- Department of Pediatrics, Xiangya Hospital, Central South University, Changsha, China
Objective: The limitations of adrenocorticotrophic hormone (ACTH) treatment for infantile spasms (ISs), such as high costs, limited availability, and adverse effects (AEs), make it necessary to explore whether corticosteroids are optimal alternatives. Many other compelling treatments have gone through trials due to the suboptimal effectiveness of hormonal therapy. A systematic review and meta-analysis were performed to evaluate the effectiveness and safety of hormonal therapy for patients with ISs.
Methods: EMBASE, Ovid MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and online registers were searched through April 2021 for randomized controlled trials (RCTs).
Results: A total of 19 RCTs (N = 1,279) were included. There was no significant difference in the effectiveness of oral corticosteroids and ACTH in electro-clinical response (risk ratio [RR] = 0.85, 95% CI 0.41–1.76). Low-dose ACTH had similar effectiveness in electro-clinical response compared to usual-dose group (RR = 0.94, 95% CI 0.60–1.47) but conferred a lower risk of AEs (RR = 1.71, 95% CI 1.08–2.71). ACTH was more beneficial in controlling spasms than vigabatrin (VGB) (RR = 1.31, 95% CI 1.05–1.64) for patients without tuberous sclerosis complex (TSC). All RCTs were connected through network meta-analysis, and we found that ketogenic diet (KD), zonisamide, methylprednisolone, or combined treatment of hormonal therapy with topiramate (TPM) or pyridoxine was not different in electro-clinical response compared to usual-dose ACTH.
Conclusion: Our analysis showed that oral corticosteroids could be optional alternatives when ACTH is not applicable, and ACTH is more beneficial for patients without TSC. Moreover, low-dose ACTH is recommended due to comparative effectiveness but lower risk of AEs. However, due to the high heterogeneity of included patients and treatment protocols, these results must be interpreted with caution. RCTs with multicentric involvement and larger sample size are needed for solid evaluation of other alternative treatments.
Introduction
Infantile spasms (ISs), also known as West Syndrome, with a minimal incidence of 0.25 per 1,000 live births (1), are early-onset developmental and epileptic encephalopathy. It is characterized by a triad of epileptic spasms, neurodevelopmental regression, and hypsarrhythmia on the electroencephalogram (EEG), mainly occurring at the age of 3–12 months, with a peak occurrence around 4–6 months (2). After developmental arrest or regression, about 70% of infants become severely disabled both physically and intellectually and can develop multiple types of refractory seizures in the future. Currently, hormonal therapy [adrenocorticotrophic hormone (ACTH) and corticosteroids] and vigabatrin (VGB) are the most effective treatments for ISs. ACTH is the preferred first-line treatment for most patients with ISs, but ACTH cost is continuing to rise, and it is difficult to obtain in some countries (3, 4). Moreover, the incidence of adverse effects (AEs) of ACTH treatments is high, and thus, corticosteroids are substitutes that are well worth being explored for the treatment effectiveness, especially for low- and middle-income countries. However, hormonal therapy is partially effective. The responder rate of hormonal therapy varied from 37 to 87%, and the relapse rate can be up to 66% (5–12). Therefore, other treatments, including antiepileptic drugs (AEDs) and the ketogenic diet (KD), are also actively investigated. To date, optimal treatments of ISs are still open for investigation and discussion. Thus, we performed this systematic review and meta-analysis to provide more evidence for clinical decisions in choosing other treatments for ISs over ACTH.
Methods
Inclusion Criteria of Studies for Our Analysis
Participants of Studies
Patients who were newly diagnosed with ISs without receiving any hormonal treatments before enrollment were eligible for our review and analysis.
Intervention and Trial Design of Studies
Based on our knowledge about hormonal therapy for ISs, the topics of relevant clinical trials could be grouped into categories as follows: (1) ACTH vs. oral or intravenous corticosteroids, (2) comparison of different dosages of hormonal therapy, (3) hormonal therapy vs. VGB, (4) hormonal therapy vs. other treatments, and (5) hormonal therapy alone vs. combined treatments. Randomized controlled trials (RCTs) with double-blind, single-blind, and open-label designs that studied the above topics were considered for inclusion.
Outcomes of Studies
Studies included in our analysis must report the complete cessation of spasms as one of the clinical end points. Remission of hypsarrhythmia on EEG, relapses, as well as developmental outcomes were expected to report, but these outcomes were dispensable for a study to be included or not.
Safety and Tolerance
The AEs were documented.
Data Sources
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). The electronic databases, including EMBASE, Ovid MEDLINE, and Cochrane Central Register of Controlled Trials (CENTRAL), were searched from database inception to April 26, 2021, to identify the published data. The search strategies (shown in Supplementary Material 1) were developed specially for each database, and the keywords were adapted according to the configuration of each database. Online trial registers, including NIH ClinicalTrials.gov, metaRegister of Controlled Trials, and WHO International Clinical Trials Registry Platform (ICTRP), were searched to identify ongoing trials or unpublished trials. The search terms, including “infantile spasms” and “West Syndrome,” were used when searching online registers. Searches were not limited by language, date, or publication status.
Assessment of Risk of Bias, Data Extraction, and Synthesis
The risk of bias of RCTs was assessed by two independent authors using the Cochrane Collaboration's tool (14). Briefly, six domains of bias, namely, selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias, were rated. Studies with all domains to be rated as low risk were considered to have a low risk of bias. If one or more domains were rated as unclear risk, and the other domains were at low risk of bias, the studies were considered as having unclear risk of bias. One study was considered to have a high risk of bias if one or more domains were rated as having a high risk of bias.
The data, including authors, publication year, sex ratio, treatment protocols, the number of patients on each treatment arm, the number of patients achieving spasm cessation and electro-clinical response, as well as AEs, were extracted by one author and confirmed by two other review authors. The electro-clinical response was defined as the complete cessation of spasms with EEG remission of hypsarrhythmia. Review Manager version 5.3 was used for calculations of data synthesis. For dichotomous outcomes, such as electro-clinical response and AEs, the risk ratio (RR) with 95% CI was calculated after data synthesis. Statistical significance for RR was defined as 95% CI not containing 1. For crossover RCTs, only results of the initial stage were synthesized and analyzed.
Network Meta-Analysis
To gain a better understanding of the performance of treatments with no head-to-head trials with ACTH, a network was generated through joint treatments. Network meta-analysis was conducted using MetaXL (version 5.3). Each direct comparison was wrapped in the MAInputTable function (IOType = “NumRR” and Method = “IVhet”), and then the MANetwork function was used to perform network meta-analysis. Consistency H was calculated at the same time.
