
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 11 March 2022
Sec. Neuroinfectious Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.819594
This article is part of the Research TopicDiagnostic Imaging for HIV-associated Neurocognitive DisordersView all 9 articles
 Benedictor Alexander Nguchu1†
Benedictor Alexander Nguchu1† Jing Zhao2,3†
Jing Zhao2,3† Yanming Wang1
Yanming Wang1 Jean de Dieu Uwisengeyimana1
Jean de Dieu Uwisengeyimana1 Xiaoxiao Wang1
Xiaoxiao Wang1 Bensheng Qiu1*
Bensheng Qiu1* Hongjun Li2,3*
Hongjun Li2,3*Objective: The brain relies on the glymphatic system to clear metabolic wastes and maintain brain homeostasis to fulfill its functions better. Yet, the complexity of the glymphatic flow and clearance and its changes in HIV infection and its role in neurocognitive dysfunction remain poorly understood. This study aims to explore the impact of HIV and combination antiretroviral therapy (cART) on the glymphatic system and establish a potential biomarker of HIV-associated neurocognitive disorders (HAND).
Methods: Here, we examined the glymphatic profiles of middle-aged virosuppressed patients with HIV (n = 27) receiving cART over 1–6 years and healthy controls (n = 28) along the perivascular space (PVS) using diffusion tensor image analysis along the perivascular space (ALPS) with guided and unguided approaches. We later combined data from these analyses to investigate MRI glymphatic correlates of cognitive impairment and other clinical tests of HIV (CD4+ T-cell counts and CD4+/CD8+ ratio).
Results: We found that glymphatic function as measured by the ALPS index increased significantly in the right and left PVSs of patients with HIV having cART. On antiretroviral therapy, a changing pattern in glymphatic clearance function in patients with HIV having cART correlated with attention and working memory. Duration on cART was also associated with cognitive performances of abstract and executive function and learning and memory.
Conclusion: These findings provide MRI evidence of the presence of HIV-induced changes in the glymphatic flow and clearance, which might underlie cognitive impairment among patients with HIV having cART. An increase in the glymphatic activity might reflect a compensatory mechanism to regulate microenvironment homeostasis compromised by HIV. This compensation might be necessary to maintain the proper functioning of the brain while coping with HIV pathology. These findings also shed light on the clinical importance of evaluating glymphatic function based on the ALPS index and suggest that improving the glymphatic system may serve as an alternative therapeutic strategy for HAND.
Human immunodeficiency virus neuroinflammation has been associated with the pathogenesis of HIV-associated neurocognitive disorders (HAND) (1). Studies have shown that combination antiretroviral therapy (cART) can reduce these inflammations (2), enhance functional circuits (3, 4), and consequently improve cognitive functions (5). However, despite antiretroviral therapy, several studies have reported persisting cognitive dysfunction in patients with HIV having cART, especially in attention and working memory, executive function, motor control, and visual processing speed (6–8). We hypothesize that the disruption of homeostasis of the neural microenvironment, resulting from HIV-induced neurotoxins and elevated metabolites, may contribute to subsequent cognitive declines in HIV-infected individuals.
The regulation of fluid homeostasis in the brain is achieved through the glymphatic system. The glymphatic system refers to the drainage pathways through which metabolic waste products and other undesirable components get flushed out of the brain, thereby stabilizing microenvironmental homeostasis and providing suitable working conditions for the brain parenchymal cells (9, 10). The system relies on the bulk flow of cerebrospinal fluid (CSF) produced by the choroid plexus. The CSF-pressure and cerebrovascular pulsatility drive CSF into the deep para-arterial spaces where CSF-interstitial fluid (ISF) exchange occurs. CSF-ISF exchange is enabled by the polarization processes of aquaporin-4 (AQP4) water channels at the end-feet of astrocytes. This exchange facilitates the drainage of metabolic wastes and other soluble particles such as glucose, lipids, signaling molecules, and apolipoprotein E (apoE) via paravenous channels (11, 12).
Presently, the role that the glymphatic system plays in cART-treated HIV individuals is poorly known. Previous studies have documented the mechanisms of the glymphatic system and its roles in most neurodegenerative diseases (10, 13). An impairment in the glymphatic clearance function has been shown to accelerate the accumulation of abluminal protein deposits, α-synuclein, Aβ and tau, and huntingtin, which, respectively, underpin small vessel disease, Parkinson's disease, Alzheimer's disease (AD), and Huntington's disease (13, 14).
Studies have also hypothesized that the building up of these protein aggregates can reciprocally further impair glymphatic functioning (15). In a variety of research tests, impaired glymphatic clearance has been associated with cognitive dysfunction, with aged individuals being at higher risk due to age-related alterations such as weakening of arterial pulsatility and AQP4 polarization dysfunctions, and stiffness or flexibility of large elastic arteries (16). A recent work published in 2020 by Liu et al. provided evidence that restoring cognitive impairments was only possible when the underlying disruptions of the glymphatic system were normalized (10), suggesting a solid interdependence between the two (17).
Ways to enhance brain-to-blood clearance include promoting protein expressions for: (1) increasing CSF bulk flow; (2) stimulating Aβ phagocytosis; and (3) reducing microglia inflammatory activation (18–21). For example, osteopontin expressions promoted by glatiramer acetate immunization prove effective for macrophage-mediated Aβ clearance (20). Lipoprotein receptor-related protein 1 (LRP1) expressions promoted by omega-3 polyunsaturated fatty acids and lithium chloride can upgrade CSF-bulk flow and accelerate Aβ42-clearance (19, 21).
Whether there is a failure in the glymphatic functioning across patients with HIV that might contribute to HIV-associated cognitive decline among patients with HIV having cART remains unexplored. It is also yet to be determined what magnitude of glymphatic influx and clearance plays a pivotal role in cART strategies to reverse neural dysfunction. Here, using diffusion tensor imaging (DTI), we investigate whether the state of the glymphatic system in HIV-infected individuals provides additional prognostic information about the cause of HAND. To this end, middle-aged, virologically suppressed patients (age range, 36–55 years) were enrolled. The evaluation of glymphatic clearance functioning was performed using “DTI-analysis along the perivascular space” (DTI-ALPS) index. The DTI-ALPS index has proven effective for the estimation of glymphatic function in earlier studies (22–24). We hypothesize that the DTI-ALPS index would offer new insights into understanding the impact of HIV and cART on the glymphatic system and the role played by glymphatic clearance in the pathogenicity of HAND.
