- 1Stroke Center and Department of Neurology, National Taiwan University Hospital, Taipei, Taiwan
- 2Department of Neurology, Landseed International Hospital, Taoyuan, Taiwan
- 3Department of Neurology, National Cheng Kung University Hospital, Tainan, Taiwan
- 4Division of Neurology, Department of Internal Medicine, Ditmanson Medical Foundation Chiayi Christian Hospital, Chiayi, Taiwan
- 5Department of Neurology, Chi Mei Medical Center, Tainan, Taiwan
- 6Department of Neurology, China Medical University Hospital, Taichung, Taiwan
- 7Department of Neurology, Mackay Memorial Hospital, Taipei, Taiwan
- 8Department of Neurology, En Chu Kong Hospital, New Taipei City, Taiwan
- 9Department of Neurology, Taichung Veterans General Hospital, Taichung, Taiwan
- 10Department of Neurology and Stroke Center, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan
- 11Department of Neurology, Chang Bing Show Chwan Memorial Hospital, Changhwa, Taiwan
- 12Department of Neurology, Tri Service General Hospital, Taipei, Taiwan
- 13Department of Neurology, Tainan Sin-Lau Hospital, Tainan, Taiwan
- 14Department of Neurology and Department of Critical Care Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 15Department of Neurology, Shin Kong WHS Memorial Hospital, Taipei, Taiwan
Background: The efficacy and safety of intravenous alteplase administered 3–4.5 h after acute ischemic stroke have been demonstrated. However, whether responses differ between low-dose and standard-dose alteplase during this time window and whether certain subgroups benefit more remain unknown.
Patients and Methods: The current analysis was based on a multicenter matched-cohort study conducted in Taiwan. The treatment group comprised 378 patients receiving intravenous alteplase 3–4.5 h after stroke onset, and the control group comprised 378 age- and sex-matched patients who did not receive alteplase treatment during the same period. Standard- and low-dose alteplase was administered to patients at the physician's discretion.
Results: Overall, patients receiving alteplase exhibited more favorable outcomes than did controls [34.0 vs. 22.7%; odds ratio (OR): 1.75, 95% confidence interval (CI): 1.27–1.42], and the effectiveness was consistent in all subgroups. Although patients in the standard-dose group (n = 182) were younger than those in the low-dose (n = 192) group, the proportions of patients with favorable outcomes (36.3 vs. 31.8%; OR: 1.22, 95% CI: 0.80–1.88) and symptomatic hemorrhage (2.8 vs 4.2%; OR: 0.65, 95% CI: 0.21–2.02) were consistently comparable in a covariate-adjusted model and an age-matched cohort. In the subgroup analysis, patients with cardioembolism, atrial fibrillation, and hypercholesterolemia were more likely to achieve favorable outcomes after receiving standard-dose than low-dose alteplase.
Conclusion: In the 3–4.5 h time window, the effectiveness and safety of standard-dose and low-dose alteplase may be comparable. A standard dose may be selected for patients with cardioembolism, atrial fibrillation, or hypercholesterolemia.
Introduction
The efficacy and safety of intravenous thrombolysis with 0.9 mg/kg alteplase at 3–4.5 h after acute ischemic stroke (AIS) were first demonstrated in the European Cooperative Acute Stroke Study III [ECASS III; (1)] and have been subsequently verified by a meta-analysis (2) and several real-world studies (3–5). Intravenous alteplase treatment for AIS within 4.5 h of symptom onset is currently recommended by various professional organizations (6–9). However, the Food and Drug Administrations in the United States and Taiwan have yet to approve the use of alteplase in the time window of 3–4.5 h.
Whether a low dose of alteplase reduces the risk of intracerebral hemorrhage (ICH) with similar effectiveness as that of a standard dose has long been debated. The ENhanced Control of Hypertension And Thrombolysis strokE stuDy (ENCHANTED) demonstrated that although low-dose alteplase was not non-inferior to standard-dose alteplase in reducing death and disability when used within 4.5 h of stroke onset, significantly fewer symptomatic ICH (sICH) events were reported in the low-dose group than in the standard-dose group (10). Currently, 0.6 mg/kg is the only approved low dose for alteplase in Japan; moreover, low-dose alteplase is commonly used in several other Asian countries, including Taiwan, for safety and cost reduction (11).
Most studies comparing standard-dose and low-dose alteplase have included patients treated within 3 or 4.5 h; however, few studies have specifically emphasized the time window of 3–4.5 h. Because the response to alteplase treatment may gradually decrease with time, whether a low dose can achieve similar effectiveness as that of a standard dose in the time window of 3–4.5 h remains unknown. Additionally, whether certain patient subgroups may benefit more from standard-dose or low-dose alteplase merits investigation. Therefore, in this study, we analyzed data of a published multicenter matched-cohort study in Taiwan that had demonstrated the real-world effectiveness of alteplase administered in the time window of 3–4.5 h after symptom onset (12).
Methods
Study Design
This study involved the analysis of data from a multicenter, retrospective, matched-cohort study initiated by the Taiwan Stroke Society to evaluate the effectiveness and safety of intravenous alteplase at 3–4.5 h after symptom onset in patients with AIS. The detailed study protocol and results have been published, and the present study is a subgroup analysis of the primary study (12). Briefly, data were extracted from 16 hospitals participating in the Taiwan Stroke Registry, which contains prospectively collected data on patients' basic demographic characteristics and risk factors, clinical course and treatment, and etiology and outcomes of stroke. The study period was from January 2008 to December 2017. The use of data from the Taiwan Stroke Registry was approved by the Institutional Review Board of National Taiwan University Hospital (Research Ethical Committee No. 201801064RINC) and informed consent was waived because this was a retrospective analysis of the prospective stroke registry. All study methods were performed in accordance with the Declaration of Helsinki. The data used in the present study can be obtained from the corresponding author on reasonable request.
