
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 07 February 2022
Sec. Neurotrauma
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.724641
Objective: The study aimed to compare outcomes of traumatic brain injury (TBI) in patients on pre-injury antiplatelet drugs vs. those, not on any antiplatelet or anticoagulant drugs.
Methods: PubMed, Embase, and Google Scholar databases were searched up to 15th May 2021. All cohort studies comparing outcomes of TBI between antiplatelet users vs. non-users were included.
Results: Twenty studies were included. On comparison of data of 2,447 patients on pre-injury antiplatelet drugs with 4,814 controls, our analysis revealed no statistically significant difference in early mortality between the two groups (OR: 1.30 95% CI: 0.85, 1.98 I2 = 80% p = 0.23). Meta-analysis of adjusted data also revealed no statistically significant difference in early mortality between antiplatelet users vs. controls (OR: 1.24 95% CI: 0.93, 1.65 I2 = 41% p = 0.14). Results were similar for subgroup analysis of aspirin users and clopidogrel users. Data on functional outcomes was scarce and only descriptive analysis could be carried out. For the need for surgical intervention, pooled analysis did not demonstrate any statistically significant difference between the two groups (OR: 1.11 95% CI: 0.83, 1.48 I2 = 55% p = 0.50). Length of hospital stay (LOS) was also not found to be significantly different between antiplatelet users vs. non-users (MD: −1.00 95% CI: −2.17, 0.17 I2 = 97% p = 0.09).
Conclusion: Our results demonstrate that patients on pre-injury antiplatelet drugs do not have worse early mortality rates as compared to patients, not on any antiplatelet or anticoagulant drugs. The use of antiplatelets is not associated with an increased need for neurosurgical intervention and prolonged LOS.
Traumatic brain injury (TBI) is often described as an insult due to a bump, blow or jolt to the head which leads to impairment of brain function (1). The spectrum of TBI can range from mild alteration in consciousness to a severe comatose state or death with or without evidence of intracranial hemorrhage (ICH) (2). It is an important medical, economic and social problem that affects all countries globally with an annual incidence rate of 349 per 100,000 person-years (3). TBI is commonly seen after falls or motor-vehicle accidents and it is one of the leading causes of mortality and disability in Western countries (4).
On account of the increasingly aging global population, there has been a rise in elderly patients sustaining TBI (5). An important aspect in treating older adults is to consider the associated comorbidities and the effect of several drugs to manage them (6). Antiplatelet and anticoagulant drugs are commonly prescribed after several conditions like myocardial infarction, interventional cardiac procedures, atrial fibrillation, knee or hip joint replacement, deep vein thrombosis, or pulmonary embolism to reduce the risk of systemic thromboembolism (7, 8). The use of such drugs complicates the management of TBI patients due to an increased risk of bleeding which may significantly alter patient prognosis (9, 10).
Of the two classes of antithrombotics, anticoagulants are known to cause greater alteration of the coagulation profile as compared to antiplatelet drugs. Indeed, studies have found that the risk of bleeding is significantly higher with anticoagulants as compared to antiplatelets alone (11). In the case of patients sustaining TBI, research suggests that preinjury use of anticoagulants (12) is a significant risk factor for developing ICH after TBI whereas corresponding evidence for antiplatelet drugs is ambiguous (13, 14). Recent meta-analysis studies have assessed the impact of preinjury anticoagulation on outcomes of general trauma and TBI patients and have noted significantly increased mortality in anticoagulant users as compared to controls (1, 10). However, similar evidence for antiplatelet drugs is scarce. To the best of our knowledge, only one meta-analysis published in 2013 has evaluated the role of pre-injury antiplatelet drugs on outcomes of TBI (15). The study reported that pre-injury antiplatelet users have a non-significant but slightly increased risk of mortality after TBI. An important limitation of the review was that only five studies were available for the pooled analysis. Since then, several researchers have published data on the outcomes of TBI with antiplatelet use and there is a need for more updated and comprehensive evidence (16–18). Therefore, we hereby aimed to conduct this systematic review and meta-analysis to assess the impact of preinjury use of antiplatelet drugs on outcomes of TBI.
The predefined research question for the review was: Do outcomes of TBI patients on pre-injury antiplatelet drugs differ from those not on any anticoagulant or antiplatelet therapy?. We primarily aimed to assess the impact of antiplatelet drugs on the early and long-term mortality of patients with TBI. Secondary objectives were to assess the impact of antiplatelet drugs on the need for surgical intervention, functional outcomes, and length of hospital stay (LOS).
