- 1Department of Radiology, The General Hospital of Northern Theatre Command, Shenyang, China
- 2Jinzhou Medical University General Hospital of Northern Theatre Command Postgraduate Training Base, Shenyang, China
- 3Department of General Surgery, The General Hospital of Northern Theatre Command, Shenyang, China
- 4Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang, China
- 5Department of Radiology, TongDe Hospital of Zhejiang Province, Hangzhou, China
Objective: To quantitatively evaluate the impaired perfusion status of patients with symptomatic internal carotid artery (ICA) steno-occlusive disease and to explore the risk factors of impaired perfusion with computed tomography perfusion (CTP).
Methods: The clinical and imaging data of 187 patients with ICA steno-occlusive disease were retrospectively analyzed. The ICA stenosis rate was divided into Grades I–IV (70–79%; 80–89%; 90–99%; 100%), and the circle of Willis was classified as four types (types I–IV). According to the literature, the value of cerebral blood flow/cerebral blood volume (CBF/CBV) of 7.55/min was used as cut-off to predict symptomatic patients. All patients were categorized into two groups: those with impaired perfusion [n = 99 (52.9%)] and those without impaired perfusion [n = 88 (47.1%)]. Symmetrical bilateral internal watershed areas were selected as the regions of interest (ROIs). Statistical analysis was made on the status of impaired perfusion and the risk factors of impaired perfusion.
Results: Univariate analysis revealed that systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), types of the circle of Willis, and clinical features at admission differed between the two groups (patients with or without impaired perfusion) (p < 0.05). Multiple logistic stepwise regression analysis showed that MAP [odds ratio (OR) = 0.946, 95% confidential interval (CI) = 0.917–0.974, p < 0.001] and type IV (type I vs. IV: OR = 4.987, 95% CI = 1.955–12.723, p = 0.001) at admission were independently associated with impaired perfusion in the internal watershed areas.
Conclusion: MAP and the type of circle of Willis at admission are independent risk factors associated with the impaired perfusion in patients with ICA steno-occlusive disease.
Introduction
Internal carotid artery (ICA) steno-occlusive disease is a leading cause of cerebral ischemia or infarction, especially in the internal watershed areas (1–3). Watershed brain tissues are more sensitive to impaired perfusion than other brain tissues, with the hypoperfusion state being the basis of its pathogenesis. Clinicians quantify the degree of perfusion impairment to identify early brain tissue injury status, transient cerebral ischemia (TIA), or acute brain infraction.
Several studies have identified that hemodynamic impairment of patients with severe stenosis or occlusion of ICA can predict the occurrence of stroke (4–6). Several studies have demonstrated that a decreased cerebral blood flow/cerebral blood volume (CBF/CBV) (<7.6/min) is a validated risk factor for symptomatic stroke (4, 7, 8). Computed tomography perfusion (CTP) is considered to have a similar diagnostic value to positron emission tomography (PET), which is the gold standard for evaluating cerebral hemodynamic impairment (9). CTP is sensitive in finding the abnormal perfusion area in the early stages of cerebral infarction, and can provide relevant hemodynamic functional information through various parameters and their ratios, which is helpful for clinicians to formulate an appropriate treatment plan for patients with ICA steno-occlusive disease. Patients with ICA steno-occlusive disease exhibiting hemodynamic impairment and insufficient collateral circulation could benefit from vascular recanalization surgery (10, 11).
Previous studies mainly focused on predicting the risk factors of stroke, but reports on risk factors affecting brain perfusion are relatively scarce. Moreover, the relationship between the circle of Willis and impaired perfusion had not been adequately investigated. Thus, we aimed to identify the independent risk factors of the impaired perfusion in the internal watershed areas induced by the occurrence of unilateral ICA steno-occlusive disease. This may help to determine the ideal candidates for further intravascular therapy.
Materials and Methods
Patients
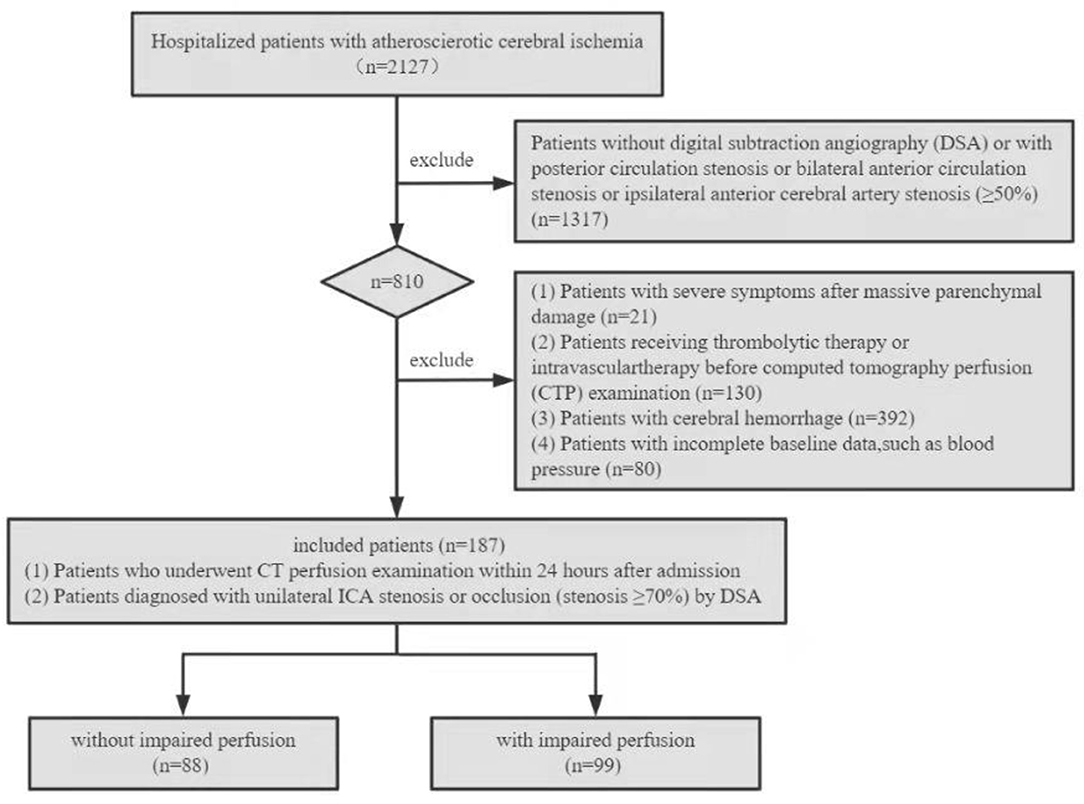
This retrospective study was approved by the Institutional Review Board of the General Hospital of the Northern Theater Command, and the study patients or their family members provided written informed consent. The clinical and imaging data of patients with unilateral ICA steno-occlusive disease from 1 January 2019 to 30 December 2021 were collected and reviewed. The inclusion criteria were specified as follows: (1) patients who underwent CTP within 24 h after admission and (2) patients with unilateral ICA stenosis or occlusion (stenosis ≥70%) diagnosed by digital subtraction anwwgiography (DSA). The following exclusion criteria were specified: (1) patients without DSA or with posterior circulation stenosis or bilateral anterior circulation stenosis or ipsilateral anterior cerebral artery (ACA) stenosis (≥50%); (2) patients with severe symptoms after massive parenchymal damage; (3) patients with previous intravenous or intra-arterial thrombolytic therapy, intracranial or extracranial arterial angioplasty, or stent implantation before CTP; (4) cerebral hemorrhage; and (5) patients with incomplete baseline data, such as blood pressure (BP). Figure 1 shows the flow chart of patient enrollment in this study.
