- 1College of Physical Education, Shenzhen University, Shenzhen, China
- 2Department of Sports Medicine and Rehabilitation, Peking University Shenzhen Hospital, Shenzhen, China
- 3School of Physical Education and Sports Exercise, South China Normal University, Guangzhou, China
- 4College of Physical Education, Southwest University, Chongqing, China
Introduction: To investigate the effect of exercise on the walking economy (WE) of patients with chronic neurological conditions (CNCs) and to determine the type of physical activity that best improves the WE of patients with CNCs.
Methods: Four electronic databases were searched until December 2022 (Web of Science, PubMed, Cochrane, and CINAHL). Studies were screened using the following inclusion criteria: 1. randomized controlled or non-randomized controlled trials; 2. exercise interventions >4 weeks in duration; 3. patients aged ≥18 years with a diagnosis of CNCs. 4. walking economy of patients measured before and after the intervention. The PEDro scale was used to assess the methodological quality of the included studies.
Results and discussion: Twenty-two studies met the inclusion criteria. Meta-analysis results showed that exercise significantly improved WE (g = −0.352, 95% CI, −0.625 to −0.078, P = 0.012). Subgroup analysis revealed that patients who received exercise showed better WE compared with those who underwent no control intervention (g = −0.474, 95% CI, −0.636 to −0.311, P < 0.001). However, exercise therapy did not show a significant improvement of WE compared with control groups (g = −0.192, 95% CI, −0.451 to 0.067, P = 0.146). In addition, we found that endurance combined with resistance, high-intensity intermittent, and other training modalities resulted in better WE compared with the pre-intervention. Of these, interval training has the greatest effect on improving WE. In conclusion, exercise can improve WE in patients with CNCs. More randomized controlled trials are necessary for the future.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022361455, identifier: CRD42022361455.
1. Introduction
Globally, approximately 1 billion people suffer from chronic neurological conditions (CNCs), which have been becoming the main cause of death and disability in the world (1). Epidemiological studies have shown that the prevalence of CNCs has continued to increase over the years (2). The ability to exercise is considered an essential challenge in patients with CNCs. Lower exercise ability usually leads to health deterioration and worse quality of life for patients with CNCs (3–6).
For patients with CNCs, the intervention may be lifelong (7). In some patients, internal surgery is expensive and risky, and the use of other medications is associated with side effects and some of them do not efficiently restore body functions and improve daily activities (8). Therefore, exercise training is increasingly being used in the field of rehabilitation of exercise ability for patients with CNCs. It can improve various exercise functions such as balance, walking performance, and gait parameters in patients with CNCs such as stroke, Parkinson's disease (PD), and multiple sclerosis (MS) (9–14). Exercise for patients with CNCs can be broadly classified as endurance training (ET), resistance training (RT), endurance combined with resistance training (ERT), intermittent training (IT), or other training modalities (OTM). OTM is used to target specific functional impairments, for example, using the treadmill or ground-based walking exercises to improve walking ability in patients with stroke (15). Compared with usual care, exercise training exists for more intense physical activity and, more significantly, they are more economical and lifelong participation. A recent meta-analysis showed that exercise improved motor participation in patients with multiple sclerosis (15). It can, therefore, provide sustainable patient recovery.
Walking economy (WE) was defined as the steady-state aerobic demand at a given submaximal speed or distance, which usually was used to measure the energy cost while walking (16–18). A higher WE indicates that a patient can walk further per unit of time and distance. WE is influenced by age, and a meta-analysis reported a 17% increase in net metabolic cost in older adults compared with healthy younger adults (19). Disorders caused by neurological disorders increase energy expenditure during walking (16, 20, 21), making patients more prone to fatigue when walking (16, 22). Patients with stroke (22), PD (16), Alzheimer's disease (AD) (23), MS (24), and spinal cord injury (SCI) (25) exhibit higher oxygen consumption compared with healthy individuals. The mechanisms underlying the high energy cost in patients with CNCs may include tremors (16), walking biomechanics (26), and neural mechanisms (27). A poor WE may increase the risk of fatigue in patients with CNCs, which in turn causes functional limitations and reduces their quality of life and social participation (28–30). Therefore, strategies meant to improve WE have been explored to improve the recovery of motor ability in patients. However, to date, no high-quality studies have systematically reviewed the effects of exercise on WE. Several studies have explored whether exercise therapy can improve cardiopulmonary function in patients with CNCs. However, such studies did not test the value of peak oxygen consumption (VO2peak) improvement as a potential physiological indicator of cardiopulmonary function (10, 12, 14, 31–33), and this aspect has not been sufficiently reviewed as a primary outcome.