A total of 28 middle-aged healthy controls (mean ± SD age = 41.35 ± 4.85 years, range = 36–52 years) and 27 age-matched patients with HIV-1 seropositive taking cART [tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV)] (mean ± SD age = 40.30 ± 4.80 years, range = 36–53 years) were enrolled at Beijing YouAn Hospital, the capita Hospital, between March 2016 and November 2016. A set of clinical tests for blood and neuropsychological assessment were administered to these participants. The reports of the demographics, blood, and neuropsychological profiles are given in Table 1. Each patient sustained at least 1 year of stable cART-regimen. All the patients had undetectable viral loads (copies/ml). The blood test reports included CD4+ T-cell counts and CD4+/CD8+ ratio, which serve as potential indicators of HIV disease severity (25). The individuals who demonstrated any record of illicit drugs and alcohol use, cerebral atrophy, brain lesions, head injury, or neurological disorders were excluded from participation. This study was conducted in compliance with the code of Ethics of the World Medical Association (Declaration of Helsinki) for human experiments. Each participant's written informed consent was obtained before participation. The Ethical Committee of the Capital Medical University and the University of Science and Technology of China reviewed and approved this study.
A battery of neuropsychological (NP) tests for six cognitive domains was administered to all patients according to Frascati recommendations (26). The Continuous Performance Test Identical Pairs (CPT-IP), the Wechsler Memory Scale-III (WMS-III), and Paced Auditory Serial Addition Test (PASAT) were used to assess attention and working memory. The category fluency and animal naming tests were used for testing verbal and language, the Grooved Pegboard test for motor function, and the Wisconsin Card Sorting Test-64 (WCST-64) for abstract and executive function. The Hopkins Verbal Learning Test-Revised (HVLT-R) and the Brief Visuospatial Memory Test-Revised (BVMT-R) tested for learning and recall, while the trail-making test part A evaluated the information processing speed. Demographically adjusted T-scores were obtained by standardizing the raw scores of each test. For the cognitive domain assessed by multiple tests, an average composite T-score was obtained across tests. NP test showing cognitive impairment in at least two cognitive domains with normal day-to-day functioning suggested ANI.
Neuroimaging data were obtained using a Siemens 3T MRI Scanner (Allegra, Siemens Medical System, Erlangen, Germany) equipped with a 32-channel head coil. Imaging protocols include a 3D-T1-weighted anatomical image [TR/TE = 1,900 ms/2.52 ms, inversion time = 900 ms, flip angle = 9°, field of view (FVO) = 250 mm2 × 250 mm2, matrix size = 246 × 256, slice thickness = 1 mm, and voxel size =1 mm3 × 0.977 mm3 × 0.977 mm3] and a diffusion-weighted imaging [60 diffusion-encoded (b = 1,000 s/mm2), 3 references (b = 0 s/mm2), TR = 3,300 ms, TE = 90 ms, flip angle = 90°, slice thickness = 4.2 mm, voxel size = 2 mm3 × 2 mm3 × 4.2 mm3].
Data pre-processing was achieved using the FMRIB Software Library (FSL) (https://fsl.fmrib.ox.ac.uk/) (27), which involves correcting for susceptibility-induced distortions, eddy currents, and subject movements. Diffusion tensor maps were generated using the DTI-tensor fitting of the FSL. Other diffusivity maps, i.e., fractional anisotropy (FA) and mean diffusivity (MD), were also produced. Because all the neuroimaging data were collected from middle-aged adults, each subject's FA map was registered to the Illinois Institute of Technology (IIT) version 3.0 template of IIT Human Brain Atlas (28), using FMRIB's Linear/Non-linear Image Registration Tools (FLIRT/FNIRT), part of the FSL version 5.09 (27). The IIT v.3.0 template of the adult human brain is a population-based FA template having a high signal-to-noise ratio (SNR) and FA values and high image sharpness with visible small white matter structures and spatial features (28). It also has smaller intergroup FA differences and higher intersubject DTI spatial normalization accuracy compared with other DTI brain templates (28). Coregistration's accuracy was confirmed by visual inspection. Diffusion tensor maps of all of the subjects were normalized to IIT version 3.0 template using the transformation matrix obtained from the normalization of FA images to the IIT version 3.0 template.
Evaluation of diffusion tensor image analysis along the perivascular space index (DTI-ALPS-index) was concordant with earlier studies (24, 29, 30). We first registered JHU-ICBM DTI-81 labels, including the areas of projection fiber (superior corona radiata) and association fiber (superior longitudinal fasciculus), to IIT version 3.0 template. Then, DTI-ALPS index was computed as the estimate of water diffusivity along the x-axis of projection and association fiber areas (Dxpro, Dxasc) modulated by diffusivity along the y-axis of the projection and z-axis of the association fibers (Dypro, Dzasc), reflecting the glymphatic system in the medullary veins (Figure 1) (24, 32). The DTI-ALPS index is given by “mean (Dxpro, Dxasc)/mean (Dypro, Dzasc).” The DTI-ALPS index was calculated based on three paradigms. (1) Using the DTI-unguided atlas-based approach (Method 1), in which the regions of interest (ROIs) were set in the whole areas of the projection and association fibers as delineated by the JHU-ICBM DTI-81 Atlas (Figure 2A). (2) Using DTI-guided manually delineated ROIs (Method 2) of these fiber areas, with color-coded FA modulated by Illinois Institute of Technology (IIT) median eigenvector 1 (V1) (IITmedian_V1) used as reference (Figure 2B). (3) Using 5-mm-diameter spherical ROIs (Method 3) placed in the areas of the projection and association fibers, with the reference slice being the center of these ROIs (Figure 2C). Notice that the term DTI-guided refers to the use of FA color-coded maps to delineate the ROIs, while DTI-unguided means otherwise. The DTI-ALPS index was first evaluated separately for both the left and the right hemispheres at the locations where the direction of the deep medullary veins was perpendicular to the ventricle body. Then, the “bilateral DTI-ALPS index,” a joint DTI index of the right and left PVSs, was recorded.