Clinical Information
The following data were collected from each study patient: age, sex, the presence of hypertension, hyperlipidemia, or hyperhomocysteinemia (HHcy), history of diabetes, coronary heart disease (CHD), atrial fibrillation (AF), stroke, current smoking status, alcohol use, left ventricular ejection fraction (LVEF), and admission BP [within 24 h before CTP, including systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP)]. According to the clinical features, patients were divided into two groups: those in the symptomatic group with TIA or contralateral limb dysfunction within 6 months, and those in the asymptomatic group with no definite symptoms of nervous system ischemia or only mild discomfort (12).
Imaging Protocols
Computed tomography perfusion examination was performed using a 64-slice spiral CT system (GE Discovery CT 750 HD, GE, USA), and the auditory-canthus-line was used as the baseline for scanning. A high-pressure injection indwelling needle was placed in the right cubital vein, and a high-pressure syringe was connected for plain CT and CTP. An injection of 40 ml iohexol (350 mg/ml, Xenetix; Guerbet, Aulnay-sous-Bois, France) and 20 ml normal saline was delivered through a high-pressure syringe at 5 ml/s through the cubital vein for CTP imaging. The scanning parameters used included: tube voltage 120 kV, tube current 250 mA, matrix 512 × 512, tube ball rotation time ≤ 1 s, delayed scanning for 5 s, dynamic scanning for 50 s, a layer thickness of 10 mm, 14 layers, and finally 420 whole brain perfusion images were obtained. The NeroPerfusion software package (Aquarius i Ntuition Edition Ver 4.4.6, Tyrus Imaging Company, USA) provided by the Perfusion Workstation was used to evaluate the data from perfusion imaging. The basilar artery and superior sagittal sinus were selected as the input artery and output vein, respectively. The dose length product (DLP) of a patient was 803.04 mGy·cm (13).
Imaging Analysis
Evaluation of ICA Stenosis
Evaluation of ICA stenosis by DSA: According to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (14), the ICA stenosis rate was measured and divided into four grades: Grades I (70–79% stenosis rate), II (80–89% stenosis rate), III (90–99% stenosis rate), and IV (100% stenosis rate).
Measurement of CTP Parameters of Regions of Interest
Regions of interest (ROIs): ROI measurements were based on the two types of internal watershed infarction (IWI) described by classical neuropathological studies, an infarction involving the corona radiata and the other involving the centrum semiovale (15). Because stenosis of the middle cerebral artery (MCA) was more commonly associated with the corona radiata, the centrum semiovale, which is the first layer after the disappearance of bilateral ventricles, was selected for measurement on CTP imaging. The ROIs excluded the following: blood vessels, calcifications, and necrotic tissues.
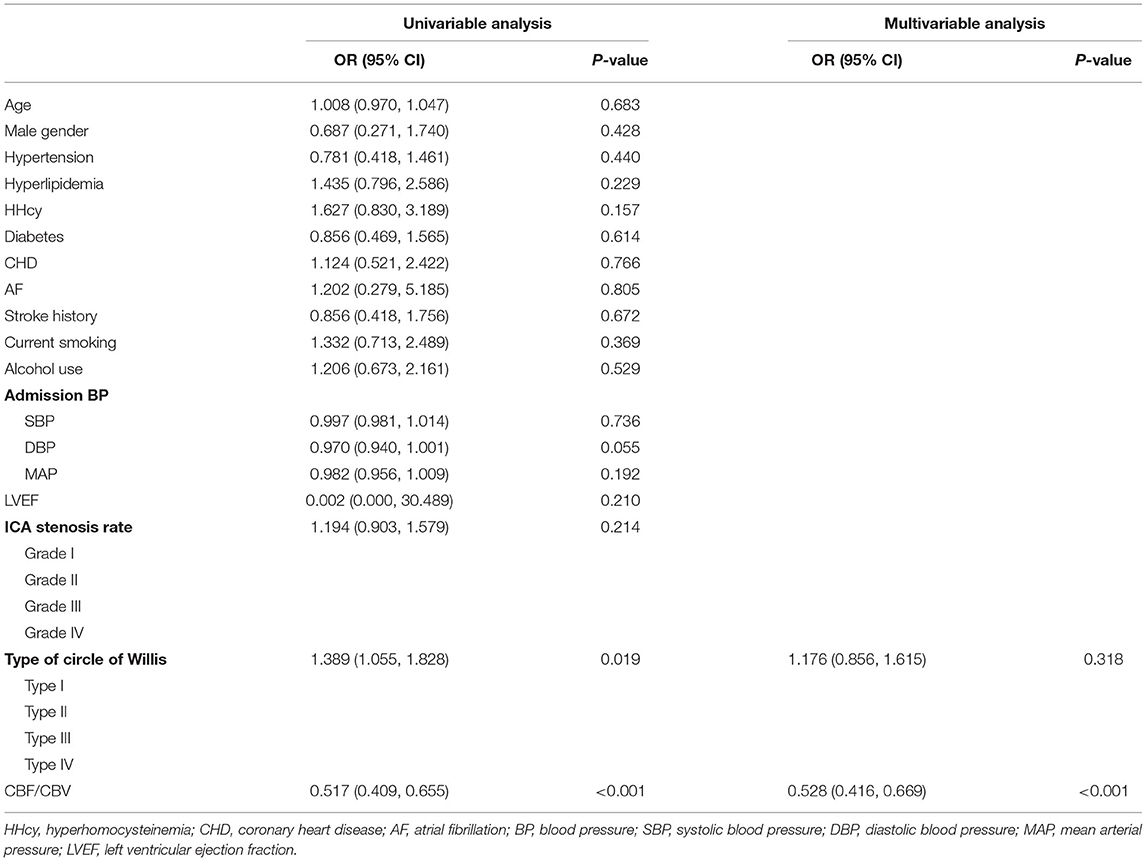
CTP parameters included CBF and CBV. Multiple ROIs (each ROI, 2 cm2) located within the centrum semiovale on the affected side were selected synchronously on the CBF and CBV parameter map, the midline of the brain was considered as the center line, and the symmetrical ROIs on the healthy side were automatically generated using the CTP software (Figure 2) (16–18). The mean value was calculated as the final CTP parameter value of the corresponding ROI.
