
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 20 May 2022
Sec. Neurorehabilitation
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.793253
Transcranial Magnetic Stimulation (TMS) has widespread use in research and clinical application. For psychiatric applications, such as depression or OCD, repetitive TMS protocols (rTMS) are an established and globally applied treatment option. While promising, rTMS is not yet as common in treating neurological diseases, except for neurorehabilitation after (motor) stroke and neuropathic pain treatment. This may soon change. New clinical studies testing the potential of rTMS in various other neurological conditions appear at a rapid pace. This can prove challenging for both practitioners and clinical researchers. Although most of these neurological applications have not yet received the same level of scientific/empirical scrutiny as motor stroke and neuropathic pain, the results are encouraging, opening new doors for TMS in neurology. We here review the latest clinical evidence for rTMS in pioneering neurological applications including movement disorders, Alzheimer's disease/mild cognitive impairment, epilepsy, multiple sclerosis, and disorders of consciousness.
Transcranial magnetic stimulation (TMS) is a non-invasive, safe and painless procedure to activate or modulate cortical targets in the central nervous system (CNS) (1, 2). TMS is based on Faraday's law of electromagnetic induction, whereby an electrical current is discharged into a TMS coil, generating a perpendicular magnetic field that transcranially and thus noninvasively reaches the brain where it, due to its time-varying characterstics, generates an electric field and electrical currents in the targeted brain tissue (3, 4). If sufficiently strong, such induced electrical currents depolarize the neurons and result in TMS-induced action potentials (neural firing) measurable with electroencephalogram (EEG) (5) and/or with motor evoked potentials(MEPs) (2, 6), or also indirectly with functional magnetic resonance imaging (fMRI) (7).
The effects of rTMS on cortical excitability depend on the precise parameters selected in the so-called rTMS protocols (Table 1) as well as coil geometry (Table 2) (8). As a rule of thumb, low frequency [LF ≤ 1 hertz (Hz)] rTMS decreases cortical excitability and high frequency rTMS (HF ≥ 5–20 Hz) increases excitability (6). For example, when applied to the motor cortex, LF-TMS reduces MEP amplitudes and increases the duration of the cortical silent period, while HF-rTMS leads to opposite effects.
TMS helps to study the neural pathways in various CNS pathologies. Single pulse TMS (sTMS) evokes immediate sensory or motor responses and can therefore help assess the efficacy or speed of conduction of a particular neural pathway (1). Repetitive TMS (rTMS) modulates brain function in such a way that effects last beyond the period of stimulation. The magnetic and electrical fields generated by rTMS bring about many changes in the human brain that may confer therapeutic benefit (2). For instance, since rTMS can have lasting effects on cortical excitability through induced synaptic plasticity mechanisms, it is likely to help in the treatment of various psychiatric and CNS disorders where cortical excitability is one of the primary underlying pathologies (1, 2, 6, 8, 13).
The therapeutic potential and applications of rTMS have received much attention in recent years. Especially in the field of psychiatry, rTMS is now a widely recognized and applied treatment option for the therapy of major depressive disorder (14, 15) or obsessive compulsive disorder (16, 17) and shown to be clinically effective and reimbursed by health insurances (18, 19).
The clinical applications of TMS seem to be less successfully applied in neurology, which is somehow surprising and not necessarily straightforward, as TMS is deeply rooted as a diagnostic technique in neurology and clinical neurophysiology (20–23). It is therefore encouraging to see that in the recent update of the evidence-based guidelines by Lefaucheur (24) on the therapeutic use of rTMS, two neurological applications (neurorehabilitation after motor stroke and the treatment of neuropathic pain) received the highest level of evidence rating, namely “level A evidence” (definite efficacy). This rating was on par with the rating used for TMS application in depression treatment.
There are other currently applied rTMS treatments in neurology, for instance in the acute treatment of migraine and migraine prevention, non-motor stroke, other CNS pain syndromes, and H-coil deep TMS for poststroke aphasia (6, 25, 26). But beyond motor stroke and neuropathic pain, few rTMS protocols and applications have yet received the same level of scientific/emperical scrutiny. For some potential applications, there is no recommendation, or classification of efficacy, based on evidence from randomized controlled trials, systematic reviews and meta-analyses (27). However, it is important to realize that the lack of a formal recommendation, or classification of efficacy, does not necessarily mean that an rTMS application has no promise. Sometimes, the lack of such a recommendation is due to underpowered, or inconsistent, evidence from clinical studies. However, other times, the evidence that exists is in fact very promising, but not yet of sufficient size or scope that recommendations are warranted. It is therefore crucial to continue monitoring the state of evidence for these less investigated yet pioneering rTMS applications. Here, we focus on those “other” or “underinvestigated” neurological disorders, by reviewing the latest clinical evidence for the potential of rTMS in the treatment of movement disorders, Alzheimer's disease/mild cognitive impairment (MCI), epilepsy, multiple sclerosis (MS), and disorders of consciousness.
General literature search, Google Scholar and MEDLINE search was carried out until December 25, 2020, by using the following search terms in various combinations: “transcranial magnetic stimulation,” “treatment,” “neurological diseases,” “Alzheimer's disease,” “Parkinson's disease,” “post stroke,” “multiple sclerosis,” “epilepsy,” “dystonia,” “Tourette syndrome,” “chronic tic disorders,” “Huntington's disease,” “choreas.” Only English language publications covering therapeutic benefits and challenges of TMS in neurological conditions of the CNS were considered. Literature covering pathophysiological and diagnostics aspects of TMS were excluded. Similarly studies looking at therapeutic benefits and challenges in neurobehavioral, psychiatric and chronic pain conditions were not included in the narrative review.
rTMS has been shown to bring about some level of improvement in movement disorders, such as Parkinson's disease (PD), dystonia, Huntington's disease, and Tourette syndrome (Table 3) (8).
It has been suggested by experimental research that changes in neurotransmitter release, transsynaptic efficiency, signaling pathways and gene transcription are induced by rTMS (58–62). Additionally, current research suggests that repetitive transcranial magnetic stimulation (rTMS) stimulates neurogenesis, neuronal survival, and the release of neuroprotective chemicals in Parkinson's disease patients (58, 63–65). One possible mechanism of action may relate to high-frequency rTMS-enhanced activity in the caudate nucleus as well as a relief of dopamine deficiency in nigrostriatal-thalamo-cortical circuitry (66–68). For instance, rTMS over M1 seems to affect dopamine release in nigrostriatal regions (24, 69).
Literature shows that rTMS could potentially be used as an important adjunctive treatment for PD (Table 3) (8, 34, 35, 70). Bradykinesia and tremor are two of the most debilitating motor symptoms in PD and thought to be related to abnormal oscillations in the subthalamic nucleus (STN) (71). Literature suggests that rTMS, especially the bilateral delivery over motor cortical regions, helps in improving motor symptoms (8, 13, 24, 27, 70). In these patients, favorable targets for high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) include primary motor cortex (M1), less focal motor cortex (MC) stimulation such as to leg or bilateral hand MC, and dorsolateral prefrontal cortex (DLPFC), while supplementary motor area (SMA) was found to be the most favorable low-frequency repetitive transcranial magnetic stimulation (LF-rTMS target) (8, 13, 70). rTMS to these targets has also been found effective for levodopa-induced dyskinesia (LID) (37). However, Lefaucheur (24) felt that these benefits were sometimes the results of a single session, and the prolonged clinical benefit needs to be investigated. Additionally, literature reporting the beneficial effect of HF-rTMS of the left DLPFC in treating non-motor depressive symptoms in PD has been covered in many review articles (8, 13, 70). However, a randomized trial failed to show any significant benefit in mood upliftment (24, 72).
In their evidence based guidelines on the therapeutic use of rTMS, Lefaucheur (24) suggest that of the various targets studied, M1 stimulation may be recommended for treating motor symptoms in PD with repeated HF-rTMS. A large double-cone coil applied to M1 leg area may help improve freezing-of-gait. However, specific recommendations for the use of rTMS in PD could not be made without further research.
Repetitive transcranial magnetic stimulation (rTMS) has been suggested as a potential treatment for cognitive impairment in Parkinson's disease (PD), with effects that appear to be additive to dopaminergic medicines (73). While it is difficult to pinpoint the exact role of pathological neural oscillations in certain aspects of motor and cognitive function, current research clearly suggests that these pathological oscillations interact and contribute to the motor and cognitive deficits seen in Parkinson's disease (74). Another study found that repetitive transcranial magnetic stimulation (rTMS) over (motor region) M1 is beneficial for motor function and may have a slight favorable effect on cognition (73). The efficacy of TMS on depression and cognition in Parkinson's disease has yielded promising preliminary results. Although it is unknown if these effects are transient, what the underlying processes are, and if such neuromodulation might transfer to real-world settings, a small study found that TMS can improve working memory in PD patients (75).
The exact mechanisms of action of rTMS in alleviating dystonia remain unknown (76–80). Although motor cortex hyperexcitability appears to be the cause of aberrant co-contraction and overflow to adjacent muscles, several studies have shown that plasticity processes and integrated sensorimotor processing are also likely to be involved (76, 78, 81, 82).