Study Population
Although this was not a randomized controlled trial, we enrolled patients at a 1:1 ratio according to whether they received intravenous alteplase (treatment group) or not (control group) within the specified time window. Patients ≥18 years old with a clinical diagnosis of AIS were included in the analysis. Patients in the treatment group received intravenous alteplase within a time window of 3–4.5 h after stroke onset; their treatment complied with the regulations of the Taiwan Food and Drug Administration and the reimbursement criteria of the National Health Insurance program in Taiwan (13). For each patient in the treatment group, one age- and sex-matched patient arriving at the emergency room in the same hospital within 2–4.5 h, but not receiving intravenous alteplase, was enrolled into the control group. The rationale behind selecting 2 h as a lower limit of onset-to-door time was that thrombolysis could not be administered to these patients within 3 h. In addition, patients were assigned to the control group only if they did not have any obvious contraindication to intravenous alteplase. Patients who received any other reperfusion therapy such as intra-arterial thrombolysis or endovascular thrombectomy were excluded from the current analysis.
The Taiwan Food and Drug Administration has approved the administration of 0.9 mg/kg intravenous alteplase for AIS as the standard dose in clinical practice. In addition, the Taiwan Food and Drug Administration has recommended that low-dose (0.6 mg/kg) intravenous alteplase may be associated with lower sICH risk on the basis of the results in the ENCHANTED and Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) trials (10, 14). In clinical practice, a standard or low dose is used according to the treating physicians' initial evaluation of the patient and professional discretion. Generally, physicians in Taiwan prefer using low-dose alteplase in patients >70 years old on the basis of the results of the TTT-AIS study (14).
Clinical Characteristics and Outcome Measures
The demographic profile, body weight, and vascular risk factors (namely hypertension, diabetes mellitus, hyperlipidemia, previous ischemic stroke, ischemic heart disease, atrial fibrillation, and ever or current smoking) of the study patients were documented. Furthermore, the initial National Institute of Health Stroke Scale (NIHSS) score, blood pressure, and laboratory data (including glucose, creatinine, platelets, and international normalized ratio) at the index stroke event were recorded. The NIHSS score was recorded at least once every day in the first 3 days and then recorded according to regional clinical practice. Hyperlipidemia was defined as receiving of lipid-lowering agents or having one of the following: fasting serum total cholesterol ≥ 200 mg/dl, fasting serum low-density lipoprotein cholesterol ≥ 130 mg/dl, fasting serum high-density lipoprotein <40 mg/dl, or fasting serum triglyceride ≥ 150 mg/dl. Hyperlipidemia was further classified as hypercholesterolemia (total cholesterol ≥ 200 mg/dl or low-density lipoprotein cholesterol ≥ 130 mg/dl) or hypertriglyceridemia (triglyceride ≥ 150 mg/dl). Ischemic stroke was classified into the subtypes large-artery atherosclerosis, small vessel occlusion, cardioembolism, and others on the basis of the Trial of Org 10172 in the Acute Stroke Treatment (TOAST) classification (15).
The primary effectiveness outcome was the percentage of patients with favorable functional outcomes as defined by modified Rankin Scale (mRS) scores of 01 at 90 days after index stroke event. The secondary effectiveness outcomes were the percentage of patients with mRS scores of 02 at 90 days and early neurological deterioration (END), which was defined as an increase in the NIHSS score by two or more points from the initial or the lowest NIHSS score between the time of admission and 72 h. The safety outcomes were any ICH event after thrombolysis and sICH occurrence as defined by the ECASS III criteria (1). To detect ICH, a brain computed tomography or magnetic resonance imaging scan was routinely performed at 24–36 h after thrombolysis.
Statistical Analysis
The original study consisted of 748 eligible patients (original cohort), of whom 374 received alteplase (alteplase cohort) and 374 did not receive alteplase (control cohort). In the original cohort, the baseline characteristics were comparable, as reported in the published article [Supplemental Table 1; (12)]. The demographic, clinical, and laboratory profiles were compared between the standard-dose and low-dose groups in the alteplase cohort by using the Mann–Whitney U test or chi-square test, as appropriate. An additional comparison was performed between patients treated with alteplase (standard and low doses) and controls. Because a considerable age gap existed between patients treated with standard and low doses of alteplase, the cohorts were age- and NIHSS score–matched by using the SAS PSMATCH procedure.
The effectiveness and safety outcomes (expressed in percentage) of the standard-dose and low-dose groups in the alteplase cohort were plotted by age at 10-year intervals (<50, 5059, 6069, 7079, and ≥80), and the trend between the age groups and alteplase dose was tested using the generalized linear mixed model. To compare clinical outcomes between the standard-dose and low-dose groups, logistic regression analyses were performed with effectiveness (mRS scores 01, mRS scores 02, and END) and safety outcomes (any ICH and sICH event) as dependent variables. First, an unadjusted analysis was performed, and the crude odds ratio (OR) was calculated. Subsequently, a multivariable analysis was performed and adjusted for covariates that were significantly associated with outcomes in the univariate analysis (Supplemental Table 2). The covariates were age, NIHSS score, diabetes mellitus, previous ischemic stroke, and atrial fibrillation for mRS score 01; NIHSS score, diabetes mellitus, and atrial fibrillation for END; and male sex, NIHSS score, diabetes mellitus, and atrial fibrillation for any ICH event. Symptomatic ICH was not adjusted for in the analysis owing to its rarity. Furthermore, unadjusted logistic regression analyses were performed between the matched patients of the standard-dose and low-dose groups.
To explore which subgroup may benefit more from the treatment (alteplase vs. control or standard dose vs. low dose), logistic regression analyses were performed with effectiveness outcomes as the dependent variable and clinical variables, treatment, and interaction terms between the clinical variables and treatment as the predictors. Statistically significant interaction terms in the subgroup analyses implied that treatment effects may differ in the subgroup. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA), and a P-value of < 0.05 indicated significance.
Results
Patient Characteristics
Baseline demographics, vascular risk factors, laboratory results, and stroke profiles of patients in the standard-dose alteplase (n = 182), low-dose alteplase (n = 192), and control (n = 374) groups are summarized in Table 1. Patients in the low-dose group were significantly older than those in the standard-dose group (69.5 ± 12.4 vs. 62.5 ± 13.1 years, P < 0.0001). The door-to-needle time was comparable between the two groups, whereas the onset-to-needle time was longer in the low-dose group. The age- and NIHSS score–matched cohorts comprised 65 patients each at a 1:1 ratio. The median age was 68 years in both the groups, and their demographic profiles were comparable (Supplemental Table 3).