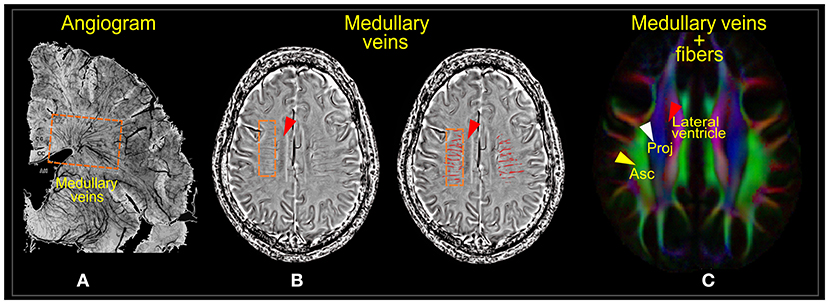
Figure 1. Medullary veins. (A) Angiogram of the parenchymal venous system. (B) Phase images of susceptibility-weighted imaging (SWI) showing the deep medullary venous structures before (left) and after segmentation (right). (C) Diffusion tensor image showing the projection (Proj) and association (Asc) fiber areas where the medullary veins run. Images (A,B) were adapted, with permission from Yan et al. (31).
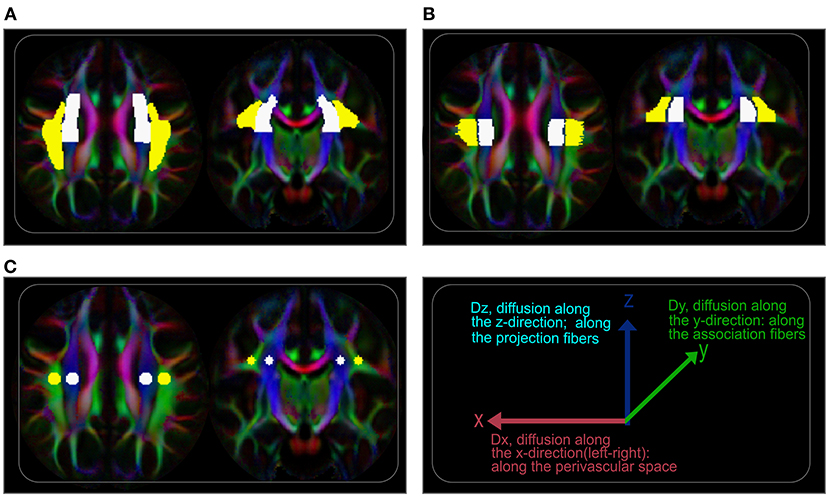
Figure 2. Regions of interest (ROIs) for ALPS index evaluation. (A) Axial (left) and coronal (right) visualization of the ROIs showing labels of the projection and association fiber areas defined by the labels of JHU-ICBM DTI-81 atlas. On the atlas-based approach (unguided), these labels were used to extract ALPS values from coregistered tensor maps. (B) Regions of the projection and association fiber areas along the periventricular space manually delineated, guided by diffusion maps (color-coded FA). (C) 5-mm-diameter spherical regions placed on the projection and association fiber areas with the centers on the reference slice.
For an intuitive understanding of the ALPS index at the ventricular level, it is essential to note the organization of the white matter (WM) fibers and their directions. The perivascular WM fibers (on the axial plane; Figure 1C) consist of two dominating fibers—the projection fibers (in the z-direction; blue; Figure 1C) and association fibers (in the y-direction; green; Figure 1C)—through which medullary veins run (Figure 1B). The direction of the PVS at the ventricular level is the direction of the medullary veins, which is also the x-direction (left/right or right/left), parallel to the walls of lateral ventricles but perpendicular to the projection and association fibers. Thus, the PVS can be well-described by differences in water molecule characteristics of the x-direction of the dominant fiber areas (Dxpro, Dxasc) and other perpendicular directions (Dypro, Dzasc) (22). This difference is mathematically presented as the ratio of the mean of the x-direction diffusivities [mean (Dxpro, Dxasc)] to the mean of the y- and z-direction diffusivities [mean (Dypro, Dzasc)].
Statistical analyses were performed on SPSS software [IBM SPSS Statistics for Windows, version 20.0 (IBM Corporation, Armonk, New York, USA)]. Group differences in the DTI-ALPS index were determined using the two-sample t-tests. Corrections for multiple comparisons were performed using a false discovery rate (p < 0.05, FDR). Correlation analyses between imaging markers and clinical measures were determined using Pearson's correlations.
Table 1 shows the demographic and clinical data of participants. A total of 55 participants (HIV: 27, HC: 28, male: 100%) were included. The average age of patients with HIV having cART and healthy controls was 40.30 ± 4.80 and 41.35 ± 4.85 years old, respectively, which did not reach the significant level for the difference (p = 0.483: p > 0.05). The average duration on cART for patients with HIV was 1,119.82 ± 671.265 days. All the patients expressed an undetectable viral load. CD4+ cell counts (cells/μl) and CD4+/CD8+ ratios of patients were 503.15 ± 170.700 and 0.72 ± 0.508, respectively. Note that the reference ranges used for CD4 cell counts and CD4+/CD8+ ratios were 544 to 1,212 cells/μl and 0.71 to 2.78, respectively. The neuropsychological test scores of cognitive domains were: learning and recall: 45.29 ± 8.85, motor function: 44.98 ± 13.26, abstract and executive function: 59.15 ± 9.73, verbal and language: 48.30 ± 7.77, attention and working memory: 47.43 ± 7.73, and information processing speed: 47.84 ± 8.36. These neuropsychological test scores did not indicate detectable changes in cognitive functioning per Frascati criteria.
As Figure 4 shows, duration of patients on cART was significantly related to cognitive scores of the domains of the abstract and executive function (p = 0.0009, r = 0.669, Figure 4A), and learning and recall (p = 0.0338, r = 0.444, Figure 4B). Neither CD4+ T-cell counts nor CD4+/CD8+ ratio demonstrated a significant relationship with the duration of patients on cART (p > 0.05).
