). An epidemiological meta-analysis of MGD showed that the overall clinical prevalence rate was 35.8%. The prevalence rate of men was significantly higher than that of women (odds ratio = 1.24) (2). The MG is one of the components of the ocular surface microenvironment and its secretion plays an important role in tear film homeostasis (3). MGD was regarded as the leading cause of evaporative dry eye (DE) worldwide (4), especially related to the evaporative DE which is the most common ocular surface disease worldwide. Long-term MG lesions can cause inflammatory reactions in the ocular surface, which in turn lead to corresponding changes in the cornea and conjunctiva (5). In severe cases, visual acuity declines, resulting in a poor prognosis. At present, there is no uniform diagnostic standard for MGD (6). The widely accepted definition of “MGD” is a chronic diffuse abnormality of the MG, usually characterized by terminal duct obstruction and/or qualitative or quantitative changes in glandular secretions. This can lead to tear film changes, eye irritation, clinical inflammation, and ocular surface diseases. The commonly used diagnostic method is the generic diagnosis and systematic assessments, and its main inclusion indicators include the patient's symptoms, altered gland secretions, and changes in tear film (6, 7). Any of the above signs in combination with ocular symptoms are indicative of MGD. Due to the lack of symptomatic specificity assigned to MGD, it is often misdiagnosed and mistreated. These limitations in arriving at accurate diagnosis are prompting increasing attention by researchers in gaining insight into the pathophysiology underlying MGD.
The meibum, secretions of the MG, spreads onto the tear film to reduce the evaporation of the tear component and protects the eye from dust and microbial agents. The lipids secreted by MGs played a critical role in the homeostasis of the tear film. According to the related studies, the contents of triglycerides, cholesterol, and monounsaturated fatty acids in lipids in patients with MGD changed significantly (8). The meibum consists of an extremely complex mixture of lipids that is mainly composed of wax lipids, cholesterol lipids, free fatty acids, phospholipids, and a small amount of protein. More than 100 components of meibum lipids have been found, and hundreds of subtypes have not been identified thus far (9–11). Accurate evaluation of the changes in lipid expression profile is conducive to the treatment effect or disease progression of patients with MGD.
Numbers of therapeutics have been applied to treat MGD, such as antiinflammatory agents, immunosuppressors, tetracycline, lubricant ointments, artificial lubricants, eyelid hygiene, eyelid warming, massage (12), IPL (13), etc. However, there was not a standardized treatment for MGD. IPL is a noncoherent polychromatic light source with a broad wavelength spectrum of 500–1,200 nm (14, 15). As an established commercial technology, IPL treatment is broadly used in diseases involving facial rosacea. It has been shown that IPL is effective for the treatment of the eyelid sebaceous gland, also termed MG (16–18). Thus, IPL is a promising new therapy for MGD. Currently, IPL treatment is regarded as a more time-efficient and effective method to ameliorate the symptoms of DE (13). Although the efficacy and safety of IPL have been confirmed by ocular surface symptoms (18), the exact therapeutic effect of IPL on the MG remains unclear, especially the lipid metabolism. In this study, a comprehensive evaluation of clinical signs and changes in the lipid composition of meibum were conducted in patients with MGD after at least three exposures to IPL. A comparison of clinical assessment and alteration of the meibum were also conducted in patients treated with IPL and those without MGD. The results of this study would be helpful to understand the mechanisms of IPL in MGD treatment by providing insightful data.
Adult patients, who were diagnosed with MGD and had not received any alternative treatments for at least 3 months, were enrolled in the study. The inclusion criteria were as follows: diagnosis of MGD according to the Japanese MGD diagnostic criteria (9), including ocular symptoms, plugged gland orifices, vascularity of lid margins, irregularity of lid margins, and decreased meibum quality and quantity. The exclusion criteria were as follows: previous ocular surgery or trauma, blepharal dysraphism, history of blepharal and periorbital skin disease within 1 month prior to enrollment, acute inflammation, rheumatic immune systemic diseases, excessive sun exposure within 1 month prior to enrollment, history of herpes zoster infection, pregnancy, and use of photosensitive drugs or foods.
Informed consent was provided by all patients following an explanation of the nature and possible consequences of the study. This study was approved by the Institutional Review Board of the Xin Hua Hospital of Shanghai Jiao Tong University School of Medicine (Shanghai, China) and was registered with the Chinese Clinical Trial Registry prior to enrollment of the first patient. This study adhered to the tenets of the Declaration of Helsinki. All examiners were blinded to the treatment group. The study was enlisted in the clinical trial registry (trial registration no.: ChiCTR2000033454).
Each patient received a series of three treatments at 3-week intervals. The patients had to use proparacaine hydrochloride eye drops (Benoxil, Santen Pharmaceutical Co., Ltd. Japan) at least 2 days prior to each treatment (19). A modular laser multiapplication platform (Quantum™, Lumenis, USA) was used to administer treatment to the periorbital area. During the IPL procedure, patients were required to wear opaque goggles, and the coolant was applied around the eyelids under both eyes. Makeup and contact lenses were removed before treatment. Depending on the patient tolerance, each subject in the patient group received 2–3 light pulses. The intensity of IPL treatment (14–16 J/cm2) depends on the Fitzpatrick skin with a 590-nm filter (19).
Visual acuity and intraocular pressure were recorded in the case-report form. The standard patient evaluation of eye dryness questionnaire was completed by each patient. Schirmer I test, corneal staining, meibum quality, and meibomian gland expressibility (MGE), lid margin abnormality, redness of conjunctival (CR), MG opening position, and corneal fluorescein staining (CF) were measured through slit-lamp microscopy. A noncontact infrared meibography system was used to assess tear breakup time (TBUT), MG dropout, and tear meniscus height (TH).