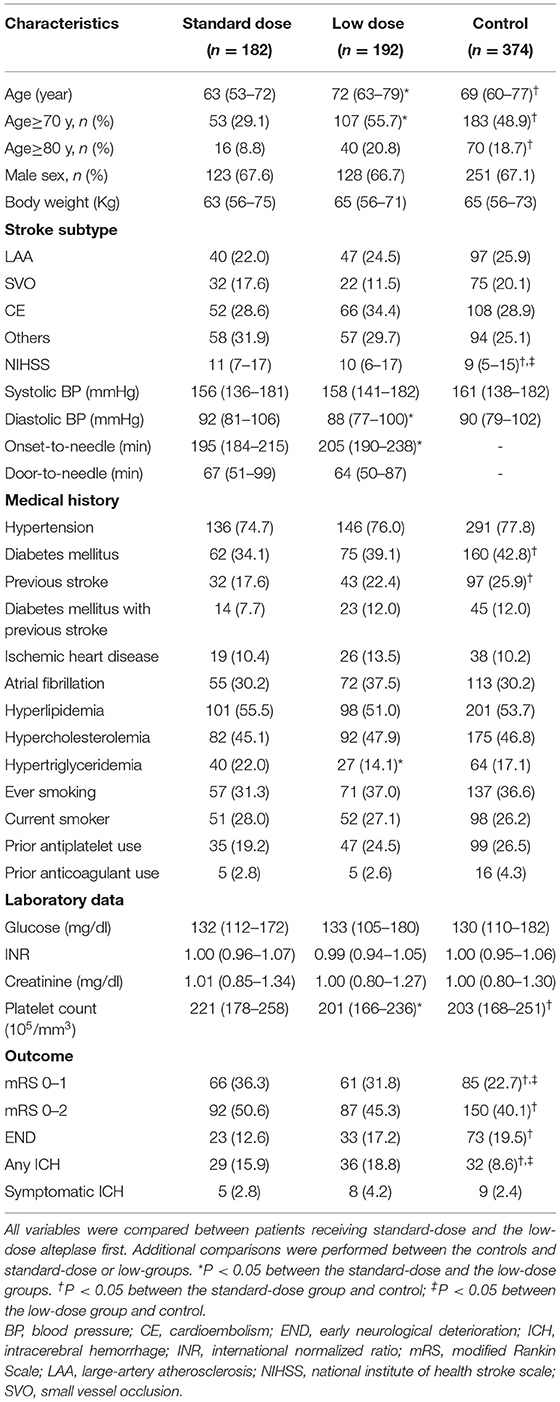
Table 1. Baseline characteristics of patients who were given standard-dose and low-dose alteplase vs. controls.
Clinical Outcomes Between Patients Treated With Standard-Dose and Low-Dose Alteplase
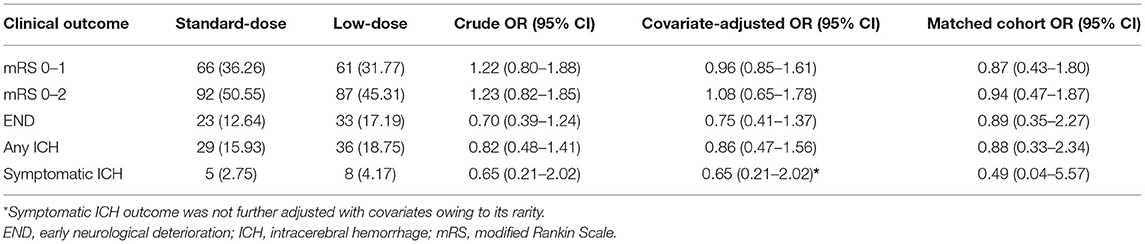
Figure 1 presents the distribution of favorable functional outcomes (mRS scores 01), END, any ICH event, and sICH by age at the 10-year intervals between the standard-dose and low-dose groups. Despite the trend of fewer favorable outcomes (Ptrend = 0.0002) and more ICH events (Ptrend = 0.06) with increasing age, no significant age and dose interaction effect was observed for all clinical outcomes. For mRS scores 0–1, 36.3% and 31.8% of patients exhibited the primary effectiveness outcome in the standard-dose and low-dose groups, respectively (OR = 1.22, 95% confidence interval [CI] = 0.80–1.88, P = 0.36). For mRS scores 02, the corresponding proportions were 50.6% and 45.3% (OR = 1.23, 95% CI = 0.82–1.85). Compared with the low-dose group, fewer patients reported END, any ICH event, and sICH in the standard-dose group, although the results were nonsignificant (Table 2).
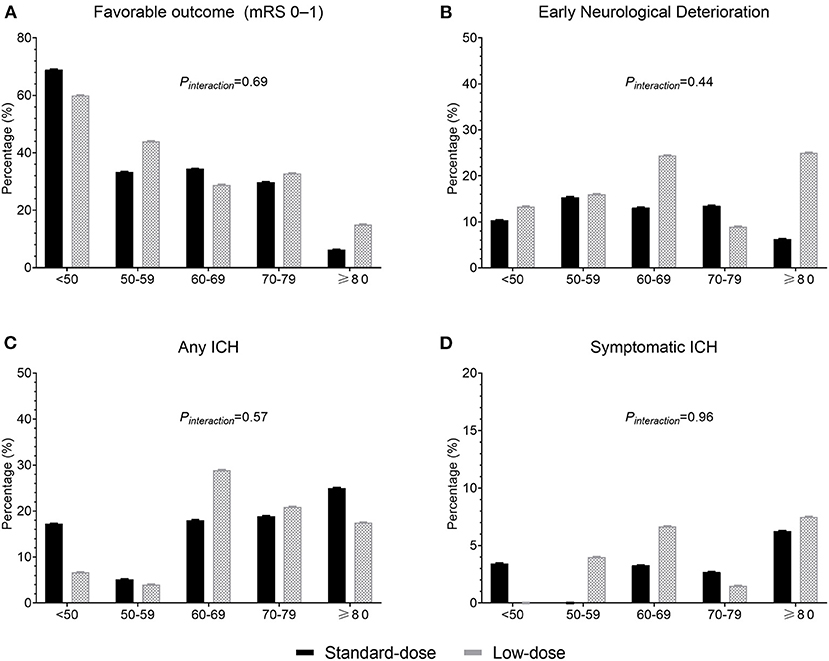
Figure 1. Proportions of clinical outcomes between the standard-dose and low-dose groups according to age. (A) Favorable outcome (modified Rankin Scale score 01); (B) early neurological deterioration; (C) any intracerebral hemorrhage (ICH); and (D) symptomatic ICH.
