
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 March 2022
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.823611
 Anran Wang1
Anran Wang1 Yapeng Li1
Yapeng Li1 Lue Zhou1
Lue Zhou1 Kai Liu1
Kai Liu1 Shaohua Li1
Shaohua Li1 Bo Song1
Bo Song1 Yuan Gao1
Yuan Gao1 Yusheng Li1
Yusheng Li1 Jie Lu2
Jie Lu2 Chuansheng Tian3
Chuansheng Tian3 Yuming Xu1*
Yuming Xu1* Longde Wang1,4*
Longde Wang1,4*Background: Carotid plaque plays an important role in the development of stroke. The triglyceride-glucose (TyG) index is a reliable alternative marker of insulin resistance. However, there are limited data regarding the relationship between TyG index and carotid plaque and its stability in nondiabetic adults.
Methods: This study was carried out on 24,895 urban workers (10,978 men and 13,917 women) aged 20 years or older who participated in a comprehensive health screening between January 2016 and December 2017 at the First Affiliated Hospital of Zhengzhou University, China. Carotid plaque was assessed using ultrasonography. TyG index was calculated as ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL) /2]. Logistic regression models and restricted cubic spline (RCS) models were used to estimate the association of the TyG index with carotid plaque and its stability by odds ratios (ORs) and 95% confidence intervals (CIs).
Results: Carotid plaque was detected in 5,668 (22.8%) respondents, with stable and unstable plaque accounting for 2,511 (10.1%) and 3,158 (12.7%), respectively. There was a significant positive association between the prevalence of carotid plaque and TyG index quartile levels, and the same associations were observed for the prevalence of stable and unstable carotid plaque (P for trend <0.0001). The multivariable-adjusted ORs (95% CIs) for the highest vs. lowest quartile of TyG index were 1.30 (1.15–1.47) for carotid plaque, 1.38 (1.17–1.63) for stable carotid plaque, and 1.24 (1.07–1.43) for unstable carotid plaque. The RCS analysis showed a linear association between TyG index and carotid plaque, and linear associations were also observed between TyG index and both stable carotid plaque and unstable carotid plaque (P for linearity<0.05).
Conclusion: Our findings suggested that the TyG index was significantly associated with carotid plaque and might be a useful indicator for the early identification of carotid plaque in nondiabetic subjects.
Stroke has become a leading cause of disability and mortality worldwide (1). Carotid plaque plays an important role in the development of stroke. Approximately 18–25% of stroke thromboembolisms arise from carotid plaque (2), and carotid plaque was also reported to be an important cause of cryptogenic stroke (3). Rupture of carotid plaque can lead to thrombosis, resulting in vessel occlusion and downstream tissue infarction. A recent systematic review and meta-analysis of the global prevalence of carotid plaque revealed a huge global burden, with 21.1% of people aged 30 to 79 years in 2020 having carotid plaque, equivalent to 815.76 million people (4).
Many studies have shown that early preventive treatment and risk factor intervention are beneficial (5–7). However, carotid ultrasound screening in the general population is not recommended in the current guidelines (8). Therefore, easily accessible and inexpensive biomarkers of carotid plaque can help to identify high-risk individuals who may benefit from early intervention.
Insulin resistance (IR) has been considered as one of the important risk factors for atherosclerosis (9, 10). Studies have shown that the triglyceride-glucose (TyG) index, calculated as ln [fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2], is a reliable alternative marker of IR compared to the gold standard hyperinsulinemic-glycemic clamp technique (11–13). Several previous studies indicated that the TyG index was associated with atherosclerosis (14, 15), and a growing number of studies have linked the TyG index to cerebro-cardiovascular disease (10, 16–18). However, only a few studies have examined the relationship between TyG index and carotid plaque yielding inconsistent results (15, 19, 20), and most previous studies have focused on whole or diabetic populations, with few studies on nondiabetic populations. Meanwhile, studies on the relationship between TyG index and carotid plaque stability are scarce. Therefore, in the present study, we aim to investigate the relationship of the TyG index with carotid plaque and its stability in nondiabetic adults.
This study consisted of a population of 28,537 urban workers aged 20 years or older who participated in a health check-up in stroke screening sites of the First Affiliated Hospital of Zhengzhou University from January 2016 to December 2017.