Results
Study Selection and Characteristics
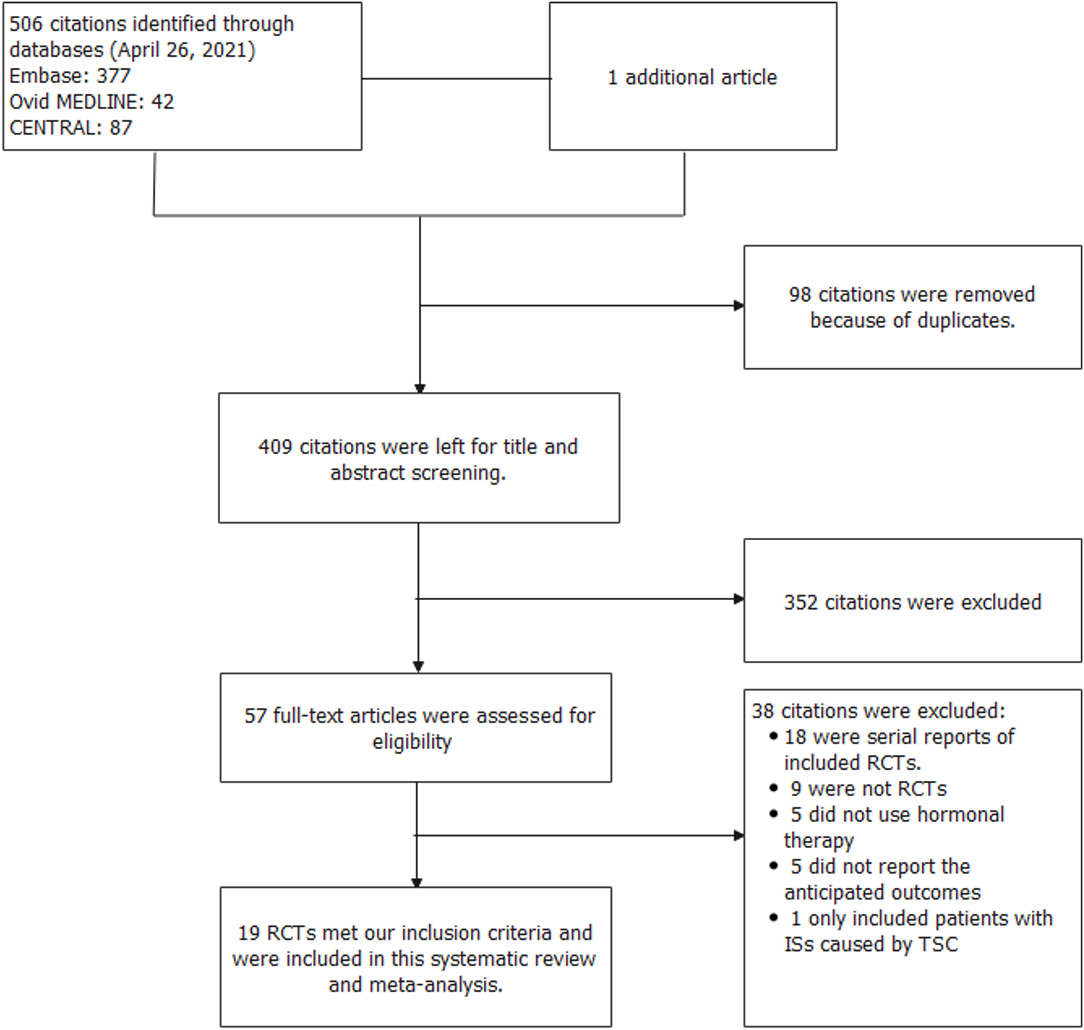
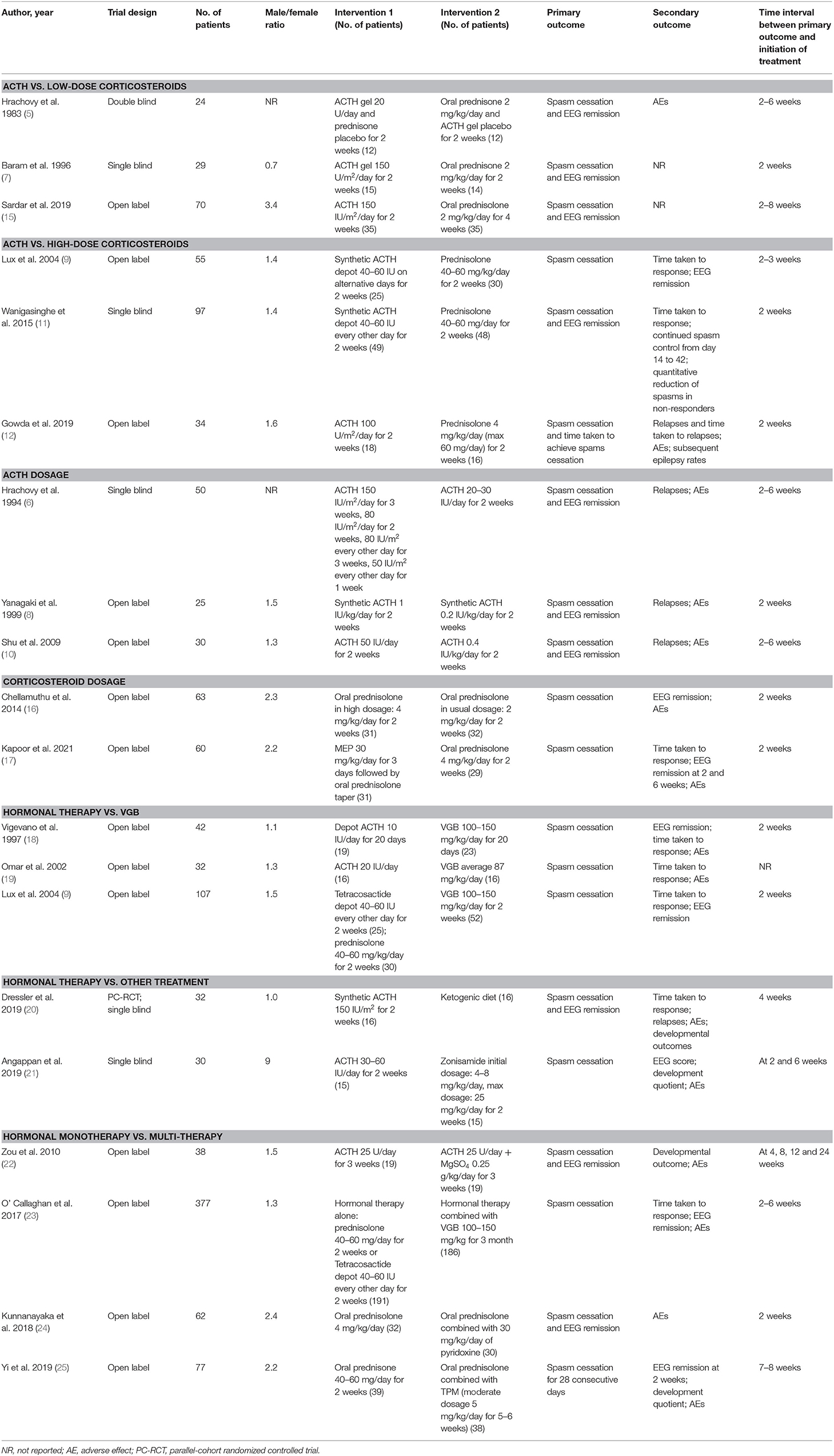
In total, 507 records were identified in the initial search; 98 were removed due to duplications. Through title and/or abstract screening, 352 were excluded. After perusing full texts, 38 records were excluded, and the reasons for exclusion are listed in Figure 1. Overall, 19 RCTs (comprising 1,279 patients), which were conducted between 1983 and 2021, met our inclusion criteria. The characteristics of the included studies are presented in Table 1. All studies were alleged as RCTs; however, only twelve and nine studies provided sufficient descriptions on random sequence generation and allocation concealment, respectively. We accepted that blinding of participants and care providers could not be done in most circumstances as two interventions were given through different routes. Only one study, Hrachovy et al. (5), used a double-blind design, and five studies applied a single-blind design by masking personnel to treatment protocols when analyzing EEG results. The remaining studies were open-label randomized trials. Judgments about each risk of bias item are presented in Figure 2. Although only nine out of 19 studies reported electro-clinical response (defined as complete cessation of spasms with EEG remission of hypsarrhythmia) as the primary outcome, corresponding EEG results were documented as one of the secondary outcomes in all other studies except for Omar et al. (19), making