The use of makeup, artificial tears, and cleaning the rim more than 3 h prior to the collection of the material was prohibited. After exposure to IPL, proparacaine hydrochloride eye drops were used again and the material was collected. The use of plastic products during sample preparation and experimentation was avoided to prevent contamination of the rouge. The steps performed for the collection of the materials were as follows: (1) the MG pressing method was used to squeeze out the secretions of the MGs with an MG expressor forceps – this is achieved by placing the thumb of one hand under the lower eyelid, squeezing the rim and rapidly pulling down, and squeezing out the secretion of the MG (the same method was used to remove the rouge from the upper eyelid); (2) a medical cotton swab was used to scrape the MG secretions, and the cotton swab was placed it in a cryotube and stored at −80°C until further analysis. The amount of rouge collected in this study was 1.77 ± 0.29 mg.
Liquid chromatography separation using CSH C18 column (1.7 μm, 2.1 × 100 mm, waters) was selected to perform reverse-phase chromatography. The lipid of meibum was redissolved in 200 μL 90% isopropanol or acetonitrile then centrifuged at 14,000 x g for 15 min, and finally, 3 μL of the sample was selected for injection. Solvent A: acetonitrile–water (6:4, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate. Solvent B: acetonitrile–isopropanol (1:9, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate. The initial mobile phase was 30% solvent B at a flow rate of 300 μL/min. It was held for 2 min and then linearly increased up to 100% solvent B in 23 min, followed by equilibrating at 5% solvent B for 10 min. Mass spectra were acquired by Q-Exactive Plus in positive and negative modes, respectively. ESI parameters were optimized and preset for all measurements as follows: source temperature, 300°C; capillary temperature, 350°C, the ion spray voltage was set at 3,000 V, S-Lens RF Level was set at 50%, and the scan range of the instruments was set at m/z 200–1800.
“Lipid Search” is a search engine for the identification of lipid species based on MS/MS math. Lipid Search contains more than 30 lipid classes and more than 1,500,000 fragment ions in the database. Both mass tolerance for precursor and fragment were set to 5 ppm.
Data were analyzed using the SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and R (version 4.0.2; package: corrplot version 0.90, psych version 2.1.9) software. Continuous intergroup variables were analyzed using an independent t-test, and pretreatment and continuous intragroup variables were analyzed with a paired t-test. Categorical intergroup variables were analyzed with the nonparametric Kruskal–Wallis test, and intragroup analysis of categorical variables was performed using the nonparametric Wilcoxon signed-rank test. Correlations between normally distributed values and nonnormally distributed values were analyzed with the linear Pearson's correlation coefficient and the Spearman's correlation coefficient, respectively. p < 0.05 denoted statistical significance.
A total of 26 patients' right eyes were enrolled in the study. We collected the meibum before the first treatment (T0) and the third treatment (T2). All enrolled patients completed the whole examination and treatment; there was no pain or any discomfort reported during the tests. All of the patients with MGD were asked to do the lipid analysis using LC-MS/MS. In addition, we also enrolled 10 volunteers who had no MGD diseases (the work plan is illustrated as follows) (Figure 1).
Following treatment with IPL, the ocular surface disease index (OSDI) was markedly decreased from 43.10 ± 15.55 to 24.78 ± 12.85 (p < 0.01). At the same time, the TBUT (s) increased following treatment from 3.19 ± 1.13 to 5.62 ± 1.81 (p < 0.001). The CF of corneal epithelium integration and the CR inflammation were markedly improved. The CF score decreased from 1.00 ± 0.94 to 0.23 ± 0.43 (p < 0.001), and the CR scores reduced from 1.38 ± 0.62 to 0.58 ± 0.44 (p < 0.001), respectively (Table 1).
Our findings suggest that there was no statistically significant difference after the IPL treatment in terms of orifice abnormality and MG dropout (from 1.04 ± 0.99 to 0.92 ± 1.01, p = 0.679 and from 1.54 ± 0.86 to 1.54 ± 0.86, p = 1, respectively) (Table 1).
The scores of MGE and meibum quality were used to evaluate the quality and expressibility of the meibum. The MGE score and meibum quality score were significantly decreased (from 1.31 ± 0.93 to 0.79 ± 0.53, p = 0.017 and from 1.40 ± 0.85 to 0.73 ± 0.51, p = 0.001, respectively) (Table 1).
A total of 323 lipid species were identified and most of them were rich in triglycerides (TG), ceramide (Cer), phosphatidylcholine (PC), sphingomyelin (SM), simple Glc series G1 (CerG1), sphingosine (SO), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), monogalactosyldiacylglycerol (MGDG), phosphatidylserine (PS), digalactosyldiacylglycerol (DGDG), and (O-acyl)-1-hydroxy fatty acid (OAHFA). More details can be seen in Figure 2A. The results of the principal component analysis (PCA) of the groups showed that there was an obvious distinguishing trend between T0 and control groups in lipid clusters (Figure 2B). When we compared the T2 to T0 and T0 to control, there were significant differences observed between the groups in lipid quality, as shown by the volcano plot (Figures 2C,D). Moreover, when we compared the T2 to T0, most of the different lipids were decreased in the former; when we compared the T0 to the control group, most of the different lipids were increased in T0.
