- 1Biomarkers of Trauma, National Institute of Nursing Research, National Institutes of Health, Bethesda, MD, United States
- 2Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, United States
- 3School of Psychology, University of Glasgow, Glasgow, United Kingdom
- 4Naval Medical Research Center, Silver Spring, MD, United States
- 5National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
- 6VA San Diego Healthcare System, San Diego, CA, United States
- 7Department of Psychology, Pennsylvania State University, University Park, PA, United States
- 8Center for Military Psychiatry and Neuroscience, Walter Reed Army Institute of Research, Silver Spring, MD, United States
- 9Joint Artificial Intelligence Center, Arlington, VA, United States
- 10Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
- 11Oak Ridge Institute for Science and Education, Oak Ridge, TN, United States
- 12Naval Medical Research Center, Operational and Undersea Medicine Directorate, Silver Spring, MD, United States
- 13Center for Neuroscience and Regenerative Medicine, Uniformed Services of the Health Sciences, Bethesda, MD, United States
Objective: The purpose of this pilot study was to determine if military service members with histories of hundreds to thousands of low-level blast exposures (i. e., experienced breachers) had different levels of serum and neuronal-derived extracellular vesicle (EV) concentrations of interleukin (IL)-6, IL-10, and tumor necrosis factor alpha (TNFα), compared to matched controls, and if these biomarkers related to neurobehavioral symptoms.
Methods: Participants were experienced breachers (n = 20) and matched controls without blast exposures (n = 14). Neuronal-derived EVs were isolated from serum and identified with mouse anti-human CD171. Serum and neuronal-derived EVs were analyzed for IL-6, IL-10, and TNFα using an ultra-sensitive assay.
Results: Serum TNFα concentrations were decreased in breachers when compared to control concentrations (p < 0.01). There were no differences in serum concentrations of IL-6, IL-10, or the IL-6/IL-10 ratio between breachers and controls (p's > 0.01). In neuronal-derived EVs, TNFα and IL-6 levels were increased in breachers compared to controls (p's < 0.01), and IL-10 levels were decreased in the breacher group compared to controls (p < 0.01). In breachers the IL-6/IL-10 ratio in neuronal-derived EVs was higher compared to controls, which correlated with higher total Rivermead Post-concussion Questionnaire (RPQ) scores (p's < 0.05).
Conclusions: These findings suggest that exposure of personnel to high numbers of low-level blast over a career may result in enduring central inflammation that is associated with chronic neurological symptoms. The data also suggest that peripheral markers of inflammation are not necessarily adequate surrogates for central neuroinflammation.
Introduction
Military personnel and law enforcement breachers represent a unique population who experience repetitive, low-level blast exposures during training and occupational duties, often over a prolonged period (1). While these low-level exposures do not result in a diagnosed concussion or traumatic brain injury (TBI), the cumulative effect of these exposures may be linked to cognitive and behavioral symptoms (2). Previously, we have reported elevated serum cytokines following acute exposure to blast from military personnel in close temporal proximity to multiple blast events of varying intensities (3). In this study, we turn our attention to the assessment of potential long-term manifestations in a population of personnel exposed to hundreds to thousands of blast events over a career and with no acute exposure to understand the cumulative effect of repetitive blast on central inflammation which may be associated with cognitive and neurobehavioral symptoms in a manner similar to military personnel with diagnosed TBIs (4, 5).
Extracellular vesicles (EVs) are formed within cells and can transfer cargo by binding to other cells and releasing the cargo, such as cytokines, altering cellular activity, including inflammatory pathways. Neuronally-derived EVs can be isolated from blood, and thus provide insights into central cell-to-cell activity (6, 7). Several studies have found exosomal changes in biomarkers isolated from serum after TBI (8–10). Recently, higher levels of interleukin (IL)-6 were reported in neuronal-derived exosomes of Veterans with multiple mild TBIs and chronic neurological symptoms, including possible blast exposures (10), suggesting central inflammatory activities relate to mild TBIs. However, the impact of exposure to a high number of low-level blast exposures over a prolonged career on central inflammation remains to be determined. The purpose of this pilot study was to examine relationships of serum and neuronal-derived EV concentrations of IL-6, IL-10, and tumor necrosis factor alpha (TNFα) with repetitive, low-level blast exposures and neurological symptoms in an experienced breacher population and a cohort of well-matched control subjects. We hypothesize that EV inflammatory markers correlate with neurologic effects from repetitive, low level blast exposure and that expression of EV inflammatory markers differ from serum inflammatory markers.
Materials and Methods
Standard Protocol Approvals, Registrations, and Subject Consents
The National Institutes of Health (NIH) and Naval Medical Research Center (NMRC) Institutional Review Boards granted approval for this study. Participants provided written informed consent prior to enrolling in the study for all procedures performed.