Cortical hyperexcitability in dystonia is thought to be caused by two abnormalities in the sensorimotor system (83). The inhibitory systems are less excited and there is an increase in the plasticity of neural connections. Hence, rTMS may be a useful therapeutic tool for dystonia if it can increase intracortical inhibition and reduce excessive cortical plasticity. LF-rTMS and cTBS protocols (continuous theta burst stimulation; a patterned inhibitory rTMS protocol thought to have analogous effects to LF-rTMS) have been investigated in dystonia by targeting M1, PMC, SMA, primary somatosensory cortex, and cerebellum. Different protocols were used for writer's cramp and craniocervical dystonia (Table 3) (84). A literature review by Erro et al. (84) found mixed evidence of benefit. While some studies reported short-lasting objective or subjective improvement in dystonia, others did not (Table 3).
The dopaminergic system, particularly in the frontal brain, can be affected by rTMS. TMS can cause an increase in the flow of dopamine to numerous parts of the brain, including the nucleus accumbens and the dorsal striatum, due to the connection between dopaminergic pathways in the cortex and those sub cortical structures (85–88). These dopaminergic pathways are the likely mediators in the beneficial effects of rTMS in Huntington disease patients (85, 87, 88).
There are currently only very limited, very small, studies (<10 patients in a study) (Table 3) reporting inconclusive evidence of the beneficial effect of TMS in ameliorating motor symptoms in Huntington's Disease (51–53). SMA is believed to play a key role in maintaining the executive aspects of motor control in Huntington's Disease (89). A small study did report a benefit in uplifting mood (non-motor symptom) (53).
Very little is known about mechanisms of action of rTMS in Tourette syndrome (90, 91). It was suggested that low frequency rTMS may help with tics and obsessive behaviors by resetting a hyperactive motor cortex (90, 92). But there are currently a limited number of rTMS studies in adult Tourette syndrome, overall showing mixed results (Table 3). Some LF-rTMS (1 Hz) and HF-rTMS (15 Hz) studies targeting motor and premotor cortical sites demonstrated no success or a limited benefit in severe Tourette syndrome (54, 55, 93). On the other hand, several open-label studies targeting SMA with LF-rTMS (1 Hz) demonstrated a decrease in the frequency and intensity of tics (56, 94–97).
rTMS can regulate brain functions through plasticity effects and it has been targeted to the tremor network to achieve therapeutic effects (93, 98–101). One rTMS protocol that has been tested in clinical trials is LF-rTMS of the cerebellum (99, 102, 103). However, this protocol did not show any improvement in tremor variables in essential tremor (103) or in resting tremor in PD (104). Other researchers tried stimulating the left M1 or premotor cortical targets but did not find any appreciable benefit in tremor reduction (105, 106).
However, a double-blind sham controlled study (N = 10; five essential tremor, five sham) investigating LF-rTMS of the pre-SMA found significant reduction in tremors after 15 daily sessions. Though tremor reductions were also seen in the sham group (26% in essential tremor and 19% of patients in sham), sustained effects at 4 and 8 week follow-up were only seen in the essential tremor group (107).
Of the various movement disorders discussed, rTMS may currently be considered an emerging strategy in ameliorating certain motor symptoms in Parkinson's disease, with moreover an effect in uplifting mood. There remains a need to increase the effectiveness of rTMS in Parkinson's disease by finding optimal stimulation strategies. When it comes to the other movement disorders covered here, the few studies showing therapeutic benefit of rTMS in dystonia seem too small to yield conclusive evidence. Though SMA has shown some promise as an effective target in Huntington's disease, also there, rTMS trials have been small and results inconclusive. The rTMS trials in Tourette syndrome show a lack of significant effects, raising doubt about the possible efficacy of rTMS. In tremor, though LF-rTMS to cerebellum and pre-SMA has shown some benefit in essential tremor, overall, the available data from small samples remains inconclusive. Larger, well- designed trials assessing rTMS efficacy in treating each of these disorders are required. Also, there is a need to reduce variability in the TMS protocols evaluated for any particular movement disorder. Another issue is that the same TMS protocol may give different results in different individuals (inter-individual variability) and also in the same individual at different times (intra-individual variability). In a recent article, our group has described the possible determinants causing these intra- and inter-subject variability, hindering its reliability, and efficacy. Among differences in general TMS reactivity due to differnces in, e.g., scalp-cortex distances or cortical excitability, recent findings suggest a systematic state-dependence of rTMS in which the cognitive but also spontaneous oscillatory brain state can modulate the size and direction of rTMS effects in the brain (108). Hopefully, the overview of currently available evidence provided here can help inform further clinical work.
Protocols of rTMS are based on persistently enhancing cortical excitability by repetitive high-frequency stimulation (109, 110). Long-term potentiation (LTP)-like changes in synaptic strength, which are commonly assumed to be a major cellular mechanism of learning and memory, are thought to be involved in such facilitation (109–111). The expression of plasticity-related neurotrophins like brain-derived neurotrophic factor (BDNF) which diminishes in the hippocampus of Alzheimer's disease patients, is regulated by neuronal activity and LTP (109, 112). Hence rTMS can considerably increase BDNF levels. By the correction or blunting of impaired LTP-like plasticity and associated signaling defects seen in AD, rTMS may provide clinical benefit (109, 113, 114). rTMS has also shown to be an inhibitory neuron function modifier as the studies show that GABAergic synaptic strength on principal neurons is reduced by 10 Hz stimulation, confirming a concept in which GABAergic synapses influence overall inhibitory/excitatory balance (109, 115, 116).
Two recent meta-analyses showed that rTMS may be an effective therapy to improve cognitive ability in patients with mild to moderate AD including MCI. In the first meta-analysis (15 RCTs; N = 240), rTMS was found to be an effective therapy to improve cognitive impairment in AD (117). rTMS significantly improved cognition in AD compared to sham (P = 0.0006). A subgroup analysis suggested that rTMS on multiple sites and multiple sessions (>10) provided more significant cognitive enhancement than rTMS on single site for ≤ 10 sessions. 20 Hz was more effective than 10 or 1 Hz frequencies. Concurrent cognitive training and/or patients with higher education seemed to confer higher benefit than single therapy, or in patients with lower education or severe dementia (117).
The other meta-analysis (12 studies including 8 RCTs; N = 231) also suggested that rTMS significantly improved cognition in AD compared to sham (P < 0.0001) (118). The sub-analysis moreover showed that multiple targets had better effects than single (0.86 vs. 0.13) and ≥5 sessions had better effect than ≤ 3 sessions (2.77 vs. 0.29). However, this meta-analysis did not find any benefit of concurrent cognitive training (118).
Thus, the meta-analyses showed that rTMS to multiple sites [Broca, right/left DLPFC, Wernicke, right/ left parietal somatosensory association cortex (pSAC), inferior frontal gyrus] and long-term treatment yields better cognitive performance than single site or short duration rTMS (109, 117, 118). However, while encouraging, this cannot be considered as a conclusive evidence as the studies included in both meta-analyses (Table 4) had small sample size, and some were not sham-controlled.
There is no recommendation yet for therapeutic use of TMS in AD and MCI (109). There are limited studies showing long-term efficacy. The clinical trials reporting positive effects on cognitive outcome measures in AD are very small and there are no clear neurobiological mechanisms to explain the benefit of rTMS in AD (109). In their evidence based guidelines, Lefaucheur (24) note that multisite rTMS with concurrent cognitive training in AD may possibly improve cognition, memory, apathy, and language in mild and early stage AD (including MCI). However, they do not recommend its clinical use until long-term observational studies show that multisite rTMS with cognitive training is more beneficial than single-site focused rTMS with cognitive training. They also stress the need for neurophysiological and imaging studies to improve the understanding of the neural mechanisms of action. Additionally, a TMS strategy that may show positive effects in young adults may have detrimental effects in older adults or in patients with brain affected by AD pathology, so one should proceed with caution.
MS is usually treated with disease-modifying therapies. However, despite treatment, patients develop relapsing/remitting MS (RRMS) and secondary progressing MS (SPMS). Since TMS has no known interaction with MS drugs, it can be used as an adjunctive treatment for management of motor and sensory symptoms of MS (8). It is believed that some of the MS symptoms are related to neuronal transmissionin the brain (6). LF-rTMS of a single neuron can cause prolonged inhibition of neuronal transmission while HF-rTMS can improve neuronal transmission (132) Thus trains of rTMS pulses modify activity in the targeted region of brain lasting for minutes or even hours (132). Thus, TMS may alleviate debilitating MS symptoms such as fatigue, spasticity, and gait abnormalities and manual dexterity, which affect quality of life (QoL), especially in patients with RRMS and SPMS (Table 5) (2, 62).
Agüera et al. (141) reported a case of RRMS (33 years, female) not responding to medications prescribed over 9 years and rapidly progressing disease. The patient benefited from rTMS which was prescribed as a compassionate treatment as no other treatment was producing any benefit. Post rTMS, there was improvement in her neuropsychological functions and blood tests showed a reduction in oxidative stress after 4 months of treatment (141).