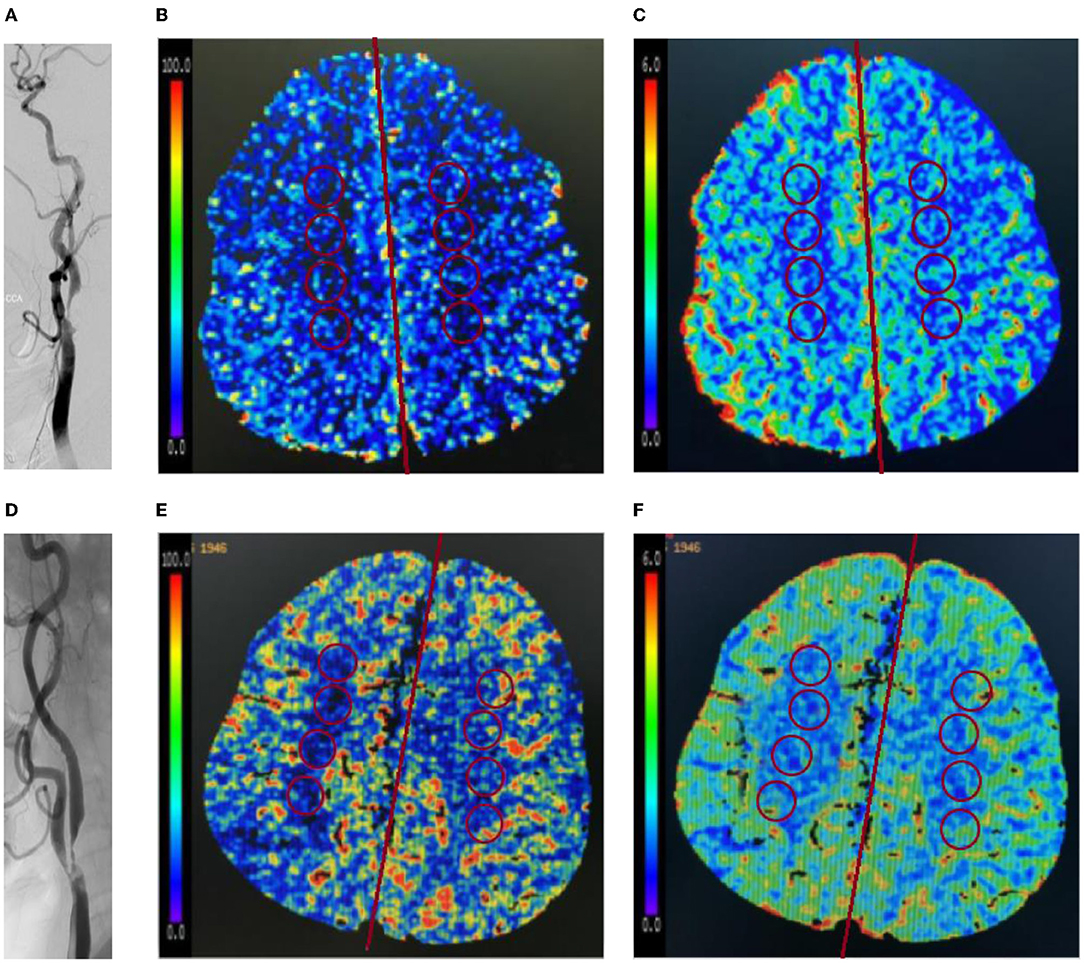
Figure 2. Representative images of patients without or with impaired perfusion. (A–C) A 65-year-old female patient was hospitalized with mild headache and was diagnosed as severe stenosis of the right internal carotid artery (ICA) by digital subtraction angiography (DSA) (A). The patient had no impaired perfusion. In the right internal watershed, cerebral blood flow (CBF) (B) decreased slightly, cerebral blood volume (CBV) (C) increased slightly, and CBF/CBV > 7.55/min. (D–F) A 73-year-old male patient was hospitalized with poor left limb activity, and was diagnosed as severe stenosis of the right ICA by DSA (D). The patient had impaired perfusion. In the right internal watershed, CBF (E) decreased, and CBV (F) decreased slightly, and CBF/CBV < 7.55/min.
In contrast to CBF and CBV taken separately, CBF/CBV would be a more reliable indicator to evaluate the state of cerebral perfusion. The decreased CBF/CBV (<7.55/min) in hemispheres with ICA steno-occlusive disease was categorized as impaired perfusion, which is consistent with the previous research results (7, 8). Based on the “penumbral hypothesis” (8, 19), the patients were divided into two groups: those with impaired perfusion group (CBF/CBV < 7.55/min) and those without impaired perfusion group (CBF/CBV > 7.55/min). The images were independently analyzed by two senior neuroradiologists who were blinded to all the clinical and procedural information of the patients (NZ and MQ). Figure 2 shows ROI measurements of images of a typical patient without impaired perfusion and a typical patient with impaired perfusion.
Definitions of Circle of Willis Morphology
The computed tomography perfusion source imaging (CTP-SI) evaluation of the circle of Willis was based on the classification criteria reported by Hartkamp et al. (20). The classification included four types of the circle of Willis: type I, complete anterior and posterior circulation; type II, incomplete posterior circulation and complete anterior circulation; type III, incomplete anterior circulation and complete posterior circulation; and type IV, incomplete anterior and posterior circulation. If the diameters of the first segment of the anterior cerebral artery (ACA-A1) and the first segment of the posterior cerebral artery (PCA-P1) were <1 mm, indicating its inability to act as collateral circulation, we regarded it as absent. The segments of the anterior communicating artery (ACoA) and posterior communicating artery (PCoA) that were not visible on the CTP-SI were considered as missing (21).
A fetal-type posterior (FTP) cerebral artery is defined as a PCoA originating from the ICA and extending directly to the posterior communicating segment of the ipsilateral posterior cerebral artery (PCA), and the diameter of the PCoA is larger than the P1 segment of the ipsilateral PCA originating from the basilar artery (22).