Methodology
Participants in this study included 20 current or former active duty military or civilian law enforcement breachers. Breaching involves the use of explosives to gain entry to hardened structures and military and law enforcement populations involved with breaching over a career are exposed to potentially hundreds of explosive events in the course of training and operations. Inclusion criteria for breachers consisted of at least 4 years of experience in the breaching profession and active involvement (at least annually) in training or operations related to breaching. Former breachers qualified for the study with a self-reported history of exposure to 400 or more breaching blasts within their career. A total of 14 matched controls were enrolled in the study who were matched in age, gender, and operational experience to the breacher group. Controls must have had at least 4 years of experience in the military or civilian law enforcement profession, be actively involved in military or civilian law enforcement training or operations and have a blast exposure history of fewer than 40 individual blasts over their career. Exclusion criteria for both breachers and controls included a history of moderate to severe brain injury with loss of consciousness over 5 min or a central nervous system, cardiac, respiratory condition or other medical condition known to impact cerebral metabolism.
Demographics, Clinical History, and Psychometric Testing
Assessments were performed on the NIH Campus, which was part of a larger effort to understand clinical impacts of blast exposures. A description of the full battery of neurological and functional tests, neuroimaging protocols, and biomarker analysis is described elsewhere (11), as well as overall study findings. During the evaluation, interviews were conducted to collect demographics and clinical history. A battery of psychometric tests was administered, including the Posttraumatic Stress Disorder Checklist—Military version (PCL-M) and the Rivermead Postconcussion Questionnaire (RPQ) used for analysis in this study. The PCL-M is a self-report measure that assesses 20 symptoms of post-traumatic stress disorder (PTSD) as identified in the DSM-IV (12). The RPQ consists of 16 items measuring the extent of physical, cognitive, and behavioral post-concussive symptoms compared to before the injury (13). Each item is rated on a 5-point ordinal scale from 0 (symptom not experienced) to 4 (severe), with a total score of 0–64 (least to most symptomatic). The modified RPQ further breaks down the questionnaire into physical and cognitive/behavioral symptom clusters (14). The RPQ-3 measures 3 items in the physical symptoms cluster (headaches, feelings of dizziness, nausea and/or vomiting) with a total score of 0–12 (best to worst). The RPQ-3 physical symptoms cluster tends to be associated with early post-concussive symptoms immediately following injury. The RPQ-13 measures 13 items in the cognitive/behavioral symptom cluster (noise sensitivity, sleep disturbance, fatigue, irritability, depression, frustration, forgetfulness, poor concentration, taking longer to think, blurred vision, light sensitivity, double vision, and restlessness), with a total score of 0–52 (best to worst). The RPQ-13 cognitive/behavioral symptoms cluster is associated with late post-concussive symptoms occurring in the days and weeks following injury, although RPQ-3 physical symptoms may continue into this late stage as well.
Blood Biomarkers
Non-fasting blood samples were processed for serum within 1 h using standard protocols (15) between 9:00 am and 12:00 pm prior to interviews and the psychometric testing, and stored at −80°C until batch assay processing.
Neuronal-Derived EV Isolation
EVs were isolated from thawed samples as previously published (10). Briefly, thawed samples were centrifuged at 3,000 × g for 15 min to remove cells and cell debris. The supernatant was added with an appropriate volume of ExoQuick reagent, mixed well and incubated 60 min at + 4°C. The ExoQuick/sample mixture was then centrifuged at 1,500 × g for 30 min. The resulting pellet containing all EVs was resuspended in equal volume of 1 x PBS.
To isolate neuronal-specific EVs, 50–100 μL of 3% BSA in 1 x PBS was added to the EV suspension with biotinylated mouse anti-human CD171 [L1 cell adhesion molecule (L1CAM)] biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) and incubated for 60 min at 4°C, followed by addition of 15 μl of streptavidin-agarose Ultralink resin (Thermo Scientific, Inc.) in 40 μL of 3% BSA and incubation for 30 min at 4°C with continuous mixing. After centrifugation at 200 × g for 10 min at 4°C and removal of the supernatant, the resin pellet was washed 3 times with PBS. Each pellet was re-suspended in equal volume of Ab/Ag dissociation buffer (Pierce™ Gentle Ag/Ab Elution Buffer 21,027, pH 6.6) by mixing for 10 s and centrifuged at 4,500 × g for 10 min at 40°C. Supernatants were transferred to clean tubes for further testing. EVs from breacher and control serum samples were characterized using MACSplex assay, and FlowLogic and MACSQuant softwares (Miltenyi Biotec) were used to analyze flow cytometric data, as described in detail previously (16).