There is mixed evidence of benefit in fatigue. In 34 patients with secondary progressive MS, HF-rTMS (20 Hz) and intermittent TBS (iTBS), a patterned excitatory protocol with after-effects analogous to HF-rTMS was used for spasticity management. HF-rTMS and iTBS significantly showed significant reduction in spasticity on the Modified Ashworth Scale compared to sham stimulation (142). Intermittent theta burst stimulation (iTBS) had longer-lasting effect on the Subjective Evaluating Spasticity Scale (SESS) and when given after HF-rTMS resulted in reduction in pain and fatigue. However, a systematic review and meta-analysis comparing transcranial direct current stimulation (tDCS), TMS, and transcranial random noise stimulation (tRNS) did not find TMS to be beneficial in fatigue (143). The analysis included 207 patients from 14 studies (11 for tDCS, 2 for TMS, and 1 for tRNS). The analysis reported that tDCS had significant short-term and long-term treatment effects compared to sham stimulation but TMS and tRNS were not found to be superior to sham stimulation (143). However, Gaede et al. (144) reported some benefit of H-coil HF-rTMS deep brain stimulation to motor cortex in 37 patients with MS related fatigue. There was significant sustained median Fatigue Severity Scale (FSS) decrease of 1.0 point (95%CI, 0.45, 1.65). However, some participants discontinued treatment due to minor side effects and the study size was too small to make any conclusive suggestion.
There is no conclusive recommendation yet for therapeutic use of TMS in MS. In their evidence based guidelines, Lefaucheur (24) suggests that iTBS targeted to the leg motor cortex may be recommended to treat lower limb spasticity in MS. However, they do not recommend using iTBS to the hand motor cortex for improving manual dexterity. Nor do they recommend using H-coil HF-rTMS deep brain stimulation to motor cortex to improve fatigue. Since iTBS and H-coil HF-rTMS has shown some benefit in MS, large studies with set iTBS and H-coil HF-rTMS protocols in MS will be required to identify how TMS can be effectively, therapeutically and routinely used in MS.
Though antiepileptic drugs (AEDs) are the mainstay of epilepsy treatment, one third of patients on AEDs develop drug resistance. Of these, many patients are not suitable candidates for surgical ablation. This patient group, which is at increased risk of morbidity, may respond to LF-TMS (8). rTMS could reduce likelihood of seizures in this patient population probably due to its ability to cause prolonged inhibitory effect on synaptic potential and focal cortical excitability (6). TMS has also been used to study the effects of AEDs on the brain (145). In patients who are candidates for surgical ablation, TMS helps identify the brain areas which are more seizure prone. Alternatively, TMS helps identify areas of cortical excitability in various epilepsy syndromes (145).
A Cochrane review of seven pilot studies from different regions of the world showed that, in all studies, TMS was used in patients with drug-resistant epilepsy (146). However, the definition of drug resistance differed between studies and ranged from ≤ 1 complex partial/secondarily generalized seizure per month to ≥3 seizures per month. Additionally patient should have had ≤ 2 unchanging AEDs. All studies used figure 8 coil though the sham TMS methods varied (146).
A meta-analysis of 11 studies (n = 164) evaluating the efficacy of LF-rTMS in medically intractable epilepsy found a significant effect size in seizure frequency [effect size: 0.34, 95% confidence interval (CI) 0.10-0.57] (147). Seizure reduction was significantly higher in patients with neocortical epilepsy or cortical dysplasia than those with other epileptic disorders (effect size of 0.71 vs. 0.22) (147).
In their systematic review, Cooper et al. (132) included 12 studies in patients with drug-resistant epilepsy being treated with LF-rTMS. Meta-analysis of the five studies with individual participant data (IPD) (n = 34) showed that patients with temporal seizure focus had significantly more favorable response than patients with extratemporal epilepsy (50 vs. 14%, p = 0.045). Stimulation with a figure-8 coil resulted in significantly more favorable response than stimulation with other types of coils (47 vs. 0%, p = 0.01). Meta-analysis of seven studies without IPD (n = 212) showed that seizure reduction rates were significantly higher in patients with mean age ≤ 21 years than those older than 21 years (69 vs. 18%) and in patients treated with targeted stimulation vs. those treated without targeted stimulation (47 vs. 14–20%). The pooled rate of 50% seizure reduction with LF-rTMS was 30% (95% CI 12–57%) (132).
There is no recommendation yet for therapeutic use of TMS in epilepsy. Though the Cochrane review found rTMS to be safe and effective in reducing epileptiform discharges, the review could not find clear evidence of the efficacy of rTMS in reducing seizure frequency (146). There is currently too much variability in the TMS techniques used in studies, in the outcomes reported and in the definition of drug-resistant epilepsy (147, 148).
Disorders of consciousness (DOC) mainly include minimally conscious state (MCS) and the “vegetative state,” clinically known as the unresponsive wakefulness syndrome (UWS). The clinical efficacy of rTMS has been studied in these patients using different HF-rTMS protocols, mainly targeting the left M1 (149–151) and the right and left DLPFC (152–154) or iTBS to left DLPFC (155). Cincotta et al. (149) (N = 11 patients with UWS) and Liu et al. (150) (N-7 patients with DOC and 11 healthy controls) tested 20 Hz rTMS of the M1 but found no evidence of therapeutic effect. He et al. (151) posted results of a randomized sham controlled study of six patients with DOC treated with 20 Hz rTMS of the M1 for five consecutive days. rTMS resulted in long-lasting behavioral and neurophysiological modifications in one patient with traumatic brain injury while five other patients showed localized brain reactivity at several electrodes, but no significant electroencephalography changes. This was a very small study with inconclusive evidence of efficacy of HF-rTMS pf M1 in DOC.
Similarly, targeting DLPFC did not provide any conclusive evidence of effect in DOC. Naro et al. (152) (N = 10 postanoxic UWS and 10 healthy controls) did not find any significant clinical change after a single session of 10 Hz rTMS to the right DLPFC. However, three patients showed short-lasting clinical improvement caused by a significant transient effect induced by rTMS (152). On the contrary, Xia et al. (153), found clinically significant benefit of 10-Hz rTMS to the left DLPFC for 20 consecutive sessions in 16 patients (5 MCS and 11 UWS). In another 2017 study, Xia et al. reported reduced EEG signal power in low-frequency and increased signal power in the high-frequency bands (154).
A study using iTBS (600 pulses per session at 80% of active motor threshold) to the left DLPFC for 5 consecutive days in eight patients with MCS or UWS reported some clinical benefit after rTMS but the benefit was statistically significant only after a week. This was a small study with no sham control.
There is no recommendation yet for therapeutic use of TMS in DOC. Though some clinical benefit in consciousness level was seen using HF-rTMS of the left M1 or after HF-rTMS or iTBS of the left DLPFC, the small sample size of these studies limits the generalization of the results. Also, contrary results from other studies raise a level of doubt regarding efficacy in DOC. Further studies testing the efficacy of TMS protocols to left M1 or DLPFC are required to determine whether TMS has any efficacy in DOC.
TMS is a relatively safe procedure. A systematic review of 93 RCTs found the TMS group had 2.60 times higher (95% CI 1.75 3.86) odds of experiencing an adverse event (AE) than placebo (p < 0.00001). Headache and dizziness were the most common AEs. However, the overall pooled estimate of treatment discontinuation due to an AE was 2.5% (95% CI 1.9-3.2%) with TMS and 2.7% (95% CI 2.0-3.5%) with placebo (156).
A meta-analytic utility prediction study including 35 studies investigating treatment of focal epilepsy (N = 6,398; 28 AEDs and 7 rTMS studies; AEDs n = 4,919; rTMS n = 136 and placebo n = 1,343) found that adjunctive rTMS provided superior QoL as compared to AEDs (148). However, there was no difference in seizure reductions between AEDs and rTMS (p = 0.94). Reduction of seizure frequency from baseline to final treatment follow-up with AED, rTMS and placebo was 36.1 ± 15.2%, 36.2 ± 7.2% and 19.6 ± 8.5%, respectively. The superior QoL was due to fewer side effects, most of which were considered mild (148). There was no difference in adverse effects between rTMS and sham TMS groups. This suggests that adjustments in the treatment environment may have mitigated the TMS related adverse effects. On the other hand, adverse effects were the main reason for treatment discontinuations in the AEDs arms of the studies (148).
A Cochrane review of seven studies analyzing the effect of TMS in epilepsy found that adverse effects were uncommon; most reported adverse effects were headache, dizziness, and tinnitus and did not lead to a significant change in medications (146). A meta-analysis of 17 RCTs evaluating rTMS to left DLPFC (10 Hz) at 60-110% resting motor threshold (rMT) reported a significant incidence of headache in the treatment group.
TMS use in epilepsy may induce seizures in patients with a known neurological disorder and clinicians should be aware and alert about this complication (6, 157). However, the risk is very low, most incidences are transient and self-limiting and do not have any long-term sequelae (157).
Cerebellar low frequency TMS works by lowering the inhibitory regulation of the cerebellar cortex over the dentate nucleus, hence potentiating some of the impaired functionality of dentate nucleus. Furthermore, a reduced inhibitory signal from Purkinje cells may boost the activation of the vestibular nuclei, resulting in improved balance in patients with cerebellar ataxias (158–162).
Dysfunction of the cerebellum and its connected neural networks causes a neurodegenerative disorder known as spinocerebellar ataxia (SCA). In a randomized, double-blinded and sham-controlled study significant improvement in clinical and kinematic outcomes of postural control in standing were observed in patients who completed a 4-week rTMS intervention with 1-month follow up as compared to the patients receiving sham intervention (159).