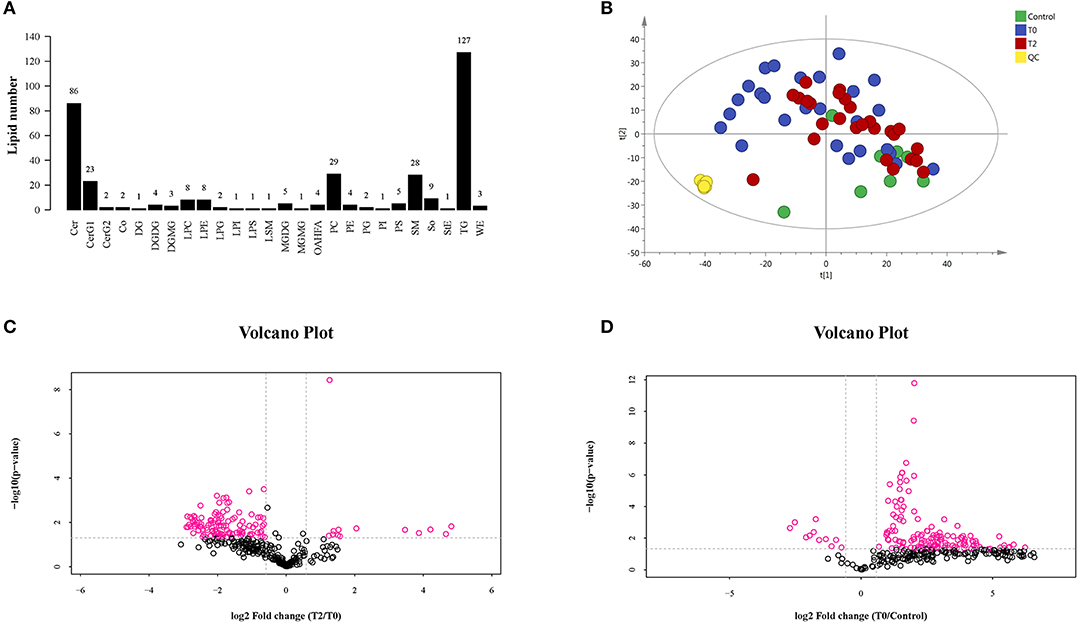
Figure 2. The LC-MS/MS analysis of human meibum in normal control (n = 10), before the first treatment of IPL (T0, n = 26) and the third treatment (T2, n = 26). (A) The identification of the lipid species. (B) PCA diagram among T0 group, T2 group, control group, and QC group. There was a significant trend distinction between T0 and control groups. (C,D), respectively). The volcano plot of the lipids' quality of T2 vs. T0 (C) and T0 vs. control (D).
At the individual lipid compound level, the increased lipids in T0 (i.e., CerG1, CerG2, Co, DG, LPC, LPE, LPG, LPI, LPS, OAHFA, StE, and TG) were decreased following IPL treatment (T2) (Figures 3A1–A12). There were no significant differences in Cer, DGDG, LSM, MGDG, PG, PI, PS, SM, SO, and WE that increased in T0 and T2 (Figures 3B1–B10). The decrease in DGMG and MGMG in T0 could be reversed by IPL treatment (T2) (Figures 3C1,C2). Furthermore, the levels of PC and PE in T2 were higher than those measured in T0 (Figures 3D1,D2).
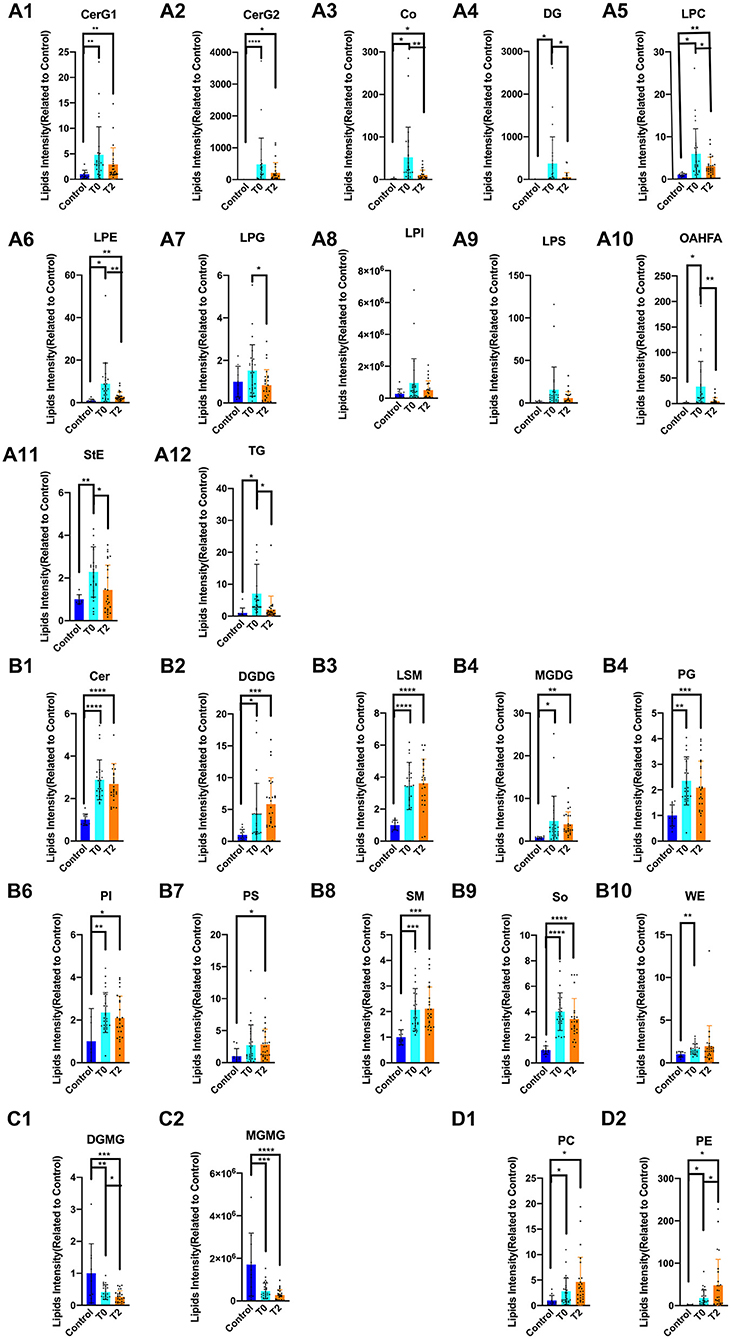
Figure 3. The alternation of lipid compound involved in IPL treatment. * p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001. Control (n = 10), T0 (n = 26), T2 (n = 26). (A1–A12) The increased lipids in T0 of CerG1, CerG2, Co, DG, LPC, LPE, LPG, LPI, LPS, OAHFA, StE, and TG were decreased after treatment of IPL(T2). (B1–B10) There was no significant difference in Cer, DGDG, LSM, MGDG, PG, PI, PS, and SM, So, WE that increased in T0 and T2. (C1–C2) The decreased lipids in T0 of DGMG and MGMG could be reversed by treatment of IPL(T2). (D1–D2) Even more, the PC and PE were higher than T0 in T2.