According to a previous study (23), the variants of the circle of Willis were further divided into the following six subtypes: complete circle of Willis, no ipsilateral PCoA, ipsilateral FTP, no ipsilateral ACA-A1, no ipsilateral PCoA and incomplete anterior circulation, ipsilateral FTP, and incomplete anterior circulation. Other deformities were not considered. Figure 3 shows the morphology variants of the circle of Willis.
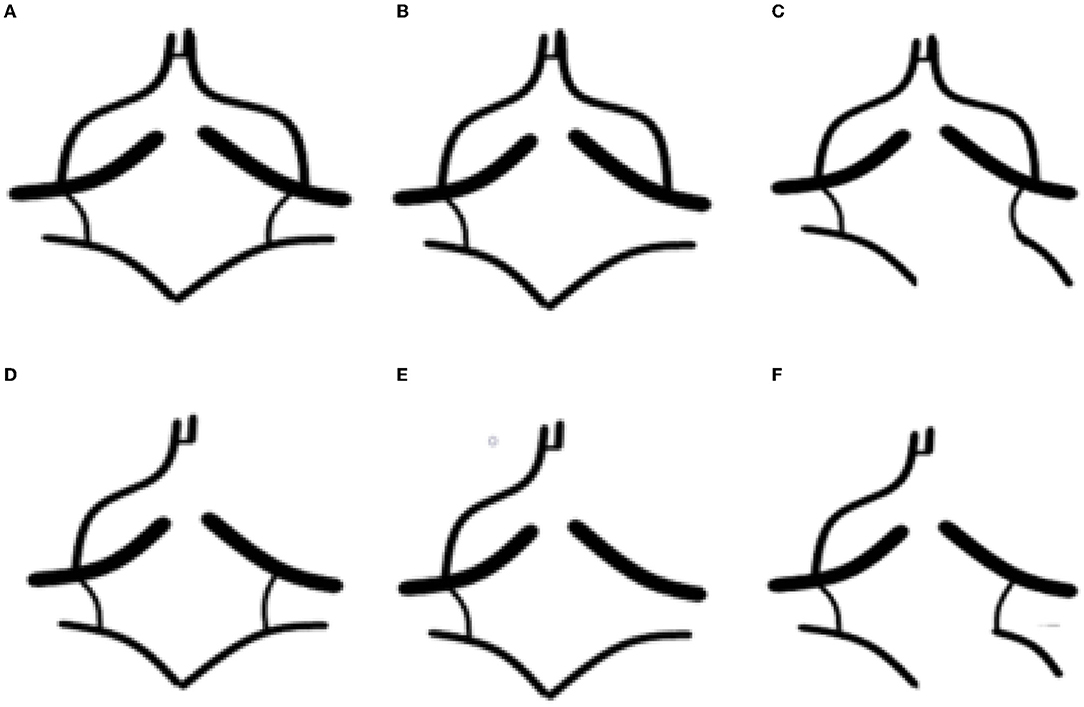
Figure 3. The morphology variants of the circle of Willis in a patient with severe stenosis of left ICA. (A) Complete circle of Willis (type I); (B) no ipsilateral posterior communicating artery (PCoA) (type II); (C) ipsilateral fetal-type posterior cerebral artery (FTP) (type II); (D) no ipsilateral in the first segment of the anterior cerebral artery (ACA-A1) (type III); (E) no ipsilateral PCoA and incomplete anterior circulation (type IV); and (F) ipsilateral fetal-type posterior (FTP) and incomplete anterior circulation (type IV).
Statistical Analysis
Data were analyzed by using the Statistical Package for Social Sciences for Windows, Version 20 (IBM Corporation, Armonk, NY, USA). Normally distributed data were described as means and standard deviation (SD). Categorical variables were described as frequency or percentage. Independent sample t-test was used for comparisons of measurements that were consistent with normal distribution. The Pearson chi-squared test and Fisher's exact test were used to analyze categorical variables. Univariate and multivariate logistic regression analyses were applied for CTP parameters in healthy and affected sides. Univariate and multivariate logistic regression analyses were applied for CTP parameters and clinical data in the symptomatic group and the asymptomatic group. Multiple logistic stepwise regression analysis was performed to identify independent risk factors associated with impaired perfusion. Univariate analysis of variance (ANOVA) was used to compare the differences of the CBF/CBV value among different vascular variants of the circle of Willis, and then the least significant difference (LSD) method was used for multiple pairwise comparisons. Interobserver agreement was assessed using a single measure statistic (intraclass correlation efficient, ICC). ICC > 0.75 was considered as excellent (24). Statistical significance was defined when p < 0.05.
Results
In this study, there were 187 patients (men, 167) with unilateral ICA steno-occlusive disease (mean age, 65 ± 8 years). Among them, 88 were in the group without impaired perfusion (34 were symptomatic), and 99 were in the group with impaired perfusion (75 were symptomatic).
Of 109 symptomatic patients, the mean CBF of affected and healthy sides was 10.30 ± 3.07 and 14.55 ± 4.07 ml/100 g/min; the mean CBV of affected and healthy sides was 1.54 ± 0.49 and 1.54 ± 0.49 ml/100 g; and the mean CBF/CBV of affected and healthy sides was 6.85 ± 1.44/min and 9.74 ± 2.07/min, respectively.
Of 78 asymptomatic patients, the mean CBF of affected and healthy sides was 13.00 ± 3.93 and 15.23 ± 4.39 ml/100 g/min; the mean CBV of affected and healthy sides was 1.43 ± 0.34 and 1.50 ± 0.41 ml/100 g; and the mean CBF/CBV of affected and healthy sides was 9.22 ± 2.98/min and 10.43 ± 2.56/min, respectively.
The degree of agreement between readers was excellent for CBF (ICC = 0.957) and CBV (ICC = 0.960).
