
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 16 January 2023
Sec. Movement Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1099862
 Ahro Kim1
Ahro Kim1 Han-Joon Kim2
Han-Joon Kim2 Aryun Kim3
Aryun Kim3 Yoon Kim4
Yoon Kim4 Ahwon Kim2
Ahwon Kim2 Jed Noel A. Ong5
Jed Noel A. Ong5 Hye Ran Park6
Hye Ran Park6 Sun Ha Paek7*
Sun Ha Paek7* Beomseok Jeon2*
Beomseok Jeon2*Background: Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is effective in improving motor function in patients with Parkinson's disease (PD). This study aimed to investigate mortality associated with bilateral STN DBS in patients with PD and to assess the factors associated with mortality and causes of death after DBS.
Methods: We reviewed the medical records of 257 patients with PD who underwent bilateral STN DBS at the Movement Disorder Center at Seoul National University Hospital between March 2005 and November 2018. Patients were evaluated preoperatively, at 3, 6, and 12 months after surgery and annually thereafter. The cause and date of death were obtained from interviews with caregivers or from medical certificates at the last follow-up.
Results: Of the 257 patients with PD, 48 patients (18.7%) died, with a median time of death of 11.2 years after surgery. Pneumonia was the most common cause of death. Older age of disease onset, preoperative falling score while on medication, and higher preoperative total levodopa equivalent daily dose were associated with a higher risk of mortality in time-dependent Cox regression analysis.
Conclusion: These results confirm the mortality outcome of STN DBS in patients with advanced PD.
Parkinson's disease (PD) is one of the most common neurodegenerative diseases worldwide. Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is effective in improving motor function in patients with PD (1, 2). This effect of DBS is known to persist for at least 5–10 years, although the effect decreases as axial signs appear, with the progression of PD (3–7). It has been recently reported that STN DBS remains effective at treating motor complications 15 years after surgery (8). Long-term outcomes of DBS, with a focus on mortality, have been reported in previous studies from various countries (9–12). The mortality outcomes of DBS in patients with PD were diverse, ranging from 4.3 to 34% (see Supplementary Table). This variation may be explained by the fact that DBS was performed in different countries or on patients of different races. However, only one article has been reported in Korea (13). We, therefore, report a larger number of cohorts from our center. The aims of this study were as follows: (1) to investigate the mortality of bilateral STN DBS in patients with PD, (2) to assess the factors associated with mortality, and (3) to assess the cause of death after DBS.
We conducted a retrospective longitudinal observational study of patients with DBS. We reviewed the medical records of patients with PD who underwent bilateral STN DBS at the movement disorder center (MDC) at the Seoul National University Hospital (SNUH) between March 2005 and May 2018. A PD diagnosis was made based on the United Kingdom Parkinson's Disease Society Brain Bank Criteria (14), and all patients were examined by experienced movement disorder specialists (BJ and HJK). In total, 269 patients were included in this study. Among them, 10 patients who were referred to other hospitals for reoperation were excluded. One patient was further excluded due to DBS of the ventral intermediate nucleus, and another patient was excluded due to thalamotomy surgery. A total of 257 patients who underwent STN DBS surgery were included. Patients who underwent a second operation at our hospital were included, and the time from symptom onset to surgery or surgery to death was calculated based on the first operation.
Preoperative and postoperative assessments were performed according to the SNUH MDC protocol (15). The Unified Parkinson's Disease Rating Scale (UPDRS) parts I, II, and III (motor subscales) and the Hoehn and Yahr (HY) stages were assessed in on-medication and off-medication states. We also measured axial scores, which is the sum of the following motor subscores from the UPDRS-III: stance (item 27), posture (item 28), postural instability (item 29), and gait (item 30) (16). We defined the presence of UPDRS I and II items (e.g., the presence of hallucination, swallowing difficulty, and falling) by a cutoff value of score 2.