Cytokine Quantification
Serum samples and isolated neuronal-derived EVs were removed from the −80°C freezer and thawed on ice. EVs were lysed with equal volume of mammalian protein extraction reagent (M-PER) (Thermo-Fisher Scientific Inc., Rockford, IL, USA) with cOmplete™ Protease Inhibitor Cocktail (Roche). All proteomic analyses were measured using the high-definition (HD-1), single-molecule array technology (SIMOA™, Quanterix, Lexington, MA). Serum samples were analyzed for cytokines: IL-6, IL-10 and TNFα (Simoa Cytokine 3-Plex Advantage Kit, Quanterix, Lexington, MA) using the method previously described by Rissin et al. (17). The Simoa™ analyzer runs ultra-sensitive, paramagnetic digital, enzyme-linked immunosorbent assays and analyzes each sample in duplicate (18). The coefficient of variation for all concentration values were <20%. The lower limit of detection (LLoD) for each assay are as follows: IL-6, 0.006 pg/mL; IL-10, 0.0022 pg/mL; TNFα, 0.011 pg/mL. Values below LLoD were excluded from analyses.
Statistical Analysis
Statistical testing was conducted with IBM SPSS Build 1.0.0.1298 (Armonk, NY: IBM Corp.). GraphPad Prism version 8 (La Jolla, CA: GraphPad Software) was used to create figures. Clinical and demographic differences were calculated using chi square and independent samples t-test. Biomarker concentrations were first natural log-transformed (ln) prior to comparing the two groups (breachers and controls) with one-way ANOVA; presented results include mean (SD) and F-statistic with p-value for significant results. Graphical representations of log-transformed biomarker concentrations include horizontal lines for mean and standard deviation values. Within the breacher group, Pearson correlations were conducted to determine the relationship between RPQ scores and log-transformed biomarker concentrations; significant values are presented as graphs including line of best fit.
Results
Demographic and Clinical Characteristics
There were 34 male participants included in the study (20 breachers and 14 controls) with a mean age of 39 years. The majority of participants were white (85%) military personnel (65–71%). No differences in age, education, or prior service were observed between the breacher and control groups (Table 1). While we found a significant difference in the number of head injuries, this number was self-reported. Participants were excluded for moderate or severe TBI. Breachers had higher scores on the PCL-M and on the RPQ-3 section of the Rivermead Postconcussive Questionnaire (Table 1).
Serum and EV Cytokine Results
In the breacher group, the log-transformed (ln) TNFα concentrations in serum were decreased in breachers (1.281 ± 0.2601) when compared to controls (1.797 ± 0.4972) (F = 15.574, p = 0.0004) (Figure 1A), with no differences in serum concentrations of IL-6 (p = 0.137), IL-10 (p = 0.437), or the IL-6/IL-10 ratio (p = 0.121) between these groups (Figures 1B–D). Concentrations of neuronal EV-specific TNFα was increased in breachers (1.797 ± 0.6143) compared to controls (1.120 ± 0.5504) (F = 9.092, p = 0.006) (Figure 2A), as well as IL-6 (1.780 ± 0.6651) vs. (0.829 ± 0.7484) (F = 13.919, p = 0.001) (Figure 2B). However, EV-specific IL-10 concentrations were decreased in the breachers (0.343 ± 0.8123) compared to controls (1.150 ± 0.7900) (F = 7.970, p = 0.008) (Figure 2C). Lastly, there was a higher IL-6/IL-10 ratio in neuronal-derived EVs in the breachers (1.3484 ± 1.1737) when compared to the controls (−0.230 ± 0.9758) (F = 15.772, p = 0.0005) (Figure 2D).
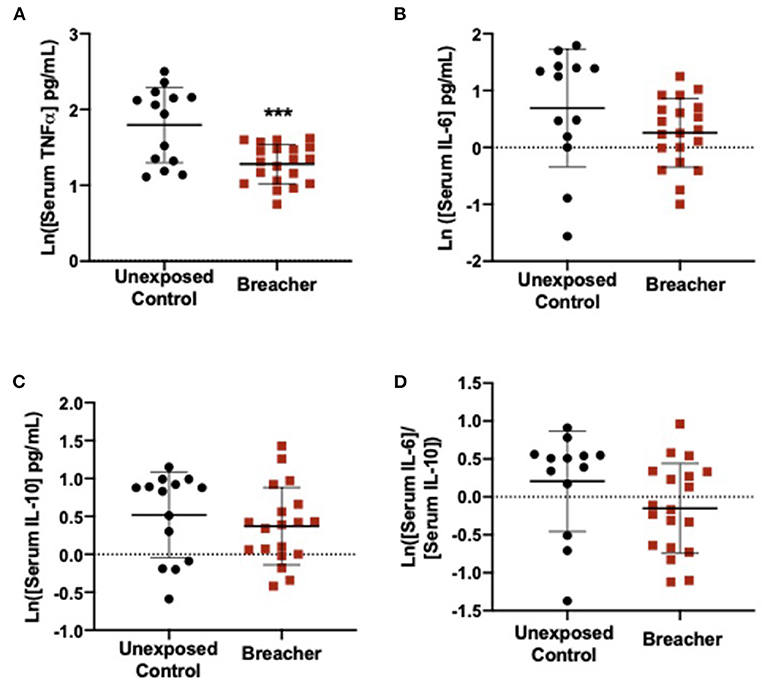
Figure 1. Serum levels of pro-inflammatory cytokine TNFα decreased in breachers. (A) Serum TNFα is decreased in breachers as compared to unexposed controls, (B) No change in serum IL-6 in breachers as compared to unexposed controls, (C) No change in serum IL-10 between groups, (D) No change in the IL-6/IL-10 ratio between groups. Significant p-values are represented as: ***p < 0.001.