Univariate and Multivariate Analyses of Factors Associated With Impaired Perfusion
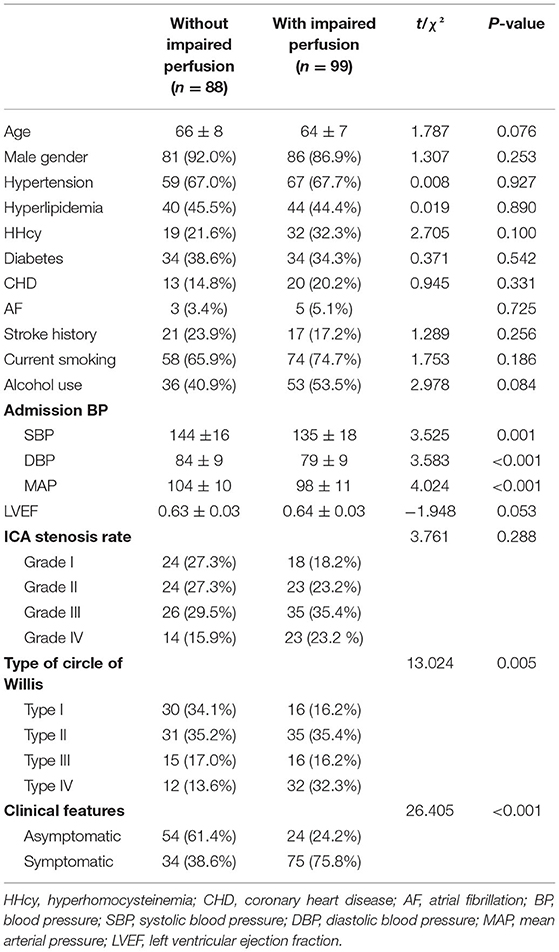
Univariate analysis identified the differences between the two patient groups (without/with impaired perfusion), with respect to admission BP (SBP, DBP, and MAP), types of the circle of Willis, and clinical features (p < 0.05). No differences in age, sex, hypertension, hyperlipidemia, HHcy, diabetes, CHD, AF, stroke, current smoking status, alcohol use, ICA stenosis rate, and LVEF were found between the two groups (p > 0.05) (Table 1).
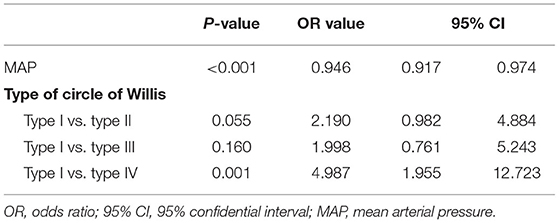
Multiple logistic stepwise regression analysis showed that admission MAP [odds ratio (OR) = 0.946, 95% confidential interval (CI) = 0.917–0.974, p < 0.001] and the type of circle of Willis [type IV (type I vs. type IV: OR = 4.987, 95% CI = 1.955–12.723, p = 0.001)] were independently associated with impaired perfusion of the internal watershed (Table 2).
Comparison of the CTP Parameters Between Healthy and Affected Sides
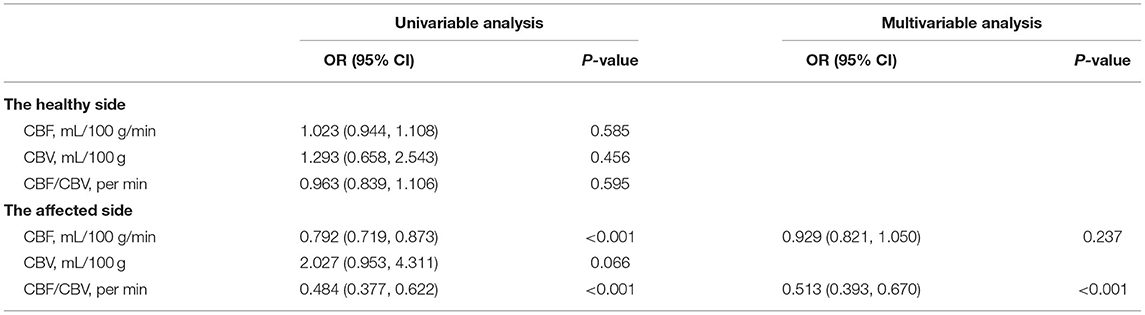
From the univariate and multiple logistic regression analyses, CBF and CBF/CBV in the affected side of symptomatic patients were significantly lower than those in the healthy side of symptomatic patients (OR = 0.807, 95% CI = 0.723–0.902, p < 0.001; OR = 0.408, 95% CI = 0.309–0.539, p < 0.001) (Table 3).
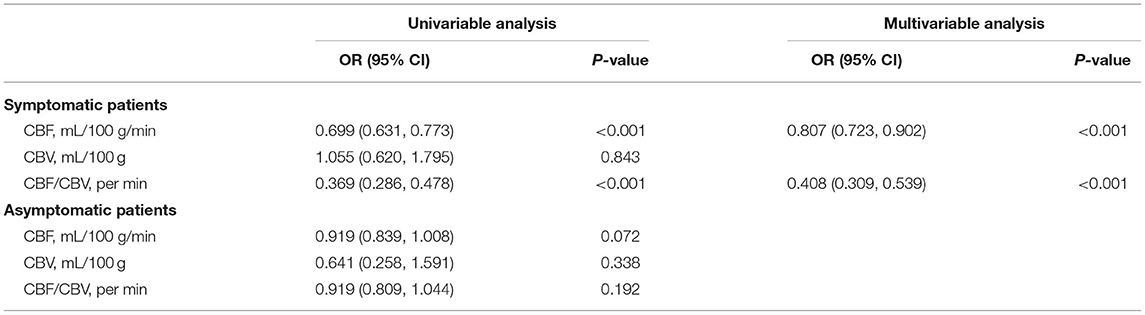
Table 3. Comparison of computed tomography perfusion (CTP) parameters between healthy and affected sides.
Univariate and Multivariate Analyses of Factors Associated With Symptomatic Patients
The CBF/CBV of the affected side is lower in the symptomatic group than in the asymptomatic group (OR = 0.513, 95% CI = 0.393–0.670, p < 0.001) (Table 4). Univariate and multiple logistic regression suggested that decreased CBF/CBV was independently associated with symptomatic patients (OR = 0.528, 95% CI = 0.416–0.669, p < 0.001) (Table 5).
The CBF/CBV value of 7.55/min was the best cut-off value for predicting symptomatic patients as shown by the receiver operating characteristic (ROC; area under the curve (AUC): 0.800, sensitivity: 0.690, and specificity: 0.747) (Figure 4).
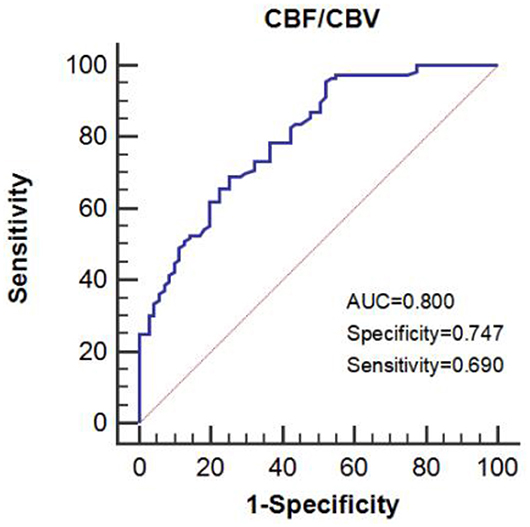
Figure 4. The area under the receiver operating characteristic (ROC) curve predicts impaired perfusion in patients with symptomatic ICA steno-occlusive disease.