Given the importance of WE in the daily life of patients with CNCs, we think a review and analysis of the current literature is necessary. Therefore, the main objectives of this meta-analysis were (1) to assess the impact of using exercise on WE in patients with CNCs and (2) to different exercise modalities in an attempt to find an intervention that improves WE optimally.
2. Methods
The meta-analysis is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The systematic evaluation program is registered in PROSPERO (CRD42022361455).
2.1. Literature search
A search was performed on the following electronic databases: PubMed, Web of Science, Cochrane, and CINAHL. The search was conducted from the earliest record to December 2022 using the following terms: (Central nervous system condition OR Central nervous system disease OR Stroke OR Multiple sclerosis OR Parkinson* disease OR Incomplete spinal cord injury OR Alzheimer* disease) AND (Exercise OR Training OR Physical activity OR Rehabilitation) AND [(Walking OR Gait OR Locomotor) AND (Speed OR Velocity OR Economy OR Expenditure OR Energy or Oxygen)]. All published peer-reviewed articles written in English were retrieved. In addition, reference lists of the retrieved studies were also reviewed. All articles identified were screened by two researchers by reading the title and abstract and evaluated against the eligibility criteria mentioned in the subsequent section.
2.2. Inclusion and exclusion criteria
2.2.1. Inclusion criteria
The inclusion criteria were as follows: (1) Participants with a diagnosis of chronic neurological diseases, such as stroke, MS, PD, SCI, and AD. Participants were able to walk alone or with appropriate assistance. (2) Included studies were longitudinal interventional studies, whether randomized controlled trials (RCT), non-randomized controlled trials (N-RCT). (3) Intervention group was based on exercise training, which lasted at least 4 weeks. (4) Walking at self-selected speed (SSS) or absolute speed tested walking economy and standardized for weight or speed. (5) Articles are written in English.
2.2.2. Exclusion criteria
The inclusion criteria were as follows: (1) We excluded studies on exercise combined with other non-physical training on intervention, such as the combination of electrical stimulation, virtual reality, and robot-assisted training. (2) Conference abstracts and posters were excluded.
2.3. Data extraction and quality assessment
The search results were downloaded and imported into EndNote software. Duplicates were removed, as well as filter titles, abstracts, and full-text articles. Two authors independently screened the titles, abstracts, and full-text articles. A third author was consulted if there was any discrepancy between the results obtained by the two authors to achieve consensus. The data were independently extracted by two researchers: extraction study design; participant characteristics; intervention description; and oxygen uptake outcome indicators. For the selection of WE, if some studies performed multiple speed measurements simultaneously, for example, 80, 100, and 120% self-selected speed, we included only the 100% group of selected speeds that were most comfortable and closest to life for the participants to ensure homogeneity of results. The article's corresponding author was contacted to clarify or obtain incomplete or missing data.