In the covariate-adjusted model, the results were overall comparable when the point estimates moved toward null as expected. However, in the matched cohort, the proportions of patients exhibiting favorable outcome were 33.9% and 36.9% in the standard-dose and low-dose groups, respectively (OR = 0.87, 95% CI = 0.43–1.80; Figure 2). The point estimates of other outcomes were similar to those of the alteplase cohort.
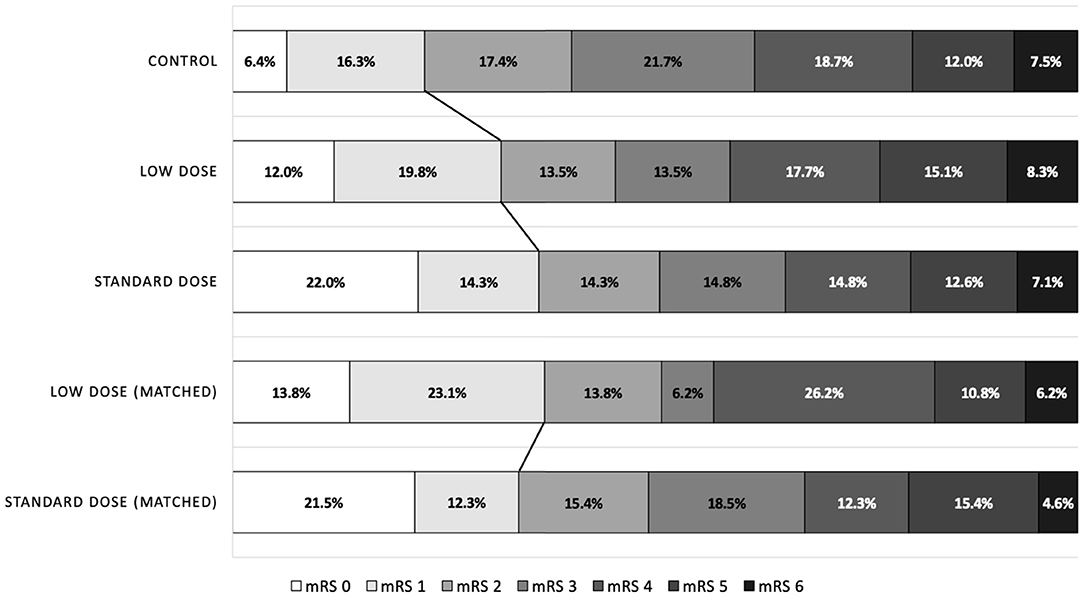
Figure 2. Distribution of modified Rankin Scale scores at 90 days after stroke, according to the treatment groups.
Subgroup Analysis of Effectiveness Outcomes
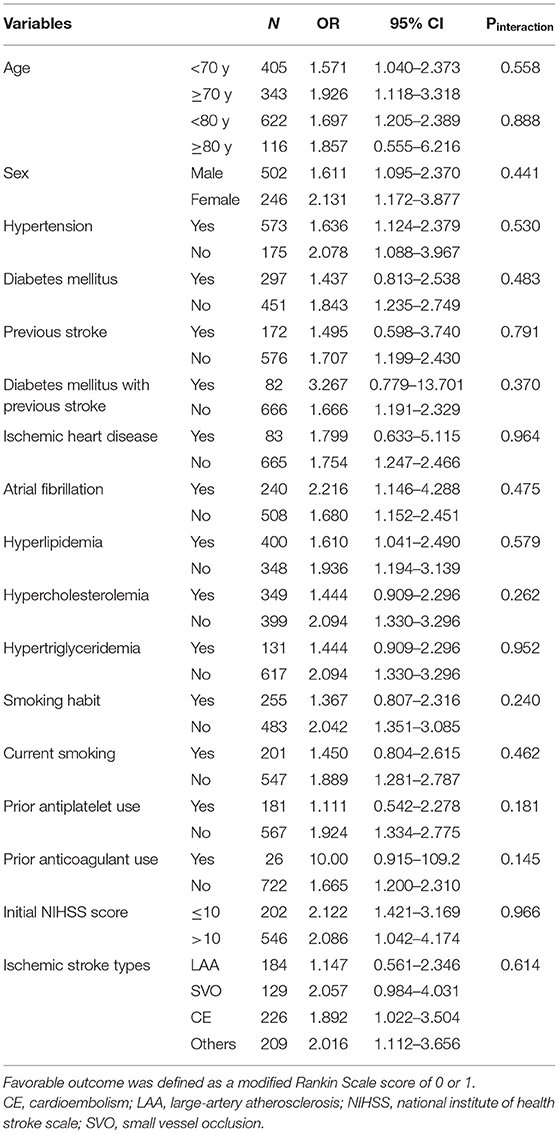
In the original cohort (alteplase vs. control), no clinical variable significantly influenced the primary outcome (all Pinteraction > 0.05; Table 3 and Figure 3A), indicating that the effectiveness of alteplase was consistent in all patient subgroups. These included subgroups that would otherwise be excluded from the ECASS III trial, such as patients aged >80 years (Pinteraction = 0.89), those with concomitant diabetes mellitus and prior stroke (Pinteraction = 0.37), and those using oral anticoagulants (Pinteraction = 0.15).