A total of 41 lipid species were significantly different in patients with MGD (T0) vs. healthy controls (Figure 4A). The difference between patients with MGD and controls included TG, SM, Cer, OAHFA, PC, DGMG, SO, PS, LPE, LSM, CerG1, CO, MGMG, and WE (Table 2). Following IPL treatment (T2), 24 lipid species were significantly different compared with T0: TG, LPC, OAHFA, Cer, SM, and PE (Table 3) (Figure 4B). The change in the lipid metabolic pattern was highly related to the improvement in the clinical index (Figures 5, 6; Table 5). The correlation was rich in TBUT, CR, and meibum quality, and some lipids were crossrelated to them. Notably, 4 lipid clusters were significantly increased in T0, whereas they were decreased following IPL treatment. The different lipids were OAHFA, LPC, TG, and Cer. More details can be seen in Table 4.
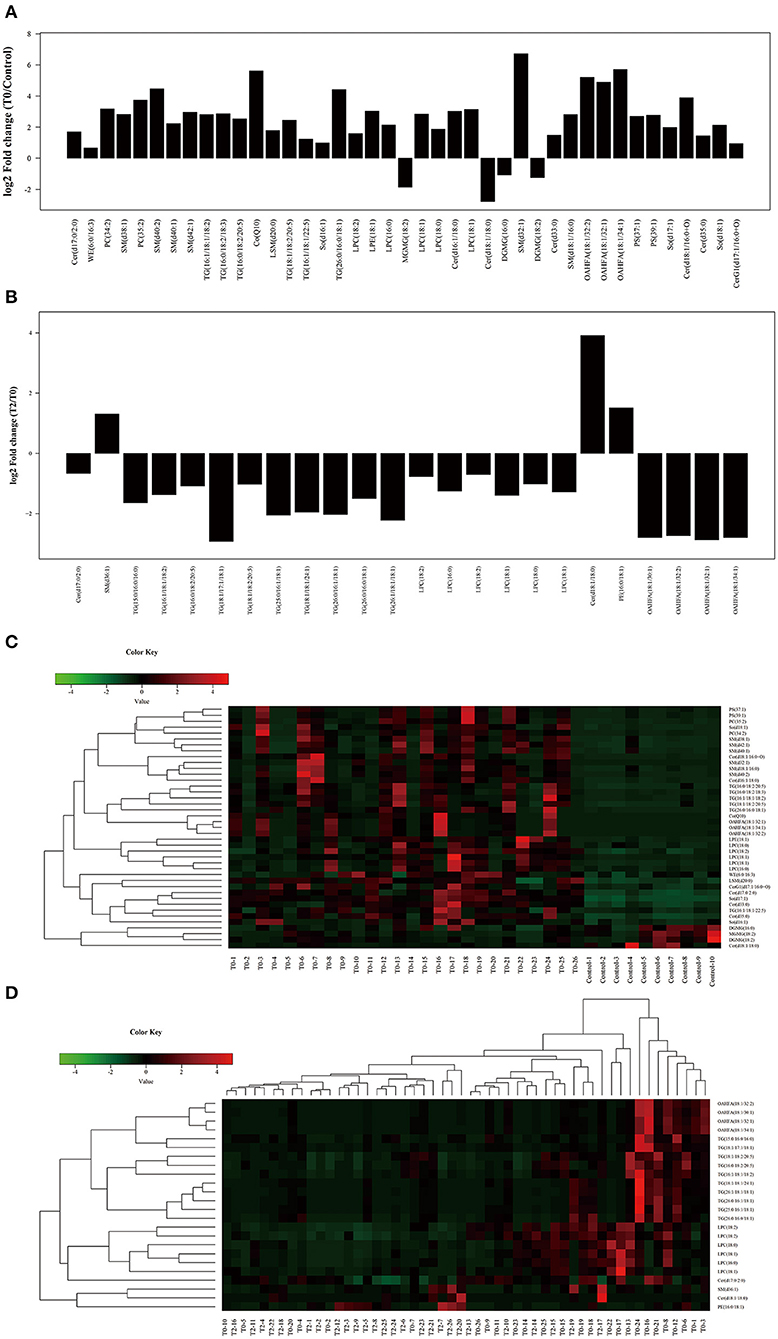
Figure 4. Significant different subclasses of lipids involved in IPL treatment. The fold change (A) and heatmap (C) of T0 vs. C; the fold change (B) and heatmap (D) of T2 vs. T0. Control (n = 10), T0 (n = 26), T2 (n = 26).