The levodopa equivalent daily dose (LEDD) (17) and levodopa doses were calculated at each visit. A diagnosis of PD dementia was made when a patient met the Movement Disorder Society Task Force clinical criteria for PDD. Preoperative cognitive function was evaluated using the Korean Mini-Mental State Examination (MMSE), and depression scores were evaluated using the Beck Depression Inventory with a cutoff score of 16.
Postoperative evaluations were performed at 3, 6, and 12 months after STN DBS, and annually thereafter. Follow-up evaluations were carried out as inpatient procedures at the MDC, with video recordings and diary entries (15). The primary outcomes were survival status and cause of death. The cause and date of death of each patient were obtained from interviews with caregivers or medical certificates at the last follow-up. The cause of death was categorized based on previous studies (10, 12).
A descriptive analysis was used to examine the DBS group. Demographic characteristics are presented as mean values ± standard deviations (SD) or numbers (%), when applicable. The Kaplan–Meier method was used for a survival analysis. Univariate and Cox regression analyses were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). We included sex, age at onset, disease duration at surgery, preoperative body mass index (BMI), depression, UPDRS-III score (off-medication score, on-medication score, on-medication score/stimulation on), axial score, falling score, and HY stage as covariates for Cox regression analysis. The proportional hazards assumption considers that HR for comparison groups is constant over the follow-up period, and the proportional hazards assumption was tested by the time-dependent Cox model, including an interaction term with time. HR varied over time in this model, which violated the proportional hazards assumption for two variables (preoperative falling on-medication score and preoperative depression). Thus, we used time-dependent Cox regression analysis by including interaction terms with time (T_COV_* preoperative falling on-medication score, T_COV_*preoperative depression). The other variables satisfied the proportional hazards assumption. SPSS ver. 20 (IBM Corp, Armonk, NY) and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses, and a P-values of below 0.05 were considered statistically significant.
Table 1 shows the demographic and baseline clinical characteristics of the patients with PD who underwent DBS surgery. Of the 257 patients, 142 (55.3%) were women. The mean ± SD age of PD onset was 46.9 ± 9.5 years, and the mean disease duration at the time of surgery was 11.5 ± 4.6 years. Preoperative HY stage on medication in all patients except one was under 3. One patient was classified as grade 4 due to diphasic dyskinesia. The mean MMSE score at baseline was 27.3 ± 2.5, and no patients had dementia. 58.8% of patients had depression. The mean ± SD preoperative UPDRS-III motor scores on medication and off medication were 21.1 ± 11.7 and 41.6 ± 14.9, respectively. The mean ± SD preoperative axial symptom score on medication and off medication was 2.9 ± 2.2 and 6.5 ± 3.4, respectively. The mean ± SD levodopa-induced dyskinesia (hour) and wearing off time were 7.2 ± 3.8 and 5.1 ± 4.4, respectively.
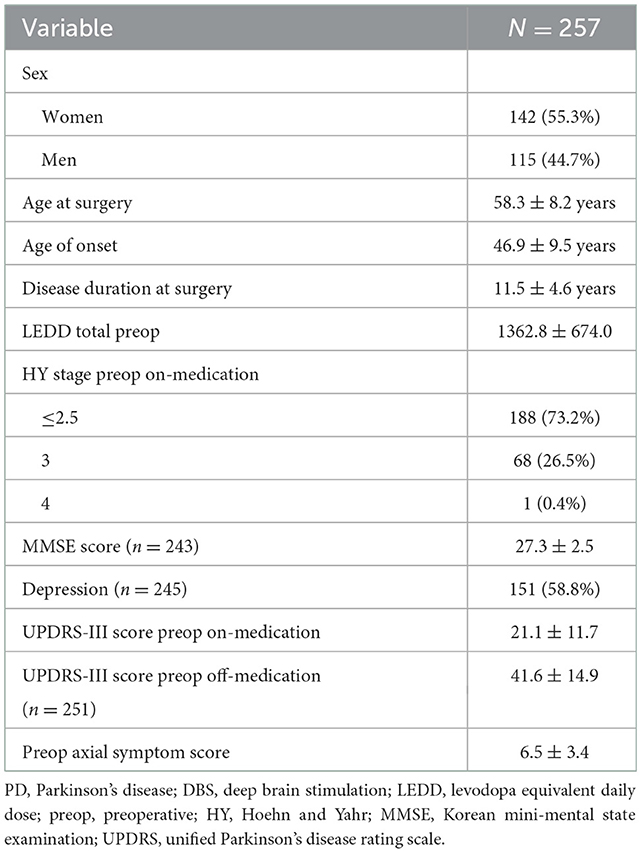
Table 1. Demographic and baseline clinical characteristics of patients with PD who underwent DBS surgery.