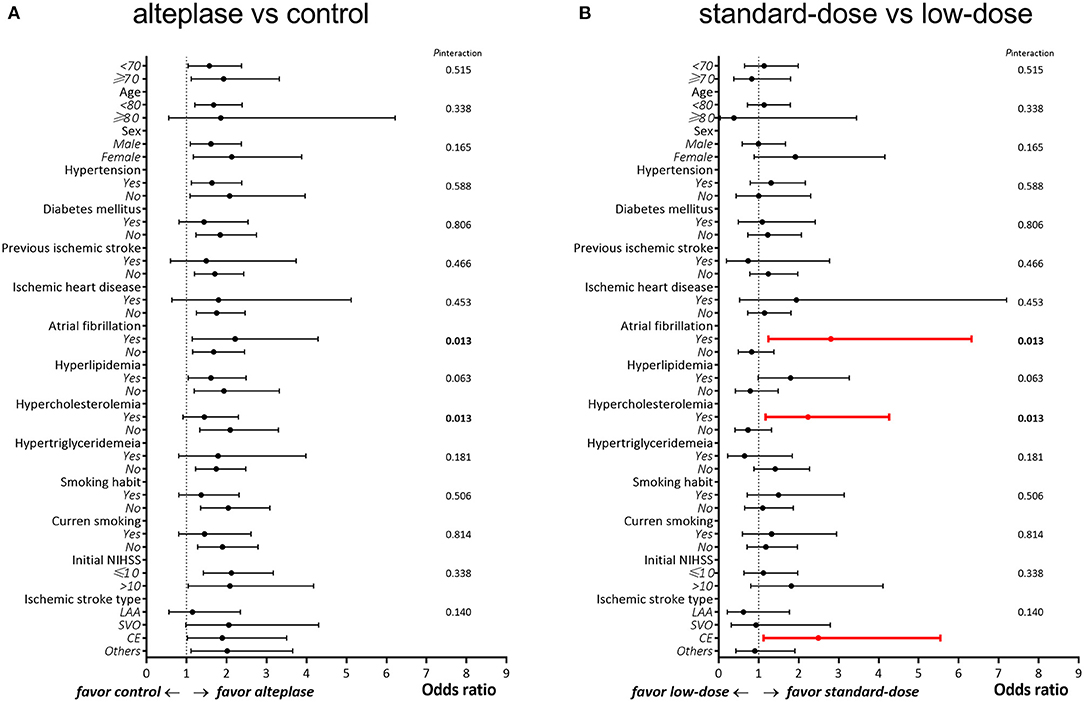
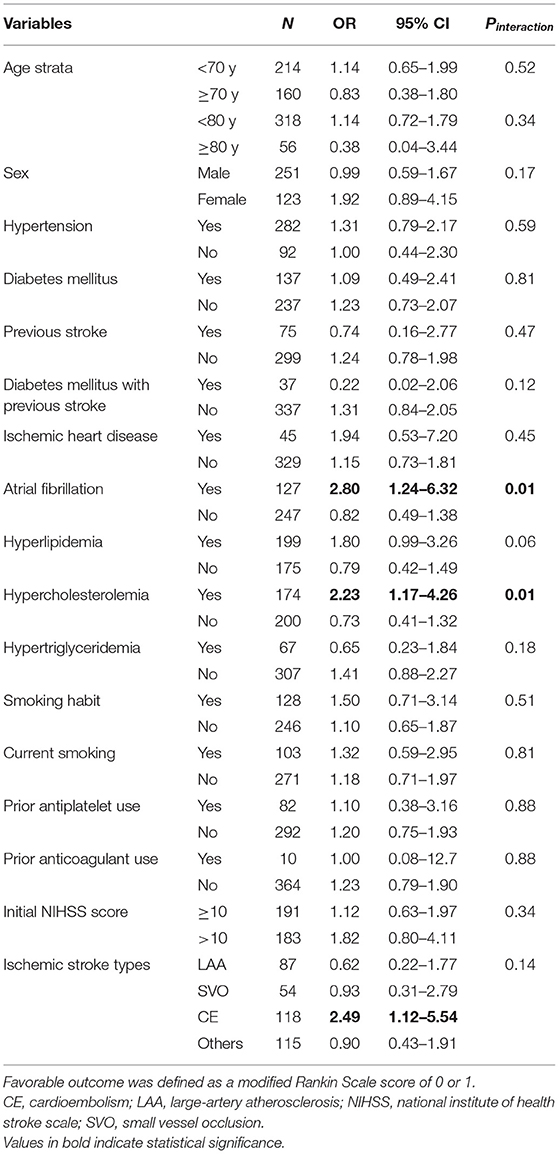
Figure 3. Subgroup analysis between patients treated with (A) alteplase vs. control and (B) standard-dose vs. low-dose alteplase.