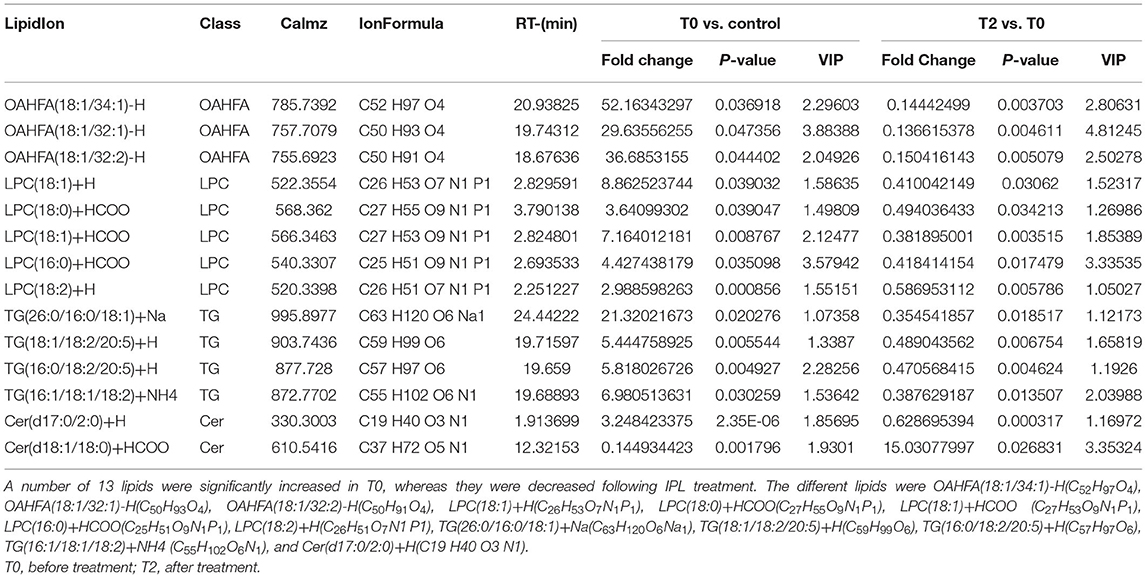
Table 4. Significant different subclasses of lipids of T0 (n = 26) verse control (n = 10) and T2 (n = 26) verse T0 (n = 26).
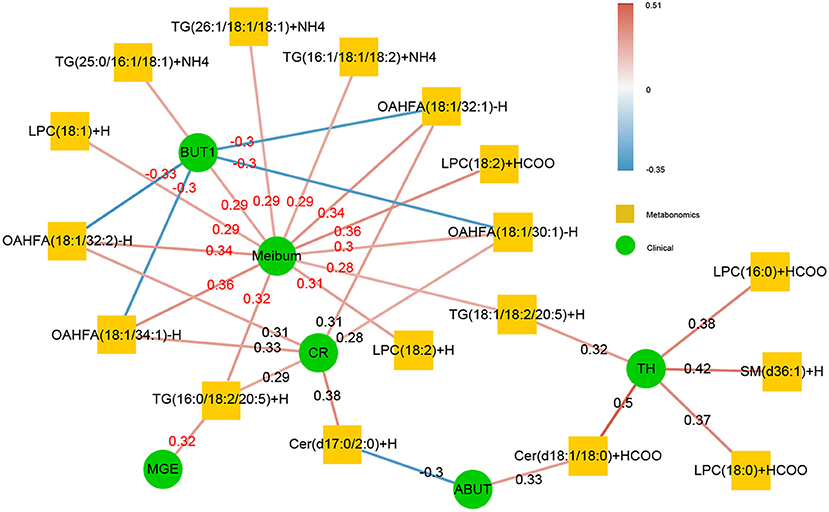
Figure 5. The correlation of significant subclasses of lipids with the clinical syndrome. The change in the lipid metabolic was highly related to the improvement in the clinical index. The correlation was rich in TBUT, CR, and meibum quality, and some lipids were crossrelated to them. The green in the middle represents the clinical index data, and the orange in the outer circle is the differential metabolic lipid. The darker the color of the line was, the stronger the correlation was, and the number on the line was the r-value of its correlation coefficient. The red line indicated that there was a positive correlation between the two, whereas the blue line represented a negative correlation between the two.
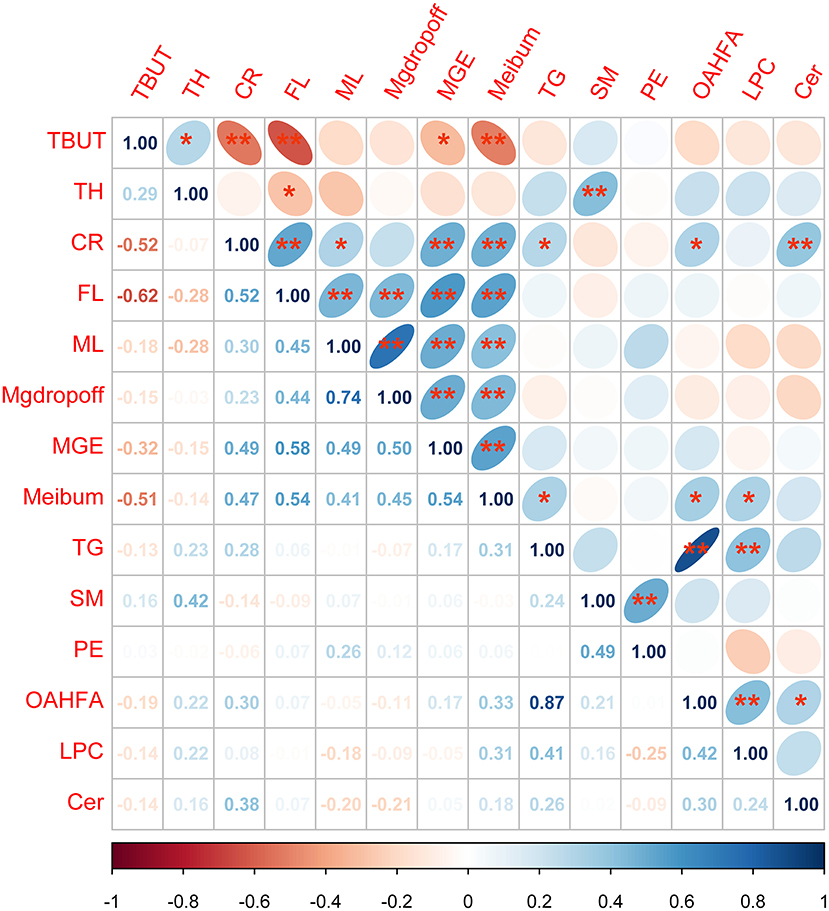
Figure 6. Analysis of the correlation between tear film lipid clusters and ophthalmic clinical indexes. *p < 0.05; **p < 0.01 Spearman's correlation analysis between all lipid cluster components and ophthalmic clinical indicators, the lower-left corner number is the r-value of correlation, the darker the color, the stronger the correlation. The ellipse in the upper right corner facing to the left is a positive correlation while facing to the right is a negative correlation. Blue represented a positive correlation between the two indicators, whereas red represented a negative correlation between the two indicators.