A median follow-up duration was 60 months. One hundred forty-two patients with PD were followed up for 5 years, and 51 patients were followed up for 10 years. Of the 257 patients with PD, 48 patients (18.7%) died, with a median time of death of 11.2 years after surgery. The disease duration was 16.08 years from onset to death. The mean age at death was 67.7 years. For the survival group, the age and duration of PD at the time of the study were 62.2 ± 8.6 and 16.4 ± 5.4 years. Survival curves are shown in Figure 1. The results showed a 1-year survival rate of 98.4%, a 3-year survival rate of 96.8 %, a 5-year survival rate of 89.8%, and a 10-year survival rate of 69.2% for patients with PD who underwent DBS treatment at the study center (Figure 1A). Kaplan–Meier plots illustrated survival probability according to the presence of preoperative falling on medication, age group at onset, and preoperative LEDD group (P < 0.05) (Figures 1B–D).
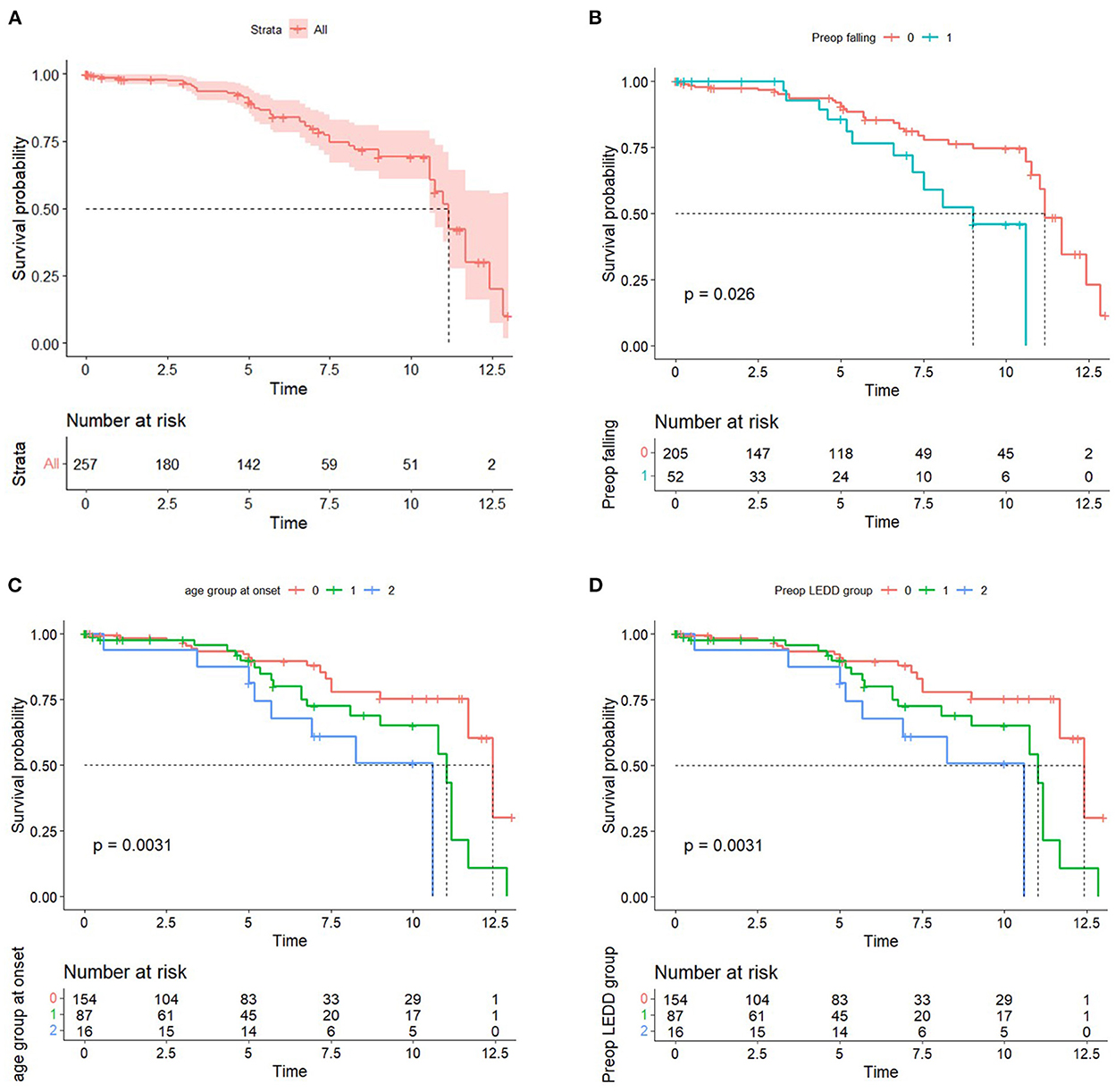
Figure 1. (A) Kaplan–Meier curve of the DBS group. (B) Kaplan–Meier curve by the presence of preoperative falling on-medication score. (C) Kaplan–Meier curve by the age group at onset. 0: <50, 1: 50–59, 2: ≥60. (D) Kaplan–Meier curve by the preoperative LEDD group. 0: first tertile, 1: second tertile, and 2: third tertile.
A time-dependent Cox regression analysis revealed that older age of onset (HR = 1.048; 95% CI = 1.005–1.093; P = 0.028), higher preoperative total LEDD (HR = 1.001, 95% CI, 1.000–1.001; P = 0.038), and the interaction term with Time (T_COV_* preoperative falling on-medication score) (HR = 1.361; 95% CI = 1.001–1.850; P = 0.049) were associated with a higher risk of mortality (Table 