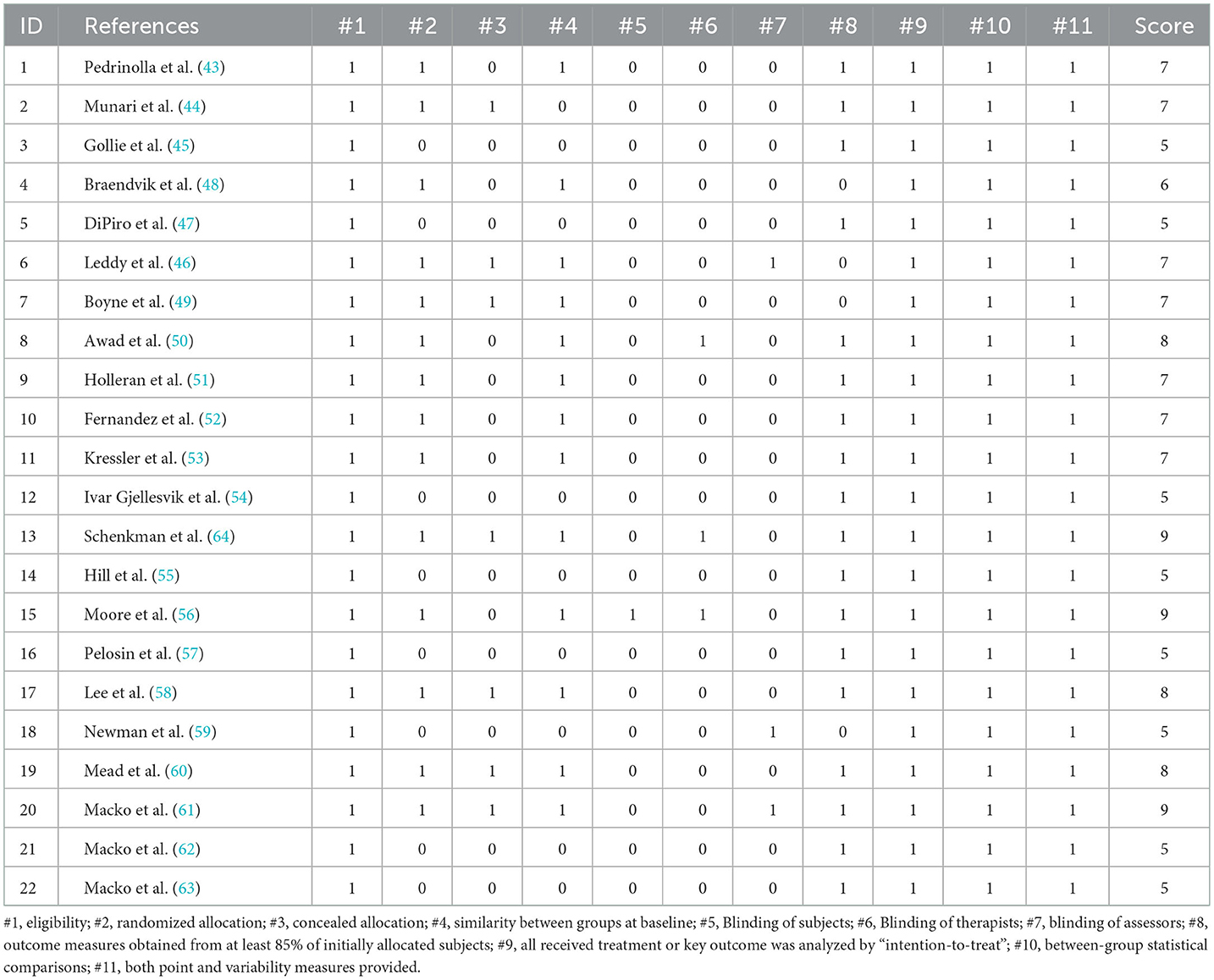
The methodological quality of the studies was assessed using the original PEDro scale (34). This scale has 11 entries that can be used to assess the methodological quality of physiotherapy. Overall, PEDro has been found to be a valid measure of the methodological quality of clinical trials (35). The evaluation criteria were as follows: eligibility criteria, randomization, concealed allocation, baseline equivalence, blinding of participants, blinding of instructors, blinding of assessors, retention rate of 85%, missing data management (intent-to-treat analysis), between-group analysis, and measures of variability. If the aforementioned information was clear in the study, 1 point was awarded; if not, 0 points were awarded. The maximum score for each study was 11 points. According to the scores, the quality of these studies was divided into four grades: excellent (>9 points), good (6 to 8 points), fair (4 to 5 points), and poor (<4 points) quality.
2.4. Exercise definition
The type of exercises was classified into five categories according to the following definitions: (1) ET is defined by the ACSM guideline as a continuous and rhythmic exercise sustained for a period that requires a substantial activation of large skeletal muscles (36), such as treadmill walking or running, and stationary cycling training. (2) RT is defined as a few dynamic muscle contractions against external loads, with sufficient progression (36). (3) ERT is defined as training that includes both endurance and resistance exercises. (4) IT involves repeated high-intensity exercise interspersed with periods of active or inactive recovery (37). (5) OTM is defined as being used to target specific functional disorders. In this study, OTM focuses on the participant's gait at a lower intensity and is designed to restore the patient's ability to walk.
2.5. Data synthesis and analysis
Statistical analyses were performed using Comprehensive Meta-Analysis, version 3.0 (Englewood, NJ, USA), with the level of statistical significance set at p < 0.05. ES values were calculated from the mean and standard deviation data before and after the exercise intervention or between the experimental and control groups. The effect size was calculated using two methods: (I) For controlled trials, we calculated effect size as the change in the mean of the exercise group before and after the intervention minus the change in the mean of the control group, divided by the combined standard deviation before the intervention, and adjusted for sample size. For studies that included a control group and multiple intervention groups, the sample size of the control group was proportionally reduced. (II) For before-and-after controlled clinical studies without a control group, the effect size was calculated as the mean change before and after the intervention divided by the standard deviation before the intervention, which was presented as Hedges' g and 95% confidence interval (CI).