Results of the group differences in the ALPS index are shown in Figure 3. All three paradigms of ALPS index evaluation reported significant group differences between patients with HIV having cART and healthy controls (HCs). Specifically, there was a significant increase in the ALPS index in the right, left, and bilateral PVSs of the HIV group (Figures 3A–C). The ALPS index computed using manually delineated guided-ROIs (Method 2, Figure 3B) offered the highest group difference with p = 0.0367, t = 2.16 (left); p = 0.0046, t = 2.99 (right); and p = 0.0090, t = 2.74 (bilateral), followed by 5-mm-diameter guided ROIs (Method 3, Figure 3C) with p = 0.0409, t = 2.11 (left); p = 0.0068, t = 2.85(right); and p = 0.0110, t = 2.66 (bilateral), and lastly the atlas-based DTI-unguided ROIs (Method 1, Figure 3A) with p = 0.0490, t = 2.03 (left); p = 0.0212, t = 2.12 (right); p = 0.0219, t = 2.19.
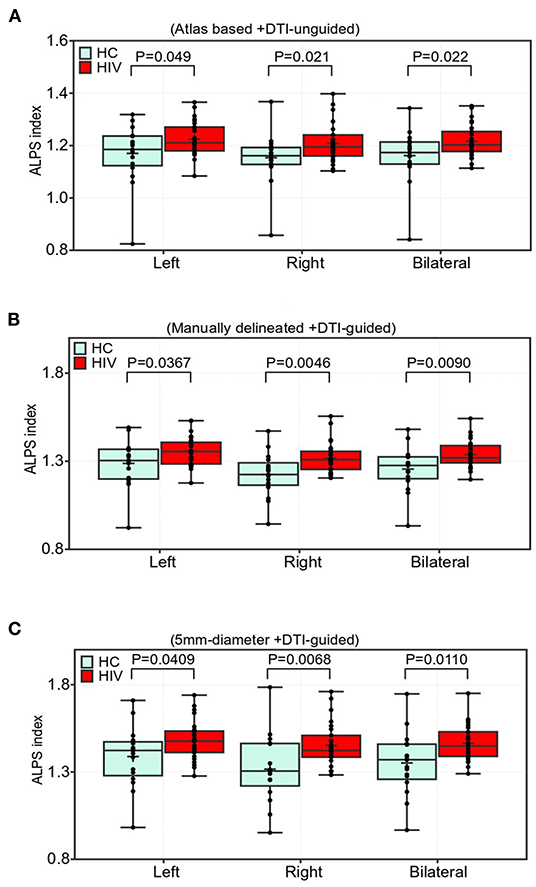
Figure 3. Comparisons of the ALPS indexes in the periventricular white matter (PVWM) veins between the HIV and HC groups. Two sample t-test with false discovery rate correction was used (FDR, p < 0.05). The group differences were based on (A) atlas-based approach, i.e., using labels from JHU-ICBM DTI-atlas and manual approaches using, (B) manually delineated regions of the lateral PVWM areas with reference to color-coded FA map, and (C) using 5-mm-diameter spherical ROIs. Participants in the cART-treated HIV group showed a significantly higher ALPS index than those in the HC group (A–C). Boxes indicate the 25th−75th percentiles of the ALPS index, and the lines and whiskers show the median and range of the ALPS index, respectively.
The results of correlations between the ALPS index and neuropsychological test scores are given in Figure 4. The diffusion characteristics, as measured by the DTI-ALPS index, along the PVWM were significantly associated with cognitive functions of cART-treated patients with HIV. The significant correlations were mainly between the ALPS index of the right and bilateral PVSs and attention and working memory (Figures 4C–E). From the DTI-unguided atlas-based approach (Method 1), the right ALPS index and bilateral ALPS index had significant correlations (p = 0.0128, r = 0.521; and p = 0.0184, r = 0.498, respectively) with attention and working memory (Figure 4C). From the manual whole-ROI DTI-guided approach (Method 2), the ALPS indexes of the right and bilateral reported respective associations (p = 0.0337, r = 0.454; and p = 0.0328, r = 0.456) with attention and working memory (Figure 4D). Similarly, the right and bilateral ALPS indexes of the 5-mm-ROI-based approach (Method 3) demonstrated significant associations with attention and working memory with p = 0.0096, r = 0.539; and p = 0.0288, r = 0.466 (Figure 4E). None of the other cognitive domains indicated significant correlations with the ALPS index.

Figure 4. Correlation results. (A,B) Correlations of duration on cART with neuropsychological scores of patients with HIV. The duration of cART treatment was positively correlated with cognitive performances of (A) abstract and learning function and (B) learning and recall. (C–E) Correlations between the ALPS indexes and cognitive functions in HIV patients. (C) With JHU-ICBM DTI-81 atlas-based ROIs, the right (R, gray) and bilateral (B, green) ALPS indexes demonstrated positive correlations with attention and working memory. (D) With manual DTI-guided ROIs, positive relationships were also exhibited between attention/working memory and ALPS indexes of the right and the combined perivascular spaces (PVSs). (E) With 5-mm-diameter ROIs, similar correlations were manifested between the right and bilateral indexes and the attention and working memory neuropsychological scores. All tests in correlation analyses were significant at p < 0.05. R, right; B, bilateral. The term “bilateral index” refers to an index computed by combining ROIs of both sides (right and left) of the PVSs.
Figure 5 shows the results of the correlation between the duration on cART and ALPS index. The duration on cART exhibited a significant correlation with the ALPS index of the patients with HIV. As with cognitive score and ALPS index, the significant correlations of the duration on cART were predominantly with the ALPS index of the right and bilateral PVSs. The respective correlations scores were (p = 0.0044, r = 0.608, right) and (p = 0.0272, r = 0.493, bilateral) for ALPS indexes of Method 1 (Figure 5A); (p = 0.0022, r = 0.643, right) and (p = 0.0059, r = 0.592, bilateral) for ALPS indexes of Method 2 (Figure 5B) and (p = 0.0032, r = 0.625, right) and (p = 0.0104, r = 0.559, bilateral) for ALPS indexes of Method 3 (Figure 5C).