2). A significant interaction term with time indicates that the effect of preoperative falling on HR changed over time. In detail, the direction of HR (<1 or >1) flip over at ~4 years and then increase more markedly as the follow-up period increase. Figure 1B also supports these results. Sex, preoperative presence of depression, disease duration at surgery, presence of preoperative axial symptoms while on medication, preoperative HY stage, and preoperative BMI were not significantly associated with mortality. However, UPDRS-III preoperative improvement score (UPDRS-III off-medication score minus UPDRS-III on-medication score) and the UPDRS-III postoperative improvement score (preoperative UPDRS-III on-medication score minus UPDRS-III on-medication/stimulation-on score) were not significantly associated with mortality (data not shown). Stimulation parameters, such as amplitude, frequency, and pulse width, were not significantly associated with mortality (data not presented). The causes of death are listed in Table 3. Causes of death were grouped into six categories: (1) progression of PD; (2) psychiatric complications; (3) accidental death; (4) unknown; (5) cancer; and (6) medical complications. Pneumonia was the most common cause of death (26, 54.2%). Four patients (8.3%) died of various cancers, four (8.3%) died by suicide, four (8.3%) died of car accidents, drowning, or falling, two (4.2%) died of other medical complications, and seven (14.6%) died of unknown causes. We also analyzed the causes of death within 5 years after surgery (Table 4). Seven patients (38.9%) died of pneumonia, while four (8.3%) died by suicide 1, 3, 13, and 30 months, respectively, after surgery.