The type of control group and different exercise types were classified. Subgroup analyses were performed based on each classification, which contained studies larger than two articles. We divided the exercise intervention into pre-exercise and control groups compared with exercise according to the study design. In addition, we also addressed the compliance of the included studies with the published Physical Activity Guidelines (PAG) (38). For studies to meet the PAG, the following conditions had to be met: 150 min/week of moderate-intensity exercise or 75 min/week of vigorous exercise, or roughly a combination of moderate and vigorous exercises. For studies that met the PAG, exercise intensity and duration had to be reported. Moderate intensity was defined as maximum heart rate = 55–70%, maximum oxygen uptake = 40–60%, heart rate reserve = 40–60%, or ratings of perceived exertion (RPE) of 11–13 on the Borg scale. Vigorous intensity was defined as the maximum heart rate of >70%, maximum oxygen uptake of >60%, heart rate reserve of >59%, RPE of >13 on the Borg scale (39).
The magnitude of Hedges' g was interpreted using Cohen's (1988) (40) convention as small (0.2–0.5), medium (0.5–0.8), and large (>0.8). We used the I-squared (I2) test to assess the statistical heterogeneity of treatment effects between studies, with I2 of > 50% considered heterogeneous. Since the participants included in the study were from different groups of diseases. Therefore, the summary results of the hypotheses are based on the random effects model. The effect of the categorical moderators was based on the significance of the QB statistic. The QB statistic indicated the statistical significance of the difference between the levels of the moderator variables. The effect of publication bias on the primary meta-analyses was addressed by combining a funnel plot assessment with Duval and Tweedie's trim and fill correction (41). Sensitivity analysis uses an exclusion-by-exclusion approach to observe whether there is a significant change in the outcome results.