meta-analysis of electro-clinical response possible. Time intervals between treatment initiation and electro-clinical response varied, but 2 weeks after treatment initiation was the most common time point to evaluate treatment effectiveness.
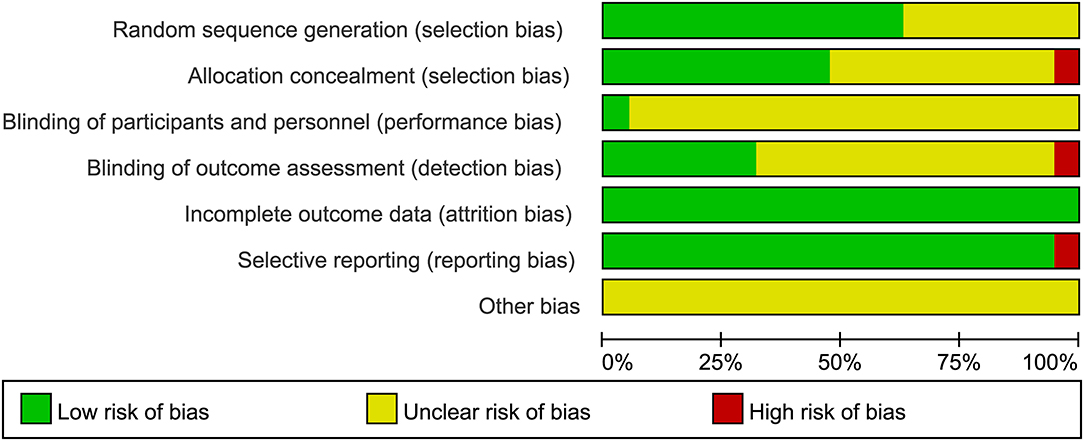
Figure 2. Judgments about each risk of bias item presented as percentages across all included studies.
Adrenocorticotrophic Hormone vs. Oral Corticosteroids
Six RCTs that compared ACTH with oral corticosteroids for the treatment of ISs, with 323 participants included, met our inclusion criteria (5, 7, 9, 11, 12, 15). Three of these RCTs, namely, Hrachovy et al. (5), Baram et al. (7), and Sardar et al. (15), used low-dose corticosteroids (2 mg/kg/day of prednisone or prednisolone). The other three RCTs, namely, Lux et al. (9), Wanigasinghe et al. (11), and Gowda et al. (12), applied high-dose prednisolone (4 mg/kg/day or 40–60 mg/day). The forms (natural or synthetic analogs) and dosages of ACTH varied in these trials. The primary comparison of Lux et al. (9) was between hormonal therapy and VGB, so the secondary comparison of ACTH and prednisolone might be underpowered. Meta-analysis revealed that the effectiveness of ACTH and corticosteroids on electro-clinical response was comparable, regardless of the dosage of corticosteroids (Figure 3).
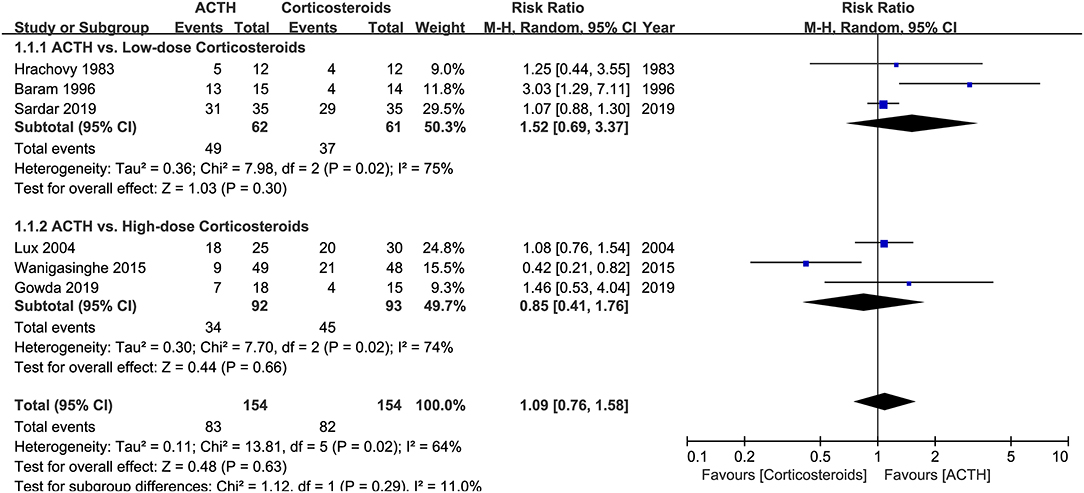
Figure 3. Forest plot showing risk ratio (RR) and 95% CI of electro-clinical response between adrenocorticotrophic hormone (ACTH) and oral corticosteroids.