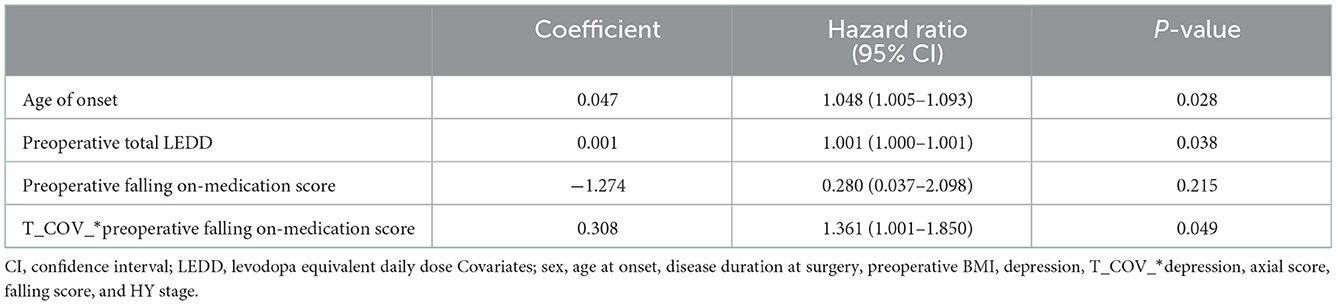
Table 2. Associations of clinical features with mortality after deep brain stimulation in patients determined by a time-dependent Cox proportional hazard model with time-dependent variables.
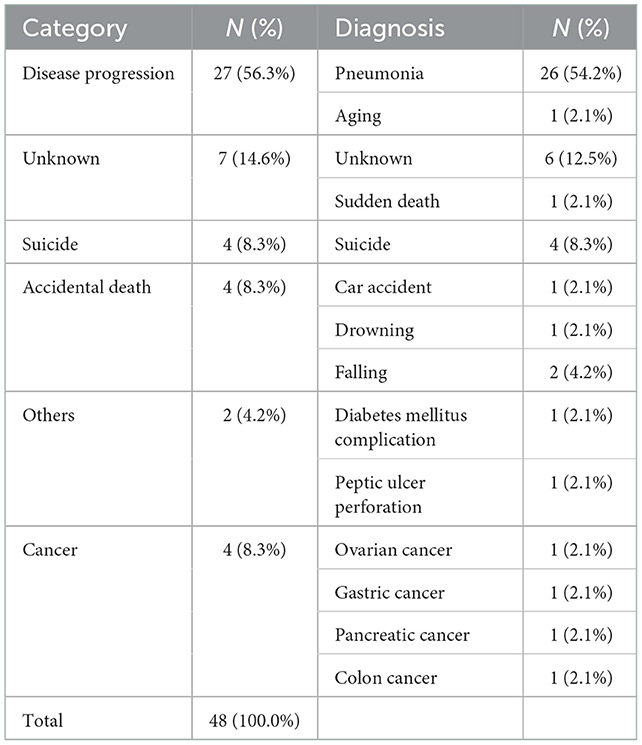
Table 3. Causes of death of patients with Parkinson's disease after undergoing deep brain stimulation.
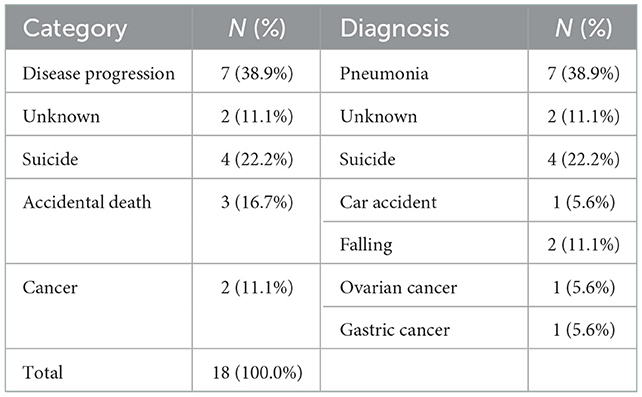
Table 4. Causes of death of patients with Parkinson's disease within 5 years of undergoing deep brain stimulation.