CBF/CBV Value and Different Variants of the Circle of Willis
Univariate ANOVA showed that there were significant differences in the CBF/CBV of patients with different vascular variants of the circle of Willis (F = 3.131, p = 0.010). Pairwise comparisons indicated that the CBF/CBV in patients with an ipsilateral FTP and patients with an ipsilateral FTP and incomplete anterior circulation was significantly lower than that in patients with a complete circle of Willis (7.03 ± 1.69 vs. 8.69 ± 2.53, p = 0.004 and 6.74 ± 2.37 vs. 8.69 ± 2.53, p = 0.002) (Figure 5).
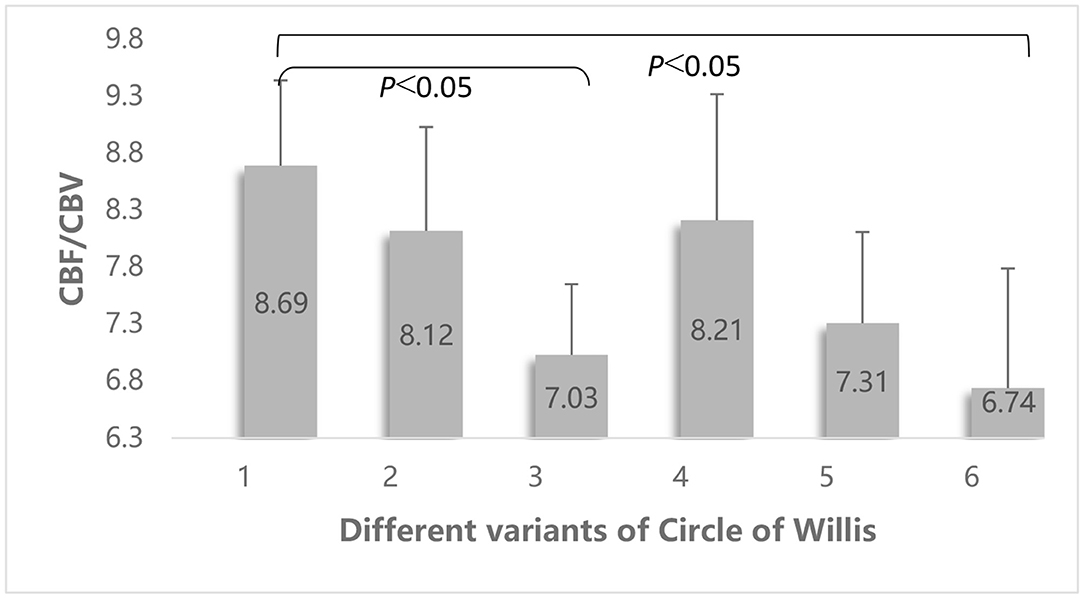
Figure 5. Comparison of the differences of the CBF/CBV value among different vascular variants of the circle of Willis. Different vascular variants of the circle of Willis: 1 = no variation; 2 = no ipsilateral PCoA; 3 = ipsilateral FTP; 4 = no ipsilateral ACA-A1; 5 = no ipsilateral PCoA and incomplete anterior circulation; 6 = ipsilateral FTP and incomplete anterior circulation.
Discussion
The evaluation of the status of cerebral perfusion in patients with a stenotic or occluded ICA before treatment is essential. A few studies have shown that patients with impaired perfusion in the hemisphere ipsilateral to a stenotic ICA may have an increased risk of ischemic stroke than those with normal perfusion (25, 26). Our study found that in symptomatic patients CBF and CBF/CBV in the affected hemisphere was lower than that in the healthy hemisphere. Compared with asymptomatic patients, the CBF/CBV value of symptomatic patients with unilateral ICA steno-occlusive disease decreased correspondingly. Our study also found that lower MAP levels on admission and the type of circle of Willis (type IV) were independent risk factors of impaired perfusion when the unilateral ICA was severely stenotic or occluded. Moreover, we also assessed the FTP variants that were associated with decreased CBF/CBV.
In this study, impaired perfusion in the internal watershed area was associated with decreased MAP levels. This finding is consistent with that of Yamauchi's finding that effective BP management could facilitate reperfusion and play a protective role in ischemic brain tissue (7). As an index of local cerebral perfusion pressure, CBF/CBV is directly related to the systemic MAP. Within a certain MAP range, the relationships between CBF/CBV and MAP appear to be linear (8). A decrease in BP may directly lead to a further decrease in the perfusion of the cerebrum that was originally hypoperfused, thus eventually lead to cerebral ischemia or infarction, especially in the internal watershed area that was already sensitive to changes in BP. A few studies have shown that BP management is a vital component of the treatment of hypoperfusion cerebral infarction (27–29). However, in these studies, the specific range of BP that is considered moderate was not clearly defined. Using the Cox regression analysis, Wohlfahrt (30) found that patients with an admission MAP < 100 mm Hg may be at an increased risk of hemodynamic stroke. Maddula (31) reported that the maintenance of a SBP ranging 130–150 mm Hg was effective in avoiding cerebral hypoperfusion.
From the findings of our study, we think that impaired perfusion was not associated with the ICA stenosis rate, but impaired perfusion was associated with the type of circle of Willis (type IV) and FTP. Thus, we hypothesized that when the unilateral ICA was severely stenosed or occluded, the restricted ipsilateral carotid blood flow might be compensated by the circle of Willis and an adaptive expansion of cerebral vessels. The carotid stenosis rate may not fully reflect the cerebral perfusion situation or an ischemic event (32). As we know, the internal watershed area is located deep in the white matter, which is in the terminal zone of the ICA, the compensation provided by the leptomeningeal collateral circulation is insufficient, which is mainly compensated by the primary collateral circulation. Whenever the ICA is stenosed or occluded, a circle of Willis with an abnormal morphology increases the risk of cerebral ischemia or infarction (33, 34). Theoretically, a complete circle of Willis can reperfuse the ischemic region before irreversible damage occurs. The compensatory effect on the internal watershed areas remains uncertain because of insufficient research on this area. Our study found that an excellent primary collateral circulation was protective of the internal watershed area, and that decreased CBF/CBV in the internal watershed area was strongly associated with type IV circle of Willis.
Medullary arterioles in the watershed areas showed insufficient self-regulation, and there was no compensatory dilation of pial vessels. If a patient had an incomplete circle of Willis, the redistribution of blood flow would be affected and result in a markedly negative effect (35).