Hrachovy et al. (5) reported that six (25%) patients had hypertension (>140/90 mmHg) during the initial and crossover stages, of which four developed at both stages and two developed only when receiving prednisone. After 2 weeks of the initial stages, 10 of 16 patients who received serial CT scans showed evidence of cerebral shrinkage, such as increased ventricular size and/or increased subarachnoid space, but no comparisons were made between treatment groups. Lux et al. (9) reported that 11 (36.7%) patients in the prednisolone group and 8 (32.0%) in the ACTH group had hypertension (>120/90 mmHg). Glycosuria was found in one patient in the ACTH group. In Gowda et al. (12), the incidence of AEs in the prednisolone group was 20% compared to 16.6% in the ACTH group, but details about the profile of AEs were not reported. Wanigasinghe et al. (11) found a higher incidence of abdominal distension in the prednisolone group (21.1% vs. 0, adjusted p-value <0.00294), whereas the other AEs, such as increased appetite, weight gain, Cushingoid features, insomnolence, hypertension, increased susceptibility to infection, and electrolyte imbalances, were not different between two groups.
Adrenocorticotrophic Hormone Dosage
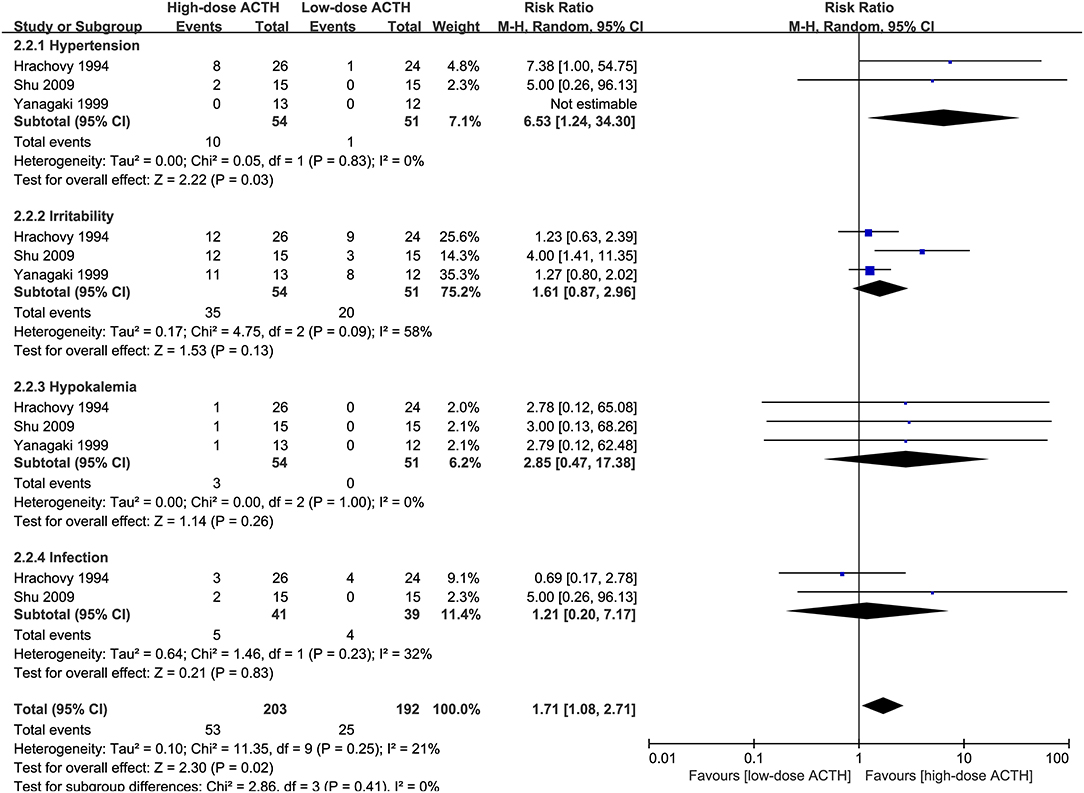
Three RCTs that compared the effectiveness and safety of different dosages of ACTH were included in our analysis (6, 8, 10). There was no significant difference in the proportion of patients achieving electro-clinical response between high- and low-dose groups (RR = 0.94, 95% CI 0.60–1.47, Figure 4). However, the patients in the high-dose ACTH treatment group had a higher incidence rate of AEs (RR = 1.71, 95% CI 1.08–2.71, Figure 5). Except for the AEs shown in Figure 5, Yanagaki et al. (8) and Shu et al. (10) also reported brain shrinkage but in different measurement methods. Shu et al. (10) reported one case of brain shrinkage in the high-dose ACTH group but no case in the low-dose group, whereas Yanagaki et al. (8) evaluated the volume difference of lateral ventricles, showing brain shrinkage was significantly milder in the low-dose group than in the high-dose group (6.6 ± 4.6 vs. 12.4 ± 5.7%; p < 0.05).
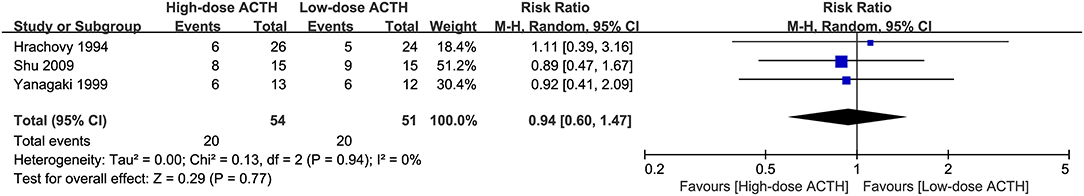
Figure 4. Forest plot showing RR and 95% CI of electro-clinical response between high and low dose of ACTH.
Corticosteroid Dosage
An open-label RCT, Chellamuthu et al. (16), compared the effectiveness and safety of high- (4 mg/kg/day) and usual-dose (2 mg/kg/day) prednisolone for the treatment of ISs. A total of 63 participants, aged 3–24 months, were enrolled, with 31 randomly allocated to the high-dose group and 32 to the usual-dose group. On day 14 after the initiation of treatment, 12 (38.7%) and 7 (21.9%) of participants achieved electro-clinical response in high- and usual-dose groups, respectively (p = 0.15). The occurrence of AEs, including increased appetite, weight gain, Cushingoid features, and infections, was higher in the high-dose group but did not reach a statistical significance.