We studied a large cohort of patients with PD over a mean duration of 5 years after STN DBS surgery. We found that 48 patients with PD (18.7%) of 257 died, with a median time of death of 11.2 years after surgery, and that pneumonia was the most common cause of death. The mortality rate in the present study was 18.7%. The present study was compared with previous studies that reported the mortality of patients with DBS in different countries and groups (Supplementary Table). The mortality outcomes of DBS in patients with PD were diverse, ranging from 4.3 to 34%. This variation may be explained by the fact that DBS was performed in different countries, on patients of different races, with different disease durations at the time of surgery.
Pneumonia was the most common cause of death reported in the present study; many previous studies have also reported pneumonia as a common cause of death (7, 11, 13, 18–20). Cancer, suicide, and falling rates have also been reported in our study. The causes of death showed a pattern similar to that reported in other studies. Lau et al. (16) reported that the most common cause of death was a progression of PD, followed by asphyxiation or aspiration pneumonia. They also reported suicide and death due to cancer. Rocha et al. (20) reported that bacterial pneumonia was the most common cause of death, followed by death due to cancer (9.0%) and suicide (6.6%). Zhang et al. (19) reported that pneumonia and asphyxia were the most common causes of death. Castrioto et al. (7) also reported that aspiration pneumonia was the most common cause of death. A high prevalence of swallowing-related diseases, the severity of which is associated with the duration and severity of PD, has also been reported (21). We considered pneumonia to be a consequence of swallowing-related disorders associated with PD progression. Furthermore, since patients without dementia were initially considered indications for DBS, the onset of dementia was classified as PD progression. Therefore, it was easy to predict that pneumonia would be the most common cause of death.
The suicide rate reported in the present study was higher than that in other studies; however, this is consistent with other studies' reports that suicidal behaviors can occur after STN DBS, especially during the first 3 years (22, 23). Thus, we should regularly assess and treat postoperative depression to prevent suicide, which is a preventable cause of death. Other studies have also reported cancer as a cause of death after STN DBS (5, 11, 12, 16, 18, 24). Thus, by focusing only on the progression of PD, we may neglect other diseases, such as cancer. Regular cancer screening in patients with PD should not be overlooked.
Older age of onset, the occurrence of preoperative falls on medication, and higher total preoperative LEDD were also associated with a higher risk of mortality in the present study. Older age at the time of surgery is a predictor of mortality in patients with DBS, which is in line with the results of previous studies (5, 9). In our study, the presence of preoperative falling on medication, which is considered an axial symptom, was associated with a higher risk of mortality. This is consistent with other studies. Lau et al. (16) suggested that the level of axial disability was the only symptom that significantly predicted death. Higher age of disease onset was reported to be associated with a faster progression rate of axial symptoms and falls in patients with PD (25, 26). Thus, older age at the time of surgery and a high preoperative falling score on medication may indicate high mortality rates. Sex differences did not influence mortality in our patients with DBS, in contrast to other studies (12, 13). Previous studies regarding sex differences did not propose any biological explanation, and thus, further study is needed.
The present study additionally found that a higher total preoperative LEDD was associated with a higher risk of mortality. Furthermore, Fasano et al. (6) reported that the subgroup of patients who developed remarkable worsening of postural stability 8 years after DBS also had marked postural instability at baseline (before surgery) in both the off-medication and on-medication conditions and showed a significantly greater intake of antiparkinsonian medication at baseline. In light of the results of the present study, it can be assumed that the group with a high baseline LEDD had more postural instability, which influenced mortality.