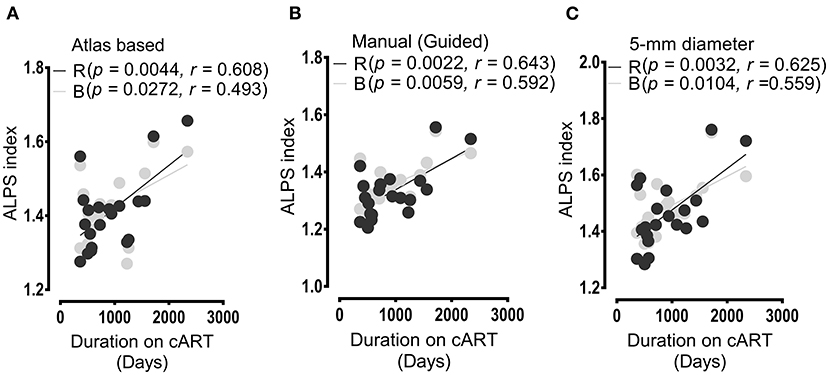
Figure 5. Results of correlations between the duration of cART treatment and ALPS index. In all analyses (A,B), the duration of cART treatment correlated positively with the ALPS index of the right (R) and bilateral (B) PVSs. (A) Correlation outcomes as evaluated between the cART period and ALPS index computed within the areas defined by JHU-ICBM atlas-based ROIs. (B) Correlations as assessed between ALPS index calculated within areas determined by manual DTI-guided ROIs and duration of cART treatment. (C) Correlation outcome of the duration of therapy and ALPS index measured within areas of 5-mm diameter. All correlation results were significant at p< 0.05. In the figure legend, R, right; B, bilateral.
The glymphatic characteristics did not demonstrate any significant correlations (p > 0.05) with the HIV immunologic functions; that is, neither CD4+T-cell counts nor CD4+/CD8+ ratios were correlated with the ALPS index.
In this study, we evaluated the glymphatic system in cART-treated patients with HIV using the DTI-ALPS index, which is the ratio of diffusivity toward the PVS. First, we assessed the DTI-ALPS index with the directionality consistent with earlier studies (22), wherein the evaluation was conducted on the left sides of the participants' brains, which are considered to have thicker fibers as all subjects were right-handed. Second, this study added the evaluation of the right PVS, considering that patients with HIV are susceptible to HIV pathology in both hemispheres rather than one side (6, 33). In addition, we evaluated the average ALPS index of bilateral sides in line with Zhang et al. (24), which we referred to as bilateral ALPS index.
Herein, we found that the ALPS index of the right PVS showed a higher group difference than that of the left PVS, suggesting that a glymphatic change occurs differentially across PVSs, depending on the severity of HIV injury, adding to the point that a complete understanding of the neurobiology and status of glymphatic system requires an extensive assessment of both hemispherical PVSs than previously appreciated (22, 23). Nevertheless, we cannot exclude that other factors such as cART penetration might also account for the differences in glymphatic clearance function observed between the left and right sides. It is also worth noting that, of the three paradigms for evaluating glymphatic clearance function, method 2 and method 3 (DTI-guided approaches) offered higher statistical power than method 1 [DTI-unguided, also referred to as atlas-based method (30)]. This was expected since the DTI-unguided approach (Method 1) often incorporates diffusivities of water molecules in areas beyond the PVS (34), which could moderate the ALPS index values.
Generally, the authors of this study have found that the ALPS index, as evaluated by all three paradigms, increased significantly in patients with HIV having cART compared to healthy controls. One possible interpretation is that the increase in the ALPS index suggests HIV-necessitated changes in glymphatic clearance functioning, possibly as a compensatory mechanism to maintain fluid homeostasis compromised by HIV.
Several previous studies have reported disruption of fluid homeostasis due to HIV (35, 36), wherein HIV has been shown to elevate levels of CSF quinolinic acids and induce abnormal microglial activation and neuroinflammation (also see Figure 6). These processes generate excess viral proteins in CNS, which attack neural and glial functions (35, 36) and metabolic regulatory system (37), and in the long run cause the death of the parenchymal cells (38). We hypothesize that such disruption of fluid homeostasis and the functional impairment of the glymphatic system might underlie cognitive deficits often seen among patients with HIV, especially in attention and working memory, executive function, and learning and memory (39, 40). Meanwhile, we also underscore that higher-order mechanisms of periventricular AQP4 polarization, arterial pulsatility, and cerebrovascular compliance play a valuable role in cognitively normal cART-treated patients with HIV to detox and cleanse the brain from HIV-associated neurotoxins and metabolites to maintain fluid milieu for normal functioning.
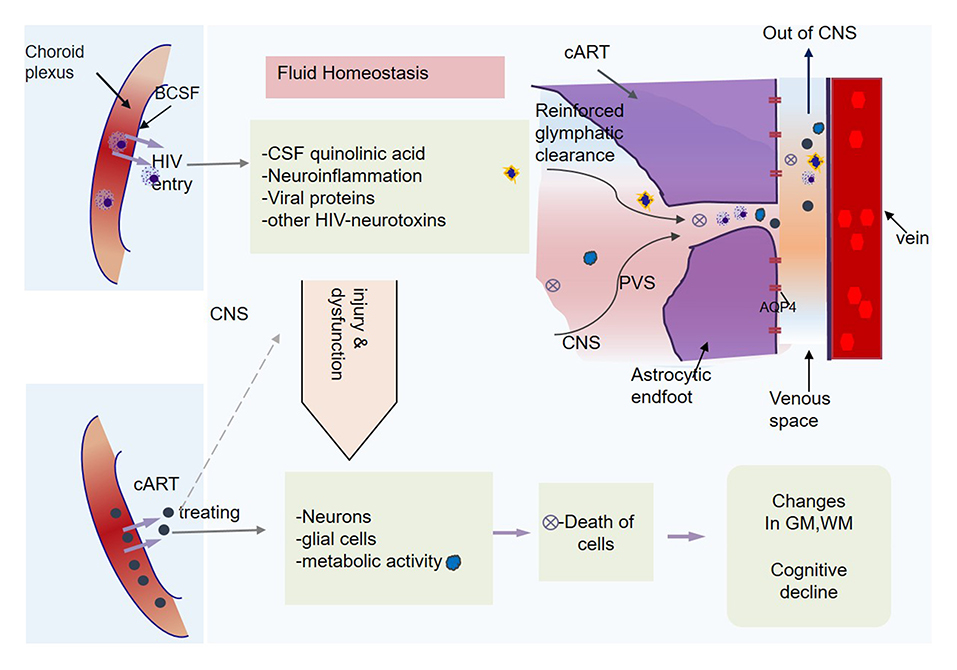
Figure 6. Central nervous system (CNS) homeostasis and glymphatic clearance. HIV elevates the cerebrospinal fluid (CSF) quinolinic acid levels. It also induces neuroinflammation, viral proteins that cause injury to neuronal and glial cells, including astrocytes, leading to the death of some cells. The metabolic activity and functions of CNS cells become subject to ongoing HIV injuries. In response to the toxic homeostasis and infectious chemicals, the brain might recruit higher-order mechanisms (AQP4 polarization processes, pulsatility, and cerebral vascular compliance) for homeostasis restoration.