A recent open-label RCT, Kapoor et al. (17) explored the feasibility of shortening the treatment course by using methylprednisolone pulses (MEP). By day 14, no difference was found in the proportion of patients achieving spasm cessation (17/31 vs. 20/29, p = 0.26) and electro-clinical response between MEP and oral prednisolone groups; however, no details about EEG remission were given. Patients in the MEP group took less time to achieve remission (mean 5.4 ± 0.9 vs. 9.5 ± 2.6 days, p < 0.0001). However, 6 (19.4%) patients who received MEP had spasm recurrence, compared to none in the oral prednisolone group. Therefore, by 6 weeks, the proportion of patients who had EEG remission was significantly lower in the MEP group (45.2 vs. 75.9%, p < 0.015). Increased appetite (65.5 vs. 3.2%) and weight gain (75.9 vs. 16.1%) were more common in oral prednisolone group (p < 0.05), whereas sleep disturbance (61.3 vs. 20.7%, p = 0.0014), hypertension (35.5 and 10.3%, p = 0.032), and irritability (74.2 vs. 31%, p = 0.0008) were more frequent in MEP group.
Hormonal Therapy vs. VGB
Of three included RCTs that compared hormonal therapy to VGB (9, 19, 26), Vigevano and Cilio (26) included 4 patients with tuberous sclerosis complex (TSC), with three assigned to VGB arm and one assigned to hormone therapy arm and all four patients responded to treatments, whereas Omar et al. (19) included 2 patients with TSC without reporting treatment assignment and response. The combined result showed that ACTH had a more favorable effect than VGB on cessation of spasms (RR = 1.31, 95% CI 1.05–1.64, Figure 6). Both Vigevano and Cilio (26) and Lux et al. (9) summarized the short-term AEs, demonstrating that the number of overall AEs was similar to hormonal therapy (n = 62) and VGB (n = 55). Patients showed hypertension, increased appetite, irritability on hormonal therapy, and more drowsiness on VGB. No visual effects were detected during 2 weeks of monitoring. Omar et al. (19) did a long-term follow-up with visual evoked responses and electroretinography carried out by an ophthalmologist and found no significant changes.

Figure 6. Forest plot showing RR and 95% CI of spasm cessation between hormonal therapy and vigabatrin.
Adrenocorticotrophic Hormone vs. Other Treatments
Dressler et al. (20) was a parallel-cohort (PC) RCT comparing KD and high-dose synthetic ACTH for ISs with 32 patients assigned in the RCT (16 KD, 16 ACTH). The other 69 patients who were not eligible for the RCT were allocated in the PC (37 KD and 32 ACTH). The proportion of patients achieving the primary outcome, i.e., electro-clinical remission, was similar between KD and ACTH groups (RCT 62 vs. 69%; PC 41 vs. 38%; combined cohort 47 vs. 48%). There was also no difference in time taken to electro-clinical remission between KD and ACTH groups. The relapse rate was similar between treatment arms in the RCT but lower for KD in the PC (KD 0 vs. ACTH 50%, p < 0.001). The frequency of overall AEs was not different between KD and ACTH groups (42 vs. 45%), but medical interventions to treat AEs were significantly less required in the KD group (30 vs. 94%, p < 0.001).
Another RCT investigated the effectiveness of oral zonisamide for ISs compared to ACTH (21). At 14 days of the initiation of treatment, the proportions of children with electro-clinical responses were not statistically different in the two groups (zonisamide 3/15 vs. ACTH 5/15, p = 0.68). At 6 weeks of treatment, more patients had EEG remission (zonisamide 5/14 vs. ACTH 10/14, p = 0.14). Patients of the ACTH group had significantly better hypsarrhythmia scores, which were assessed via the Jeavons scoring system. Predominant AEs of patients receiving zonisamide were lethargy and irritability. One case required dose reduction due to metabolic acidosis, and one case withdrew the medication due to significant loss of appetite.
Monotherapy vs. Multitherapy
Zou et al. (22) was an open-label RCT with a 24-week follow-up duration, comparing the effectiveness of ACTH monotherapy and ACTH with MgSO4. At 4 weeks of the initiation of treatment, the proportion of patients with spasm cessation was higher in ACTH + MgSO4 group compared to the ACTH group (12/19 vs. 8/19, p = 0.003), but the proportions of patients with electro-clinical response were not statistically different in the two groups. Developmental quotients (DQs) measured by the Gesell tests showed no difference between the two groups at baseline and after 24 weeks of treatment. However, patients of the combination therapy group showed a significant improvement of mean DQ at personal-social dimension compared to baseline level (combination therapy group: from 48.6 to 65.2, p < 0.05; ACTH group: from 47.7 to 49.9). AEs were reported in 31.6% and 42.1% of patients in the combination therapy group and ACTH group, respectively, but were not significantly different between the two groups.
O'Callaghan et al. (23) was an open-label RCT that studied whether the combination of hormonal therapy and VGB was superior to hormonal therapy alone. The type of hormonal therapy (i.e., prednisolone or tetracosactide depot) was randomly assigned or chosen by parents. Spasm cessation on days 13 and 14 was achieved in 166 (89%) of 186 patients treated with combination therapy compared to 132 (69%) of 191 patients on hormonal therapy (95% CI 11.8–28.6; χ2 = 23.2; p < 0.001). Electro-clinical remission was achieved in 123 (66%) patients on combination therapy compared to 104 (55%) who were allocated to hormonal therapy (95% CI 1.4–21.6; χ2 = 5.2; p = 0.023). Time taken to reach spasm-free was shorter on combination therapy than hormonal therapy [median 2 days (interquartile range (IQR) 2–4) vs. median 4 days (IQR 3–6); z = 6.04; p < 0.001]. No significant difference in AEs was found between groups (117 in the combination therapy group and 111 in the hormonal therapy group).
Kunnanayaka et al. (24) was an open-label RCT to explore whether the addition of pyridoxine to prednisolone could offer more benefits to patients with ISs. All 66 eligible patients received 30 mg/kg/day of pyridoxine for 3 days before the commencement of the study. Four patients were excluded due to marked improvement after the initial pyridoxine trial. The proportions of children with electro-clinical remission on day 14 were not significantly different (monotherapy 28.1% vs. combination therapy 30.0%, p = 0.87). The AEs in both groups included increased appetite, irritability, weight gain, Cushingoid facies, and excessive daytime sleepiness, but no patients were required to withdraw the treatments. Follow-up of the patients who were spasm-free showed that 4 patients relapsed in the prednisolone group and one relapsed in the combination therapy group after 1 month of treatment.