The results of correlation analysis between tear film lipid cluster components and ophthalmic clinical indexes showed that there was a significant correlation between SM and TH; TG and meibum; OAHFA and meibum, CR; LPC and meibum; and Cer and CR (all p < 0.05). Details can be found in Figure 6.
The finding of this study confirmed the therapeutic effect of IPL on MGD by symptoms and lipidomics analysis of the meibum. IPL treatment provides a novel approach that improves the management of MG diseases in a clinical setting, including improvement in the ocular surface health resulting from increasing tear production, stabilizing the tear film, improving corneal epithelial cell contacts, decreasing the conjunctival inflammation, and improving the quality and expressibility of the meibum from the MGs. Additionally, the lipids, such as LPC, OAHFA, TG, Cer, etc., were revealed alteration in patients with MGD after exposure to intense pulsed light (IPL) analyzed by lipidomics profiles. What is more, some of the lipids were highly related to the symptoms of MGD. These findings could be a valuable method to diagnose, prevent, and design personalized medical methods for MGD.
Intense pulsed light is a popular procedure for treating skin and ocular diseases. The mechanism of action of IPL systems is based on the principle of selective thermolysis, according to which certain targets, termed chromophores, are capable of absorbing and subsequently converting through nonradiative transfer the impinging light into heat energy. IPL treatment has been utilized for years in dermatological clinical practice (20, 21). Recently, this procedure was also applied in ophthalmology to treat MGD (22–24). Its use for this purpose stemmed from declines in DE symptoms noted in patients who underwent IPL treatment for facial rosacea. Previous research studies confirmed the efficacy of IPL treatment in relieving the symptoms and signs of MGD (23, 24). In our study, the patient's IPL treatment resulted in marked increases in the TBUT values. As previously reported, these rises occurred as a result of improved tear film stability and a reduction in the tear evaporation rate. These effects are in accord with our measurement of a significant increase in tear production. Furthermore, we found that the ocular surface health improved based on declines in bulbar redness and CF. All supported that IPL was performed as an effective treatment method for MGD.
It is understood that there is still a lack of specific reference data for evaluating the specific efficacy of IPL in the treatment of MGD. Through our study, we can quantitatively analyze the changes in lipid composition of the MG in patients with MGD, to infer its curative effect. We hypothesized that we could find the potential utility of novel biomarkers for predictive, preventive, and personalized medicine strategy for MGD through analysis of the alternation of the meibum lipidomics profile induced by IPL. The dysfunction of the MG was the initial factor of evaporative DE (9). Previous studies have demonstrated abnormal MGs, MG dropout, and abnormal keratosis in apolipoprotein E knockout mice. The corresponding microenvironmental cytokines all significantly changed. Studies showed that the increased expression of inflammatory factors such as IL-6 and TNF- α in acinar cells, the activation of NF- κ B signal pathway, and the enhancement of oxidative stress further simulated the histopathological, functional, and inflammatory changes in MGD (25). In addition, OAHFAs and their derivatives contribute to the formation of lipid polar gradients and participate in the connection between the tear lipid layer and the water layer to maintain tear film stability (26). LPC played an important role in maintaining the stability of tear film because of its chemical structure with both hydrophilic and lipophilic groups. These enzyme activities affected the regeneration of oxide membrane phospholipids and the maintenance of intracellular redox balance (27). Based on the effects of IPL treatment on the principal component analysis of patients with MGD, this procedure reversed several changes in the lipidomics profile toward patterns characteristic of those seen in healthy subjects. The moderate declines induced by IPL treatment in the MGD symptomology were associated with reversal of the hyperlipidemia in 12 of the 27 classes of lipids. It is possible that the restoration of the normal healthy profiles in this group of lipids contributed to the suppression of MGD symptomology (13). Of note, IPL treatment did not alter any of the other 15 classes of lipids changed by MGD. Furthermore, two sub classes (PC and PE) were higher than those in the untreated MGD group. Collectively, these results indicate that IPL treatment cannot completely reverse MGD symptomology, which is in accord with the failure of this procedure to fully reverse all of the changes in the lipidomics profile induced by MGD. Despite the reversal of the changes in an appreciable number of lipid classes by IPL treatment, there was an appreciable number of insensitive lipid classes that could underlie the failure of the treatment to fully reverse all of the MGD symptomologies. Nevertheless, we found that the increases in 13 subclasses of lipids in MGD could be reversed by IPL. They were rich in OAHFA, LPC, TG, and Cer. These lipids may be the suitable markers for monitoring responses to IPL treatment. TBUT, CR, and meibum quality are three clinical indices that were vindicated as being relevant markers to assess IPL treatment efficacy. In a word, the lipidomics profile can be regarded as a promised method to evaluate the effectiveness of treatment of MGD. What is more, the changes in OAHFA, LPC, TG, and Cer represented that the IPL can significantly change the composition of lipidomics profile in subjects, thus significantly improving the clinical symptoms of patients with MGD.