Our results differ from two earlier studies that examined the causes of cognitive impairment in patients with type 2 diabetes mellitus (23) or idiopathic normal pressure hydrocephalus (22) which found a lowered ALPS index. This may be explained by the fact that cART-treated patients with HIV in this study did not manifest observable cognitive changes in the neuropsychological battery tests, which may suggest that brain injury might occur early before deficits could be detected in the neuropsychological battery tests or might suggest stable cognitive reserve in patients with HIV despite HIV neuropathology, possibly favored by actions of the combination of [tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV)]. This is contrary to the patients (such as Alzheimer's, diabetic, or small vessel diseases) who are typically affected by constant accumulation and deposition of Aβ, which affects the ISF bulk flow (13), leading to a substantial decline of glymphatic functioning observed in these diseases (24).
Previous studies have demonstrated that cART can successfully penetrate the blood-cerebrospinal fluid barrier (BCSFB) and subsequently promote neutrophil and phagocytic activity, which is essential for the clearance of inflammatory immune complexes (41). The cART has also been shown to reduce the abnormal activity of viral proteins and glial activation, which normally have a deleterious effect on synoptodentritic structure and function (5, 42). So cART in our patients who have been on cART for over 1–6 years might have been protective against severe damage to cognitive reserve and thus allowed the brain to recruit other strategies—including glymphatic system, to cope with neuropathological damage due to HIV. Less protected CNS is at higher risk of developing PVS inflammation, vascular dysfunctions, and abnormal astrocytic AQP4 polarization, leading to later suppression of the glymphatic pathways (10), which collectively would weaken the brain capacity to modulate glymphatic clearance functioning. Moreover, it is essential to note that enhanced glymphatic functioning seen in our patients with HIV having cART could be imperative to clean the day-to-day remnants of the cART-drugs that crossed the CNS (43).
Earlier studies of the glymphatic system have already shown that the basal ganglia are entirely drained by deep medullary veins, indicating the more close relationship between the glymphatic system in the basal ganglia and the glymphatic clearance function calculated from the diffusion of PVSs around the deep medullary veins (24). The basal ganglia and the surrounding structures have also been reported to be the primary targets of HIV infection (7, 44–46). For example, following HIV infection, structural MRI studies have found tissues worn away in the structures of basal ganglia, including the caudate nucleus, and in the walls of lateral ventricles and the structures of the thalamus and corpus callosum (44–48). Learning and memory-related deficits in patients with HIV have been associated with injuries in these structures, including the caudate nucleus (49, 50). A study of white matter microstructures conducted in the USA in patients over 60 years old has also detected HIV injury in the dominant fibers (projection: corona radiata; and association: superior longitudinal fasciculus) crossed by these medullary veins, leading to abnormal diffusion and microstructural integrity (51).
Our results support these earlier findings, and provide further evidence of the drainage pathways of the lost tissues in the basal ganglia (see Figure 6) and other regions next to the medullary veins, which leads to reduction of basal ganglia and thalamic volumes (44, 52), expansion of the lateral ventricles (48), and thinning of the corpus callosum (46), which are usually detected in among patients with HIV. The authors of this study also infer that HIV injury might prolong to initiate infection in axons, which may further lead to abnormal flow or diffusion of water molecules in white matter tracts, and these changes may at least partly underpin changes in glymphatic clearance.
The team of this study also found that increased glymphatic function in patients with HIV having cART was related to the cognitive function of attention and working. Previous studies have already documented the relationship between glymphatic function and cognitive function in patients with cerebral small vessel disease, diabetes, and AD (18, 22–24). Increasing activity or functions related to the cognitive function of attention and working memory in patients with HIV has previously been reported in a study performed by Chang et al., which investigated the neural correlates of attention and working memory deficits (53). The authors found that greater activation of the key brain regions such as frontal areas was required to perform more complex attention and working memory tasks, suggesting that neural correlates of attentional deficits due to HIV injury may be increased attentional modulation. Therefore, we infer that increasing glymphatic function in patients with HIV having cART might also reflect excessive modulation required for attention and working memory following HIV neuropathology.
Again, from correlation analyses, we also observed a significant correlation between the duration of cART treatment and cognitive performance of the patients with HIV. The longer the period on cART, the higher neuropsychological test scores of learning and memory and abstract and executive function. We speculate that this association may be mediated by cART. However, further studies are required to validate if this relationship is valid over the long term. This is because there is still an ongoing debate whether long-term use of cART has a detrimental effect on brain structure and function. For example, some studies have reported improvements in basal ganglia indicators in patients with HIV in the early use of cART, but later these improvements started to decline after the long-term use of cART, accompanied by cognitive deficits (54).
This study of middle-aged cART-treated patients with HIV did not find any relationship between glymphatic changes in the CNS compartments and HIV immunologic functions (i.e., CD4+T-cell counts and CD4+/CD8+ ratio), suggesting a generally healthy condition for these cART-treated individuals living with HIV (49).
This study has some limitations worth noting. First, all the analyses were performed using data acquired with b = 1,000 s/mm2 because: (1) water molecules with high motivity in PVSs are thought to have more significant influence in measurements when the b value is lower; (2) the signal-to-noise ratio in b = 1,000 s/mm2 is higher than in b = 2,000 s/mm2; and (3) the cognitive function has previously been demonstrated to correlate with ALPS index evaluated with b = 1,000 s/mm2 data than b = 1,000 s/mm2 data (23, 24, 29). However, the absence of comparisons with b = 2,000 s/mm2 results may limit our findings. Second, even though ALPS-index reflects the instantaneous glymphatic function at the time of the scan, adding the glymphatic evaluation at times of sleep demonstrated to have the more active glymphatic system (55) should be considered in further studies. It is also important to note that this study did not include untreated HIV cases. Furthermore, large, both gender, longitudinal data are warranted to validate conclusions of our findings.