This systematic review was conducted following the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (19). We searched for eligible studies electronically on the databases of PubMed, Embase, and Google Scholar. Two reviewers carried out the literature search independent of each other. Search limits were from the inception of the databases to 15th May 2021. The main terms used for the literature search in various combinations were: “traumatic brain injury”, “head injury”, “intracranial hemorrhage”, “antiplatelets”, “antithrombotic”, “aspirin”, “clopidogrel”, “ticagrelor”, “dipyridamole”, “prasugrel”, and “eptifibatide”. Search strategy in detail is presented in Supplementary Table 1. After deduplication of the search results, we reviewed the output of each database by assessing the titles and abstracts of every study. We identified articles relevant to the review and extracted their full texts. The two reviewers independently evaluated these studies for final inclusion in the review. We resolved any disagreements by discussion. In the end, we reviewed the reference list of included studies for any missed references.
We structured the eligibility criteria on the PICOS (Population, Intervention, Comparison, Outcome, Study type) framework. The detailed criteria were 1. Cohort studies were conducted on patients sustaining TBI (Population). 2. Studies comparing data of patients with preinjury use of an antiplatelet drug (Intervention) with a control group not on any antiplatelet or anticoagulant drugs (Comparison). 3. Studies assessing any one of the following outcomes: mortality, functional outcome, need for surgical intervention, LOS (Outcomes).
Exclusion criteria for the review were are follows: (1) Studies on trauma patients not reporting separate data for TBI, (2) Studies including patients on both anticoagulant and antiplatelet drugs and not reporting separate data for antiplatelet drugs, (3) Single arm studies not comparing outcomes with control group, (4) Non-English language studies, case reports, and review articles, (5) Studies reporting duplicate data. In case of two or more studies were from the same healthcare setup, we included the article with the largest sample size.
Data from each study was sourced by two authors independently. We extracted details of the first author, publication year, study type, country and study location, study period, included population, study groups, sample size, age, Glasgow coma scale (GCS), number of patients undergoing reversal of antiplatelet drugs, type of antiplatelet used and study outcomes.
The methodological quality of included studies was assessed using the Newcastle-Ottawa scale (NOS) (20). This too was carried out in duplicate and independently by two study investigators. Studies were awarded points for selection of study population, comparability, and outcomes. The maximum score which can be awarded is nine.
The meta-analysis was conducted using “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre [Cochrane Collaboration], Copenhagen, Denmark; 2014). We used a random-effects model for all outcomes. Early mortality was defined as events occurring within 30 days of injury. We pooled crude mortality rates and the need for surgical intervention using odds ratios (OR) with 95% confidence intervals (CI). We also extracted multivariable-adjusted data on mortality were available and pooled them using the generic inverse variance function of the software. Mean and standard deviation (SD) data of the length of hospital stay was extracted and pooled to calculate the mean difference (MD) and 95% CI. Median, range and interquartile range data was converted into mean and standard deviation (SD) when required using the method of Wan et al. (21). A sensitivity analysis was also performed for a meta-analysis of the primary outcome. Individual studies were sequentially excluded from the meta-analysis in the software itself to check any undue influence of the study on the total effect size. A sub-group was performed for crude mortality rates based on the type of antiplatelet drug.
Heterogeneity was assessed using the I2 statistic. I2 values of 25–50% represented low, values of 50–75% medium, and more than 75% represented substantial heterogeneity. We used funnel plots to assess publication bias for the primary outcome. P ≤ 0.05 was considered statistically significant.
We identified a total of 3,678 unique articles after the literature search (Figure 1). Thirty-eight were selected for full-text analysis of which 19 were excluded as they did not fulfill the inclusion criteria. Finally, 20 studies were included in this review (16–18, 22–38). Characteristics of included studies are presented in Table 1. The studies were published between 2002 and 2021. The majority of them originated from the USA. Except for two (17, 27), all were retrospective cohort studies. While all studies included patients with varying degrees of TBI, two studies (31, 34) included only those patients who underwent neurosurgical intervention. The included patients were of the elderly age group in most studies. The sample size of the antiplatelet group varied from 19 to 833 patients while that of the control arm varied from 37 to 1,125 patients. Aspirin and clopidogrel were the most common antiplatelets used by the patients. Only four studies (18, 24, 29, 33) reported separate data for single and double antiplatelet users. In the remaining studies, a mix of single and double drug users was compared with controls. Eleven of the 19 studies had no data on reversal of antiplatelet drugs post-TBI. Assessing the quality of included studies, the NOS score varied from 6 to 8.