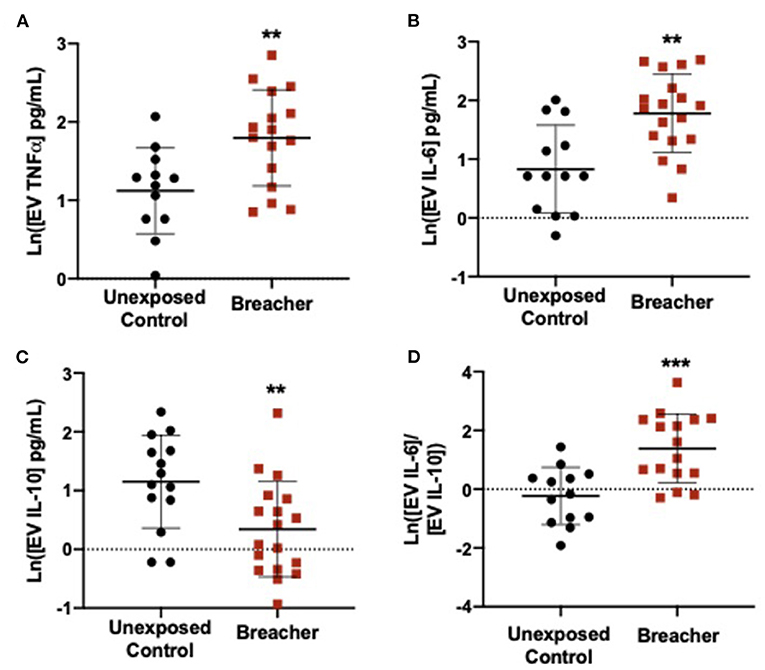
Figure 2. Neuronal-derived EV cytokines propagate a pro-inflammatory environment in the brain. (A,B) EVs derived from neurons contain elevated levels of pro-inflammatory cytokines TNFα and IL-6 in experienced breachers when compared to unexposed controls. In addition, (C) anti-inflammatory cytokine IL-10 is decreased in this population. (D) The ratio of pro/anti-inflammatory cytokines IL-6/IL-10 is higher in experienced breachers. Significant p-values are represented as: **p < 0.01, ***p < 0.001.
Cytokines Relation to Post-concussive Disorder and PTSD Symptoms With Breachers
We found correlations between higher total RPQ scores and increased serum IL-6/IL-10 ratios (r = 0.526, p = 0.025) as well as increased neuronal-derived EV IL-6/IL-10 ratios (r = 0.585, p = 0.022) (Figure 3; Table 2). We also identified correlations between higher RPQ-13 scores and increased IL-6/IL-10 ratios from serum (r = 0.515, p = 0.029) and EVs (r = 0.580, p = 0.023). There were no significant relationships between PTSD symptoms and cytokine concentrations.
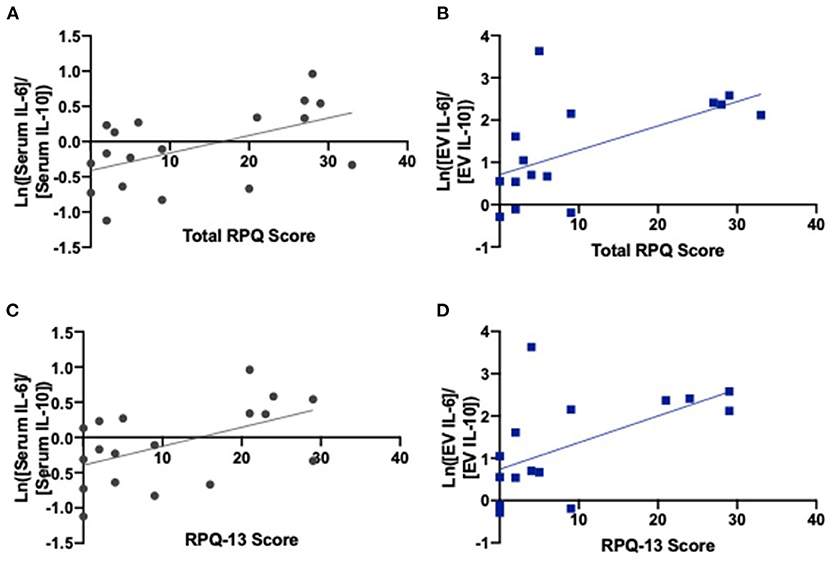
Figure 3. IL-6/IL-10 ratios correlate with late postconcussive symptoms. (A) Serum ratios of IL-6/IL-10 (r = 0.526, p = 0.025) and (B) EV ratios of IL-6/IL-10 correlate with total RPQ score (r = 0.585, p = 0.022). (C) Serum ratios of IL-6/IL-10 (r = 0.515, p = 0.029) and (D) EV ratios of IL-6/IL-10 correlate with RPQ-13 scores (r = 0.580, p = 0.023), the portion of the exam related to late postconcussive symptoms. Graphs include line of best fit.