A total of 28,522 participants completed questionnaires, physical examinations, and blood tests. We excluded 3,037 participants with a history of diabetes and 590 subjects with a history of any malignancy, acute inflammation, infectious diseases, or renal disease. Finally, a total of 24,895 participants were enrolled in the current study.
Individual information on demographic characteristics, personal medical history (hypertension, dyslipidemia, stroke, and coronary heart disease), and lifestyle factors (smoking, drinking, vegetable consumption, fruit consumption, physical activity, etc.) were obtained by trained interviewers using a standard questionnaire. Current smoking was defined as smoking 1 cigarette per day for more than 1 year. Current drinking was defined as consuming at least ≥45 g alcoholic drink each time per day during the last year. Vegetable consumption was divided into two groups (≥5 days/week, <5 days/week) using a daily consumption standard of 200 g of vegetables. Fruit consumption was divided into two groups (≥5 days/week and <5 days/week) using a standard consumption of 200 g of fruit per day. Physical activity was defined as an exercise for at least 30 min per time in no less than 3 times per week.
Physical examinations including height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were conducted by trained staff. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥28 kg/m2.
In addition, overnight fasting blood samples were drawn from each participant to test fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Fasting blood glucose was measured using the glucose oxidase method. Lipid levels including TC, TG, and HDL-C were measured using Olympus Au5400 automated biochemistry analyzer (First Chemical Co, Ltd, Japan) and commercially available kits. LDL-C was calculated by the Friedewald equation when TG was ≤4.5 mmol/L or was directly measured when TG was >4.5 mmol/L. Hypertension was defined as SBP ≥140 mmHg, DBP≥90 mmHg, self-reported hypertension, or the usage of antihypertensive medications. Dyslipidemia was defined as TG ≥2.26 mmol/L, TC ≥6.22 mmol/L, HDL-C <1.04 mmol/L, LDL-C ≥4.14 mmol/L, self-reported diagnosis of dyslipidemia, or taking lipid-lowering drugs.
Carotid plaques were evaluated by qualified sonographers with uniform training, who were unaware of the baseline characteristics and laboratory findings of the participants. Two qualified sonographers measured each participant separately; discrepancies in measurement data were resolved by consensus. We used the iU22 (Philips Healthcare), HA500 (Hitachi Healthcare), and DC-8 (Mindray) ultrasound systems. The transmission frequency of carotid ultrasound images is 5 to 10 MHz. Examination of the bilateral common carotid artery, internal carotid artery, external carotid artery and bulb for the presence of plaque in the transverse and longitudinal directions.
Carotid plaque was defined as a focal structure encroaching into the arterial lumen by at least 0.5 mm or 50% of the surrounding carotid intima–media thickness (CIMT) value, or CIMT > 1.5 mm. Stable carotid plaques were defined as having a homogeneous texture with a regular smooth morphology and high level or homogeneous echogenicity. In contrast, unstable carotid plaques were defined as having an incomplete fibrous cap or ulceration with low level or heterogeneous echogenicity (21).
The characteristics of the population are presented as frequency (%) for categorical variables and means (standard deviations [SDs]) or median (interquartile ranges [IQRs]) for continuous variables when appropriate. The population was divided into four groups based on the quartiles of the TyG index. Data were compared using chi-square analysis for categorical variables, and analysis of variance (ANOVA) or Mann–Whitney U-tests for continuous variables unless otherwise specified.
Logistic regression models were used to investigate the relationship between the TyG index and carotid plaque. To adjust for potential confounders, three models were developed: Model 1, adjusted for age and sex; Model 2, further adjusted for drinking, smoking, vegetable consumption, sports, BMI, hypertension, dyslipidemia, antihypertensive agents, and lipid-lowering agents; and Model 3, Model 2 plus further adjusted for LDL-C, and HDL-C. When participants were divided into 3 categories (no carotid plaque, stable carotid plaque, and unstable carotid plaque), multinomial logistic regression models (Model 1, adjusted for age and sex; Model 2, further adjusted for drinking, smoking, vegetable consumption, sport, BMI, hypertension, dyslipidemia, antihypertensive agents, and lipid-lowering agents; and Model 3, further adjusted for LDL-C, HDL-C) were conducted to adjust for potential confounders. The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). When the TyG index was treated as a continuous variable, a restricted cubic spline with 3 knots (at the 5th, 50th, and 95th percentiles of the TyG index) was performed to examine the non-linear association between the TyG index and carotid plaque.