Fifteen studies reported crude early mortality rates. On comparison of data of 2,447 patients on pre-injury antiplatelet drugs with 4,814 controls, our analysis revealed no statistically significant difference in early mortality between the two groups (OR: 1.30 95% CI: 0.85, 1.98 I2 = 80% p = 0.23) (Figure 2). There was no evidence of publication bias (Supplementary Figure 1). On sensitivity analysis, we found no change in the significance of the results on the exclusion of any study. Nine studies reported multivariable-adjusted data on early mortality. Meta-analysis revealed no statistically significant difference between antiplatelet users vs. controls (OR: 1.24 95% CI: 0.93, 1.65 I2 = 41% p = 0.14) (Figure 3). Results were stable on sensitivity analysis and did not change on the exclusion of any study.
Separate data for aspirin and clopidogrel could be extracted from only five and four studies, respectively. Pooled analysis failed to demonstrate any difference in mortality rates for aspirin (OR: 1.14 95% CI: 0.54, 2.42 I2 = 74% p = 0.73) or for clopidogrel (OR: 1.14 95% CI: 0.25, 5.23 I2 = 85% p = 0.87) (Figure 4). Due to a lack of data, we could not analyze the impact of antiplatelet drugs on long-term mortality rates.
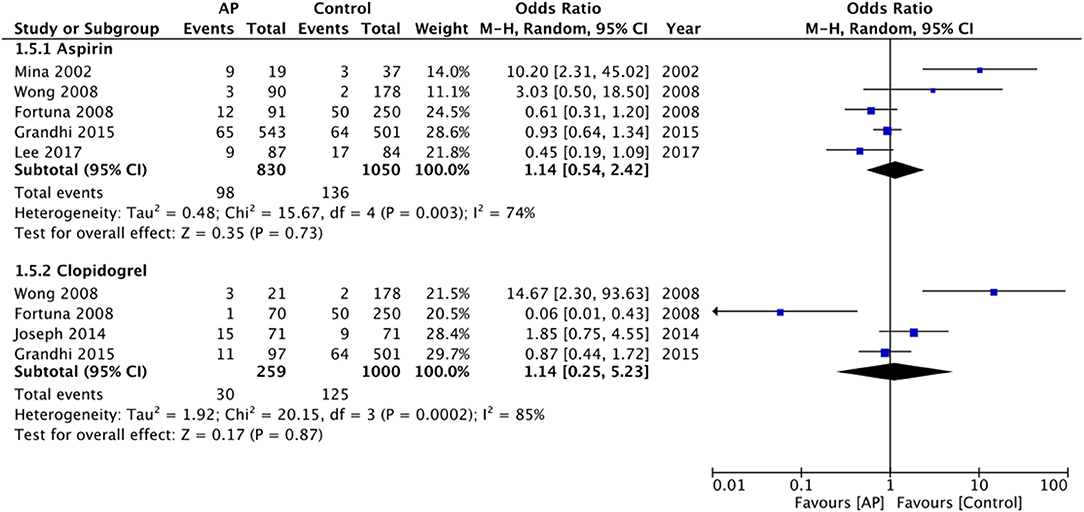
Figure 4. Meta-analysis of crude early mortality rates between antiplatelet users vs. controls based on the type of drug.
Due to limited data and variable reporting amongst included studies, pooled analysis for functional outcomes could not be carried out. Results were analyzed descriptively. Robinson et al. (35) in their analysis reported poor functional outcomes (defined as a score of ≥2 on the modified Rankin Scale) at discharge in 58% of antiplatelet users as compared to 33% controls. On multivariate analysis, antiplatelet use was not found to be a significant predictor of poor functional outcomes. Okazaki et al. (30) also reported that antiplatelet use was not independently associated with unfavorable outcomes at discharge (defined as Glasgow outcome scale [GOS] ≤ 4) (OR: 1.33 95% CI: 0.30–5.96). Similarly, Sumiyoshi et al. (33) have reported that antiplatelet use is not associated with unfavorable outcomes (GOS <4) (OR: 1.74 95% CI: 0.87–3.47). On the other hand, Scotti et al. (18) in their study found that both single antiplatelet use (OR: 1.58 95% CI: 1.01–2.49) and double antiplatelet use (OR: 3.37 95% CI: 1.52–7.45) were significant predictors of functional dependency at discharge (GOS ≤ 4). Farsi et al. (32) noted that antiplatelet users had a higher risk of moderate (5.2% vs. 1.4%) and severe disability (2.2% vs. 0.2%) as compared to controls.