Yi et al. (25) was an RCT that studied whether the addition of topiramate (TPM) to prednisone was more beneficial for patients with ISs or late-onset epileptic spasms. Cessation of spams at day 14 after treatment entry was achieved in 28 (71.8%) of 39 patients on prednisone and in 29 (76.3%) of 38 patients on combination therapy (χ2 = 0.205; p = 0.796). Among these patients who were spasm-free, 21 of 28 patients in the monotherapy group and 20 of 29 in the combination therapy group achieved electro-clinical remission. The rate of patients who stayed spasm-free in the monotherapy group and the combination therapy group at day 49 or 56 was not significantly different (71.8 vs. 65.8%, p = 0.569), so was at day 120 (61.5 vs. 50.0%, p = 0.308). There was no significant difference in the rate of patients occurring AEs between the two treatment groups.
Network Meta-Analysis
To gain a comprehensive understanding of the efficacy of all included treatments compared to usual- or high-dose ACTH, a network meta-analysis was conducted using MetaXL (version 5.3) (Figure 7A). Because ACTH usage was rather heterogeneous, we defined less than 25 U/day of ACTH as low-dose ACTH, otherwise as usual- or high-dose ACTH. The assignment of treatment groups of all studies and all comparisons of network meta-analysis are provided in Supplementary Material 2.
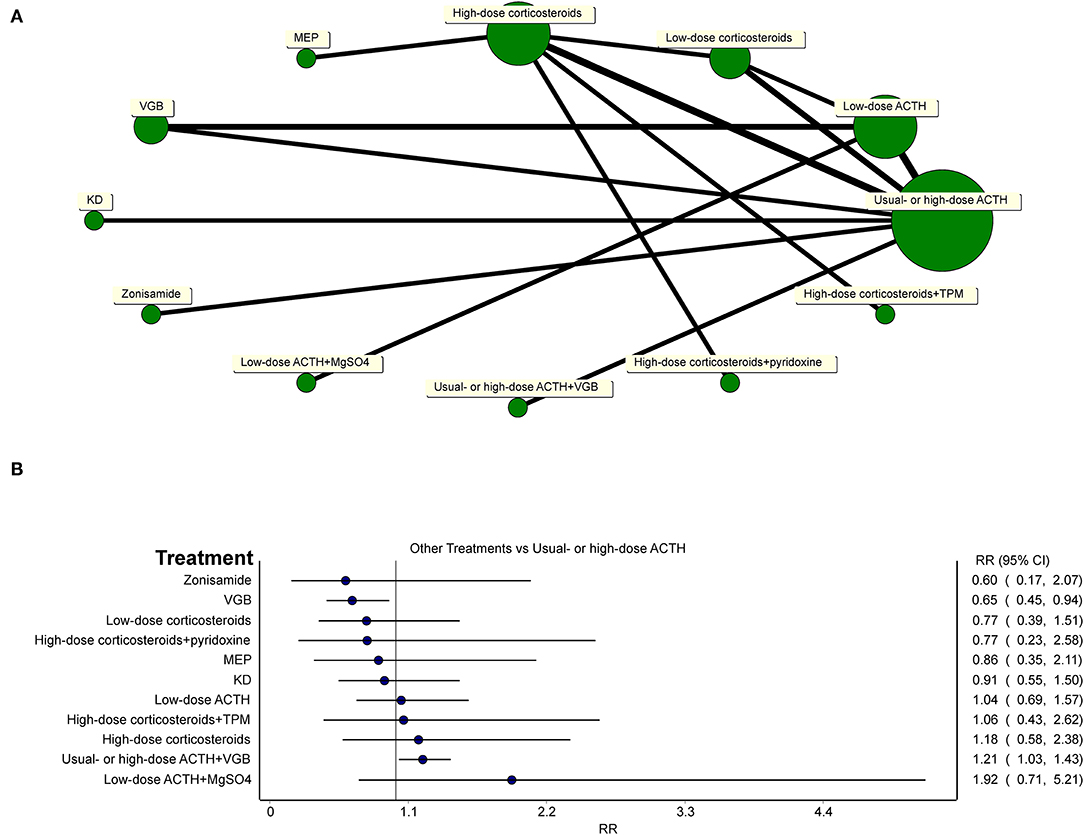
Figure 7. (A) Network of all treatments of included randomized controlled trials. (B) Network meta-analysis of all other treatments vs. usual-dose ACTH in the effectiveness of the electro-clinical response.
Network meta-analysis (Figure 7B) showed that usual- or high-dose ACTH did not superior to oral corticosteroids (regardless of the dosage of oral corticosteroids), MEP, zonisamide, and KD in the effectiveness of the electro-clinical response. Additional treatments to hormonal therapy, such as TPM, pyridoxine, and MgSO4, did not lend any benefits to patients in controlling spasms, but the combination of hormonal therapy and VGB was better than using ACTH alone (RR = 1.21, 95% CI 1.03–1.43). For general patients without TSC, ACTH had an edge over VGB (RR = 0.65, 95% CI 0.45–0.94).
Discussion
The limitations of ACTH treatment for ISs, such as high costs, availability in resource-limited countries, and severe AEs, make good alternatives of oral corticosteroids. Especially, there is currently no potent evidence showing ACTH is superior to oral corticosteroids regarding spasm cessation and EEG remission of hypsarrhythmia. Our meta-analysis showed that oral corticosteroids, prednisone, and prednisolone, regardless of low (2 mg/kg/day) or high dosage (40–60 mg/day or 4 mg/kg/day), were as effective as ACTH in controlling spasms. However, considering that infants have the inadequate ability of the HSD11B1 enzyme to reduce prednisone to prednisolone, the use of low-dose prednisone probably should not be recommended (27). Although oral corticosteroids have overcome many of the limitations associated with ACTH treatment, their overall long-period treatment (usually 4–6 weeks) is still a drawback. MEP, as another application form of corticosteroids, overcomes the drawback of long-period treatment and, thus, improves treatment compliance. No head-to-head RCT that compared ACTH and MEP was retrieved during literature searching, but through network meta-analysis, we found that MEP had similar efficacy as ACTH in the short-term spasm control (RR = 0.86, 95% CI 0.35–2.11). However, the included RCT (17) and a non-RCT (28) both reported a higher rate of reoccurrence of spasms in patients who received MEP, which should not be overlooked.