The role of TMS in diagnosis, pathophysiology and treatment interventions of genetically confirmed hereditary ataxias was studied in a critical review (23). Hereditary ataxias are a heterogeneous group of neurodegenerative disorders affecting motor cortex and the corticospinal tract. Early involvement of the corticospinal tract and motor cortex circuitry was shown by the available data and the effectiveness of cerebellar repetitive TMS (rTMS) as treatment approach was observed (23).
The efficacy of TMS over the cerebellum for inherited spinocerebellar degeneration was reported in a placebo-controlled trial (107). Patients treated with active TMS showed a significant reduction in truncal ataxia. The contraction of nuchal and shoulder muscles was evoked by active stimulation. Sham stimulation, on the other hand, generated the same noise as active stimulation, as well as some scalp sensation. The study's findings revealed that the disease type had an impact on TMS's effectiveness (163).
A study of Ihara et al. (164) compared pre and post severity of ataxia, cerebellar hemispheric blood flow (CHBF), ascorbate free radical (AFR), superoxide dismutase protein, superoxide scavenging activity, and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in cerebrospinal fluid (CSF) during an 8-week course of repetitive transcranial magnetic stimulation (rTMS) in 20 individuals with spinocerebellar degenerations (SCD). After applying rTMS, AFR and ataxia severity decreased in SCD patients and CHBF increased.
A review of Alberto et al. (165) concluded that non-invasive brain stimulation has made substantial advances in developing particular protocols of stimulation to regulate cerebellar excitability with the aim to restore the cerebellar physiological activity in ataxia patients. Literature showed that rTMS or tDCS may be useful tools for patients suffering from neurodegenerative ataxia.
In rTMS, there are substantial placebo effects. This means that efficacy of rTMS (response rates, remission rates, etc.) should be evaluated not just in isolation, but in comparison to placebo groups. Therefore, clinical studies without good blinding and placebo control provide limited information on the extent to which clinical outcomes are attributable to direct neuromodulation effects or to indirect placebo effects. This is not only highly relevant in psychiatric applications, but also in neurology as, e.g., very evident in essential tremor. Most of the studies included in this review are placebo controlled. But it remains the case that some literature on neurological disorders lacks proper controls/blinding.
This review is limited by its narrative structure. There is a high possibility of study selection bias. However, it is a comprehensive review of literature and its judicious interpretation that covers all aspects of TMS that a clinician would require for selecting the right patient population and TMS strategy for either investigating or treating a particular neurological disorder.
Several animal studies are being conducted and cerebellar stimulation is being explored to treat movement disorders (166). However, in general, TMS therapy for a particular neurological condition needs more directional exploration by standardizing study designs, end points, TMS frequency, target, coil, location of stimulus and other such variables.
This review shows that though TMS is not the first line treatment in the discussed neurological conditions, it has an important place in ameliorating symptoms and improving QoL of patients with debilitating disease not responding to drug therapy. With right patient, target and strategy selection, as summarized in Table 6, the required efficacy may be seen. However, it is too early to unambiguously recommend TMS as a therapeutic clinical option in many of these neurological conditions. To reach that stage, more clinical studies are necessary. By providing the current overview, hopefully we could contribute to informing those studies.
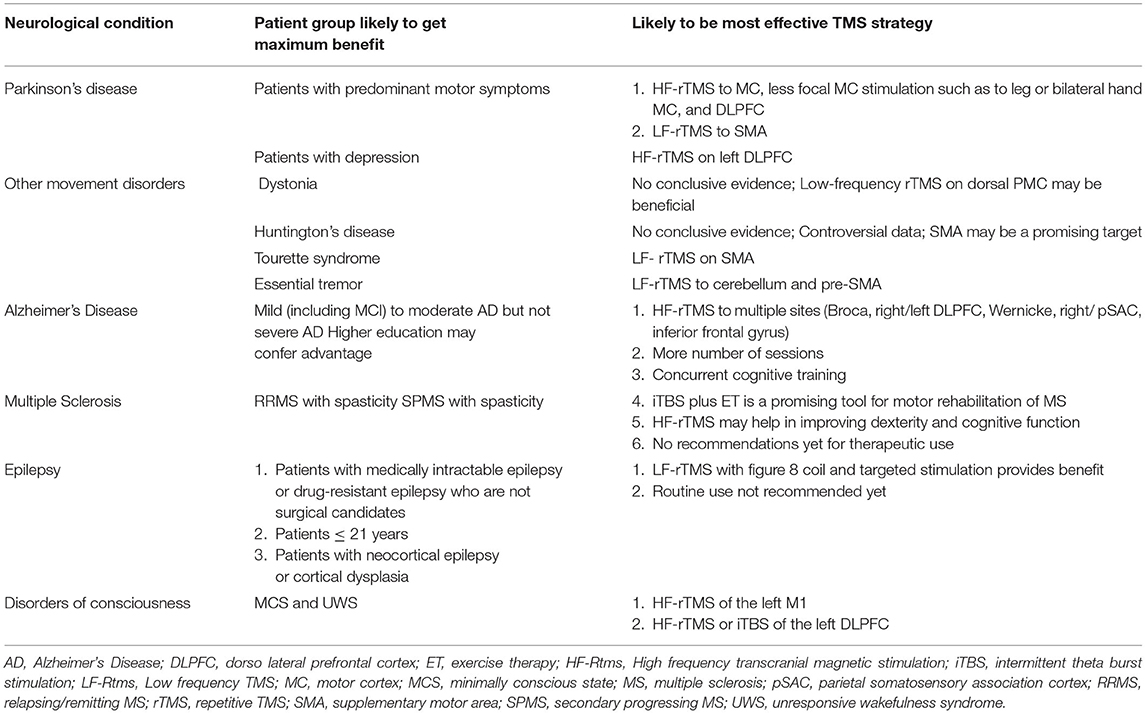
Table 6. The patient population and TMS strategy that may provide benefit and needs to investigated further in larger trials.
FS primarily did the literature review and wrote first draft. TG and AS contributed further insights and co-authored with FS the final manuscript. All authors contributed to the article and approved the submitted version.
AS is Chief Scientific Advisor for PlatoScience and Alphasys; as well as Director of the International Clinical TMS Certification Course (www.tmscourse.eu).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Seewoo BJ, Etherington SJ, Rodger J. Transcranial magnetic stimulation. eLS. (2019) 2019:1–8. doi: 10.1002/9780470015902.a0028620
2. Ruiz ML, Sospedra M, Arce SA, Tejeiro-Martínez J, Benito-León J. Current evidence on the potential therapeutic applications of transcranial magnetic stimulation in multiple sclerosis: a systematic review of the literature. Neurología. (2020) 37:199–215. doi: 10.1016/j.nrl.2018.03.023
3. Hallett M. Transcranial magnetic stimulation and the human brain. Nature. (2000) 406:147–50. doi: 10.1038/35018000
4. Tyc F, Boyadjian A. Cortical plasticity and motor activity studied with transcranial magnetic stimulation. Rev Neurosci. (2006) 17:469–96. doi: 10.1515/REVNEURO.2006.17.5.469
5. Thut G, Northoff G, Ives J, Kamitani Y, Pfennig A, Kampmann F, et al. Effects of single-pulse transcranial magnetic stimulation (TMS) on functional brain activity: a combined event-related TMS and evoked potential study. Clin Neurophysiol. (2003) 114:2071–80. doi: 10.1016/S1388-2457(03)00205-0
6. Iglesias AH. Transcranial magnetic stimulation as treatment in multiple neurologic conditions. Curr Neurol Neurosci Rep. (2020) 20:1–9. doi: 10.1007/s11910-020-1021-0
7. Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, et al. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex. (2007) 17:2841–52. doi: 10.1093/cercor/bhm013
8. Habib S, Hamid U, Jamil A, Zainab AZ, Yousuf T, Habib S, et al. Transcranial magnetic stimulation as a therapeutic option for neurologic and psychiatric illnesses. Cureus. (2018) 10:e3456. doi: 10.7759/cureus.3456
9. Rastogi P, Lee EG, Hadimani RL, Jiles DC. Transcranial magnetic stimulation: development of a novel deep-brain triple-halo coil. IEEE Magnet Lett. (2019) 10:1–5. doi: 10.1109/LMAG.2019.2903993
10. Rastogi P. Novel Coil Designs for Different Neurological Disorders in Transcranial Magnetic Stimulation. Ames: Iowa State University (2019).