The present study had several strengths. Compared with other similar long-term studies, our study examined one of the largest cohorts, followed up at the same DBS center over a longer period of time. Patients were followed by the same neurosurgeons and neurologists. We were able to contact all patients by telephone or in-person, so none were lost to follow-up, leading to more valid conclusions. However, this study also had several limitations. First, this was a single-center study conducted in South Korea; thus, these results do not represent the entire Korean population. Second, whether DBS could affect the course of PD is a debatable issue, but we do not have control data. Third, we were not able to estimate total electrical energy delivered for DBS frequency due to the unavailability of impedance. Finally, there is no genetic and clinical information such as comorbidity burden and dementia conversion in patients with PD who underwent STN DBS surgery.
In conclusion, this study highlighted the mortality outcomes of STN DBS in patients with PD; older age of PD onset, preoperative falling scores on-medication may be associated with a higher risk of mortality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No.1901-142-1005). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AhrK and BJ were involved in the conception and design of the work. H-JK, AryK, and YK recruited participants and collected the data. Data analysis and interpretation was primarily conducted by AhrK, with guidance and advice offered by AhwK, JO, HP, and SP. AhrK wrote the manuscript. BJ and SP commented on and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1099862/full#supplementary-material
1. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. (2003) 349:1925–34. doi: 10.1056/NEJMoa035275
2. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. (2006) 355:896–908. doi: 10.1056/NEJMoa060281
3. Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord. (2010) 25:578–86. doi: 10.1002/mds.22735
4. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain J Neurol. (2005) 128(Pt 10):2240–9. doi: 10.1093/brain/awh571
5. Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson's disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. (2008) 14:114–9. doi: 10.1016/j.parkreldis.2007.06.012
6. Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR, et al. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain J Neurol. (2010) 133:2664–76. doi: 10.1093/brain/awq221
7. Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. (2011) 68:1550–6. doi: 10.1001/archneurol.2011.182
8. Bove F, Mulas D, Cavallieri F, Castrioto A, Chabardès S, Meoni S, et al. Long-term outcomes (15 years) after subthalamic nucleus deep brain stimulation in patients with Parkinson disease. Neurology. (2021) 97:e254–62. doi: 10.1212/WNL.0000000000012246
9. Toft M, Lilleeng B, Ramm-Pettersen J, Skogseid IM, Gundersen V, Gerdts R, et al. Long-term efficacy and mortality in Parkinson's disease patients treated with subthalamic stimulation. Movement Disord. (2011) 26:1931–4. doi: 10.1002/mds.23817
10. Schüpbach MW, Welter ML, Bonnet AM, Elbaz A, Grossardt BR, Mesnage V, et al. Mortality in patients with Parkinson's disease treated by stimulation of the subthalamic nucleus. Mov Disord. (2007) 22:257–61. doi: 10.1002/mds.21264
11. Rocha S, Monteiro A, Linhares P, Chamadoira C, Basto MA, Reis C, et al. Long-term mortality analysis in Parkinson's disease treated with deep brain stimulation. Parkinsons Dis. (2014) 2014:717041. doi: 10.1155/2014/717041
12. Bang Henriksen M, Johnsen EL, Sunde N, Vase A, Gjelstrup MC, Østergaard K. Surviving 10 years with deep brain stimulation for Parkinson's disease: a follow-up of 79 patients. Eur J Neurol. (2016) 23:53–61. doi: 10.1111/ene.12614
13. Ryu HS, Kim MS, You S, Kim MJ, Kim YJ, Kim J, et al. Mortality of advanced Parkinson's disease patients treated with deep brain stimulation surgery. J Neurol Sci. (2016) 369:230–5. doi: 10.1016/j.jns.2016.08.041
14. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. (1992) 55:181–4.