However, variants of the circle of Willis are diverse and the compensatory capacity of different variations also varies. Therefore, we also evaluated the effect of single-vessel variation on CBF/CBV. In this study, we suggest that the presence of an FTP, with or without an incomplete anterior circulation, was a risk factor for impaired perfusion in the internal watershed area. On the single side of the FTP, the ICA supplies blood to both MCA and PCA, which affects the onset of a secondary collateral flow between the ICA and vertebrobasilar arterial system. This leads to a bilaterally imbalanced CBF, and the perfusion pressure of the affected side is relatively lower than that of the opposite side.
Patients with unilateral ICA steno-occlusive disease, combined with an ipsilateral FTP, have been reported to exhibit a marked reduction in the ipsilateral cerebral perfusion pressure. In addition, they are more likely to show decompensation and infarction involving watershed areas (36). Bisschops (37) concluded that an ACoA deficiency increased the incidence of IWI in patients with a stenotic ICA. This event might be related to the poor collateral compensation between the cortical branch of the MCA and the lenticular artery or between the MCA and superficial cortical branches of the ACA. However, unlike in our study, the FTP was not analyzed by Bisschops.
Previous studies have reported that ICA with severe stenosis or occlusion combined with elevated blood viscosity and an elevated risk of clotting may lead to a cerebral watershed infarction (CWSI) (15). However, in our study, there were no significant differences in the degree of hyperlipidemia and HHcy between the two groups. Probably, these factors promoted the occurrence of atherosclerosis by increasing the changes of blood viscosity but did not directly affect cerebral perfusion in our study.
Our results demonstrate that quantitative evaluation of the cerebral perfusion status and risk factors of impaired perfusion in patients with symptomatic unilateral ICA steno-occlusive disease is clinically relevant. If a subgroup of high-risk patients could be correctly identified by clinical doctors, the risk of irreversible cerebral infarction could be reduced. In addition, relevant studies have found that, when brain tissues have weak tolerance to ischemia and hypoxia, it is more likely to have more severe cerebral ischemia or infarction under the influence of ischemic intervention factors (38).
This study had some limitations. The study's sample size was relatively small, and the enrollment bias could not be avoided. A large sample in multiple centers is needed in future studies. Moreover, this study did not consider the effect of mild cerebral vascular stenosis on cerebral perfusion. Finally, BP was measured once at admission and included in the analysis, making it difficult to exclude the fluctuation of BP. A 24-h ambulatory BP should be considered for future studies.
In conclusion, admission MAP and the type of circle of Willis were found to be independent risk factors associated with the internal watershed impaired perfusion in patients with unilateral ICA steno-occlusive disease. Variants in the FTP were also associated with decreased CBF/CBV.
Conclusion
MAP and the type of circle of Willis at admission may be independent risk factors associated with the impaired perfusion in patients with ICA steno-occlusive disease.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of General Hospital of Northern Theater Command. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XQ, JD, and ZX conceived the project idea and wrote the manuscript. XW and YD provided critical suggestions for the design of experiments. YP, NZ, MQ, and JL collected the imaging and clinical data. ZX and BY supervised the project. All authors contributed to this article and approved the submitted version.
Funding
This study was supported by Grant No. 202054044 from the Project of Natural Science Foundation of Shenyang and by Grant No. 201602768 from the Project of Natural Science Foundation of Liaoning province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsutsui S, Nanba T, Yoshioka Y, Sasaki M, Fujiwara S, Kobayashi M, et al. Preoperative brain temperature imaging on proton magnetic resonance spectroscopy predicts hemispheric ischemia during carotid endarterectomy for unilateral carotid stenosis with inadequate collateral blood flow. Neurol Res. (2018) 40:617–23. doi: 10.1080/01616412.2018.1457130
2. Hossmann KA, Heiss WD. History of the Letzte Wiese/last meadow concept of brain ischemia. Stroke. (2016) 47:e46–50. doi: 10.1161/STROKEAHA.115.010976
3. Bladin CF, Chambers BR. Clinical features, pathogenesis, and computed tomographic characteristics of internal watershed infarction. Stroke. (1993) 24:1925–32. doi: 10.1161/01.STR.24.12.1925
4. Derdeyn CP. Hemodynamics and oxygen extraction in chronic large artery steno-occlusive disease: clinical applications for predicting stroke risk. J Cereb Blood Flow Metab. (2018) 38:1584–97. doi: 10.1177/0271678X17732884
5. Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. (2002) 125:595–607. doi: 10.1093/brain/awf047
6. Derdeyn CP, Grubb RL Jr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. (1999) 53:251–9. doi: 10.1212/WNL.53.2.251
7. Yamauchi H, Kagawa S, Kishibe Y, Takahashi M, Higashi T. Misery perfusion, blood pressure control, and 5-year stroke risk in symptomatic major cerebral artery disease. Stroke. (2015) 46:265–8. doi: 10.1161/STROKEAHA.114.007134
8. Schumann P, Touzani O, Young AR, Morello R, Baron JC, MacKenzie ET. Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain. (1998) 1998:1369–79. doi: 10.1093/brain/121.7.1369
9. Wright EA, d'Esterre CD, Morrison LB, Cockburn N, Kovacs M, Lee T-Y. Absolute cerebral blood flow infarction threshold for 3-hour ischemia time determined with CT perfusion and 18F-FFMZ-PET imaging in a porcine model of cerebral ischemia. PLoS ONE. (2016) 11:158–157. doi: 10.1371/journal.pone.0158157
10. Sebök M, Esposito G, Niftrik CHB, Fierstra J, Schubert T, Wegener S, et al. Flow augmentation STA-MCA bypass evaluation for patients with acute stroke and unilateral large vessel occlusion: a proposal for an urgent bypass flowchart. J Neurosurg. (2022). 2022:1–9. doi: 10.3171/2021.10.JNS21986
11. Naylor AR, Ricco JB. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:3–81. doi: 10.1016/j.ejvs.2018.03.023