The commonly reported serious AEs of ACTH treatment include hypertension, infection, reversible brain shrinkage, cardiac hypertrophy, and electrolyte abnormalities, which deter the extensive usage of ACTH. These AEs may be dose- and duration-dependent, so it is of great concern to strike the balance among the lower ACTH dose, fewer AEs, and maximum therapeutic effects. Our analysis showed the effectiveness of low- and usual-dose ACTH was not different, but patients using usual-dose ACTH were at higher risk of having AEs. Overall, low-dose ACTH should be considered over high-dose regimens due to comparative effectiveness and a preferable profile of AEs.
The VGB, as an inhibitor of GABA-transaminase, has been introduced as a first-choice drug in the treatment of ISs, especially it is the best choice for IS caused by TSC (29, 30). VGB is given orally and rapidly absorbed, which is a great advantage for pediatric patients. Although visual-field defect, which is usually irreversible and asymptomatic, has been reported in pediatric patients, VGB, in general, is well-tolerated in children (31). It is of utmost importance to address whether VGB has similar therapeutic effectiveness as hormonal therapy in patients with IS who do not have TSC. Our analysis showed that hormonal therapy had better efficacy in spasm cessation for general patients with ISs; compared to VGB, even a few patients with TSC were occasionally included. In addition, the only regimen in our analysis that had better efficacy than ACTH was the combined use of hormonal therapy and VGB.
Even if hormonal therapy and VGB are administered at the first-line treatments, only about 30% of patients achieve long-term remission. Many patients have the reoccurrence of spasms or develop other types of epilepsy, such as Lennox-Gastaut Syndrome. There is a strong demand for other compelling therapeutic options. A systematic review showed that at least 25% of patients, most of which failed the first-line treatments, can be expected to have a >50% reduction of spasm frequency after the treatment of zonisamide (32). A systematic review of 13 observational studies showed that 57.4% of patients achieved >50% improvement in spasm frequency with 6 months of KD treatment (33). A recent non-RCT on patients who failed hormonal therapy showed that 13.4% of patients had ≥50% spasm reduction compared to 10% in the control group (34). When addressing the effectiveness and safety of other non-first-line treatment for ISs, out of consideration of ethics, most studies used observational or uncontrolled trials, and inevitably, the included patients often have complicated medication histories of AEDs or long treatment lag. During our literature search, only two RCTs administered alternative treatments (i.e., KD and zonisamide) as the first-line treatments to patients with hormonal therapy naïve. Our network meta-analysis revealed that neither of these two treatments showed a difference in the effectiveness of short-term spasm control compared to usual-dose ACTH. Additionally, the combining use of hormonal therapy with TPM, pyridoxine, or MgSO4 did not lend any benefits to patients in spasm control, either.
The main limitation of this study is the high heterogeneity of included patients and treatment protocols. The age range, sex ratio, and the ratio of cryptogenic and symptomatic ISs varied from study to study. A variety of agents were used, such as natural ACTH gel or synthetic ACTH, in a rather wide range of treatment lengths and dosages. Although 2 weeks after initiation of treatment was the most common time point to evaluate electro-clinical response, some studies adopted longer time intervals (4–8 weeks) before the evaluation, which was another source of clinical heterogeneity. As shown in Zou et al. (22) and Angappan et al. (21), the proportion of patients with EEG remission increased at a later time point. Another limitation is the limited number and small sample size of RCTs that studied other alternative treatments, such as KD or zonisamide, thereby, making the level of evidence low. Furthermore, many of the included studies, even studies that compared hormonal therapy and VGB, did not exclude the patients with TSC, which may confound the comparison of effectiveness. Thus, our results must be interpreted with caution.
Conclusion
Our systematic review and meta-analysis showed that oral corticosteroids could be optional alternatives when ACTH is not applicable due to high costs, limited availability, or severe AEs, but for MEP as the first-line treatment, more studies are needed to explore the treatment duration and dosage and continuous effects on spasm control. Low-dose ACTH is recommended because of similar effectiveness and lower risk of AEs compared to high-dose ACTH. The evaluation of the effectiveness of other alternative treatments still needs RCTs with multicentric involvement and larger sample size to provide a higher level of evidence.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SG developed the literature search protocol, conducted data analysis, and drafted and revised the manuscript for intellectual content. SG and LM conducted a literature search. SG and LZ contributed to data extraction. FL and ZP contributed to the assessment of risks of bias. FY revised the manuscript for intellectual content. JP planned, designed, and conceptualized this study and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81771409 and 82071462).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.772333/full#supplementary-material
References
1. Jia JL, Chen S, Sivarajah V, Stephens D, Cortez MA. Latitudinal differences on the global epidemiology of infantile spasms: systematic review and meta-analysis. Orphanet J Rare Dis. (2018) 13:216. doi: 10.1186/s13023-018-0952-x
2. Cowan LD, Hudson LS. The epidemiology and natural history of infantile spasms. J Child Neurol. (1991) 6:355–64. doi: 10.1177/088307389100600412
3. Wray CD, Benke TA. Effect of price increase of adrenocorticotropic hormone on treatment practices of infantile spasms. Pediatr Neurol. (2010) 43:163–6. doi: 10.1016/j.pediatrneurol.2010.04.005
4. Hartung DM, Johnston K, Van Leuven S, Deodhar A, Cohen DM, Bourdette DN. Trends and characteristics of US medicare spending on repository corticotropin. JAMA Intern Med. (2017) 177:1680–2. doi: 10.1001/jamainternmed.2017.3631
5. Hrachovy RA, Frost Jr. JD, Kellaway P, Zion TE. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. (1983) 103:641–5. doi: 10.1016/S0022-3476(83)80606-4
6. Hrachovy RA, Frost Jr. JD, Glaze DG. High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. J Pediatr. (1994) 124:803–6. doi: 10.1016/S0022-3476(05)81379-4
7. Baram TZ, Mitchell WG, Tournay A, Snead IOC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. (1996) 97:375–9. doi: 10.1542/peds.97.3.375
8. Yanagaki S, Oguni H, Hayashi K, Imai K, Funatuka M, Tanaka T, et al. A comparative study of high-dose and low-dose ACTH therapy for West syndrome. Brain Develop. (1999) 21:461–7. doi: 10.1016/S0387-7604(99)00053-4
9. Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet (london, england). (2004) 364:1773–8. doi: 10.1016/S0140-6736(04)17400-X
10. Shu XM, Li J, Zhang GP, Mao Q. A comparative study of conventional dose and low dose adrenocorticotrophic hormone therapy for West syndrome. Chin J Contemp Pediatr. (2009) 11:445–8.