Additionally, sensitivity analyses were performed by excluding participants with hypertension, and dyslipidemia.
All analyses above were conducted using R software (version 3.6.3). A two-sided P < 0.05 was considered statistically significant.
Among the 24,895 participants, 10,978 (41.1%) participants were men and 13,917 (58.9%) were women. The median (IQR) age of overall participants was 45 (38–52) years, and the median TyG index was 8.50 (IQR 8.15–8.90). Compared with participants in the lowest quartile, those with higher TyG index were more likely to be older and male; to be current smokers, current drinkers, vegetable consumption, fruit consumption, active physical activity, obese, and to have a higher prevalence of dyslipidemia, hypertension, stroke, coronary heart disease, more likely to take antihypertensive agents and lipid-lowering agents, to have a higher level of SBP, DBP, FBG, TC, TG, LDL -C, and more likely to have a lower level of HDL-C. The characteristics of participants according to quartiles of the TyG index are presented in Table 1.
Carotid plaque was detected in 5,668 (22.8%) respondents, with stable and unstable plaque accounting for 2,511 (10.1%) and 3,158 (12.7%), respectively. There was a significant positive association between the prevalence of carotid plaque and TyG index quartile levels, and the same trend was observed for the prevalence of stable and unstable carotid plaque (P for trend <0.0001; Figure 1).
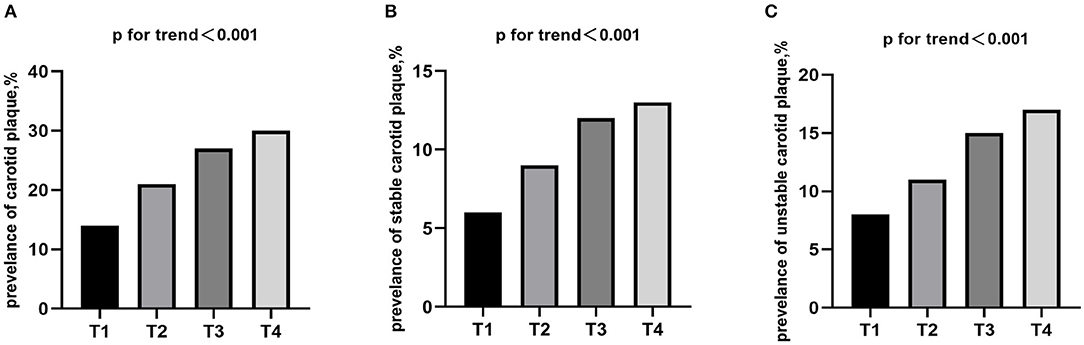
Figure 1. Prevalence of (A) carotid plaque, (B) stable and (C) unstable stratified by quartiles of the TyG index. TyG index, triglyceride-glucose index.
Data were categorized by no carotid plaque, or carotid plaque. The multivariate-adjusted logistic regression models showed that TyG index levels were positively associated with carotid plaque. In model 3, the adjusted ORs (95% CIs) were 1.07 (0.95–1.19) for the second quartile, 1.16 (1.03–1.30) for the third quartile, and 1.30 (1.15–1.47) for the highest quartile compared with the lowest quartile of TyG index. When treated as a continuous variable, every 1 unit increase in the TyG index was associated with a 22% increased prevalent risk of carotid plaque (Table 2). Restricted cubic splines showed a similar trend (Figure 2A).