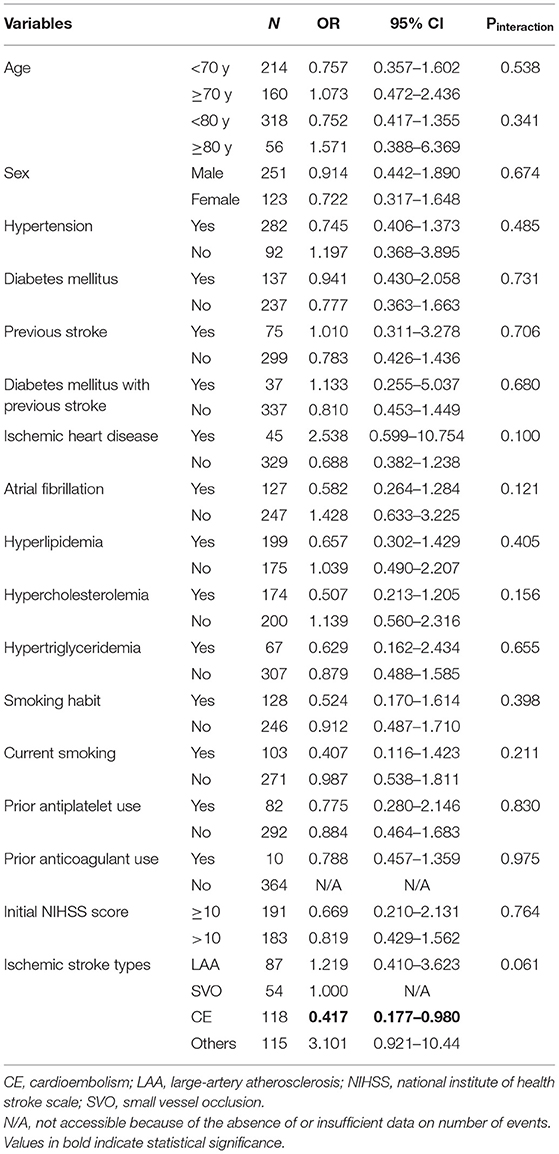
In the alteplase cohort (standard dose vs. low dose), significant interactions were observed between the alteplase dose and the presence of atrial fibrillation (Pinteraction = 0.01) as well as between the alteplase dose and the presence of hypercholesterolemia (Pinteraction = 0.01; Table 4 and Figure 3B). In patients with atrial fibrillation, administration of a standard dose resulted in higher odds of favorable outcomes than using a low dose (OR = 2.80, 95% CI = 1.24–6.32); in patients without atrial fibrillation, opposite results were obtained (OR = 0.82, 95% CI = 0.49–1.38). Furthermore, administration of a standard dose was associated with favorable outcome in patients with hypercholesterolemia (OR = 2.23, 95% CI = 1.17–4.26) but not in patients without hypercholesterolemia (OR = 0.73, 95% CI = 0.41–1.32). Regarding ischemic stroke type, administration of standard-dose alteplase was associated with favorable outcome only in patients with the cardioembolism subtype (OR = 2.49, 95% CI = 1.12–5.54). The interaction effect between the alteplase dose and atrial fibrillation remained significant even after adjustment for age and NIHSS score (Pinteraction = 0.03) or in the matched cohort (Pinteraction = 0.04). On the other hand, the interaction effect between the dose and hypercholesterolemia persisted in the age- and NIHSS score–adjusted model (Pinteraction = 0.04) but not in the matched cohort (Pinteraction = 0.38). No significant interaction was noted between the occurrence of any ICH event and clinical variables (all Pinteraction > 0.05; Table 5).
Discussion
The present study demonstrated that in the time window of 3–4.5 h after AIS onset, patients who received thrombolysis with intravenous alteplase exhibited functional improvement compared with controls, and the results were consistent across patient subgroups. Moreover, standard-dose and low-dose alteplase exhibited comparable effectiveness and safety in this time window, although the standard dose may be preferred in patients with atrial fibrillation or hypercholesterolemia.
The main novelty of our study is the comparison of the effectiveness of standard-dose and low-dose alteplase in the late time window, which has never been specifically investigated before. Japan proposed the use of low-dose alteplase at 0.6 mg/kg in 2006 on the basis of the results of an uncontrolled, open-label parallel study, in which the thrombolytic agent was administered within 3 h (16). This low-dose approach was soon adopted by neighboring Asian countries such as China, Korea, and Taiwan; however, several observational studies have reported contrary results regarding the benefit of the low-dose regimen (14, 17, 18). Notably, comparative studies using standard- or low-dose alteplase unquestionably extended the time limit to 4.5 h following the publication of the ECASS III trial in 2008, although the ECASS III trial only demonstrated the benefit of 0.9 mg/kg alteplase within 3–4.5 h (1).
To date, the strongest evidence of the effectiveness of low-dose alteplase administration within a window of 3–4.5 h was provided by the ENCHANTED trial published in 2016, which included patients who were given alteplase within 4.5 h of stroke onset (10). In the subgroup analysis of the ENCHANTED trial, no significant interaction was observed between the alteplase dose and time from onset to randomization (<3 vs. ≥3 h). In the ≥3-h subgroup (i.e., 3–4.5 h), the proportion of death and disability was 51.1% in the low-dose group and 50.1% in the standard-dose group (OR = 1.04, 95% CI = 0.84–1.30). In a study published in 2019 that included data of 6,250 patients from nine stroke registries in six Asian countries (the largest real-world data–based study to date) and compared the effectiveness of low-dose and standard-dose alteplase (11), the adjusted odds of death and disability or sICH were not significantly different between the low-dose and standard-dose groups. Although no significant interaction was observed between the alteplase dose and time (<3 vs. ≥3 h; Pinteraction = 0.395), a trend favoring low-dose alteplase was noted in the ≥3-h subgroup (OR = 0.75 for death and disability, 95% CI = 0.51–1.10) compared with the <3-h subgroup (OR = 1.13, 95% CI = 0.93–1.37).
In comparison, our study only enrolled patients treated within a window of 3–4.5 h; this study might be the first matched-cohort study in Asia with a quasi-randomized control design to specifically consider this indication. The original goal of this study was to demonstrate the benefit of alteplase administered within a window of 3–4.5 h in comparison with the control. Further subgroup analyses revealed that the effectiveness and safety outcome were numerically higher in patients who were given standard-dose alteplase than in those who were given low-dose alteplase. Because of the observational study design, confounding by indication is inevitable, and physicians tend to prescribe low-dose alteplase for older patients. This is partly attributed to a finding reported by a multicenter study in Taiwan that a low dose of 0.6 mg/kg is associated with improved functional outcomes in elderly patients [71–80 years; (14)]. In our study, the overall effectiveness diminished with increasing age, irrespective of the alteplase dose used. Thus, more favorable outcomes may be observed in the standard-dose group. To overcome this selection bias, an age- and severity-matched cohort was created, and the results revealed a slightly greater number of favorable outcomes in the low-dose group than in the standard-dose group (36.9 vs. 33.9%). However, the aforementioned results were nonsignificant. Nevertheless, the present results do not preclude the use of a standard dose in older patients within the 3–4.5-h time window, even in those of Asian ethnicity.