11. Wanigasinghe J, Arambepola C, Sri Ranganathan S, Sumanasena S, Attanapola G. Randomized, single-blind, parallel clinical trial on efficacy of oral prednisolone versus intramuscular corticotropin on immediate and continued spasm control in west syndrome. Pediatr Neurol. (2015) 53:193–9. doi: 10.1016/j.pediatrneurol.2015.05.004
12. Gowda VK, Narayanaswamy V, Shivappa SK, Benakappa N, Benakappa A. Corticotrophin-ACTH in comparison to prednisolone in west syndrome–a randomized study. Indian J Pediatr. (2019) 86:165–70. doi: 10.1007/s12098-018-2782-1
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
14. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
15. Sardar H, Khan AA, Sultan T. Treatment of infantile spasms in resource limited settings: a randomized controlled trial. J Int Child Neurol Assoc. (2019) 1. doi: 10.17724/jicna.2019.163
16. Chellamuthu P, Sharma S, Jain P, Kaushik JS, Seth A, Aneja S. High dose (4 mg/kg/day) versus usual dose (2 mg/kg/day) oral prednisolone for treatment of infantile spasms: an open-label, randomized controlled trial. Epilepsy Res. (2014) 108:1378–84. doi: 10.1016/j.eplepsyres.2014.06.019
17. Kapoor D, Sharma S, Garg D, Samaddar S, Panda I, Patra B, et al. Intravenous methylprednisolone versus oral prednisolone for west syndrome: a randomized open-label trial. Indian J Pediatr. (2021). doi: 10.1007/s12098-020-03630-3
18. Vigevano F, Cilio MR, Claps D, Faberi A, Gisondi A. Vigabatrin versus ACTH as first line therapy in West syndrome. Bollettino–lega italiana contro l'epilessia. (1994) 86:113–4.
19. Omar FZ, Al-AbdulWahab NO, Ali BM, Karashi FA, Al-Musallam SA. Vigabatrin versus ACTH in the treatment of infantile spasms. Neurosciences. (2002) 7:18–21.
20. Dressler A, Benninger F, Trimmel-Schwahofer P, Gröppel G, Porsche B, Abraham K, et al. Efficacy and tolerability of the ketogenic diet versus high-dose adrenocorticotropic hormone for infantile spasms: a single-center parallel-cohort randomized controlled trial. Epilepsia. (2019) 60:441–51. doi: 10.1111/epi.14679
21. Angappan D, Sahu JK, Malhi P, Singhi P. Safety, tolerability, and effectiveness of oral zonisamide therapy in comparison with intramuscular adrenocorticotropic hormone therapy in infants with West syndrome. Eur J Paediat Neurol. (2019) 23:136–42. doi: 10.1016/j.ejpn.2018.09.006
22. Zou LP, Wang X, Dong CH, Chen CH, Zhao W, Zhao RY. Three-week combination treatment with ACTH + magnesium sulfate versus ACTH monotherapy for infantile spasms: a 24-week, randomized, open-label, follow-up study in China. Clin Therapeut. (2010) 32:692–700. doi: 10.1016/j.clinthera.2010.04.008
23. O'Callaghan FJ, Edwards SW, Alber FD, Hancock E, Johnson AL, Kennedy CR, et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. (2017) 16:33–42. doi: 10.1016/S1474-4422(16)30294-0
24. Kunnanayaka V, Jain P, Sharma S, Seth A, Aneja S. Addition of pyridoxine to prednisolone in the treatment of infantile spasms: a pilot, randomized controlled trial. Neurol India. (2018) 66:385–90. doi: 10.4103/0028-3886.227281
25. Yi Z, Wu H, Yu X, Zha J, Chen H, Chen Y, et al. High-dose prednisone therapy for infantile spasms and late-onset epileptic spasms in China: the addition of topiramate provides no benefit. Seizure. (2019) 71:174–8. doi: 10.1016/j.seizure.2019.07.016
26. Vigevano F, Cilio MR. Vigabatrin versus ACTH as first-line treatment for infantile spasms: a randomized, prospective study. Epilepsia. (1997) 38:1270. doi: 10.1111/j.1528-1157.1997.tb00063.x
27. Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Datab System Rev. (2013) 6:CD001770. doi: 10.1002/14651858.CD001770.pub3
28. Rajpurohit M, Gupta A, Madaan P, Sahu JK, Singhi P. Safety, feasibility and effectiveness of pulse methylprednisolone therapy in comparison with intramuscular adrenocorticotropic hormone in children with west syndrome. Indian J Pediatr. (2021) 88:663–67. doi: 10.1007/s12098-020-03521-7
29. Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. (1999) 14:71–4. doi: 10.1177/088307389901400201
30. Fejerman N, Cersósimo R, Caraballo R, Grippo J, Corral S, Martino RH, et al. Vigabatrin as a first-choice drug in the treatment of West syndrome. J Child Neurol. (2000) 15:161–5. doi: 10.1177/088307380001500304
31. Riikonen R, Rener-Primec Z, Carmant L, Dorofeeva M, Hollody K, Szabo I, et al. Does vigabatrin treatment for infantile spasms cause visual field defects? An international multicentre study. Dev Med Child Neurol. (2015) 57:60–7. doi: 10.1111/dmcn.12573
32. Panda PK, Sharawat IK, Panda P, Dawman L. Efficacy, tolerability, and safety of zonisamide in children with epileptic spasms: a systematic review and meta-analysis. Seizure. (2021) 91:374–83. doi: 10.1016/j.seizure.2021.07.017
33. Prezioso G, Carlone G, Zaccara G, Verrotti A. Efficacy of ketogenic diet for infantile spasms: a systematic review. Acta Neurol Scand. (2018) 137:4–11. doi: 10.1111/ane.12830
Keywords: infantile spasms, west syndrome, hormonal therapy, ACTH, corticosteroids
Citation: Guang S, Mao L, Zhong L, Liu F, Pan Z, Yin F and Peng J (2022) Hormonal Therapy for Infantile Spasms: A Systematic Review and Meta-Analysis. Front. Neurol. 13:772333. doi: 10.3389/fneur.2022.772333
Received: 08 September 2021; Accepted: 06 January 2022;
Published: 10 February 2022.
Edited by:
Sergiusz Jozwiak, Medical University of Warsaw, PolandReviewed by:
Rinze Neuteboom, Erasmus Medical Center, NetherlandsBarbara Steinborn, Poznan University of Medical Sciences, Poland
Maurizio Elia, IRCCS Oasi Maria SS, Italy
Bruria Ben-Zeev, Sheba Medical Center, Israel
Copyright © 2022 Guang, Mao, Zhong, Liu, Pan, Yin and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Peng, pengjing627@126.com
 Shiqi Guang
Shiqi Guang