11. Deng Z-D, Lisanby SH, Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. (2013) 6:1–13. doi: 10.1016/j.brs.2012.02.005
12. Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. (2002) 19:361–70. doi: 10.1097/00004691-200208000-00008
13. Latorre A, Rocchi L, Berardelli A, Bhatia KP, Rothwell JC. The use of transcranial magnetic stimulation as a treatment for movement disorders: a critical review. Movement Disord. (2019) 34:769–82. doi: 10.1002/mds.27705
14. Voigt J, Carpenter L, Leuchter A. A systematic literature review of the clinical efficacy of repetitive transcranial magnetic stimulation (rTMS) in non-treatment resistant patients with major depressive disorder. BMC Psychiatry. (2019) 19:1–11. doi: 10.1186/s12888-018-1989-z
15. Garnaat SL, Yuan S, Wang H, Philip NS, Carpenter LL. Updates on transcranial magnetic stimulation therapy for major depressive disorder. Psychiatr Clin. (2018) 41:419–31. doi: 10.1016/j.psc.2018.04.006
16. Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of obsessive-compulsive disorder (OCD). Psychiatr Quarter. (2018) 89:645–65. doi: 10.1007/s11126-018-9566-7
17. Cocchi L, Zalesky A, Nott Z, Whybird G, Fitzgerald PB, Breakspear M. Transcranial magnetic stimulation in obsessive-compulsive disorder: a focus on network mechanisms and state dependence. NeuroImage. (2018) 19:661–74. doi: 10.1016/j.nicl.2018.05.029
18. Voigt J, Carpenter L, Leuchter A. Cost effectiveness analysis comparing repetitive transcranial magnetic stimulation to antidepressant medications after a first treatment failure for major depressive disorder in newly diagnosed patients–a lifetime analysis. PLoS ONE. (2017) 12:e0186950. doi: 10.1371/journal.pone.0186950
19. Reti IM. A rational insurance coverage policy for repetitive transcranial magnetic stimulation for major depression. J ECT. (2013) 29:e27–8. doi: 10.1097/YCT.0b013e3182801cd7
20. Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen L, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. (2012) 123:858–82. doi: 10.1016/j.clinph.2012.01.010
21. Dharmadasa T, Huynh W, Kiernan MC. Transcranial magnetic stimulation in the cortical exploration of dementia. Diagnosis and Management in Dementia. Cambridge, MA: Elsevier (2020). p. 327–43.
22. Giuffre A, Kahl CK, Zewdie E, Wrightson JG, Bourgeois A, Condliffe EG, et al. Reliability of robotic transcranial magnetic stimulation motor mapping. J Neurophysiol. (2021) 125:74–85. doi: 10.1152/jn.00527.2020
23. Rodríguez-Labrada R, Velázquez-Pérez L, Ziemann U. Transcranial magnetic stimulation in hereditary ataxias: diagnostic utility, pathophysiological insight and treatment. Clin Neurophysiol. (2018) 129:1688–98. doi: 10.1016/j.clinph.2018.06.003
24. Lefaucheur J-P. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2020.02.003
25. Starling AJ, Tepper SJ, Marmura MJ, Shamim EA, Robbins MS, Hindiyeh NA, et al. A multicenter, prospective, single arm, open label, post-market, observational study to evaluate the use of sTMS in reduction of Migraine Headache (ESPOUSE Study). Age. (2017) 42:1038–48. doi: 10.1177/0333102418762525
26. McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. (2017) 78:16cs10905. doi: 10.4088/JCP.16cs10905
27. Lefaucheur J., Andr é-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. (2014) 125:2150–206. doi: 10.1016/j.clinph.2014.05.021
28. Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson's disease. Movement Disord. (2006) 21:325–31. doi: 10.1002/mds.20713
29. Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson's disease. Movement Disord. (2006) 21:2201–5. doi: 10.1002/mds.21089
30. Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Foly K N, Hamdy A. Dopamine Levels After Repetitive Transcranial Magnetic Stimulation of Motor Cortex in Patients With Parkinson's Disease: Preliminary Results. Milwaukee: Wiley Online Library (2007).
31. Hamada M, Ugawa Y, Tsuji S. High-frequency rTMS over the supplementary motor area for treatment of Parkinson's disease. Movement Disord. (2008) 23:1524–31. doi: 10.1002/mds.22168
32. Filipović SR, Rothwell JC, van de Warrenburg BP, Bhatia K. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson's disease. Movement Disord. (2009) 24:246–53. doi: 10.1002/mds.22348
33. Filipović SR, Rothwell JC, Bhatia K. Slow (1 Hz) repetitive transcranial magnetic stimulation (rTMS) induces a sustained change in cortical excitability in patients with Parkinson's disease. Clin Neurophysiol. (2010) 121:1129–37. doi: 10.1016/j.clinph.2010.01.031
34. Chou Y-h, Hickey PT, Sundman M, Song AW, Chen N-k. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. (2015) 72:432–40. doi: 10.1001/jamaneurol.2014.4380
35. Zanjani A, Zakzanis KK, Daskalakis ZJ, Chen R. Repetitive transcranial magnetic stimulation of the primary motor cortex in the treatment of motor signs in Parkinson's disease: a quantitative review of the literature. Movement Disord. (2015) 30:750–8. doi: 10.1002/mds.26206
36. Fricke C, Duesmann C, Woost TB, von Hofen-Hohloch J, Rumpf J-J, Weise D, et al. Dual-site transcranial magnetic stimulation for the treatment of Parkinson's disease. Front Neurol. (2019) 10:174. doi: 10.3389/fneur.2019.00174
37. Alemam AI, Eltantawi MA. Repetitive transcranial magnetic stimulation in treatment of levodopa-induced dyskinesia in Parkinson's Disease. J Neurol Res. (2019) 9:28–34. doi: 10.14740/jnr512
38. Khedr EM, Mohamed KO, Soliman RK, Hassan AM, Rothwell JC. The effect of high-frequency repetitive transcranial magnetic stimulation on advancing Parkinson's disease with dysphagia: double blind randomized clinical trial. Neurorehabil Neural Repair. (2019) 33:442–52. doi: 10.1177/1545968319847968
39. Mi T-M, Garg S, Ba F, Liu A-P, Liang P-P, Gao L-L, et al. Repetitive transcranial magnetic stimulation improves Parkinson's freezing of gait via normalizing brain connectivity. NPJ Parkinsons Dis. (2020) 6:1–9. doi: 10.1038/s41531-020-0118-0
40. Siebner HR, Filipovic SR, Rowe JB, Cordivari C, Gerschlager W, Rothwell JC, et al. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain. (2003) 126:2710–25. doi: 10.1093/brain/awg282
41. Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. (2005) 128:104–15. doi: 10.1093/brain/awh315
42. Borich M, Arora S, Kimberley TJ. Lasting effects of repeated rTMS application in focal hand dystonia. Restor Neurol Neurosci. (2009) 27:55–65. doi: 10.3233/RNN-2009-0461
43. Havrankova P, Jech R, Walker ND, Operto G, Tauchmanova J, Vymazal J, et al. Repetitive TMS of the somatosensory cortex improves writer's cramp and enhances cortical activity. Neuroendocrinol Lett. (2010) 31:73–86.
44. Huang Y-Z, Lu C-S, Rothwell JC, Lo C-C, Chuang W-L, Weng Y-H, et al. Modulation of the disturbed motor network in dystonia by multisession suppression of premotor cortex. PLoS ONE. (2012) 7:e47574. doi: 10.1371/journal.pone.0047574
45. Kimberley TJ, Borich MR, Arora S, Siebner HR. Multiple sessions of low-frequency repetitive transcranial magnetic stimulation in focal hand dystonia: clinical and physiological effects. Restor Neurol Neurosci. (2013) 31:533–42. doi: 10.3233/RNN-120259
46. Kimberley TJ, Schmidt R, Chen M, Dykstra DD, Buetefisch CM. Mixed effectiveness of rTMS and retraining in the treatment of focal hand dystonia. Front Hum Neurosci. (2015) 9:385. doi: 10.3389/fnhum.2015.00385
47. Kranz G, Shamim E, Lin P, Kranz G, Hallett M. Transcranial magnetic brain stimulation modulates blepharospasm: a randomized controlled study. Neurology. (2010) 75:1465–71. doi: 10.1212/WNL.0b013e3181f8814d
48. Koch G, Porcacchia P, Ponzo V, Carrillo F, Cáceres-Redondo MT, Brusa L, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. (2014) 7:564–72. doi: 10.1016/j.brs.2014.05.002
49. Pirio Richardson S, Tinaz S, Chen R. Repetitive transcranial magnetic stimulation in cervical dystonia: effect of site and repetition in a randomized pilot trial. PLoS ONE. (2015) 10:e0124937. doi: 10.1371/journal.pone.0124937
50. Zittel S, Helmich R, Demiralay C, Münchau A, Bäumer T. Normalization of sensorimotor integration by repetitive transcranial magnetic stimulation in cervical dystonia. J Neurol. (2015) 262:1883–9. doi: 10.1007/s00415-015-7789-1
51. Brusa L, Versace V, Koch G, Bernardi G, Iani C, Stanzione P, et al. Improvement of choreic movements by 1Hz repetitive transcranial magnetic stimulation in Huntington's disease patients. Annals Neurol. (2005) 58:655–6. doi: 10.1002/ana.20613
52. Shukla A, Jayarajan RN, Muralidharan K, Jain S. Repetitive transcranial magnetic stimulation not beneficial in severe choreiform movements of Huntington disease. J ECT. (2013) 29:e16–7. doi: 10.1097/YCT.0b013e3182711dfc
53. Groiss SJ, Netz J, Lange HW, Buetefisch CM. Frequency dependent effects of rTMS on motor and cognitive functions in Huntington's disease. Basal Ganglia. (2012) 2:41–8. doi: 10.1016/j.baga.2011.12.001
54. Münchau A, Bloem B, Thilo K, Trimble M, Rothwell J, Robertson M. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. (2002) 59:1789–91. doi: 10.1212/01.WNL.0000036615.25044.50
55. Orth M, Kirby R, Richardson M, Snijders A, Rothwell J, Trimble M, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. (2005) 116:764–8. doi: 10.1016/j.clinph.2004.10.003
56. Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive–compulsive disorder (OCD) and Tourette's syndrome (TS). Int J Neuropsychopharmacol. (2006) 9:95–100. doi: 10.1017/S1461145705005729
57. Landeros-Weisenberger A, Mantovani A, Motlagh MG, de Alvarenga PG, Katsovich L, Leckman JF, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimul. (2015) 8:574–81. doi: 10.1016/j.brs.2014.11.015
58. Arias-Carrión O. Basic mechanisms of rTMS: implications in Parkinson's disease. Int Arch Med. (2008) 1:1–8. doi: 10.1186/1755-7682-1-2
59. Lamusuo S, Hirvonen J, Lindholm P, Martikainen I, Hagelberg N, Parkkola R, et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation–positron emission tomography evidence for release of endogenous opioids. Eur J Pain. (2017) 21:1505–15. doi: 10.1002/ejp.1052
60. Marra HLD, Myczkowski ML, Memória CM, Arnaut D, Ribeiro PL, Mansur CGS, et al. Transcranial magnetic stimulation to address mild cognitive impairment in the elderly: a randomized controlled study. Behav Neurol. (2015) 2015:287843. doi: 10.1155/2015/287843
61. Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. (2013) 154:2563–8. doi: 10.1016/j.pain.2013.07.042
62. Hausmann A, Weis C, Marksteiner J, Hinterhuber H, Humpel C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Mol Brain Res. (2000) 76:355–62. doi: 10.1016/S0169-328X(00)00024-3
63. Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. (2015) 9:303. doi: 10.3389/fnhum.2015.00303
64. Dong Q, Wang Y, Gu P, Shao R, Zhao L, Liu X, et al. The neuroprotective mechanism of low-frequency rTMS on nigral dopaminergic neurons of Parkinson's disease model mice. Parkinsons Dis. (2015) 2015:564095. doi: 10.1155/2015/564095
65. Lisanby S, Arango V, Underwood M, Perara T, Dwork A, Sackeim H editors. Hippocampal Plasticity Following Chronic Repetitive Transcranial Magnetic Stimulation. Philadelphia, PA: Lippincott Williams & Wilkins (2000).