In conclusion, we found that the DTI-ALPS index in patients with HIV having cART was high. The higher ALPS index in cART-treated patients with HIV suggests enhanced glymphatic function. Moreover, the higher ALPS index was also associated with the cognitive scores of attention and working memory. Authors infer that HIV-neurotoxins disrupt the microenvironmental fluid homeostasis, which necessitates changes in glymphatic flow and clearance. Such changes in fluid homeostasis and glymphatic functioning might underlie HAND seen among patients with HIV having cART. These findings also suggest that the therapeutic initiatives aiming at improving functions of paravascular fluid exchange between CSF and ISF, cerebral arterial pulsatility, and perivascular AQP4 polarization would be the best strategies to maintain or normalize cognition despite the HIV pathology of an individual.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of the Capital Medical University and the University of Science and Technology of China. The patients/participants provided their written informed consent to participate in this study.
BN, JZ, YW, and XW: substantial contributions to conceptualization, methodology, data collection, image analysis, and writing—original draft preparation. BN, JZ, YW, JD, and HL: contributions to the data collection, image analysis, and interpretation of data. BN, XW, HL, and BQ: draft the work or review, edit, and revise it critically for important intellectual content. HL and BQ: final approval of the version submitted. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grants 61936013, 81771806, and 21876041), the Ministry of Science and Technology of China (SQ2019YF013267), and the China Primary Health Care Foundation-Youan Foundation of Liver Disease and AIDS (BJYAYY-CY2019-04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Howdle GC, Quidé Y, Kassem MS, Johnson K, Rae CD, Brew BJ, et al. Brain amyloid in virally suppressed HIV-associated neurocognitive disorder. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e739. doi: 10.1212/NXI.0000000000000739
2. Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. (2010) 202:161–70. doi: 10.1086/653213
3. Toniolo S, Cercignani M, Mora-Peris B, Underwood J, Alagaratnam J, Bozzali M, et al. Changes in functional connectivity in people with HIV switching antiretroviral therapy. J Neurovirol. (2020) 26:754–63. doi: 10.1007/s13365-020-00853-0
4. Zhuang Y, Qiu X, Wang L, Ma Q, Mapstone M, Luque A, et al. Combination antiretroviral therapy improves cognitive performance and functional connectivity in treatment-naïve HIV-infected individuals. J Neurovirol. (2017) 23:704–12. doi: 10.1007/s13365-017-0553-9
5. Ortega M, Brier MR, Ances BM. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS. (2015) 29:703–12. doi: 10.1097/QAD.0000000000000611
6. Lew BJ, McDermott TJ, Wiesman AI, O'Neill J, Mills MS, Robertson KR, et al. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology. (2018) 91:E1860–9. doi: 10.1212/WNL.0000000000006504
7. Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4(+) T lymphocyte decline. Proc Natl Acad Sci USA. (2005) 102:15647–52. doi: 10.1073/pnas.0502548102
8. Wiesman AI, O'Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, et al. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain. (2018) 141:1678–90. doi: 10.1093/brain/awy097
9. Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, et al. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife. (2018) 7:e34028. doi: 10.7554/eLife.34028.016
10. Liu X, Hao J, Yao E, Cao J, Zheng X, Yao D, et al. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav Immun. (2020) 89:357–70. doi: 10.1016/j.bbi.2020.07.022
11. Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. (2020) 143:2576–93. doi: 10.1093/brain/awaa179
12. Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. (2017) 140:2691–705. doi: 10.1093/brain/awx191
13. Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci. (2017) 131:2257–74. doi: 10.1042/CS20160381
14. Liu Q, Yan L, Huang M, Zeng H, Satyanarayanan SK, Shi Z, et al. Experimental alcoholism primes structural and functional impairment of the glymphatic pathway. Brain Behav Immun. (2020) 85:106–19. doi: 10.1016/j.bbi.2019.06.029
15. Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner's guide. Neurochem Res. (2015) 40:2583–99. doi: 10.1007/s11064-015-1581-6
16. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. (2014) 76:845–61. doi: 10.1002/ana.24271
17. He X-F, Liu D-X, Zhang Q, Liang F-Y, Dai G-Y, Zeng J-S, et al. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci. (2017) 10:144. doi: 10.3389/fnmol.2017.00144
18. Dempsey C, Araiz AR, Bryson K, Finucane O, Larkin C, Mills E, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun. (2017) 61:306–16. doi: 10.1016/j.bbi
19. Pan Y, Short JL, Newman SA, Choy KH, Tiwari D, Yap C, et al. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of β-amyloid. Brain Behav Immun. (2018) 70:36–47. doi: 10.1016/j.bbi.2018.03.007
20. Rentsendorj A, Sheyn J, Fuchs D-T, Daley D, Salumbides BC, Schubloom HE, et al. A novel role for osteopontin in macrophage-mediated amyloid-β clearance in Alzheimer's models. Brain Behav Immun. (2018) 67:163–80. doi: 10.1016/j.bbi.2017.08.019
21. Yan L, Xie Y, Satyanarayanan SK, Zeng H, Liu Q, Huang M, et al. Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer's disease. Brain Behav Immun. (2020) 85:35–45. doi: 10.1016/j.bbi.2019.05.033
22. Bae YJ, Choi BS, Kim J-M, Choi J-H, Cho SJ, Kim JH. Altered glymphatic system in idiopathic normal pressure hydrocephalus. Parkin Relat Disord. (2021) 82:56–60. doi: 10.1016/j.parkreldis.2020.11.009
23. Yang G, Deng N, Liu Y, Gu Y, Yao X. Evaluation of glymphatic system using diffusion MR technique in T2DM cases. Front Hum Neurosci. (2020) 14:300. doi: 10.3389/fnhum.2020.00300
24. Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. (2021) 238:118257. doi: 10.1016/j.neuroimage.2021.118257