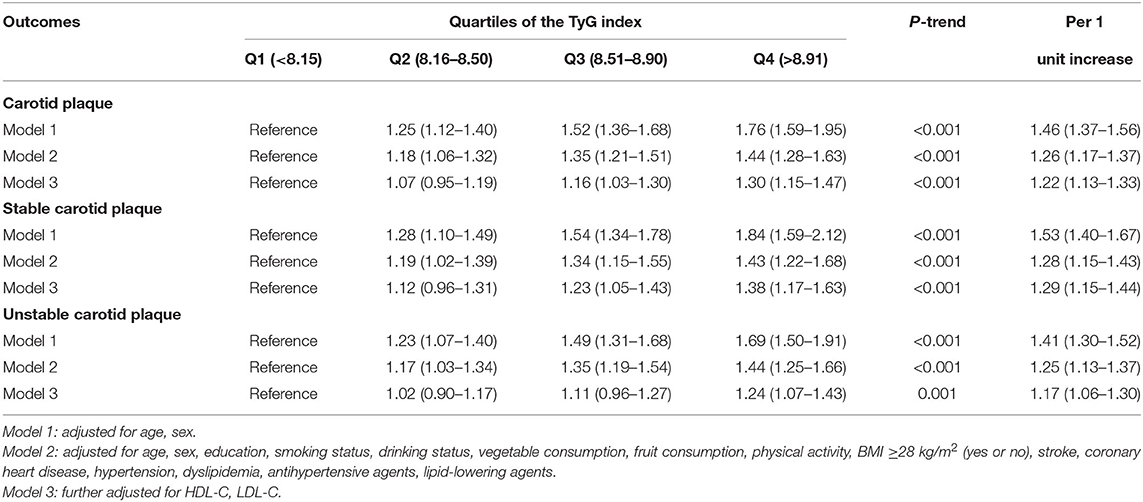
Table 2. Multivariate-adjusted ORs (95% CI) of the association of triglyceride-glucose index with carotid plaque and its stability.
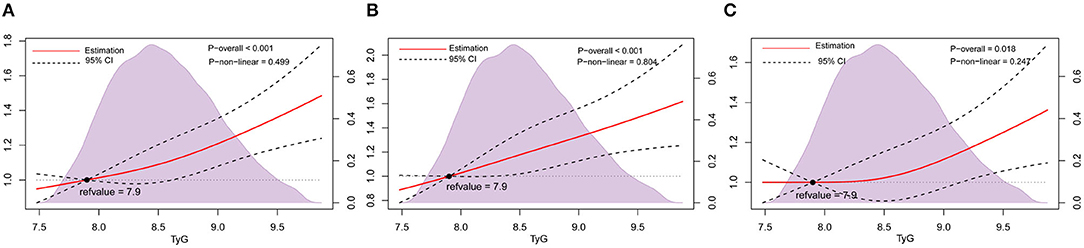
Figure 2. Association of TyG index with carotid plaque and its stability among nondiabetic adults. Odds ratios and 95% CIs derived from restricted cubic spline regression, with knots placed at 5th, 50th, and 95th percentiles of the distribution of TyG index. The reference point for the TyG index is the midpoint (7.9) of the reference group from the categorical analysis. Odds ratios were adjusted for the same variables as Model 3 in Table 2. (A), Carotid plaque. (B) Stable carotid plaque. (C) Unstable carotid plaque. TyG index, triglyceride-glucose index.
Data were further classified by no carotid plaque, stable carotid plaque and unstable carotid plaque. In multinomial logistic regression models, there was a statistically significant difference between stable carotid plaques and no carotid plaque, and the adjusted ORs (95% CIs) in model 3 were 1.12 (0.96–1.31) for the second quartile, 1.23 (1.05–1.43) for the third quartile, and 1.38 (1.17–1.63) for highest quartile compared with the lowest quartile of TyG index. Furthermore, for every 1 unit increase in the TyG index, the prevalence of stable carotid plaque increased by 1.30 times (Table 2). Restricted cubic splines adjusted for multiple variables showed a similar trend (Figure 2B).
In model 3 compared to the lowest quartile, only the highest quartile showed a statisticant difference between unstable carotid plaque and no carotid plaque, and the adjusted ORs (95% CIs) were 1.02 (0.90–1.17) for the second quartile, 1.11 (0.96–1.27) for the third quartile, 1.24 (1.07–1.43) for the highest quartile compared to the lowest quartile of TyG index. When the TyG index was considered a continuous variable, the prevalence of unstable carotid plaque increased by 1.17 times for every 1 unit increase in the TyG index (Table 2). Restricted cubic splines adjusted for multiple variables showed a similar trend (Figure 2C).
Sensitivity analyses were performed to assess the relationship between TyG and carotid plaque by excluding participants using lipid-lowering drugs, antihypertensive medications, dyslipidemia and hypertension. After multivariable adjustment for the risk factors, sensitivity analyses showed similar results (Figure 3).