Except for the omega-3, one of OAHFAs, dietary supplementation, most of the treatment strategies for MGD were symptomatic treatment. However, it was controversial about omega-3 dietary supplementation (28, 29). What is more, OAHFAs were detected decreased in MGD in our results and other researchers reported (30). Lam et al. reported that OAHFA level was positively correlated with TBUT, reduction in eye evaporation rate, and degree of eye discomfort; LPC also had a similar trend and this is consistent with our study (31). The results of thin-layer chromatography analysis showed that the content of phosphatidylinositol in tears of patients with MGD decreased significantly, but increased significantly after IPL treatment, which coincided with our results, and our study further confirmed that there was a significant positive correlation between LPC and meibum, so effectively increasing the content of LPC lipid components in tear film can improve their ocular symptoms (32). Our research shows that there is a strong correlation between OAHFA, LPC, and ML, FL, ABUT and other clinical indicators. Emerging treatment options for MGD should be developed. Based on our results, from the changes in lipidomics profile, the decrease taken of OAHFAs, TGs and LPCs may be a benefit for treating MGD.
However, this study has potential limitations. First of all, the sample size of this study was small, and we would further expand the scale of the study to verify the reliability of our conclusions. Moreover, adolescents and children were not included in the study group. Further studies should expand the sample size to obtain data for all ages. In addition, we will do further basic research on how the changes in lipid composition before and after IPL treatment affect the pathological mechanism of MGD and observe how IPL regulates the signal pathway of lipid composition changes.
In this study, we found that IPL treatment could reverse some of the MGD-induced hyperlipidemia. These changes alleviated some of the MGD symptomologies by stabilizing the tear film and improving the ocular surface health. Therefore, IPL can be used as an effective regimen for the treatment of MGD. The pertinent lipid changes include LPC, OAHFA, TG, Cer, etc. in patients with MGD can be used as the biomarkers for taking personalized medical methods for MGD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Xinhua Hospital Affiliated to Medical College of Shanghai Jiao Tong University. The patients/participants provided their written informed consent to participate in this study.
HZ and S-NW carried out conceptualization. YS and L-YT involved in methodology. HZ, DX, and ZC provided resources. S-NW and JP performed visualization. HZ supervised the study. HZ contributed in funding acquisition. All authors have read and agreed to the published version of the manuscript.
This study was supported in part by the Medicine & Engineering Collaboration Research Fund of Shanghai Jiao Tong University (ZH2018QNB27). The funders have no role in the study design, data collection and analysis, decision on publishing, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
IPL, intense pulsed light; MGD, meibomian gland dysfunction; T0, before treatment; T2, after treatment; TBUT, tear breakup time; MGE, meibomian gland expressibility; DE, dry eye; UHPLC-MS/MS, ultrahigh pressure liquid chromatography liquid chromatography-tandem mass spectrometry; CR, redness of conjunctival; CF, fluorescein staining; TG, triglyceride; Cer, ceramide; PC, phosphatidylcholine; SM, sphingomyelin; CerG1, simple Glc series G1; SO, sphingosine; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; MGDG, monogalactosyldiacylglycerol; PS, phosphatidylserine; DGDG, digalactosyldiacylglycerol; OAHFA, (O-acyl)-1-hydroxy fatty acid; PE, phosphatidylethanolamine; DGMG, digalactosylmonoacylglycerol; WE, wax ester; CerG2, simple Glc series G2; CO, coenzyme; LPG, lysophosphatidylglycerol; PG, phosphatidylglycerol; DG, diglyceride; LPI, lysophosphatidylinositol; LPS, lysophosphatidylserine; LSM, lysosphingomyelin; MGMG, monogalactosylmonoacylglycerol; PI, phosphatidylinositol; StE, stigmasterol ester; PCA, principal component analysis.