A low-dose regimen, however, was associated with significantly lower sICH occurrence rate (1.0 vs. 2.1%, P = 0.01) in the ENCHANTED trial (10). Furthermore, a multicenter study in Taiwan revealed that in elderly patients (7180 years), the rate of sICH occurrence increased significantly as the dose increased (14). Our study, however, found that the rates of any ICH event and sICH occurrence were nonsignificantly higher in the low-dose group than in the standard-dose group. This may be related to the higher age in the low-dose group. However, no obvious interaction effect between age and the alteplase dose on ICH was observed (Figure 1), and the result remained consistent in age-adjusted or age-matched analyses. Certain unobserved factors in the low-dose group may have contributed to a higher sICH rate, or it could have occurred by chance given the low event rates. Our study results indicate that standard-dose alteplase within 3–4.5 h of stroke onset can be administered without causing an absolute increase in the risk of hemorrhage.
In the ECASS III trial, no heterogeneity in the treatment effect within a time window of 3–4.5 h was observed across all patient subgroups (19). Our study demonstrated similar benefits in various patient groups, including those groups that were excluded from the ECASS III trials, such as elderly patients (age > 80 years), those with concomitant diabetes mellitus and prior stroke, or those using anticoagulants. These findings are consistent with the 2019 American Stroke Association guideline for the early management of AIS, which stated that “careful analysis of available published data…indicates that these exclusion criteria from the trial may not be justified in practice” (8).
In the ENCHANTED trial, low-dose alteplase may have exerted a net benefit in patients without atrial fibrillation (20). Consistent with this finding, our study showed that patients with atrial fibrillation or hypercholesterolemia benefited more from standard-dose alteplase than from the low-dose option. In the histopathological composition of thrombi, atrial fibrillation–related cardioembolism is typically characterized by a higher percentage of fibrin with smaller fractions of red blood cells compared with noncardioembolic thrombi (21). Because alteplase specifically binds to fibrin and initiates fibrinolysis, patients with fibrin-rich thrombi may respond better to standard-dose than to low-dose alteplase. To rule out the age effect, age-adjusted or matched analyses were performed in the current study, which yielded findings similar to those of the aforementioned studies. The association between standard-dose alteplase and hypercholesterolemia is implicit and may also involve alteplase “resistance” in platelet-rich thrombi (22). Nevertheless, further studies are warranted to verify these results.
This is the first study to test the dose effect of alteplase given within the 3–4.5-h time window; this approach differs from that in subgroup analyses in previous larger studies (10, 11). Furthermore, we conducted sufficient subgroup evaluation to assess the heterogeneity of treatment effects. However, several limitations must be addressed. A major drawback of this study is the nonrandomized study design, due to which an imbalance in baseline characteristics may be observed between the treatment and control groups. However, we enrolled age- and sex-matched controls from the same hospital to minimize selection bias. Moreover, we adopted several statistical models to adjust the baseline imbalance. Second, the alteplase dose regimen was determined at the discretion of the physicians in charge, and this decision may be influenced by various clinical practices. Third, the sample size was relatively small and might limit the statistical power for detecting clinically meaningful differences. Fourth, the median door-to-needle time in our study was 65 min, which was longer than that in most clinical trials (23). However, our study started in 2008, at which time many hospitals in Taiwan had not implemented a dedicated code stroke yet (24). Physicians may also need to explain the off-label use of alteplase in the 3–4.5-h time window. These factors may significantly prolong the door-to-needle time. Nonetheless, our time metrics are comparable with the result of 65 min recorded in the participating hospitals of Get with the Guidelines—Stroke in the United States from 2006 to 2016 (25). Finally, patients treated with mechanical thrombectomy were excluded; thus, the results may not be extrapolated to patients with large vessel occlusion undergoing bridging reperfusion therapy. We did not collect information on the presence of intracranial large vessel occlusions. Therefore, we could not explore whether an interaction existed between the presence of large vessel occlusion and the efficacy of the standard-dose or low-dose alteplase, thus limiting the generalizability of our findings.
In summary, the present study demonstrated that the effectiveness of standard-dose alteplase may be comparable to that of low-dose alteplase in patients with AIS within a 3–4.5-h time window, without increasing the risk of hemorrhage. Additionally, standard-dose alteplase may be selected for patients with atrial fibrillation or hypercholesterolemia. In countries where the standard and low doses of alteplase are used in parallel, the current analysis may guide physicians in the selection of appropriate regimens.
Data Availability Statement
The raw data supporting the conclusions of this article are available upon reasonable request to the corresponding author.
Ethics Statement
The study was approved by the Institutional Review Board of National Taiwan University Hospital and informed consent was waived.