25. Ambrosioni J, Farrera J, de Lazzari E, Nicolás D, Manzardo C, Hernández-Meneses MM, et al. Immunological and virological efficacy of different antiretroviral regimens initiated during acute/recent HIV infection. AIDS. (2020) 34:2269–74. doi: 10.1097/QAD.0000000000002685
26. Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. (2007) 69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b
27. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. (2012) 62:782–90. doi: 10.1016/j.neuroimage.2011.09.015
28. Zhang S, Arfanakis K. Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. Neuroimage. (2018) 172:40–50. doi: 10.1016/j.neuroimage.2018.01.046
29. Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. (2017) 35:172–8. doi: 10.1007/s11604-017-0617-z
30. Yokota H, Vijayasarathi A, Cekic M, Hirata Y, Linetsky M, Ho M, et al. Diagnostic performance of glymphatic system evaluation using diffusion tensor imaging in idiopathic normal pressure hydrocephalus and mimickers. Curr Gerontol Geriatr Res. (2019) 2019:5675014. doi: 10.1155/2019/5675014
31. Yan S, Wan J, Zhang X, Tong L, Zhao S, Sun J, et al. Increased visibility of deep medullary veins in leukoaraiosis: a 3-T MRI study. Front Aging Neurosci. (2014) 6:144. doi: 10.3389/fnagi.2014.00144
32. Steward CE, Venkatraman VK, Lui E, Malpas CB, Ellis KA, Cyarto EV, et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J Neuroimaging. (2021) 31:569–78. doi: 10.1111/jon.12837
33. Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex. (2012) 22:2065–75. doi: 10.1093/cercor/bhr285
34. Zhang R, Zhou Y, Yan S, Zhong G, Liu C, Jiaerken Y, et al. A brain region-based deep medullary veins visual score on susceptibility weighted imaging. Front Aging Neurosci. (2017) 9:269. doi: 10.3389/fnagi.2017.00269
35. Borjabad A, Brooks AI, Volsky DJ. Gene expression profiles of HIV-1-infected glia and brain: toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders. J Neuroimmune Pharmacol. (2010) 5:44–62. doi: 10.1007/s11481-009-9167-1
36. Heyes MP, Ellis RJ, Ryan L, Childers ME, Grant I, Wolfson T, et al. Elevated cerebrospinal fluid quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain. (2001) 124:1033–42. doi: 10.1093/brain/124.5.1033
37. Bourgi K, Wanjalla C, Koethe JR. Inflammation and metabolic complications in HIV. Curr HIV AIDS Rep. (2018) 15:371–81. doi: 10.1007/s11904-018-0411-2
38. Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol. (2000) 6:S24. doi: 10.1007/978-3-642-59683-4_1
39. Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage. (2003) 19:1686–93. doi: 10.1016/S1053-8119(03)00232-5
40. Wang M, Wang Q, Ding H, Shang H. Association of hippocampal magnetic resonance imaging with learning and memory deficits in HIV-1–seropositive patients. JAIDS J Acquired Immune Defic Syndromes. (2015) 70:436–43. doi: 10.1097/QAI.0000000000000789
41. Mastroianni CM, Lichtner M, Mengoni F, D'Agostino C, Forcina G, d'Ettorre G, et al. Improvement in neutrophil and monocyte function during highly active antiretroviral treatment of HIV-1-infected patients. AIDS. (1999) 13:883–90. doi: 10.1097/00002030-199905280-00003
42. Li TS, Tubiana R, Katlama C, Calvez V, Mohand HA, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. (1998) 351:1682–6. doi: 10.1016/S0140-6736(97)10291-4
43. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. (2007) 8:33–44. doi: 10.1038/nrn2040
44. Ances B, Roc A, Wang J, Korczykowski M, Okawa J, Stern J, et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. (2006) 66:862–6. doi: 10.1212/01.wnl.0000203524.57993.e2
45. Aylward EH, Hendere JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, et al. Reduced basal ganglia volume in HIV-1-associated dementia- results J Neurol. (1993) 43: 2099–104. doi: 10.1212/WNL.43.10.2099
46. Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, et al. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. (2006) 31:12–23. doi: 10.1016/j.neuroimage.2005.11.043
47. Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, et al. Structural brain alterations can be detected early in HIV infection. Neurology. (2012) 79:2328–34. doi: 10.1212/WNL.0b013e318278b5b4
48. Wang Y, Zhang J, Gutman B, Chan TF, Becker JT, Aizenstein HJ, et al. Multivariate tensor-based morphometry on surfaces: application to mapping ventricular abnormalities in HIV/AIDS. Neuroimage. (2010) 49:2141–57. doi: 10.1016/j.neuroimage.2009.10.086
49. Janssen MA, Meulenbroek O, Steens SC, Góraj B, Bosch M, Koopmans PP, et al. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS. (2015) 29:2139–48. doi: 10.1097/QAD.0000000000000824
50. Wang G-J, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. (2004) 127:2452–8. doi: 10.1093/brain/awh269
51. Chang K, Premeaux TA, Cobigo Y, Milanini B, Hellmuth J, Rubin LH, et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS. (2020) 34:203–13. doi: 10.1097/QAD.0000000000002404
52. Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, et al. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry. (2012) 72:361–70. doi: 10.1016/j.biopsych.2012.02.018
53. Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. (2001) 57:1001–7. doi: 10.1212/WNL.57.6.1001
54. Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, et al. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc. (2008) 14:725–33. doi: 10.1017/S1355617708080910
Keywords: HIV infection, HAND, glymphatic clearance function, ALPS index, diffusion tensor imaging, fluid homeostasis
Citation: Nguchu BA, Zhao J, Wang Y, de Dieu Uwisengeyimana J, Wang X, Qiu B and Li H (2022) Altered Glymphatic System in Middle-Aged cART-Treated Patients With HIV: A Diffusion Tensor Imaging Study. Front. Neurol. 13:819594. doi: 10.3389/fneur.2022.819594
Received: 21 November 2021; Accepted: 01 February 2022;
Published: 11 March 2022.
Edited by:
Dasja Pajkrt, Academic Medical Center, NetherlandsReviewed by:
Toshiaki Taoka, Nagoya University, JapanCopyright © 2022 Nguchu, Zhao, Wang, de Dieu Uwisengeyimana, Wang, Qiu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bensheng Qiu, YnFpdUB1c3RjLmVkdS5jbg==; Hongjun Li, bGlob25nanVuMDAxMTNAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.