66. Yang YD, Allen T, Abdullahi SM, Pelphrey KA, Volkmar FR, Chapman SB. Neural mechanisms of behavioral change in young adults with high-functioning autism receiving virtual reality social cognition training: a pilot study. Autism Res. (2018) 11:713–25. doi: 10.1002/aur.1941
67. Wichmann T, Dostrovsky JO. Pathological basal ganglia activity in movement disorders. Neuroscience. (2011) 198:232–44. doi: 10.1016/j.neuroscience.2011.06.048
68. González-García N, Armony JL, Soto J, Trejo D, Alegría MA, Drucker-Colín R. Effects of rTMS on Parkinson's disease: a longitudinal fMRI study. J Neurol. (2011) 258:1268–80. doi: 10.1007/s00415-011-5923-2
69. Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. (2003) 126:2609–15. doi: 10.1093/brain/awg268
70. Machado S, Arias-Carrión O, Paes F, Vieira RT, Caixeta L, Novaes F, et al. Repetitive transcranial magnetic stimulation for clinical applications in neurological and psychiatric disorders: an overview. Eurasian J Med. (2013) 45:191. doi: 10.5152/eajm.2013.39
71. Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp Neurol. (2009) 219:58–61. doi: 10.1016/j.expneurol.2009.05.014
72. Brys M, Fox MD, Agarwal S, Biagioni M, Dacpano G, Kumar P, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology. (2016) 87:1907–15. doi: 10.1212/WNL.0000000000003279
73. Khedr EM, Mohamed KO, Ali AM, Hasan AM. The effect of repetitive transcranial magnetic stimulation on cognitive impairment in Parkinson's disease with dementia: pilot study. Restor Neurol Neurosci. (2020) 38:55–66. doi: 10.3233/RNN-190956
74. Teo W-P, Hendy AM, Goodwill AM, Loftus AM. Transcranial alternating current stimulation: a potential modulator for pathological oscillations in Parkinson's disease? Front Neurol. (2017) 8:185. doi: 10.3389/fneur.2017.00185
75. Zhang Q, Aldridge GM, Narayanan NS, Anderson SW, Uc EY. Approach to cognitive impairment in Parkinson's disease. Neurotherapeutics. (2020) 17:1495–510. doi: 10.1007/s13311-020-00963-x
76. Tyvaert L, Houdayer E, Devanne H, Monaca C, Cassim F, Derambure P. The effect of repetitive transcranial magnetic stimulation on dystonia: a clinical and pathophysiological approach. Neurophysiologie Clinique/Clin Neurophysiol. (2006) 36:135–43. doi: 10.1016/j.neucli.2006.08.007
77. Quartarone A, Rizzo V, Terranova C, Cacciola A, Milardi D, Calamuneri A, et al. Therapeutic use of non-invasive brain stimulation in dystonia. Front Neurosci. (2017) 11:423. doi: 10.3389/fnins.2017.00423
78. Sharma K, Cucca A, Lee A, Agarwal S, Frucht SJ, Biagioni MC. Transcranial magnetic stimulation therapy for focal leg dystonia: a case report. J Clin Movement Disord. (2019) 6:1–4. doi: 10.1186/s40734-019-0076-z
79. Huang YZ, Edwards MJ, Bhatia KP, Rothwell JC. One-Hz repetitive transcranial magnetic stimulation of the premotor cortex alters reciprocal inhibition in DYT1 dystonia. Movement Disord. (2004) 19:54–9. doi: 10.1002/mds.10627
80. Cohen LG, Hallett M. Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology. (1988) 38:1005. doi: 10.1212/WNL.38.7.1005
81. Betti S, Spoto A, Castiello U, Sartori L. Testing rTMS-induced neuroplasticity: a single case study of focal hand dystonia. Neural Plasticity. (2018) 2018:6464896. doi: 10.1155/2018/6464896
82. Udupa K, Bhattacharya A, Chen R. Exploring the connections between basal ganglia and cortex revealed by transcranial magnetic stimulation, evoked potential and deep brain stimulation in dystonia. Eur J Paediatr Neurol. (2022) 36:69–77. doi: 10.1016/j.ejpn.2021.12.004
83. Lozeron P, Poujois A, Richard A, Masmoudi S, Meppiel E, Woimant F, et al. Contribution of TMS and rTMS in the understanding of the pathophysiology and in the treatment of dystonia. Front Neural Circuits. (2016) 10:90. doi: 10.3389/fncir.2016.00090
84. Erro R, Tinazzi M, Morgante F, Bhatia KP. Non-invasive brain stimulation for dystonia: therapeutic implications. Eur J Neurol. (2017) 24:1228–e64. doi: 10.1111/ene.13363
85. Medina F, Tunez I. Huntington's disease: the value of transcranial meganetic stimulation. Curr Med Chem. (2010) 17:2482–91. doi: 10.2174/092986710791556078
86. Brown KE, Neva JL, Ledwell NM, Boyd LA. Use of transcranial magnetic stimulation in the treatment of selected movement disorders. Degener Neurol Neuromusc Dis. (2014) 4:133. doi: 10.2147/DNND.S70079
87. Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. (1995) 15:3896–904. doi: 10.1523/JNEUROSCI.15-05-03896.1995
88. You Z-B, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci. (1998) 18:6492–500. doi: 10.1523/JNEUROSCI.18-16-06492.1998
89. Klöppel S, Draganski B, Siebner HR, Tabrizi SJ, Weiller C, Frackowiak RS. Functional compensation of motor function in pre-symptomatic Huntington's disease. Brain. (2009) 132:1624–32. doi: 10.1093/brain/awp081
90. Khalifa N, Edebol Eeg-Olofsson K. Low-frequent Repetitive Transcranial Magnetic Stimulation (rTMS) in adolescents with Tourette syndrome. J Neonatal Pediatr Med. (2017) 3:S1013. doi: 10.4172/2572-4983.1000S1013
91. Kleimaker M, Kleimaker A, Weissbach A, Colzato LS, Beste C, Bäumer T, et al. Non-invasive brain stimulation for the treatment of gilles de la tourette syndrome. Front Neurol. (2020) 11:1539. doi: 10.3389/fneur.2020.592258
92. Grados M, Huselid R, Duque-Serrano L. Transcranial magnetic stimulation in Tourette syndrome: a historical perspective, its current use and the influence of comorbidities in treatment response. Brain Sci. (2018) 8:129. doi: 10.3390/brainsci8070129
93. Chae J-H, Nahas Z, Wassermann E, Li X, Sethuraman G, Gilbert D, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette's syndrome. Cogn Behav Neurol. (2004) 17:109–17. doi: 10.1097/01.wnn.0000116253.78804.3a
94. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. (2016) 17:557–61. doi: 10.3109/15622975.2014.964767
95. Kwon HJ, Lim WS, Lim MH, Lee SJ, Hyun JK, Chae J-H, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette's syndrome. Neurosci Lett. (2011) 492:1–4. doi: 10.1016/j.neulet.2011.01.007
96. Mantovani A, Leckman JF, Grantz H, King RA, Sporn AL, Lisanby SH. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of tourette syndrome: report of two cases. Clin Neurophysiol. (2007) 118:2314–5. doi: 10.1016/j.clinph.2007.07.011
97. Salatino A, Momo E, Nobili M, Berti A, Ricci R. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimul. (2014) 7:341–3. doi: 10.1016/j.brs.2014.01.002
98. Frey J, Hess CW, Kugler L, Wajid M, Wagle Shukla A. Transcranial magnetic stimulation in tremor syndromes: pathophysiologic insights and therapeutic role. Front Neurol. (2021) 2021:1482. doi: 10.3389/fneur.2021.700026
99. Shih LC, Pascual-Leone A. Non-invasive brain stimulation for essential tremor. Tremor Other Hyperkin Movements. (2017) 7:458. doi: 10.5334/tohm.377
100. Reis J, Swayne O, Vandermeeren Y, Camus M, Dimyan M, Harris-Love M, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. (2008) 586:325–51. doi: 10.1113/jphysiol.2007.144824
101. Karp B, Wassermann E, Porter S, Hallett M editors. Transcranial Magnetic Stimulation Acutely Decreases Motor Ties. Philadelphia, PA: Lippincott-Raven Publ (1997).