3. Result
3.1. Study characteristics
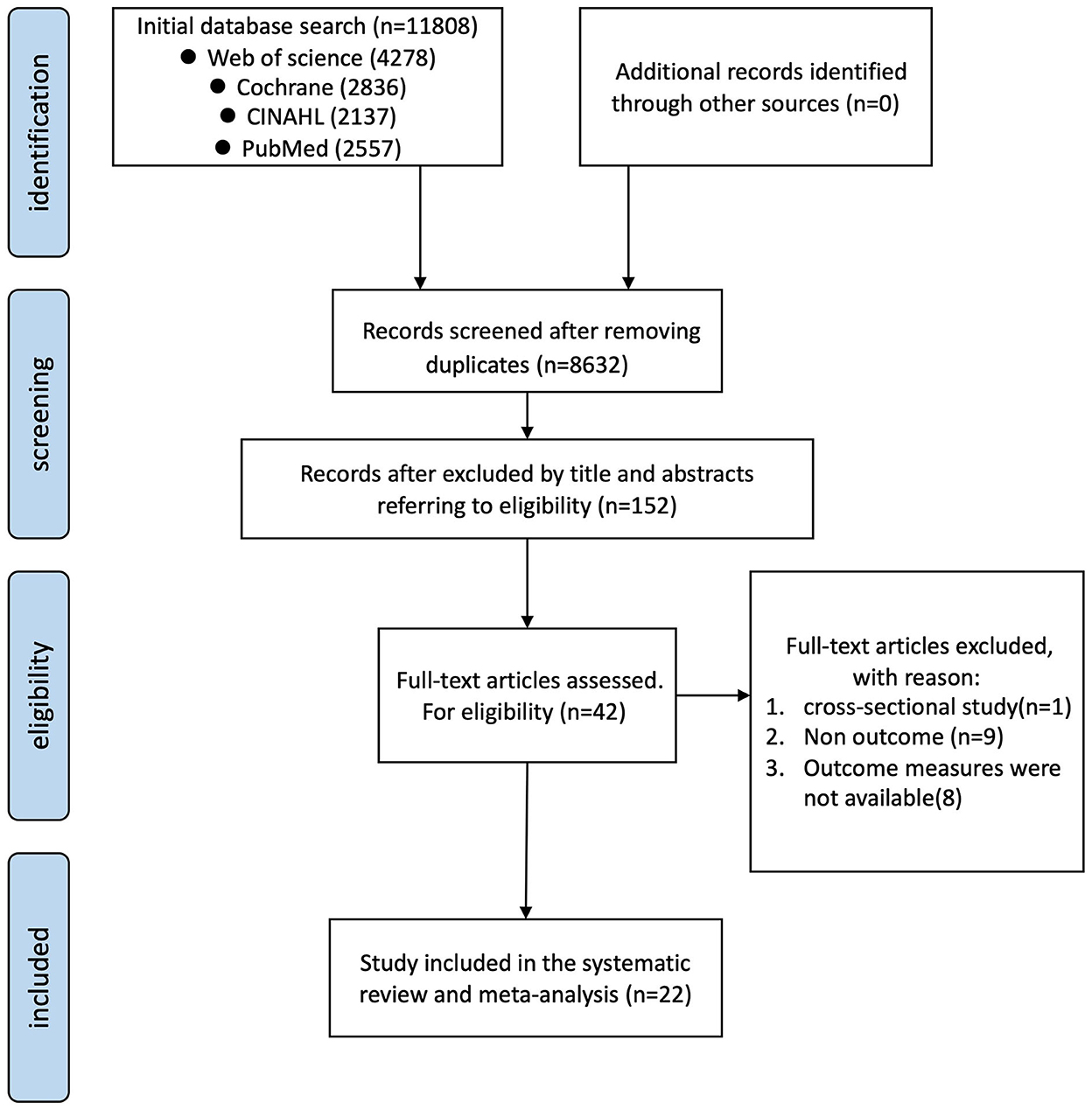
Analysis of the four databases yielded 11,808 results. Among others, 8,632 titles and abstracts were screened to remove duplicates. Figure 1 illustrates the number of articles screened and those that met the inclusion criteria. One study was excluded by consensus due to high variability within the participants' group (42). Finally, 22 studies were included in the meta-analysis, yielding a total of 30 interventions (43–64) (Figure 1).
3.2. Participants
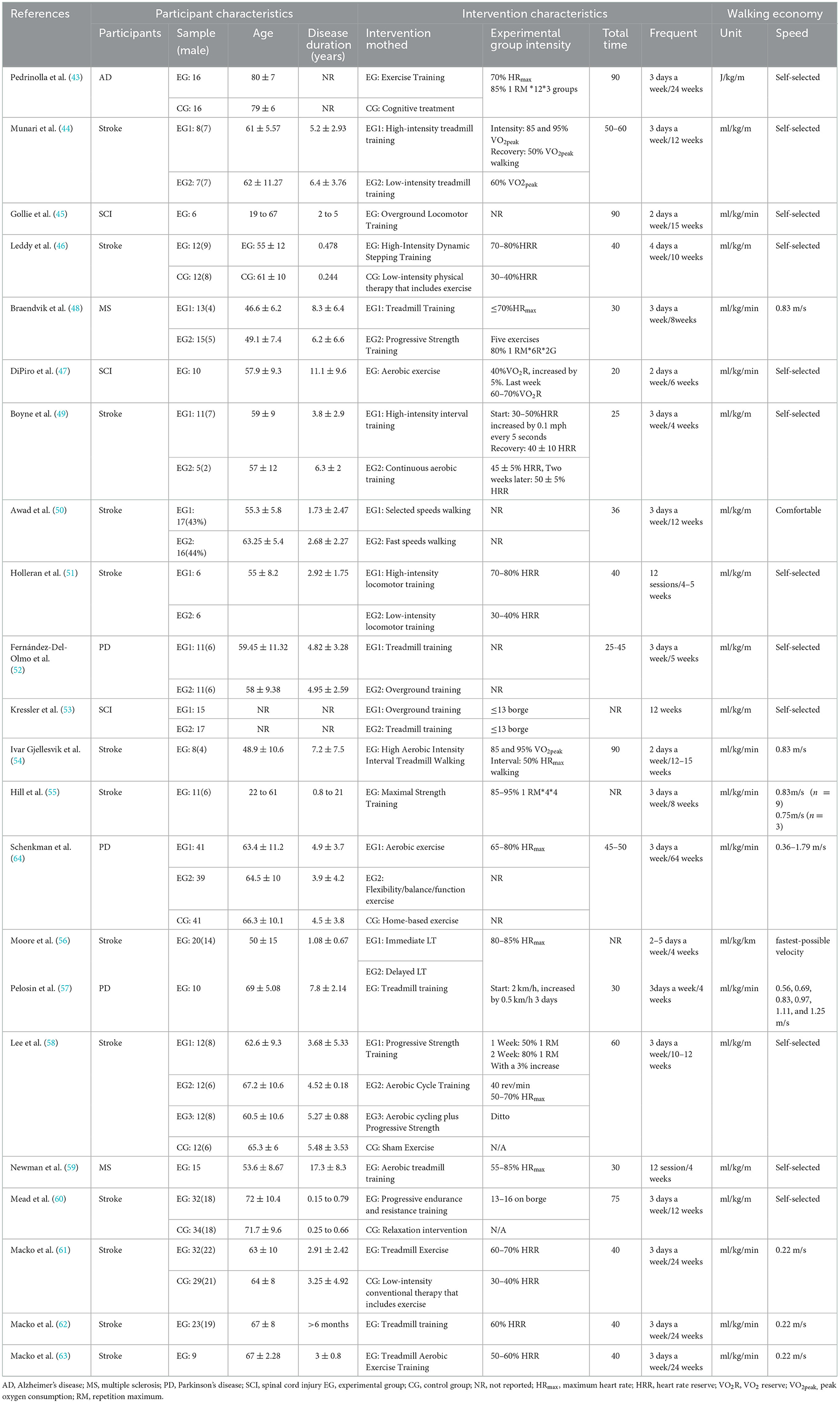
A total of 612 participants with chronic neurological disorders were included for quantitative analysis, with a mean age of 60.76 ± 8.62. Table 1 presents the participants' characteristics of all included studies. Studies involving patients with stroke (n = 13), AD (n = 1), PD (n = 3), MS (65), and SCI (n = 3) were determined. The sample size for each study ranged from 6 to 66. All included studies were supervised, non-home-based clinical intervention trials (Table 1).
3.3. Interventions
Table 1 shows the exercise details for each study. Among the studies included in the meta-analysis, the interventions recorded included IT (n = 3), RT (n = 3), ET (n = 11), ERT (n = 3), and OIMT (n = 11). The length of intervention ranged from 4 to 64 weeks. The intervention groups in all experiments were based on supervised, non-home exercise. The control group in the six RCT studies used cognitive rehabilitation or usual care (43, 46, 58, 60, 61, 64). Four RCT studies used a usual care control group that contained exercise intervention (46). Table 1 shows the specific intervention intensity and details.
Adverse events were reported in four of the 22 studies (46, 48, 60, 64). They included non-injurious falls, joint pain, and abrasions, but no serious adverse time was recorded. Moreover, these effects were not significantly different between the control and experimental groups. One study reported a fall that occurred outside of the session.