12. Nariai T, Matsushima Y, Imae S, Tanaka Y, Ishii K, Senda M, et al. Severe haemodynamic stress in selected subtypes of patients with moyamoya disease: a positron emission tomography study. J Neurol Neurosurg Psychiatry. (2005) 76:663–9. doi: 10.1136/jnnp.2003.025049
13. Zensen S, Guberina N, Opitz M, Köhrmann M, Deuschl C, Forsting M, et al. Radiation exposure of computed tomography imaging for the assessment of acute stroke. Neuroradiology. (2021) 63:511–8. doi: 10.1007/s00234-020-02548-z
14. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
15. Mangla R, Kolar B, Almast J, Ekholm SE. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics. (2011) 31:1201–14. doi: 10.1148/rg.315105014
16. Yamauchi H, Higashi T, Kagawa S, Kishibe Y, Takahashi M. Impaired perfusion modifies the relationship between blood pressure and stroke risk in major cerebral artery disease. J Neurol Neurosurg Psychiatry. (2013) 84:1226–32. doi: 10.1136/jnnp-2013-305159
17. d'Esterre CD, Aviv RI, Lee TY. The evolution of the cerebral blood volume abnormality in patients with ischemic stroke: a CT perfusion study. Acta Radiol. (2012) 53:461–7. doi: 10.1258/ar.2012.110582
18. Kim JH, Lee EJ, Lee SJ, Choi NC, Lim BH, Shin T. Comparative evaluation of cerebral blood volume and cerebral blood flow in acute ischemic stroke by using perfusion-weighted MR imaging and SPECT. Acta Radiol. (2002) 43:365–70. doi: 10.1034/j.1600-0455.2002.430404.x
19. Campbell BCV, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. (2011) 42:3435–40. doi: 10.1161/STROKEAHA.111.618355
20. Hartkamp MJ, van Der GJ, van EKJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. (1999) 30:2671–8. doi: 10.1161/01.STR.30.12.2671
21. Hindenes LB, Håberg AK, Johnsen LH, Mathiesen EB, David R, Vangberg TR. Variations in the Circle of Willis in a large population sample using 3D TOF angiography: the Tromsø Study. PLoS ONE. (2020) 15:e0241373. doi: 10.1371/journal.pone.0241373
22. Hsu CF, Chen KW, Su CH, Shen CY, Chi HY. Bilateral vertebral artery hypoplasia and fetal-type variants of the posterior cerebral artery in acute ischemic stroke. Front Neurol. (2021) 12:582149. doi: 10.3389/fneur.2021.582149
23. Westphal LP, Lohaus N, Winklhofer S, Manzolini C, Held U, Steigmiller K, et al. Circle of Willis variants and their association with outcome in patients with middle cerebral artery-M1-occlusion stroke. Eur J Neurol. (2021) 28:3682–91. doi: 10.1111/ene.15013
24. Mcgraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. (1996) 1:390. doi: 10.1037/1082-989X.1.4.390
25. Yamauchi H, Higashi T, Kagawa S, Kishibe Y, Takahashi M. Chronic hemodynamic compromise and cerebral ischemic events in asymptomatic or remote symptomatic large-artery intracranial occlusive disease. Am J Neuroradiol. (2013) 34:1704–10. doi: 10.3174/ajnr.A3491
26. Taylor RA, Kasner SE. Natural history of asymptomatic intracranial arterial stenosis. J Neuroimaging. (2009) 19:17–9. doi: 10.1111/j.1552-6569.2009.00416.x
27. Jiang B, Churilov L, Kanesan L, Dowling R, Mitchell P, Dong Q, et al. Blood pressure may be associated with arterial collateralization in anterior circulation ischemic stroke before acute reperfusion therapy. Stroke. (2017) 19:222–8. doi: 10.5853/jos.2016.01739
28. Goyal N, Tsivgoulis G, Iftikhar S, Khorchid Y, Fawad IM, Doss VT, et al. Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. Neurointery Surg. (2017) 9:451–4. doi: 10.1136/neurintsurg-2016-012386
29. Goyal N, Tsivgoulis G, Pandhi A, Chang JJ, Dillard K, Ishfaq MF, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. (2017) 89:540–7. doi: 10.1212/WNL.0000000000004184
30. Wohlfahrt P, Krajcoviechova A, Jozifova M, Mayer O, Vanek J, Filipovsky J, et al. Low blood pressure during the acute period of ischemic stroke is associated with decreased survival. Hypertens. (2015) 33:339–45. doi: 10.1097/HJH.0000000000000414
31. Maddula M, Sprigg N, Bath PM, Munshi S. Cerebral misery perfusion due to carotid occlusive disease. Stroke Vasc Neurol. (2017) 2:88–93. doi: 10.1136/svn-2017-000067
32. Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. (2000) 31:128–32. doi: 10.1161/01.STR.31.1.128
33. Zhou H, Sun J, Ji X, Lin J, Tang S, Zeng J, et al. Correlation between the integrity of the circle of willis and the severity of initial noncardiac cerebral infarction and clinical prognosis. Medicine. (2016) 95:e2892. doi: 10.1097/MD.0000000000002892
34. Jaramillo A, Góngora-RF, Labreuche J, Hauw J-J, Amarenco P. Predictors for malignant middle cerebral artery infarctions: a postmortem analysis. Neurology. (2006) 66:815–20. doi: 10.1212/01.wnl.0000203649.60211.0e
35. Mione G, Pische G, Wolff V, Tonnelet R, Humbertjean L, Richard S. Perioperative bioccipital watershed strokes in bilateral fetal posterior cerebral arteries during spinal surgery. World Neurosurg. (2016) 85:17–21. doi: 10.1016/j.wneu.2015.09.098
36. Wentland AL, Rowley HA, Vigen KK, Field AS. Fetal origin of the posterior cerebral artery produces left——right asymmetry on perfusion imaging. Am J Neuroradiol. (2010) 31:448–53. doi: 10.3174/ajnr.A1858
37. Bisschops RHC, Klijn CJM, Kappelle LJ, van Huffelen AC, van der GJ. Collateral flow and ischemic brain lesions in patients with unilateral carotid occlusion. Neurology. (2003) 60:1435–41. doi: 10.1212/01.WNL.0000061616.96745.90
Keywords: internal carotid artery, CT perfusion, impaired perfusion, internal watershed, circle of Willis
Citation: Qiao X, Duan J, Zhang N, Duan Y, Wang X, Pei Y, Xu Z, Yang B, Qi M and Li J (2022) Risk Factors of Impaired Perfusion in Patients With Symptomatic Internal Carotid Artery Steno-Occlusive Disease. Front. Neurol. 13:801413. doi: 10.3389/fneur.2022.801413
Received: 25 October 2021; Accepted: 14 March 2022;
Published: 14 April 2022.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Hyun Seok Choi, Seoul Medical Center, South KoreaMarialuisa Zedde, IRCCS Local Health Authority of Reggio Emilia, Italy
Alexander Seiler, Goethe University Frankfurt, Germany
Copyright © 2022 Qiao, Duan, Zhang, Duan, Wang, Pei, Xu, Yang, Qi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihua Xu, eHV6aGlodWEwMDFAeWVhaC5uZXQ=; Benqiang Yang, YnF5YW5nODg4QHNpbmEuY29t
†These authors have contributed equally to this work
 Xinxin Qiao1,2†
Xinxin Qiao1,2† Jinfeng Duan
Jinfeng Duan Yang Duan
Yang Duan Xinrui Wang
Xinrui Wang Zhihua Xu
Zhihua Xu Benqiang Yang
Benqiang Yang