102. Kang N, Cauraugh JH. Does non-invasive brain stimulation reduce essential tremor? A systematic review and meta-analysis. PLoS ONE. (2017) 12:e0185462. doi: 10.1371/journal.pone.0185462
103. Bologna M, Rocchi L, Leodori G, Paparella G, Conte A, Kahn N, et al. Cerebellar continuous theta burst stimulation in essential tremor. Cerebellum. (2015) 14:133–41. doi: 10.1007/s12311-014-0621-0
104. Bologna M, Di Biasio F, Conte A, Iezzi E, Modugno N, Berardelli A. Effects of cerebellar continuous theta burst stimulation on resting tremor in Parkinson's disease. Parkinson Relat Disord. (2015) 21:1061–6. doi: 10.1016/j.parkreldis.2015.06.015
105. Hellriegel H, Schulz EM, Siebner HR, Deuschl G, Raethjen JH. Continuous theta-burst stimulation of the primary motor cortex in essential tremor. Clin Neurophysiol. (2012) 123:1010–5. doi: 10.1016/j.clinph.2011.08.033
106. Chuang WL, Huang YZ, Lu CS, Chen RS. Reduced cortical plasticity and GABAergic modulation in essential tremor. Movement Disord. (2014) 29:501–7. doi: 10.1002/mds.25809
107. Badran BW, Glusman CE, Austelle CW, Jenkins S, DeVries WH, Galbraith V, et al. A double-blind, sham-controlled pilot trial of pre-supplementary motor area (Pre-SMA) 1 Hz rTMS to treat essential tremor. Brain Stimul. (2016) 9:945–7. doi: 10.1016/j.brs.2016.08.003
108. Janssens SE, Sack AT. Spontaneous fluctuations in oscillatory brain state cause differences in transcranial magnetic stimulation effects within and between individuals. Front Hum Neurosci. (2021) 15:802244. doi: 10.3389/fnhum.2021.802244
109. Weiler M, Stieger KC, Long JM, Rapp PR. Transcranial magnetic stimulation in Alzheimer's disease: are we ready? Eneuro. (2020) 7. doi: 10.1523/ENEURO.0235-19.2019
110. Guerra A, Assenza F, Bressi F, Scrascia F, Del Duca M, Ursini F, et al. Transcranial magnetic stimulation studies in Alzheimer's disease. Int J Alzheimers Dis. (2011) 2011:263817. doi: 10.4061/2011/263817
111. Lee J, Lee AY. Transcranial magnetic stimulation parameters as neurophysiological biomarkers in Alzheimer's disease. Annals Clin Neurophysiol. (2021) 23:7–16. doi: 10.14253/acn.2021.23.1.7
112. Makowiecki K, Harvey AR, Sherrard RM, Rodger J. Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice. J Neurosci. (2014) 34:10780–92. doi: 10.1523/JNEUROSCI.0723-14.2014
113. Tokay T, Holl N, Kirschstein T, Zschorlich V, Köhling R. High-frequency magnetic stimulation induces long-term potentiation in rat hippocampal slices. Neurosci Lett. (2009) 461:150–4. doi: 10.1016/j.neulet.2009.06.032
114. Kumar S, Zomorrodi R, Ghazala Z, Goodman MS, Blumberger DM, Cheam A, et al. Extent of dorsolateral prefrontal cortex plasticity and its association with working memory in patients with Alzheimer disease. JAMA Psychiatry. (2017) 74:1266–74. doi: 10.1001/jamapsychiatry.2017.3292
115. Holczer A, Németh VL, Vékony T, Vécsei L, Klivényi P, Must A. Non-invasive brain stimulation in Alzheimer's disease and mild cognitive impairment—a state-of-the-art review on methodological characteristics and stimulation parameters. Front Hum Neurosci. (2020) 14:179. doi: 10.3389/fnhum.2020.00179
116. Lenz M, Galanis C, Müller-Dahlhaus F, Opitz A, Wierenga CJ, Szabó G, et al. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat Commun. (2016) 7:1–13. doi: 10.1038/ncomms10020
117. Wang X, Mao Z, Ling Z, Yu X. Repetitive transcranial magnetic stimulation for cognitive impairment in Alzheimer's disease: a meta-analysis of randomized controlled trials. J Neurol. (2020) 267:791–801. doi: 10.1007/s00415-019-09644-y
118. Lin Y, Jiang W-J, Shan P-Y, Lu M, Wang T, Li R-H, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer's disease: a systematic review and meta-analysis. J Neurol Sci. (2019) 398:184–91. doi: 10.1016/j.jns.2019.01.038
119. Cotelli M, Manenti R, Cappa S, Zanetti O, Miniussi C. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol. (2008) 15:1286–92. doi: 10.1111/j.1468-1331.2008.02202.x
120. Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. (2011) 82:794–7. doi: 10.1136/jnnp.2009.197848
121. Bentwich J, Dobronevsky E, Aichenbaum S, Shorer R, Peretz R, Khaigrekht M, et al. Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer's disease: a proof of concept study. J Neural Transmission. (2011) 118:463–71. doi: 10.1007/s00702-010-0578-1
122. Ahmed MA, Darwish ES, Khedr EM, Ali AM. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer's dementia. J Neurol. (2012) 259:83–92. doi: 10.1007/s00415-011-6128-4
123. Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer's disease: a randomized, double-blind study. J Neural Transmission. (2013) 120:813–9. doi: 10.1007/s00702-012-0902-z
124. Rutherford G, Lithgow B, Moussavi Z. Short and long-term effects of rTMS treatment on Alzheimer's disease at different stages: a pilot study. J Exp Neurosci. (2015) 9:S24004. doi: 10.4137/JEN.S24004
125. Rabey JM, Dobronevsky E. Repetitive transcranial magnetic stimulation (rTMS) combined with cognitive training is a safe and effective modality for the treatment of Alzheimer's disease: clinical experience. J Neural Transmission. (2016) 123:1449–55. doi: 10.1007/s00702-016-1606-6
126. Lee J, Choi BH, Oh E, Sohn EH, Lee AY. Treatment of Alzheimer's disease with repetitive transcranial magnetic stimulation combined with cognitive training: a prospective, randomized, double-blind, placebo-controlled study. J Clin Neurol. (2016) 12:57–64. doi: 10.3988/jcn.2016.12.1.57
127. Nguyen J-P, Suarez A, Kemoun G, Meignier M, Le Saout E, Damier P, et al. Repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer's disease. Neurophysiologie Clinique/Clin Neurophysiol. (2017) 47:47–53. doi: 10.1016/j.neucli.2017.01.001
128. Zhao J, Li Z, Cong Y, Zhang J, Tan M, Zhang H, et al. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer's disease patients. Oncotarget. (2017) 8:33864. doi: 10.18632/oncotarget.13060
129. Koch G, Martorana A, Caltagirone C. Transcranial magnetic stimulation: emerging biomarkers and novel therapeutics in Alzheimer's disease. Neurosci Lett. (2020) 719:134355. doi: 10.1016/j.neulet.2019.134355
130. Turriziani P. Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci. (2012) 6:62. doi: 10.3389/fnhum.2012.00062
131. Eliasova I, Anderkova L, Marecek R, Rektorova I. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer's disease: a pilot study. J Neurol Sci. (2014) 346:318–22. doi: 10.1016/j.jns.2014.08.036
132. Cooper YA, Pianka ST, Alotaibi NM, Babayan D, Salavati B, Weil AG, et al. Repetitive transcranial magnetic stimulation for the treatment of drug-resistant epilepsy: a systematic review and individual participant data meta-analysis of real-world evidence. Epilep Open. (2018) 3:55–65. doi: 10.1002/epi4.12092
133. Centonze D, Petta F, Versace V, Rossi S, Torelli F, Prosperetti C, et al. Effects of motor cortex rTMS on lower urinary tract dysfunction in multiple sclerosis. Multiple Scler J. (2007) 13:269–71. doi: 10.1177/1352458506070729
134. Mori F, Codecà C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, et al. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. (2010) 17:295–300. doi: 10.1111/j.1468-1331.2009.02806.x
135. Boutière C, Rey C, Zaaraoui W, Le Troter A, Rico A, Crespy L, et al. Improvement of spasticity following intermittent theta burst stimulation in multiple sclerosis is associated with modulation of resting-state functional connectivity of the primary motor cortices. Multiple Scler J. (2017) 23:855–63. doi: 10.1177/1352458516661640
136. Mori F, Ljoka C, Magni E, Codecà C, Kusayanagi H, Monteleone F, et al. Transcranial magnetic stimulation primes the effects of exercise therapy in multiple sclerosis. J Neurol. (2011) 258:1281–7. doi: 10.1007/s00415-011-5924-1