Data on the need for surgical intervention was available from eight studies. The pooled analysis did not demonstrate any statistically significant difference between the two groups (OR: 1.11 95% CI: 0.83, 1.48 I2 = 55% p = 0.50) (Figure 5). LOS was also not found to be significantly different between antiplatelet users vs. non-users (MD: −1.00 95% CI: −2.17, 0.17 I2 = 97% p = 0.09) (Figure 6).
Much research has been conducted on the impact of preinjury use of antiplatelet and anticoagulant drugs on trauma patients (9, 10). Indeed, with a flourishing use of these medications for multiple systemic indications, a large number of trauma patients are being treated under the influence of antithrombotics. In this context, it is essential to gauge the impact of these medications on patient outcomes so that appropriate and timely diagnostic and treatment measures can be undertaken to improve patient survival and functional outcomes. The current study aims to fill this gap by providing evidence on the impact of antiplatelet drugs on outcomes of TBI patients.
In the case of patients with a head injury, any TBI leading to ICH can be a devastating and life-threatening complication (39). ICH may develop immediately or late after the initial injury and can significantly alter the patient's prognosis (40). Theoretically, any patient on antithrombotics would have an increased risk of ICH post-TBI. However, while the risk of intracranial complications post TBI is well established for oral anticoagulants (12), the role of antiplatelet drugs has been controversial. Savioli et al. (14) in a recent study involving 483 patients on antiplatelet drugs sustaining minor head injury noted no increased risk of ICH amongst antiplatelet users. Contrarily, a 2017 meta-analysis by Brand et al. (13) noted a 1.87 times increased risk of ICH in antiplatelet users sustaining a head injury. It is important to note that the severity of ICH is an important factor affecting prognosis (41). Research has indicated that preinjury use of anticoagulants is associated with increased intracranial hematoma volume and hematoma expansion as compared to patients, not under any anticoagulant therapy and both these factors can influence overall mortality rates (42). However, the same has not been proven in the case of antiplatelet drugs. An animal study has shown that antiplatelet drugs do not increase hematoma volume and have no impact on functional outcomes after experimental ICH (43). Murthy et al. (44) in a recent study on primary intracerebral hemorrhage patients have demonstrated that prior use of antiplatelets does not affect hematoma volume, hematoma expansion, the incidence of major disability, or death.
In line with these studies, our primary analysis demonstrated that preinjury use of antiplatelet drugs has no impact on early mortality rates in the case of patients with TBI. The analysis is strengthened by the fact that no study had an undue effect on the overall results of sensitivity analysis. We also noted no difference in the need for neurosurgical intervention and LOS between antiplatelet users vs. controls. Our study was unable to conduct a quantitative analysis for functional outcomes, but limited data indicate that antiplatelet drugs may not have an adverse impact on functional outcomes as well. The results of our analysis differ from the past review on this subject which noted a slightly increased risk of mortality in antiplatelet users (15). By adding 15 more studies, our review is a significant update that clarifies the impact of these drugs on outcomes of TBI patients. In comparison with other antithrombotics, Lim et al. (1) in a meta-analysis have noted an increased risk of overall mortality amongst TBI patients on preinjury anticoagulant drugs. Similar to our results, preinjury anticoagulants were not associated with an increased need for neurosurgical intervention or prolonged LOS in patients with TBI. In the case of general trauma, Lee et al. (10) in a pooled analysis of 1,365,446 patients have also demonstrated that pre-injury anticoagulation significantly increases the risk of overall mortality but does not impact the incidence of surgical intervention. This disparity between antiplatelets and antithrombotics on patient outcomes also has been noted by a recent study by Narula et al. (45) wherein only preinjury anticoagulants were found to increase mortality of trauma patients but not preinjury antiplatelets.
The differential impact of antiplatelets and anticoagulants on overall mortality in TBI patients corroborates with the contrasting evidence on the effect of these drugs on ICH severity. It is plausible that since anticoagulants are known to increase the risk of ICH and its severity in TBI unlike antiplatelets, they have a greater impact on patient prognosis as compared to antiplatelets (42, 44). It is also important to note that the studies included in this review had a heterogeneous population and patients with varying degrees of TBI were included. Nevertheless, there were no major variations in Glasgow Coma Scale (GCS) scores between antiplatelet and non-antiplatelet groups. As randomized trials are not possible to assess the impact of antiplatelets on trauma patients, one must interpret the results because there were baseline differences between the study and control groups. However, one of the strengths of our review is that the results on mortality were reiterated on a pooled analysis of adjusted data, albeit from only eight studies.