15. Lee JY, Han JH, Kim HJ, Jeon BS, Kim DG, Paek SH. STN DBS of advanced Parkinson's disease experienced in a specialized monitoring unit with a prospective protocol. J Korean Neurosurg Soc. (2008) 44:26–35. doi: 10.3340/jkns.2008.44.1.26
16. Lau B, Meier N, Serra G, Czernecki V, Schuepbach M, Navarro S, et al. Axial symptoms predict mortality in patients with Parkinson disease and subthalamic stimulation. Neurology. (2019) 92:e2559–e70. doi: 10.1212/WNL.0000000000007562
17. Thobois S. Proposed dose equivalence for rapid switch between dopamine receptor agonists in Parkinson's disease: A review of the literature. Clin Ther. (2006) 28:1–12. doi: 10.1016/j.clinthera.2005.12.003
18. Merola A, Rizzi L, Artusi CA, Zibetti M, Rizzone MG, Romagnolo A, et al. Subthalamic deep brain stimulation: clinical and neuropsychological outcomes in mild cognitive impaired parkinsonian patients. J Neurol. (2014) 261:1745–51. doi: 10.1007/s00415-014-7414-8
19. Zhang J, Wang T, Zhang C-C, Zeljic K, Zhan S, Sun B-M, et al. The safety issues and hardware-related complications of deep brain stimulation therapy: a single-center retrospective analysis of 478 patients with Parkinson's disease. Clin Interv Aging. (2017) 12:923–8. doi: 10.2147/CIA.S130882
20. Rocha AL, Oliveira A, Sousa C, Monteiro P, Rosas MJ, Vaz R. Long term mortality of patients with Parkinson's disease treated with deep brain stimulation in a reference center. Clin Neurol Neurosurg. (2021) 202:106486. doi: 10.1016/j.clineuro.2021.106486
21. Kanna SV, Bhanu K. A simple bedside test to assess the swallowing dysfunction in Parkinson's disease. Ann Indian Acad Neurol. (2014) 17:62–5. doi: 10.4103/0972-2327.128556
22. Giannini G, Francois M, Lhommée E, Polosan M, Schmitt E, Fraix V, et al. Suicide and suicide attempts after subthalamic nucleus stimulation in Parkinson disease. Neurology. (2019) 93:e97–e105. doi: 10.1212/WNL.0000000000007665
23. Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schüpbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain J Neurol. (2008) 131:2720–8. doi: 10.1093/brain/awn214
24. Weaver FM, Stroupe KT, Smith B, Gonzalez B, Huo Z, Cao L, et al. Survival in patients with Parkinson's disease after deep brain stimulation or medical management. Mov Disord. (2017) 32:1756–63. doi: 10.1002/mds.27235
25. Kempster PA, Williams DR, Selikhova M, Holton J, Revesz T, Lees AJ. Patterns of levodopa response in Parkinson's disease: a clinico-pathological study. Brain J Neurol. (2007) 130:2123–8. doi: 10.1093/brain/awm142
Keywords: mortality, Parkinson's disease, STN (subthalamic nucleus), outcome, deep brain stimulation
Citation: Kim A, Kim H-J, Kim A, Kim Y, Kim A, Ong JNA, Park HR, Paek SH and Jeon B (2023) The mortality of patients with Parkinson's disease with deep brain stimulation. Front. Neurol. 13:1099862. doi: 10.3389/fneur.2022.1099862
Received: 16 November 2022; Accepted: 19 December 2022;
Published: 16 January 2023.
Edited by:
Luigi M. Romito, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Wooyoung Jang, Gangneung Asan Hospital, Republic of KoreaCopyright © 2023 Kim, Kim, Kim, Kim, Kim, Ong, Park, Paek and Jeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beomseok Jeon,  YnJhaW5Ac251LmFjLmty; Sun Ha Paek,
YnJhaW5Ac251LmFjLmty; Sun Ha Paek,  cGFla3NoQHNudS5hYy5rcg==
cGFla3NoQHNudS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.