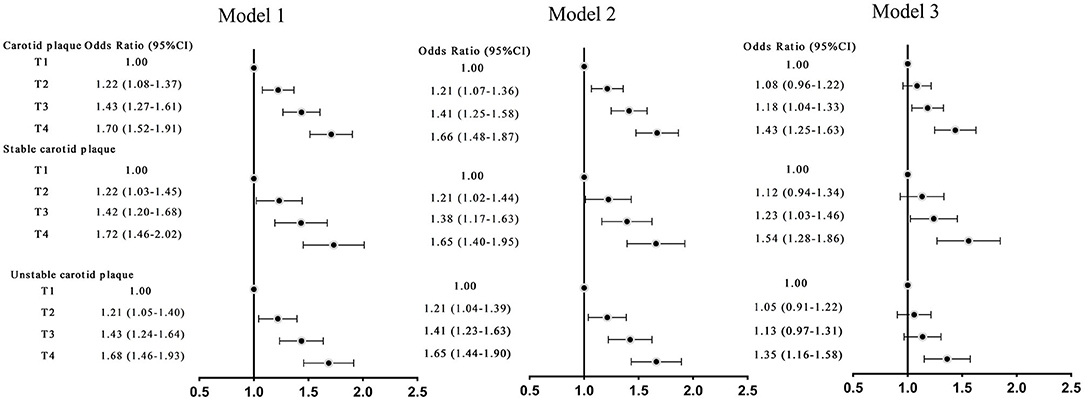
Figure 3. Odds ratios (ORs; 95% CIs) of TyG index and carotid plaque and its stability in adults without hypertension, dyslipidemia. Model 1: adjusted for age, sex. Model 2: adjusted for age, sex, education, smoking status, drinking status, vegetable consumption, fruit consumption, physical activity, BMI ≥28 kg/m2 (yes or no), stroke, coronary heart disease. Model 3: further adjusted for HDL-C, LDL-C.
This population-based study provided evidence on the association of the TyG index with carotid plaque. We found that a higher TyG index was significantly associated with carotid plaque and this relationship was observed in both stable and unstable carotid plaques. In addition, when the TyG index was used as a continuous variable, linear relationships were observed between the TyG index and carotid plaque, independent of other factors.
Plaques in the carotid arteries have been used as an indicator of systemic vascular atherosclerosis (22). The carotid area is easily accessible for non-invasive studies and is relatively accurate. Carotid plaque plays a major role in the occurrence of cerebrocardiovascular disease (23, 24).
Several previous studies indirectly supported our findings that the TyG index was associated with markers of atherosclerosis. For example, a study based on a healthy Korean population showed that TyG index was associated with increased brachial-ankle pulse wave velocity (baPWV), independent of HDL-C, LDL-C, and other risk factors for atherosclerosis (14). Similarly, a cross-sectional analysis of 4,718 Chinese adults with hypertension also found an independent positive association between the TyG index and baPWV (25). In addition, another study of postmenopausal women in Europe showed that the TyG index was associated with baPWV in lean participants (BMI ≤25 kg/m2) (26). Moreover, a recent cohort study found that a higher TyG index was more likely to lead to a higher risk of arterial stiffness (27). Furthermore, a growing number of studies have demonstrated that the TyG index is associated with an increased risk of recurrence, progression and all-cause mortality in patients with cerebrocardiovascular disease (10, 16–18, 28).
However, to date, only a few studies have examined the relationship between the TyG index and carotid plaque and the results remain inconsistent (15, 19, 20). Zhao et al. found that the TyG index was associated with a high risk of atherosclerosis, but not carotid plaque (15). Considering that the participants in the study were over 65 years of age and had a high proportion of lipid-lowering and glucose-lowering medications, the association between TyG index and carotid plaque might be masked. The Asymptomatic Polyvascular Abnormalities Community (APAC) study reported that TyG index was associated with unstable carotid plaque, but not with stable carotid plaque (20). In line with our findings, a cohort study showed that the TyG index increased the risk of carotid atherosclerosis (19). However, HDL-C, LDL-C and other blood markers, well-known risk factors for atherosclerosis, were not adjusted for as confounders in the regression models. Our study extended the current literature and demonstrated that the TyG index was independently associated with carotid plaque and its stability (stable carotid plaque, unstable carotid plaque) in nondiabetic adults.