Aspirin and clopidogrel were the most common antiplatelet drugs used in the included studies. Both the drugs inhibit platelet aggregation but by different mechanisms. While aspirin acts by inhibiting cyclooxygenase enzymes and reducing the production of thromboxane A2, clopidogrel acts by inhibiting adenosine dinucleotide phosphate (ADP) receptors and subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex (46). Clopidogrel is considered to be superior to aspirin for the prevention of thromboembolic events and is being more widely used (47). However, we noted no difference between the two drugs for their impact on mortality in TBI patients.
Another important aspect to consider is the difference between dual antiplatelet vs. single antiplatelet therapy. In the majority of the included studies, data of single and dual antiplatelet therapy were combined and compared with controls. Due to scarce data, we could not differentiate the mortality rates with these two regimens. Research indicates that as compared to single antiplatelet drugs, dual antiplatelet therapy reduces the risk of thromboembolic events in ischemic stroke patients but with a significant increase in the risk of bleeding (48). In one of the included studies, Scotti et al. (18) have demonstrated that dual antiplatelet but not single antiplatelet therapy is associated with a significantly increased risk of mortality in TBI patients. Similarly, Sumiyoshi et al. (33) also noted an increased risk of mortality with dual as compared to single antiplatelet therapy. Further studies reporting separate data are needed to clarify the impact of dual antiplatelet therapy on outcomes of TBI.
As past evidence indicated higher mortality in antiplatelet users sustaining TBI (15), the use of platelet transfusion has been practiced to improve outcomes of patients with TBI (49). In the included studies, data on antiplatelet reversal was not universally reported. Furthermore, there was significant variability in the number of patients transfused with platelets where data was available. However, recent reviews have suggested that there is no clear evidence on the benefits of platelet transfusions in TBI patients on prior antiplatelet drugs (50, 51). On the contrary, reversal of antiplatelet agents may lead to a non-significant increase in thromboembolic events (51).
Our review has some limitations. Foremost, all included studies were retrospective in nature and may be prone to selection bias. Baseline matching was not carried out by majority studies. Furthermore, multivariable-adjusted outcomes were also not reported by several studies. Since antiplatelet drugs are usually prescribed in patients with comorbidities it is plausible that several confounding factors could have influenced outcomes. Secondly, due to a lack of data were unable to assess the impact of antiplatelet drugs on functional outcomes. It is also unclear if there is any difference in long-term outcomes with these drugs. Thirdly, an important limitation of our meta-analysis is that data of single and dual antiplatelet therapy were combined and compared with controls. Since a few studies have demonstrated worse outcomes with dual antiplatelet therapy, future studies need to report separate outcomes of these regimens. Fourthly, the majority of data in our review was on aspirin and clopidogrel. It is not known how other antiplatelets like ticagrelor, dipyridamole, prasugrel impact outcomes of TBI. Lastly, the studies in our meta-analysis included a mix of TBI patients (mild, moderate and severe). Specifically, one study included only severe TBI patients (17) while another study included only mild TBI patients (29). A subgroup analysis or a meta-regression analysis assessing the relationship between TBI severity and mortality rates could not be conducted as close to 50% of the studies did not report baseline GCS scores. Furthermore, the mean and SD of GCS scores of the remaining studies were wide and they could not be divided into specific subgroups for a separate analysis. We could assess the influence of these studies only via a sensitivity analysis which did not change the study results. However, there is a need for future studies to take this variable into account while reporting outcomes.
Our results demonstrate that patients on pre-injury antiplatelet drugs do not have worse early mortality rates as compared to patients, not on any antiplatelet or anticoagulant drugs. The use of antiplatelets is not associated with an increased need for neurosurgical intervention and prolonged LOS. Further studies with baseline matching or reporting adjusted data are needed to strengthen current conclusions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LC and GC conceived and designed the study and were involved in literature search and data collection. LC and RY analyzed the data. LC and GC wrote the paper. GC and RY reviewed and edited the manuscript. All authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.724641/full#supplementary-material
1. Lim XT, Ang E, Lee ZX, Hajibandeh S, Hajibandeh S. Prognostic significance of preinjury anticoagulation in patients with traumatic brain injury: a systematic review and meta-analysis. J Trauma Acute Care Surg. (2021) 90:191–201. doi: 10.1097/TA.0000000000002976
2. Pervez M, Kitagawa RS, Chang TR. Definition of traumatic brain injury, neurosurgery, trauma orthopedics, neuroimaging, psychology, and psychiatry in mild traumatic brain injury. Neuroimaging Clin N Am. (2018) 28:1–13. doi: 10.1016/j.nic.2017.09.010
3. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. (2016) 43:774–85. doi: 10.1017/cjn.2016.290
4. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. (2020) 104:213–38. doi: 10.1016/j.mcna.2019.11.001
5. Fernández-Abinader JA, González-Colón K, Feliciano CE, Mosquera-Soler AM. Traumatic brain injury profile of an elderly population in Puerto Rico. P. R. Health Sci. J. (2017) 36:237–9. Available at: https://pubmed.ncbi.nlm.nih.gov/29220069/ (accessed May 28, 2021).