1. Nelson JD, Shimazaki J., Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. (2011) 52:1930–7. doi: 10.1167/iovs.10-6997b
2. Hassanzadeh S, Varmaghani M, Zarei-Ghanavati S, Heravian Shandiz J, Azimi Khorasani A. Global prevalence of meibomian gland dysfunction: a systematic review and meta-analysis. Ocul Immunol Inflamm. (2021) 29:66–75. doi: 10.1080/09273948.2020.1755441
3. Zhang X, M VJ, Qu Y, He X, Ou S, Bu J, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. (2017) 18:1398. doi: 10.3390/ijms18071398
4. Hagan S, Martin E. Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. (2016) 7:15. doi: 10.1186/s13167-016-0065-3
5. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. (2003) 1:107–26. doi: 10.1016/S1542-0124(12)70139-8
6. Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. (2011) 52:2006–49. doi: 10.1167/iovs.10-6997f
7. Ngo W, Gann D, Nichols JJ. Impact of the 2011 International Workshop on Meibomian Gland Dysfunction on clinical trial attributes for meibomian gland dysfunction. Ocul Surf. (2020) 18:27–30. doi: 10.1016/j.jtos.2019.10.003
8. Osae E A, Steven P, Redfern R, Hanlon S, Smith CW, Rumbaut RE, et al. Dyslipidemia and meibomian gland dysfunction: utility of lipidomics and experimental prospects with a diet-induced obesity mouse model. Int J Mol Sci. (2019) 20:3505. doi: 10.3390/ijms20143505
9. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. (2004) 2:149–65. doi: 10.1016/S1542-0124(12)70150-7
10. Butovich IA. The meibomian puzzle: combining pieces together. Prog Retin Eye Res. (2009) 28:483–98. doi: 10.1016/j.preteyeres.2009.07.002
11. Wojtowicz JC, Butovich IA, McCulley JP. Historical brief on composition of human meibum lipids. Ocul Surf. (2009) 7:145–53. doi: 10.1016/S1542-0124(12)70309-9
12. Nichols KK, Redfern RL, Jacob JT, Nelson JD, Fonn D, Forstot SL, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. (2013) 54:Tfos14-9. doi: 10.1167/iovs.13-13074
13. Rong B, Tang Y, Tu P, Liu R, Qiao J, Song W, et al. Intense pulsed light applied directly on eyelids combined with meibomian gland expression to treat meibomian gland dysfunction. Photomed Laser Surg. (2018) 36:326–32. doi: 10.1089/pho.2017.4402
14. Gupta PK, Vora GK, Matossian C, Kim M, Stinnett S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Can J Ophthalmol. (2016) 51:249–53. doi: 10.1016/j.jcjo.2016.01.005
15. Vora GK, Gupta PK. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr Opin Ophthalmol. (2015) 26:314–8. doi: 10.1097/ICU.0000000000000166
16. Yurttaser Ocak S, Karakus S, Ocak O B, Cakir A, Bolukbasi S, Erden B, et al. Intense pulse light therapy treatment for refractory dry eye disease due to meibomian gland dysfunction. Int Ophthalmol. (2020) 40:1135–41. doi: 10.1007/s10792-019-01278-3
17. Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg. (2015) 33:41–6. doi: 10.1089/pho.2014.3819
18. Tashbayev B, Yazdani M, Arita R, Fineide F, Utheim TP. Intense pulsed light treatment in meibomian gland dysfunction: a concise review. Ocul Surf. (2020) 18:583–94. doi: 10.1016/j.jtos.2020.06.002
19. Liu R, Rong B, Tu P, Tang Y, Song W, Toyos R, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. (2017) 183:81–90. doi: 10.1016/j.ajo.2017.08.021
20. Paasch U, Schwandt A, Seeber N, Kautz G, Grunewald S, Haedersdal M. New lasers and light sources - old and new risks? J Dtsch Dermatol Ges. (2017) 15:487–96. doi: 10.1111/ddg.13238
21. Yi J, Hong T, Zeng H, Li P, Li P, Wang S, et al. A meta-analysis-based assessment of intense pulsed light for treatment of melasma. Aesthetic Plast Surg. (2020) 44:947–52. doi: 10.1007/s00266-020-01637-x
22. Wladis EJ, Aakalu VK, Foster JA, Freitag SK, Sobel RK, Tao JP, et al. Intense Pulsed Light for Meibomian Gland Disease: a report by the american academy of ophthalmology. Ophthalmology. (2020) 127:1227–33. doi: 10.1016/j.ophtha.2020.03.009
23. Craig JP, Chen YH, Turnbull PR. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. (2015) 56:1965–70. doi: 10.1167/iovs.14-15764
24. Xue AL, Wang MTM, Ormonde SE, Craig JP. Randomised double-masked placebo-controlled trial of the cumulative treatment efficacy profile of intense pulsed light therapy for meibomian gland dysfunction. Ocul Surf. (2020) 18:286–97. doi: 10.1016/j.jtos.2020.01.003
25. Bu J, Wu Y, Cai X, Jiang N, Jeyalatha MV, Yu J, et al. Hyperlipidemia induces meibomian gland dysfunction. Ocul Surf. (2019) 17:777–86. doi: 10.1016/j.jtos.2019.06.002
26. Miyamoto M, Sassa T, Sawai M, Kihara A. Lipid polarity gradient formed by ω-hydroxy lipids in tear film prevents dry eye disease. Elife. (2020) 9:e53582. doi: 10.7554/eLife.53582.sa2
27. Wahlig S, Lovatt M, Mehta JS. Functional role of peroxiredoxin 6 in the eye. Free Radic Biol Med. (2018) 126:210–20. doi: 10.1016/j.freeradbiomed.2018.08.017
28. Al-Namaeh M. A systematic review of the effect of omega-3 supplements on meibomian gland dysfunction. Ther Adv Ophthalmol. (2020) 12:2515841420952188. doi: 10.1177/2515841420952188
29. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380:11–22. doi: 10.1056/NEJMoa1812792
30. Khanal S, Ngo W, Nichols K K, Wilson L, Barnes S, Nichols J J. Human meibum and tear film derived (O-acyl)-omega-hydroxy fatty acids in meibomian gland dysfunction. Ocul Surf. (2021) 21:118–28. doi: 10.1016/j.jtos.2021.05.009
31. Lam SM, Tong L, Duan X, Acharya UR, Tan JH, Petznick A, et al. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. J Lipid Res. (2014) 55:1959–69. doi: 10.1194/jlr.P051185
Keywords: intense pulsed light, dry eye, disease markers, lipidomics, meibomian gland dysfunction
Citation: Zhao H, Wu S-N, Shao Y, Xiao D, Tang L-Y, Cheng Z and Peng J (2022) Lipidomics Profiles Revealed Alterations in Patients With Meibomian Gland Dysfunction After Exposure to Intense Pulsed Light. Front. Neurol. 13:827544. doi: 10.3389/fneur.2022.827544
Received: 02 December 2021; Accepted: 04 January 2022;
Published: 15 February 2022.
Edited by:
Yuzhen Xu, Tongji University, ChinaReviewed by:
Yi Zhou, Amgen, United StatesCopyright © 2022 Zhao, Wu, Shao, Xiao, Tang, Cheng and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhao, MjA4ODc3ODdAcXEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.