Discussion
In this pilot study we report significantly higher concentrations inflammatory cytokines TNFα and IL-6 in neuronal-derived EVs, whereas concentrations of IL-10 were lower in experienced breachers compared to controls. Ratios of IL-6 and IL-10 were significantly different in breachers' neuronal-derived EVs compared to controls and that these ratios relate to post-concussive disorder symptoms, suggesting dysregulation of immune activity may relate to high blast exposures and place individuals at greater risk for chronic symptoms. Unexpectantly, we also report that serum levels of TNFα were reduced in breachers compared to controls within the context of higher concentrations in neuronal derived EVs, suggesting that inflammation may be related to central processes. These findings suggest a role for inflammatory cytokines in the effects of cumulative repetitive, low-level blast exposures and suggest that these biomarker changes relate to chronic symptoms.
Our current finding of lower serum levels of TNFα in the breacher group differs from our previous work in which we observed an increase in serum TNFα and IL-6 in breachers undergoing blast exposure in an acute setting (3). This discrepancy suggests that a career of high blast exposures may result in differential immune activity from that of acute exposure. Our findings do align with a recent report in a population of military personnel with blast TBI and non-acute serum sampling, in which pro-inflammatory markers IL-8 and MMP3 measured in serum were significantly decreased and IL-6 followed a similar decreasing pattern, though it did not reach significance (19). While previous studies have measured IL6, IL10, and TNFα from serum in breachers (3), our study is the first description of these inflammatory cytokines in neuronal-derived EVs from a unique cohort of experienced breachers.
Our data suggest that peripheral levels of circulating cytokines may not be as informative of neuroinflammatory activity which is observed in neuronal derived EV concentration of cytokines. Injury to the brain often compromises the blood-brain barrier and also activates a secondary cascade where microglia and astrocytes release a variety of factors that can damage neurons and prolong the inflammatory response within the brain for months or years (20, 21). Importantly, secondary damage to the brain may lead to chronic neuroinflammatory imbalance and continue to have adverse effects on neurological function after the primary damage has resolved (22). Thus, the ability to distinguish CNS from peripheral inflammatory response is crucial for a better understanding of how the inflammatory response may impact neurological outcomes long-term.
Notably, proteins within EVs are protected from proteinase-specific degradation, meaning EV-isolated cytokine levels are potentially more stable than those in serum and may be further suitable for clinical applications (23). Literature specific to EVs in blast exposure is limited, however, studies in other neurological processes such as TBI have successfully assessed the total content of EVs isolated from blood in military populations (24–27). For example, differential expression of EV miRNA related to inflammatory and neuronal repair pathways is observed in veterans with remote blast-related mTBI (27), supporting protein findings. EVs isolated from saliva show differential inflammatory gene expression in civilian populations with acute head trauma (28, 29). While these studies examine total EVs from blood and saliva, neuronal cell specific proteins can also be identified in total EVs isolated from cerebral spinal fluid (CSF) (30). In most contexts where use of CSF is not feasible, the isolation of neuronal-derived EVs provides enhanced specificity to the CNS processes. A sports-related mild TBI study found that protein concentrations within neuronal-derived EVs differed depending on the time since injury, including elevations in IL-6 (31). Neuronal-derived EV proteins have been shown to differ depending on cognitive impairment status, including IL-6, in veterans with remote TBI (32, 33). In addition, work is expanding to include the role of EVs from other brain parenchymal cells beyond neurons, including astrocytes, microglia, and oligodendrocytes (8, 34, 35). Future studies specific to breacher populations are warranted, such as measuring miRNA and distinguishing cytokine profiles in EVs from astrocytes and microglia.
The cytokine levels isolated from neuronal-derived EVs differed from our observations of serum cytokine levels. In breachers, we found elevated levels of IL-6 and TNFα in neuronal-derived EVs. In addition, we identified lower levels of IL-10 from neuronal-derived EVs. The balance between the pro- and anti-inflammatory activity is essential, as it creates an environment essential for neuronal health, and a proinflammatory environment has been associated with risk for the development of neurodegenerative diseases (36) and has been shown to influence cognitive behavior (37, 38). Therefore, we quantified the ratio between IL-6 and IL-10; if pro-inflammatory cytokines are upregulated compared to anti-inflammatory cytokines, or if anti-inflammatory cytokines are down-regulated, the ratio of IL-6/IL-10 will be larger. IL-6/IL-10 ratios have been used as a predictive marker for disease severity (39), but few have investigated their potential in predicting long-term health after blast exposures. Breachers had higher EV-specific IL-6/IL-10 ratios, implying the pro-inflammatory environment after repetitive, low-level blast exposures may be attributed to the chronic neuroinflammatory imbalance found in NF-κB pathway activation and reduced neurologic outcome.