The mechanism underlying the relationship between TyG and carotid plaque is unclear. The TyG index is a reliable alternative marker for IR, which may play an important role in inferring the relationship between TyG and carotid plaque. Many previous studies have demonstrated that IR plays an important role in the development of atherosclerosis and advanced plaque, by promoting increased synthesis and release of lipoproteins, growth and proliferation of vascular smooth muscle cells, and enhanced transport of LDL-C to arterial smooth muscle cells and inflammation (9, 10, 28–30). In addition, IR is associated with a group of metabolic abnormalities known as insulin resistance syndrome. Each component of insulin resistance syndrome is an independent risk factor for atherosclerosis and plaque formation (9, 31).
Our study has some limitations. First, this was a cross-sectional study and no conclusion can be drawn on the causal relationship between TyG and carotid plaque. Second, the study was carried out in a nondiabetic population, and our findings may not extend to other populations. Considering that most diabetic patients are on long-term glucose-lowering medication or insulin, it may have affected the TyG-carotid plaque relationship. Third, our study was a physical examination population, there were no data to calculate the homeostasis model assessment of insulin resistance (HOMA-IR) to explore the relationship between the HOMA-IR and carotid plaque and its stability. Finally, we did not collect data on plaque size, and the relationship of the TyG index with plaque size should be further studied.
Our findings suggested that the TyG index was significantly associated with carotid plaque and might be a useful indicator for early identification of carotid plaque in nondiabetic subjects.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YX and LW designed the research. YaL, LZ, YG, KL, JL, and SL helped with the acquisition and analysis of the data. AW wrote the article. BS, YG, CT, and YuL contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
This work was supported by a grant from the Ministry of Science and Technology of the People's Republic of China (2018YFC1311303).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the study participants and the clinical staff for their support and contribution to this study.
1. Global Regional Regional and National Burden of Stroke. 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:439–58. doi: 10.1016/s1474-4422(19)30034-1
2. Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. (2019) 18:559–72. doi: 10.1016/S1474-4422(19)30035-3
3. Kopczak A, Schindler A, Bayer-Karpinska A, Koch ML, Sepp D, Zeller J, et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Col Cardiol. (2020) 76:2212–22. doi: 10.1016/j.jacc.2020.09.532
4. Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and Modelling study. Lancet Glob Health. (2020) 8:e721–e9. doi: 10.1016/S2214-109X(20)30117-0
5. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, et al. Editor's Choice - management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the european society for vascular surgery (Esvs). Eur J Vascular Endovasc Surg. (2018) 55:3–81. doi: 10.1016/j.ejvs.2018.03.023
6. Saba L, Brinjikji W, Spence JD, Wintermark M, Castillo M, Borst GJD, et al. Roadmap consensus on carotid artery plaque imaging and impact on therapy strategies and guidelines: an international, multispecialty, expert review and position statement. AJNR Am J Neuroradiol. (2021) 42:1566–75. doi: 10.3174/ajnr.A7223
7. Ibrahimi P, Jashari F, Bajraktari G, Wester P, Henein MY. Ultrasound assessment of carotid plaque echogenicity response to statin therapy: a systematic review and meta-analysis. Int J Mol Sci. (2015) 16:10734–47. doi: 10.3390/ijms160510734
8. Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for asymptomatic carotid artery stenosis: us preventive services task force recommendation statement. JAMA. (2021) 325:476–81. doi: 10.1001/jama.2020.26988
9. Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocrine Revi. (2019) 40:1447–67. doi: 10.1210/er.2018-00141
10. Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated Tyg index predicts progression of coronary artery calcification. Diabetes Care. (2019) 42:1569–73. doi: 10.2337/dc18-1920
11. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
12. Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. Tyg index performs better than homa in a brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. (2011) 93:e98–e100. doi: 10.1016/j.diabres.2011.05.030
13. Dikaiakou E, Vlachopapadopoulou EA, Paschou SA, Athanasouli F, Panagiotopoulos I, Kafetzi M, et al. Triglycerides-glucose (Tyg) index is a sensitive marker of insulin resistance in greek children and adolescents. Endocrine. (2020) 70:58–64. doi: 10.1007/s12020-020-02374-6
14. Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in korean adults. Cardiovasc Diabetol. (2018) 17:41. doi: 10.1186/s12933-018-0692-1
15. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the northern shanghai study. Cardiovasc Diabetol. (2019) 18:95. doi: 10.1186/s12933-019-0898-x
16. da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira  C, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. (2019) 18:89. doi: 10.1186/s12933-019-0893-2
17. Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. (2021) 20:46. doi: 10.1186/s12933-021-01238-1
18. Zhou Y, Pan Y, Yan H, Wang Y, Li Z, Zhao X, et al. Triglyceride glucose index and prognosis of patients with ischemic stroke. Front Neurol. (2020) 11:456. doi: 10.3389/fneur.2020.00456
19. Wu Z, Wang J, Li Z, Han Z, Miao X, Liu X, et al. Triglyceride glucose index and carotid atherosclerosis incidence in the chinese population: a prospective cohort study. Nutr Metab Cardiovasc Dis. (2021) 31:2042–50. doi: 10.1016/j.numecd.2021.03.027
20. Wang A, Tian X, Zuo Y, Zhang X, Wu S, Zhao X. Association between the triglyceride-glucose index and carotid plaque stability in nondiabetic adults. Nutr Metab Cardiovasc Dis. (2021) 31:2921–8. doi: 10.1016/j.numecd.2021.06.019
21. Sztajzel R. Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med Weekly. (2005) 135:635–43.
22. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the american society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr. (2008) 21:93–111; quiz 89–90. doi: 10.1016/j.echo.2007.11.011
23. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. (2014) 7:1025–38. doi: 10.1016/j.jcmg.2013.11.014
24. Kamtchum-Tatuene J, Nomani AZ, Falcione S, Munsterman D, Sykes G, Joy T, et al. Non-stenotic carotid plaques in embolic stroke of unknown source. Front Neurol. (2021) 12:719329. doi: 10.3389/fneur.2021.719329
25. Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type hypertension registry study. Cardiovasc Diabetol. (2020) 19:139. doi: 10.1186/s12933-020-01124-2
26. Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, et al. The Tyg index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circulation. (2018) 27:716–24. doi: 10.1016/j.hlc.2017.05.142
27. Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. (2021) 20:146. doi: 10.1186/s12933-021-01342-2
28. Yamazoe M, Hisamatsu T, Miura K, Kadowaki S, Zaid M, Kadota A, et al. Relationship of insulin resistance to prevalence and progression of coronary artery calcification beyond metabolic syndrome components: shiga epidemiological study of subclinical atherosclerosis. Arteriosclerosis Thrombosis Vascular Biol. (2016) 36:1703–8. doi: 10.1161/ATVBAHA.116.307612
29. Pané A, Conget I, Boswell L, Ruiz S, Viñals C, Perea V, et al. Insulin resistance is associated with preclinical carotid atherosclerosis in patients with type 1 diabetes. Diabetes Metab Res Rev. (2020) 7:e3323. doi: 10.1002/dmrr.3323
30. Ke JF, Wang JW, Zhang ZH, Chen MY, Lu JX, Li LX. Insulin therapy is associated with an increased risk of carotid plaque in type 2 diabetes: a real-world study. Front Cardiovasc Med. (2021) 8:599545. doi: 10.3389/fcvm.2021.599545
Keywords: carotid plaque, plaque stability, triglyceride-glucose index, insulin resistance, nondiabetic adults
Citation: Wang A, Li Y, Zhou L, Liu K, Li S, Song B, Gao Y, Li Y, Lu J, Tian C, Xu Y and Wang L (2022) Triglyceride-Glucose Index Is Related to Carotid Plaque and Its Stability in Nondiabetic Adults: A Cross-Sectional Study. Front. Neurol. 13:823611. doi: 10.3389/fneur.2022.823611
Received: 08 December 2021; Accepted: 04 January 2022;
Published: 24 March 2022.
Edited by:
Wendell A. Lopes, State University of Maringá, BrazilReviewed by:
Chongke Zhong, Soochow University, ChinaCopyright © 2022 Wang, Li, Zhou, Liu, Li, Song, Gao, Li, Lu, Tian, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuming Xu, eHV5dW1pbmdAenp1LmVkdS5jbg==; Longde Wang, bG9uZ2VkX3dhbmdAeWVhaC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.