6. Natarajan S, Mistry T, Asnani U. Evaluation of the prevalence of comorbidities in patients reporting for dentoalveolar surgeries. Indian J Dent Res. (2019) 30:860–3. doi: 10.4103/ijdr.IJDR_142_18
7. Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. (2015) 386:281–91. doi: 10.1016/S0140-6736(15)60243-4
8. Kapil N, Datta YH, Alakbarova N, Bershad E, Selim M, Liebeskind DS, et al. Antiplatelet and Anticoagulant Therapies for Prevention of Ischemic Stroke. Clin Appl Thromb. (2017) 23:301–18. doi: 10.1177/1076029616660762
9. Lecky FE, Omar M, Bouamra O, Jenks T, Edwards A, Battle CE, et al. The effect of preinjury warfarin use on mortality rates in trauma patients: a European multicentre study. Emerg Med J. (2015) 32:916–20. doi: 10.1136/emermed-2014-203959
10. Lee ZX, Lim XT, Ang E, Hajibandeh S, Hajibandeh S. The effect of preinjury anticoagulation on mortality in trauma patients: a systematic review and meta-analysis. Injury. (2020) 51:1705–13. doi: 10.1016/j.injury.2020.06.010
11. Kuno T, Takagi H, Sugiyama T, Ando T, Miyashita S, Valentin N, et al. Antithrombotic strategies after transcatheter aortic valve implantation: Insights from a network meta-analysis. Catheter Cardiovasc Interv. (2020) 96:E177–86. doi: 10.1002/ccd.28498
12. Gulati S, Solheim O, Carlsen SM, Øie LR, Jensberg H, Gulati AM, et al. Risk of intracranial hemorrhage (rich) in users of oral antithrombotic drugs: Nationwide pharmacoepidemiological study. PLoS ONE. (2018) 13. doi: 10.1371/journal.pone.0202575
13. Van Den Brand CL, Tolido T, Rambach AH, Hunink MGM, Patka P, Jellema K. Systematic review and meta-analysis: is pre-injury antiplatelet therapy associated with traumatic intracranial hemorrhage? J. Neurotrauma. (2017) 34:1–7. doi: 10.1089/neu.2015.4393
14. Savioli G, Ceresa IF, Luzzi S, Giotta Lucifero A, Pioli Di Marco MS, Manzoni F, et al. Mild head trauma: Is antiplatelet therapy a risk factor for hemorrhagic complications? Med. (2021) 57:357. doi: 10.3390/medicina57040357
15. Batchelor JS, Grayson A. A meta-analysis to determine the effect of preinjury antiplatelet agents on mortality in patients with blunt head trauma. Br J Neurosurg. (2013) 27:12–8. doi: 10.3109/02688697.2012.705361
16. Tollefsen MH, Vik A, Skandsen T, Sandrød O, Deane SF, Rao V, et al. Patients with moderate and severe traumatic brain injury: impact of preinjury platelet inhibitor or warfarin treatment. World Neurosurg. (2018) 114:e209–17. doi: 10.1016/j.wneu.2018.02.167
17. Suehiro E, Fujiyama Y, Kiyohira M, Haji K, Ishihara H, Nomura S, et al. Risk of deterioration of geriatric traumatic brain injury in patients treated with antithrombotic drugs. World Neurosurg. (2019) 127:e1221–7. doi: 10.1016/j.wneu.2019.04.108
18. Scotti P, Séguin C, Lo BWY, de Guise E, Troquet JM, Marcoux J. Antithrombotic agents and traumatic brain injury in the elderly population: hemorrhage patterns and outcomes. J Neurosurg. (2020) 133:486–95. doi: 10.3171/2019.4.JNS19252
19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
20. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed Oct 30, 2020).
21. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
22. Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. (2002) 53:668–72. doi: 10.1097/00005373-200210000-00008
23. Wong DK, Lurie F, Wong LL. The effects of clopidogrel on elderly traumatic brain injured patients. J Trauma Inj Infect Crit Care. (2008) 65:1303–8. doi: 10.1097/TA.0b013e318185e234
24. Fortuna GR, Mueller EW, James LE, Shutter LA, Butler KL. The impact of preinjury antiplatelet and anticoagulant pharmacotherapy on outcomes in elderly patients with hemorrhagic brain injury. Surgery. (2008) 144:598–605. doi: 10.1016/j.surg.2008.06.009
25. Ivascu FA, Howells GA, Junn FS, Bair HA, Bendick PJ, Janczyk RJ. Predictors of mortality in trauma patients with intracranial hemorrhage on preinjury aspirin or clopidogrel. J Trauma Inj Infect Crit Care. (2008) 65:785–8. doi: 10.1097/TA.0b013e3181848caa
26. Peck KA, Calvo RY, Schechter MS, Sise CB, Kahl JE, Shackford MC, et al. The impact of preinjury anticoagulants and prescription antiplatelet agents on outcomes in older patients with traumatic brain injury. J Trauma Acute Care Surg. (2014) 76:431–6. doi: 10.1097/TA.0000000000000107
27. Joseph B, Pandit V, Aziz H, Kulvatunyou N, Hashmi A, Tang A, et al. Clinical outcomes in traumatic brain injury patients on preinjury clopidogrel: a prospective analysis. J Trauma Acute Care Surg. (2014) 76:817–20. doi: 10.1097/TA.0b013e3182aafcf0
28. Cull JD, Sakai LM, Sabir I, Johnson B, Tully A, Nagy K, et al. Outcomes in traumatic brain injury for patients presenting on antiplatelet therapy. Am Surg. (2015) 81:128–32. doi: 10.1177/000313481508100223
29. Grandhi R, Harrison G, Voronovich Z, Bauer J, Chen SH, Nicholas D, et al. Preinjury warfarin, but not antiplatelet medications, increases mortality in elderly traumatic brain injury patients. J Trauma Acute Care Surg. (2015) 614–21. doi: 10.1097/TA.0000000000000542
30. Okazaki T, Hifumi T, Kawakita K, Nakashima R, Matsumoto A, Shishido H, et al. Association between comorbidities, nutritional status, and anticlotting drugs and neurologic outcomes in geriatric patients with traumatic brain injury. World Neurosurg. (2016) 93:336–40. doi: 10.1016/j.wneu.2016.06.070
31. Han H, Koh EJ, Choi H, Kim B-C, Yang SY, Cho K-T. The effect of preoperative antiplatelet therapy on hemorrhagic complications after decompressive craniectomy in patients with traumatic brain injury. Korean J Neurotrauma. (2016) 12:61. doi: 10.13004/kjnt.2016.12.2.61
32. Farsi D, Karimi P, Mofidi M, Mahshidfar B, Rezai M, Hafezimoghadam P, et al. Effects of pre-injury anti-platelet agents on short-term outcome of patients with mild traumatic brain injury: a cohort study. Bull Emerg Trauma. (2017) 5:110–5. Available at: https://pubmed.ncbi.nlm.nih.gov/28507998/ (accessed May 27, 2021).
33. Sumiyoshi K, Hayakawa T, Yatsushige H, Shigeta K, Momose T, Enomoto M, et al. Outcome of traumatic brain injury in patients on antiplatelet agents: a retrospective 20-year observational study in a single neurosurgery unit. Brain Inj. (2017) 31:1445–54. doi: 10.1080/02699052.2017.1377349
34. Lee AT, Gagnidze A, Pan SR, Sookplung P, Nair B, Newman SF, et al. Preoperative low-dose aspirin exposure and outcomes after emergency neurosurgery for traumatic intracranial hemorrhage in elderly patients. Anesth Analg. (2017) 125:514–20. doi: 10.1213/ANE.0000000000002053
35. Robinson D, Pyle L, Foreman B, Ngwenya LB, Adeoye O, Woo D, et al. Antithrombotic regimens and need for critical care interventions among patients with subdural hematomas. Am J Emerg Med. (2021) 47:6–12. doi: 10.1016/j.ajem.2021.03.035
36. Rønning P, Helseth E, Skaansar O, Tverdal C, Andelic N, Bhatnagar R, et al. Impact of preinjury antithrombotic therapy on 30-day mortality in older patients hospitalized with traumatic brain injury (TBI). Front Neurol. (2021) 12:650695. doi: 10.3389/fneur.2021.650695