Author Contributions
J-SJ contributed to the conception and design of the study. C-HaC and J-SJ analyzed the data. C-HaC contributed to the first draft of the manuscript. S-CT and J-SJ provided critical revision on the manuscript. All authors contributed to the acquisition of data and agreed with the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.763963/full#supplementary-material
References
1. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
2. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
3. Liao XL, Wang CX, Wang YL, Wang CJ, Zhao XQ, Zhang LQ, et al. Implementation and outcome of thrombolysis with alteplase 3 to 4.5 h after acute stroke in Chinese patients. CNS Neurosci Ther. (2013) 19:43–7. doi: 10.1111/cns.12031
4. Morihara R, Kono S, Sato K, Hishikawa N, Ohta Y, Yamashita T, et al. Thrombolysis with low-dose tissue plasminogen activator 3-4.5 h after acute ischemic stroke in five hospital groups in Japan. Transl Stroke Res. (2016) 7:111–9. doi: 10.1007/s12975-016-0448-8
5. Deguchi I, Tanahashi N, Takao M. clinical study of intravenous, low-dose recombinant tissue plasminogen activator for acute cerebral infarction: comparison of treatment within 3 hours versus 3-4.5 hours. J Stroke Cerebrovasc Dis. (2018) 27:1033–40. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.009
6. Dong Q, Dong Y, Liu L, Xu A, Zhang Y, Zheng H, et al. The Chinese stroke association scientific statement: intravenous thrombolysis in acute ischaemic stroke. Stroke Vasc Neurol. (2017) 2:147–59. doi: 10.1136/svn-2017-000074
7. Chen CH, Hsieh HC, Sung SF, Hsieh CY, Chen PL, Tsai LK, et al. 2019 Taiwan stroke society guideline for intravenous thrombolysis in acute ischemic stroke patients. Formos J Stroke. (2019) 1:1–22. doi: 10.6318/FJS.201906_1(1).0001
8. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
9. Toyoda K, Koga M, Iguchi Y, Itabashi R, Inoue M, Okada Y, et al. Guidelines for intravenous thrombolysis (recombinant tissue-type plasminogen activator), the third edition, march 2019: a guideline from the Japan stroke society. Neurol Med Chir. (2019) 59:449–91. doi: 10.2176/nmc.st.2019-0177
10. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. (2016) 374:2313–23. doi: 10.1056/NEJMoa1515510
11. Wang X, Li J, Moullaali TJ, Lee KJ, Kim BJ, Bae HJ, et al. Low-dose versus standard-dose alteplase in acute ischemic stroke in Asian stroke registries: an individual patient data pooling study. Int J Stroke. (2019) 14:670–7. doi: 10.1177/1747493019858777
12. Chen YW, Sung SF, Chen CH, Tang SC, Tsai LK, Lin HJ, et al. Intravenous thrombolysis administration 3-45 h after acute ischemic stroke: a retrospective, multicenter study. Front Neurol. (2019) 10:1038. doi: 10.3389/fneur.2019.01038
13. Su YH, Chen CH, Lin HJ, Chen YW, Tseng MC, Hsieh HC, et al. Safety and effectiveness of intravenous thrombolysis for acute ischemic stroke outside the coverage of national health insurance in Taiwan. Acta Neurol Taiwan. (2017) 26:3–12.
14. Chao AC, Liu CK, Chen CH, Lin HJ, Liu CH, Jeng JS, et al. Different doses of recombinant tissue-type plasminogen activator for acute stroke in Chinese patients. Stroke. (2014) 45:2359–65. doi: 10.1161/STROKEAHA.114.005245
15. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
16. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan alteplase clinical trial (J-ACT). Stroke. (2006) 37:1810–15. doi: 10.1161/01.STR.0000227191.01792.e3
17. Liao X, Wang Y, Pan Y, Wang C, Zhao X, Wang DZ, et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke. (2014) 45:2354–8. doi: 10.1161/STROKEAHA.114.005989
18. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Low-versus standard-dose alteplase for ischemic strokes within 4.5 hours: a comparative effectiveness and safety study. Stroke. (2015) 46:2541–8. doi: 10.1161/STROKEAHA.115.010180
19. Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. (2009) 8:1095–102. doi: 10.1016/S1474-4422(09)70264-9
20. Wang X, Lee KJ, Moullaali TJ, Kim BJ, Li Q, Bae HJ, et al. Who will benefit more from low-dose alteplase in acute ischemic stroke? Int J Stroke. (2020) 15:39–45. doi: 10.1177/1747493019858775
21. Bacigaluppi M, Semerano A, Gullotta GS, Strambo D. Insights from thrombi retrieved in stroke due to large vessel occlusion. J Cereb Blood Flow Metab. (2019) 39:1433–51. doi: 10.1177/0271678X19856131
22. Jang IK, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, et al. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. (1989) 79:920–8. doi: 10.1161/01.CIR.79.4.920
23. LeCouffe NE, Kappelhof M, Treurniet KM, Rinkel LA, Bruggeman AE, Berkhemer OA, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. (2021) 385:1833–44. doi: 10.1056/NEJMoa2107727
24. Chen CH, Tang SC, Tsai LK, Hsieh MJ, Yeh SJ, Huang KY, et al. Stroke code improves intravenous thrombolysis administration in acute ischemic stroke. PLoS ONE. (2014) 9:e104862. doi: 10.1371/journal.pone.0104862
Keywords: thrombolysis, alteplase, rt-PA, atrial fibrillation, hypercholesterolemia
Citation: Chen C-H, Tang S-C, Chen Y-W, Chen C-H, Tsai L-K, Sung S-F, Lin H-J, Huang H-Y, Po HL, Sun Y, Chen P-L, Chan L, Wei C-Y, Lee J-T, Hsieh C-Y, Lin Y-Y, Lien L-M and Jeng J-S (2022) Effectiveness of Standard-Dose vs. Low-Dose Alteplase for Acute Ischemic Stroke Within 3–4.5 h. Front. Neurol. 13:763963. doi: 10.3389/fneur.2022.763963
Received: 24 August 2021; Accepted: 07 January 2022;
Published: 08 February 2022.
Edited by:
Peter Sporns, University Hospital of Basel, SwitzerlandReviewed by:
Mikito Hayakawa, University of Tsukuba, JapanNirav Bhatt, Emory University, United States
Copyright © 2022 Chen, Tang, Chen, Chen, Tsai, Sung, Lin, Huang, Po, Sun, Chen, Chan, Wei, Lee, Hsieh, Lin, Lien and Jeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiann-Shing Jeng, anNqZW5nQG50dS5lZHUudHc=
 Chih-Hao Chen
Chih-Hao Chen Sung-Chun Tang
Sung-Chun Tang Yu-Wei Chen
Yu-Wei Chen Chih-Hung Chen3
Chih-Hung Chen3 Li-Kai Tsai
Li-Kai Tsai Sheng-Feng Sung
Sheng-Feng Sung Huey-Juan Lin
Huey-Juan Lin Hung-Yu Huang
Hung-Yu Huang Helen L. Po
Helen L. Po Yu Sun
Yu Sun Po-Lin Chen
Po-Lin Chen Lung Chan
Lung Chan Cheng-Yu Wei
Cheng-Yu Wei Cheng-Yang Hsieh
Cheng-Yang Hsieh Li-Ming Lien
Li-Ming Lien Jiann-Shing Jeng
Jiann-Shing Jeng