Particularly, higher IL-6/IL-10 ratios in breachers correlated with higher total RPQ scores as well as RPQ-13 scores in both serum and EVs, implying that breachers who have inflammatory activity in the periphery, and possibly in the CNS, increase the risk for more chronic symptoms. However, we found no increased inflammatory response in the periphery of breachers compared to controls, highlighting the complications in utilizing peripheral cytokine levels alone to determine personnel most at risk for developing long-term symptoms. Regardless, our findings continue to suggest that neuronal-derived cytokines may contribute to a pro-inflammatory environment in the brain that could impact cognitive function by influencing injury-related processes in the CNS. Therefore, additional studies to further determine these relationships are warranted.
This study includes several strengths, including the isolation of neuronal-derived EVs from serum and the observation of increased CNS inflammation associated with neurological symptoms in a unique breacher population. This study was limited by the small sample size and the use of one time-point, preventing us from verifying the clinical significance of neuroinflammatory proteins in predicting adverse symptoms in breachers. The number of breaching exposures was estimated by participants and subject to recall bias. In addition, symptoms presented in this study are primarily self-reported and do not include any confirmation of reported symptoms through extensive testing. Although previous work suggests that certain EV cytokines (including IL-6, IL-10, and TNFα), are primarily encapsulated within EVs (40), distinguishing between EV surface-level and encapsulated cytokines was outside the scope of this study. Identification of the mechanism leading to the observed increase in inflammatory cytokines in circulating neuronal-derived EVs was also outside the scope of this study. However, these results show the importance of examining links between inflammation and neurological symptoms and call for further studies specific to experienced career breachers.
Overall, the findings suggest CNS-specific inflammatory responses following low-level blast exposures may be related to chronic symptoms. Future studies identifying biomarkers in circulating blood as well as proteins isolated from neuronal-derived EVs are warranted. In addition, research should examine whether neuroinflammatory biomarkers influence the development of unfavorable outcomes in breachers.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the National Institutes of Health (NIH) and Naval Medical Research Center (NMRC) Institutional Review Boards. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KE designed and conceptualized the study, analyzed the data, and revised the manuscript for intellectual content. JL, ES, and EW contributed substantial revisions to the manuscript. AQ and CM had a major role in the acquisition of the data and contributed the analysis of the data. EP, KD, ML, PW, and WC managed and executed the protocol, recruited the subjects, collected the samples, and contributed to the collection of data. MO'B managed and executed the protocol and contributed to the management of data. CL, B-XQ, and CD had a major role in the acquisition of the data. JS contributed substantial revisions to the manuscript, study supervision, and coordination. SA contributed study supervision and coordination, obtained funding, and contributed substantial revisions to the manuscript. JG contributed to the conception and design of the study and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Joint Program Committee-5 Development of Exposure Standards to Repeated Blast Exposure program, work unit #603115HP-3730-001-A1118. This work was also supported by National Institute of Nursing Research (NINR) and National Institute of Neurological Disease and Stroke (NINDS) Intramural Research Programs. This work was supported in part by an appointment to the Research Participation Program at the Walter Reed Army Institute of Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Army Medical Research and Development Command. Some of the authors are Military Service Members (or employees of the U.S. Government). This work was prepared as part of their official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a Military Service Member or employee of the U.S. Government as part of that person's official duties.