37. Svedung Wettervik T, Lenell S, Enblad P, Lewén A. Pre-injury antithrombotic agents predict intracranial hemorrhagic progression, but not worse clinical outcome in severe traumatic brain injury. Acta Neurochir (Wien). (2021) 163:1403–13. doi: 10.1007/s00701-021-04816-0
38. Fernando SM, Mok G, Rochwerg B, English SW, Thavorn K, McCredie VA, et al. Preadmission antiplatelet use and associated outcomes and costs among ICU patients with intracranial hemorrhage. J Intensive Care Med. (2021) 36:70–9. doi: 10.1177/0885066619885347
39. Black M, Graham DI. Sudden unexplained death in adults caused by intracranial pathology. J Clin Pathol. (2002) 55:44–50. doi: 10.1136/jcp.55.1.44
40. Khalayleh H, Lin G, Kadar Sfarad H, Mostafa M, Abu Abed N, Imam A, et al. Traumatic minor intracranial hemorrhage: management by non-neurosurgeon consultants in a regional trauma center is safe and effective. World J Surg. (2019) 43:497–503. doi: 10.1007/s00268-018-4821-5
41. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. (2001) 32:891–6. doi: 10.1161/01.STR.32.4.891
42. Seiffge DJ, Goeldlin MB, Tatlisumak T, Lyrer P, Fischer U, Engelter ST, et al. Meta-analysis of haematoma volume, haematoma expansion and mortality in intracerebral haemorrhage associated with oral anticoagulant use. J Neurol. (2019) 266:3126–35. doi: 10.1007/s00415-019-09536-1
43. Lauer A, Schlunk F, Van Cott EM, Steinmetz H, Lo EH, Foerch C. Antiplatelet pretreatment does not increase hematoma volume in experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. (2011) 31:1736–42. doi: 10.1038/jcbfm.2011.22
44. Murthy S, Roh DJ, Chatterjee A, McBee N, Parikh NS, Merkler AE, et al. Prior antiplatelet therapy and haematoma expansion after primary intracerebral haemorrhage: An individual patient-level analysis of CLEAR III, MISTIE III and VISTA-ICH. J Neurol Neurosurg Psychiatry. (2021) 92:364–9. doi: 10.1136/jnnp-2020-323458
45. Narula N, Tsikis S, Jinadasa SP, Parsons CS, Cook CH, Butt B, et al. The effect of anticoagulation and antiplatelet use in trauma patients on mortality and length of stay. Am Surg. (2021) 1:000313482198904. doi: 10.1177/0003134821989043
46. Chechik O, Thein R, Fichman G, Haim A, Tov TB, Steinberg EL. The effect of clopidogrel and aspirin on blood loss in hip fracture surgery. Injury. (2011) 42:1277–82. doi: 10.1016/j.injury.2011.01.011
47. Paciaroni M, Ince B, Hu B, Jeng JS, Kutluk K, Liu L, et al. Benefits and risks of clopidogrel vs aspirin monotherapy after recent ischemic stroke: a systematic review and meta-analysis. Cardiovasc Ther. (2019) 2019:1607181. doi: 10.1155/2019/1607181
48. Ye MB, Chen YL, Wang Q, An J, Ye F, Jing P. Aspirin plus clopidogrel versus aspirin mono-therapy for ischemic stroke: a meta-analysis. Scand Cardiovasc J. (2019) 53:169–75. doi: 10.1080/14017431.2019.1620962
49. Leong LB, David TKP. Is platelet transfusion effective in patients taking antiplatelet agents who suffer an intracranial hemorrhage? J. Emerg Med. (2015) 49:561–72. doi: 10.1016/j.jemermed.2015.02.023
50. Thorn S, Güting H, Mathes T, Schäfer N, Maegele M. The effect of platelet transfusion in patients with traumatic brain injury and concomitant antiplatelet use: a systematic review and meta-analysis. Transfusion. (2019) 59:3536–44. doi: 10.1111/trf.15526
Keywords: antiplatelets, antithrombotic, blood thinners, head injury, mortality, intracranial hemorrhage, complications
Citation: Cheng L, Cui G and Yang R (2022) The Impact of Preinjury Use of Antiplatelet Drugs on Outcomes of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Front. Neurol. 13:724641. doi: 10.3389/fneur.2022.724641
Received: 11 July 2021; Accepted: 12 January 2022;
Published: 07 February 2022.
Edited by:
András Büki, University of Pécs, HungaryReviewed by:
Eirik Helseth, Oslo University Hospital, NorwayCopyright © 2022 Cheng, Cui and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Cheng, Y2hlbmdsaTIwMjEyMDIxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.