137. Koch G, Rossi S, Prosperetti C, Codecà C, Monteleone F, Petrosini L, et al. Improvement of hand dexterity following motor cortex rTMS in multiple sclerosis patients with cerebellar impairment. Multiple Scler J. (2008) 14:995–8. doi: 10.1177/1352458508088710
138. Elzamarany E, Afifi L, El-Fayoumy NM, Salah H, Nada M. Motor cortex rTMS improves dexterity in relapsing-remitting and secondary progressive multiple sclerosis. Acta Neurologica Belgica. (2016) 116:145–50. doi: 10.1007/s13760-015-0540-y
139. Hulst H, Goldschmidt T, Nitsche M, De Wit S, Van Den Heuvel O, Barkhof F, et al. rTMS affects working memory performance, brain activation and functional connectivity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. (2017) 88:386–94. doi: 10.1136/jnnp-2016-314224
140. Burhan AM, Subramanian P, Pallaveshi L, Barnes B, Montero-Odasso M. Modulation of the left prefrontal cortex with high frequency repetitive transcranial magnetic stimulation facilitates gait in multiple sclerosis. Case Rep Neurol Med. (2015) 2015:251829. doi: 10.1155/2015/251829
141. Agüera E, Caballero-Villarraso J, Feijóo M, Escribano BM, Bahamonde MC, Conde C, et al. Impact of repetitive transcranial magnetic stimulation on neurocognition and oxidative stress in relapsing-remitting multiple sclerosis: a case report. Front Neurol. (2020) 11:817. doi: 10.3389/fneur.2020.00817
142. Korzhova J, Bakulin I, Sinitsyn D, Poydasheva A, Suponeva N, Zakharova M, et al. High-frequency repetitive transcranial magnetic stimulation and intermittent theta-burst stimulation for spasticity management in secondary progressive multiple sclerosis. Eur J Neurol. (2019) 26:680–e44. doi: 10.1111/ene.13877
143. Liu M, Fan S, Xu Y, Cui L. Non-invasive brain stimulation for fatigue in multiple sclerosis patients: a systematic review and meta-analysis. Multiple Scler Relat Disord. (2019) 36:101375. doi: 10.1016/j.msard.2019.08.017
144. Gaede G, Tiede M, Lorenz I, Brandt AU, Pfueller C, Dörr J, et al. Safety and preliminary efficacy of deep transcranial magnetic stimulation in MS-related fatigue. Neurol-Neuroimmunol Neuroinflam. (2018) 5:e423. doi: 10.1212/NXI.0000000000000423
145. Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol/Evoked Potentials Section. (1989) 74:458–62. doi: 10.1016/0168-5597(89)90036-1
146. Chen R, Spencer DC, Weston J, Nolan SJ. Transcranial magnetic stimulation for the treatment of epilepsy. Cochr Database Syst Rev. (2016) 2016:CD011025. doi: 10.1002/14651858.CD011025.pub2
147. Hsu W-Y, Cheng C-H, Lin M-W, Shih Y-H, Liao K-K, Lin Y-Y. Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation: a meta-analysis. Epilep Res. (2011) 96:231–40. doi: 10.1016/j.eplepsyres.2011.06.002
148. Mahajan UV, Parker JJ, Williams NR, Bhati MT, Ku S, Grant G, et al. Adjunctive repetitive transcranial magnetic stimulation delivers superior quality of life for focal epilepsy compared to anti-epileptic drugs: a meta-analytic utility prediction study. Brain Stimul. (2020) 13:430–2. doi: 10.1016/j.brs.2019.12.006
149. Cincotta M, Giovannelli F, Chiaramonti R, Bianco G, Godone M, Battista D, et al. No effects of 20 Hz-rTMS of the primary motor cortex in vegetative state: a randomised, sham-controlled study. Cortex. (2015) 71:368–76. doi: 10.1016/j.cortex.2015.07.027
150. Liu X, Meng F, Gao J, Zhang L, Zhou Z, Pan G, et al. Behavioral and resting state functional connectivity effects of high frequency rTMS on disorders of consciousness: a sham-controlled study. Front Neurol. (2018) 9:982. doi: 10.3389/fneur.2018.00982
151. He F, Wu M, Meng F, Hu Y, Gao J, Chen Z, et al. Effects of 20 Hz repetitive transcranial magnetic stimulation on disorders of consciousness: a resting-state electroencephalography study. Neural Plasticity. (2018) 2018:5036184. doi: 10.1155/2018/5036184
152. Naro A, Russo M, Leo A, Bramanti P, Quartarone A, Calabrò RS. A single session of repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in patients with unresponsive wakefulness syndrome: preliminary results. Neurorehabil Neural Repair. (2015) 29:603–13. doi: 10.1177/1545968314562114
153. Xia X, Bai Y, Zhou Y, Yang Y, Xu R, Gao X, et al. Effects of 10 Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Front Neurol. (2017) 8:182. doi: 10.3389/fneur.2017.00182
154. Xia X, Liu Y, Bai Y, Liu Z, Yang Y, Guo Y, et al. Long-lasting repetitive transcranial magnetic stimulation modulates electroencephalography oscillation in patients with disorders of consciousness. Neuroreport. (2017) 28:1022–9. doi: 10.1097/WNR.0000000000000886
155. Wu M, Wu Y, Yu Y, Gao J, Meng F, He F, et al. Effects of theta burst stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Brain Stimul. (2018) 11:1382–4. doi: 10.1016/j.brs.2018.07.055
156. Zis P, Shafique F, Hadjivassiliou M, Blackburn D, Venneri A, Iliodromiti S, et al. Safety, tolerability, and nocebo phenomena during transcranial magnetic stimulation: a systematic review and meta-analysis of placebo-controlled clinical trials. Neuromodulation. (2020) 23:291–300. doi: 10.1111/ner.12946
157. Loo CK, McFarquhar TF, Mitchell PB. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int J Neuropsychopharmacol. (2008) 11:131–47. doi: 10.1017/S1461145707007717
158. Farzan F, Wu Y, Manor B, Anastasio EM, Lough M, Novak V, et al. Cerebellar TMS in treatment of a patient with cerebellar ataxia: evidence from clinical, biomechanics and neurophysiological assessments. Cerebellum. (2013) 12:707–12. doi: 10.1007/s12311-013-0485-8
159. Manor B, Greenstein PE, Davila-Perez P, Wakefield S, Zhou J, Pascual-Leone A. Repetitive transcranial magnetic stimulation in spinocerebellar ataxia: a pilot randomized controlled trial. Front Neurol. (2019) 10:73. doi: 10.3389/fneur.2019.00073
160. Pauly MG, Steinmeier A, Bolte C, Hamami F, Tzvi E, Münchau A, et al. Cerebellar rTMS and PAS effectively induce cerebellar plasticity. Sci Rep. (2021) 11:1–13. doi: 10.1038/s41598-021-82496-7
161. Schwenkreis P, Tegenthoff M, Witscher K, Börnke C, Przuntek H, Malin JP, et al. Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect. Brain. (2002) 125:301–9. doi: 10.1093/brain/awf023
162. Groiss SJ, Ugawa Y. Cerebellar stimulation in ataxia. Cerebellum. (2012) 11:440–2. doi: 10.1007/s12311-011-0329-3
163. Shiga Y, Tsuda T, Itoyama Y, Shimizu H, Miyazawa K, Jin K, et al. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J Neurol Neurosurg Psychiatry. (2002) 72:124–6. doi: 10.1136/jnnp.72.1.124
164. Ihara Y, Takata H, Tanabe Y, Nobukuni K, Hayabara T. Influence of repetitive transcranial magnetic stimulation on disease severity and oxidative stress markers in the cerebrospinal fluid of patients with spinocerebellar degeneration. Neurol Res. (2005) 27:310–3. doi: 10.1179/016164105X39897
165. Benussi A, Pascual-Leone A, Borroni B. Non-invasive cerebellar stimulation in neurodegenerative ataxia: a literature review. Int J Mol Sci. (2020) 21:1948. doi: 10.3390/ijms21061948
Keywords: transcranial magnetic stimulation (TMS), Alzheimer, Parkinson, movement disorder, epilepsy, migraine, stroke
Citation: Somaa FA, de Graaf TA and Sack AT (2022) Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 13:793253. doi: 10.3389/fneur.2022.793253
Received: 11 October 2021; Accepted: 25 February 2022;
Published: 20 May 2022.
Edited by:
Sergio Machado, Federal University of Santa Maria, BrazilReviewed by:
Roberto Rodríguez-Labrada, Cuban Neuroscience Center, CubaCopyright © 2022 Somaa, de Graaf and Sack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander T. Sack, YS5zYWNrQG1hYXN0cmljaHR1bml2ZXJzaXR5Lm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.