Author Disclaimer
The views expressed in this manuscript reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Army, the Department of the Navy, Department of Defense, or the United States Government. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Reviewer MM declared a past coauthorship/collaboration with one of the authors JG.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carr W, Stone JR, Walilko T, Young LA, Snook TL, Paggi ME, et al. Repeated low-level blast exposure: a descriptive human subjects study. Mil Med. (2016) 181(Suppl. 5):28–39. doi: 10.7205/MILMED-D-15-00137
2. Carr W, Polejaeva E, Grome A, Crandall B, LaValle C, Eonta SE, et al. Relation of repeated low-level blast exposure with symptomology similar to concussion. J Head Trauma Rehabil. (2015) 30:47–55. doi: 10.1097/HTR.0000000000000064
3. Gill J, Motamedi V, Osier N, Dell K, Arcurio L, Carr W, et al. Moderate blast exposure results in increased IL-6 and TNFα in peripheral blood. Brain Behav Immun. (2017) 65:90–4. doi: 10.1016/j.bbi.2017.02.015
4. Devoto C, Arcurio L, Fetta J, Ley M, Rodney T, Kanefsky R, et al. Inflammation relates to chronic behavioral and neurological symptoms in military personnel with traumatic brain injuries. Cell Transplant. (2017) 26:1169–77. doi: 10.1177/0963689717714098
5. Edwards KA, Motamedi V, Osier ND, Kim H-S, Yun S, Cho Y-E, et al. A moderate blast exposure results in dysregulated gene network activity related to cell death, survival, structure, and metabolism. Front Neurol. (2020) 11:91. doi: 10.3389/fneur.2020.00091
6. Osier N, Motamedi V, Edwards K, Puccio A, Diaz-Arrastia R, Kenney K, et al. Exosomes in acquired neurological disorders: new insights into pathophysiology and treatment. Mol Neurobiol. (2018) 55:9280–93. doi: 10.1007/s12035-018-1054-4
7. Hornung S, Dutta S, Bitan G. CNS-derived blood exosomes as a promising source of biomarkers: opportunities and challenges. Front Mol Neurosci. (2020) 13:38. doi: 10.3389/fnmol.2020.00038
8. Winston CN, Romero HK, Ellisman M, Nauss S, Julovich DA, Conger T, et al. Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front Neurosci. (2019) 13:1005. doi: 10.3389/fnins.2019.01005
9. Kenney K, Qu BX, Lai C, Devoto C, Motamedi V, Walker WC, et al. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. (2018) 32:1276–84. doi: 10.1080/02699052.2018.1483530
10. Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, et al. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. (2018) 32:1277–84. doi: 10.1080/02699052.2018.1471738
11. Stone JR, Avants BB, Tustison NJ, Wassermann EM, Gill J, Polejaeva E, et al. Functional and structural neuroimaging correlates of repetitive low-level blast exposure in career breachers. J Neurotrauma. (2020) 37:2468–81. doi: 10.1089/neu.2020.7141
12. Weathers F, Litz B, Herman D, Huska JA, Keane T. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Paper Presented at Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX, United States (1993).
13. King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. (1995) 242:587–92. doi: 10.1007/BF00868811
14. Eyres S, Carey A, Gilworth G, Neumann V, Tennant A. Construct validity and reliability of the rivermead post-concussion symptoms questionnaire. Clin Rehabil. (2005) 19:878–87. doi: 10.1191/0269215505cr905oa
15. Olivera A, Lejbman N, Jeromin A, French LM, Kim HS, Cashion A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. (2015) 72:1109–16. doi: 10.1001/jamaneurol.2015.1383
16. Edwards KA, Greer K, Leete J, Lai C, Devoto C, Qu BX, et al. Neuronally-derived tau is increased in experienced breachers and is associated with neurobehavioral symptoms. Sci Rep. (2021) 11:19527. doi: 10.1038/s41598-021-97913-0
17. Rissin DM, Fournier DR, Piech T, Kan CW, Campbell TG, Song L, et al. Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem. (2011) 83:2279–85. doi: 10.1021/ac103161b
18. Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. (2016) 21:533–47. doi: 10.1177/2211068215589580
19. Rusiecki J, Levin LI, Wang L, Byrne C, Krishnamurthy J, Chen L, et al. Blast traumatic brain injury and serum inflammatory cytokines: a repeated measures case-control study among U.S. military service members. J Neuroinflammation. (2020) 17:20. doi: 10.1186/s12974-019-1624-z
20. Hinson HE, Rowell S, Schreiber M. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J Trauma Acute Care Surg. (2015) 78:184–91. doi: 10.1097/TA.0000000000000468
21. Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. (2013) 4:18. doi: 10.3389/fneur.2013.00018
22. DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. (2016) 139(Suppl. 2):136–53. doi: 10.1111/jnc.13607
23. Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. (2015) 9:358–67. doi: 10.1002/prca.201400114
24. Mondello S, Guedes VA, Lai C, Czeiter E, Amrein K, Kobeissy F, et al. Circulating brain injury exosomal proteins following moderate-to-severe traumatic brain injury: temporal profile, outcome prediction and therapy implications. Cells. (2020) 9. doi: 10.3390/cells9040977
25. Flynn S, Leete J, Shahim P, Pattinson C, Guedes VA, Lai C, et al. Extracellular vesicle concentrations of glial fibrillary acidic protein and neurofilament light measured 1 year after traumatic brain injury. Sci Rep. (2021) 11:3896. doi: 10.1038/s41598-021-82875-0
26. Devoto C, Lai C, Qu BX, Guedes VA, Leete J, Wilde E, et al. Exosomal MicroRNAs in military personnel with mild traumatic brain injury: preliminary results from the chronic effects of neurotrauma consortium biomarker discovery project. J Neurotrauma. (2020) 37:2482–92. doi: 10.1089/neu.2019.6933
27. Ghai V, Fallen S, Baxter D, Scherler K, Kim TK, Zhou Y, et al. Alterations in plasma microRNA and protein levels in war veterans with chronic mild traumatic brain injury. J Neurotrauma. (2020) 37:1418–30. doi: 10.1089/neu.2019.6826
28. Cheng Y, Pereira M, Raukar N, Reagan JL, Queseneberry M, Goldberg L, et al. Potential biomarkers to detect traumatic brain injury by the profiling of salivary extracellular vesicles. J Cell Physiol. (2019) 234:14377–88. doi: 10.1002/jcp.28139
29. Matuk R, Pereira M, Baird J, Dooner M, Cheng Y, Wen S, et al. The role of salivary vesicles as a potential inflammatory biomarker to detect traumatic brain injury in mixed martial artists. Sci Rep. (2021) 11:8186. doi: 10.1038/s41598-021-87180-4
30. Muraoka S, Jedrychowski MP, Tatebe H, DeLeo AM, Ikezu S, Tokuda T, et al. Proteomic profiling of extracellular vesicles isolated from cerebrospinal fluid of former national football league players at risk for chronic traumatic encephalopathy. Front Neurosci. (2019) 13:1059. doi: 10.3389/fnins.2019.01059
31. Goetzl EJ, Elahi FM, Mustapic M, Kapogiannis D, Pryhoda M, Gilmore A, et al. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. Faseb j. (2019) 33:5082–8. doi: 10.1096/fj.201802319R
32. Goetzl EJ, Peltz CB, Mustapic M, Kapogiannis D, Yaffe K. Neuron-derived plasma exosome proteins after remote traumatic brain injury. J Neurotrauma. (2020) 37:382–8. doi: 10.1089/neu.2019.6711
33. Peltz CB, Kenney K, Gill J, Diaz-Arrastia R, Gardner RC, Yaffe K. Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology. (2020) 95:e1126–33. doi: 10.1212/WNL.0000000000010087
34. Kawata K, Mitsuhashi M, Aldret R. A preliminary report on brain-derived extracellular vesicle as novel blood biomarkers for sport-related concussions. Front Neurol. (2018) 9:239. doi: 10.3389/fneur.2018.00239
35. Goetzl EJ, Yaffe K, Peltz CB, Ledreux A, Gorgens K, Davidson B, et al. Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. Faseb j. (2020) 34:3359–66. doi: 10.1096/fj.201902842R
36. Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep. (2016) 13:3391–6. doi: 10.3892/mmr.2016.4948
37. Coppens V, Morrens M, Destoop M, Dom G. The interplay of inflammatory processes and cognition in alcohol use disorders-a systematic review. Front Psychiatry. (2019) 10:632. doi: 10.3389/fpsyt.2019.00632
38. Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs. (2012) 44:206–17. doi: 10.1097/JNN.0b013e3182527690
39. Tampoia M, Abbracciavento L, Morrone M, Fumarulo R. IL-6/IL-10 ratio as a prognostic and predictive marker of the severity of inherited epidermolysis bullosa. Iran J Immunol. (2017) 14:340–9. Available online at: https://iji.sums.ac.ir/article_39329_4525.html
Keywords: extracellular vesicles, neuroinflammation, breacher, blast, military
Citation: Edwards KA, Leete JJ, Smith EG, Quick A, Modica CM, Wassermann EM, Polejaeva E, Dell KC, LoPresti M, Walker P, O'Brien M, Lai C, Qu B-X, Devoto C, Carr W, Stone JR, Ahlers ST and Gill JM (2022) Elevations in Tumor Necrosis Factor Alpha and Interleukin 6 From Neuronal-Derived Extracellular Vesicles in Repeated Low-Level Blast Exposed Personnel. Front. Neurol. 13:723923. doi: 10.3389/fneur.2022.723923
Received: 11 June 2021; Accepted: 03 February 2022;
Published: 21 April 2022.
Edited by:
Elham Rostami, Uppsala University Hospital, SwedenReviewed by:
Keisuke Kawata, Indiana University Bloomington, United StatesMaja Mustapic, Laboratory of Clinical Investigation, National Institute on Aging (NIH), United States
Copyright © 2022 Edwards, Leete, Smith, Quick, Modica, Wassermann, Polejaeva, Dell, LoPresti, Walker, O'Brien, Lai, Qu, Devoto, Carr, Stone, Ahlers and Gill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katie A. Edwards, a2F0aWUuYS5lZHdhcmRzM0BnbWFpbC5jb20=
 Katie A. Edwards
Katie A. Edwards Jacqueline J. Leete
Jacqueline J. Leete Ethan G. Smith
Ethan G. Smith Alycia Quick
Alycia Quick Claire M. Modica
Claire M. Modica Eric M. Wassermann
Eric M. Wassermann Elena Polejaeva6
Elena Polejaeva6 Kristine C. Dell
Kristine C. Dell Matthew LoPresti
Matthew LoPresti Chen Lai
Chen Lai Bao-Xi Qu
Bao-Xi Qu Christina Devoto
Christina Devoto James R. Stone
James R. Stone Stephen T. Ahlers
Stephen T. Ahlers Jessica M. Gill
Jessica M. Gill