3.4. Measurements
The WE measurement primarily involved relative intensity (n = 14) and absolute intensity (n = 8). All incorporated energy costs or WE were collected using oxygen uptake data obtained directly from indirect thermometry and further processed using body weight or speed. WE measurements under relative intensity measurements were based on self-selected or subject-perceived comfortable speeds, and WE under absolute intensity measurements was in the range of 0.22–1.25 m/s. Most studies were allowed to allow participants to use handrails while walking, or to use other assistance, with one study using 40% weight support in the intervention (56).
3.5. Quality assessment
The mean methodological quality score of the 22 included studies was 6.62 ± 1.46. Most studies were of moderate quality, and no studies were rated as low quality (<4). Because most studies used a before-and-after control design, a significant portion of the sample could not meet the requirements for concealed allocation and blinding. No studies were excluded because of methodological quality (Table 2).
3.6. Meta-analysis
A total of 22 studies were included in the meta-analysis. Analysis of the overall pooled results revealed small heterogeneity, with a small but beneficial effect of exercise on WE (g = −0.352, 95% CI: −0.625 to −0.078, P = 0.012, I2 = 43.301%) (Figure 1). Subgroup analyses were conducted according to the type of intervention in the control group. Pooled results from N-RCTs without controls showed a small effect size improvement in WE after the exercise intervention compared with before intervention (g = −0.474, 95% CI: −0.636 to −0.311, P < 0.001, I2 = 26.009%). Ten interventions from six RCT studies showed no significant beneficial effect of exercise training on WE compared with controls (g = −0.192, 95% CI: −0.451 to 0.067, P = 0.146, I2 = 59.349%) (Figure 2).
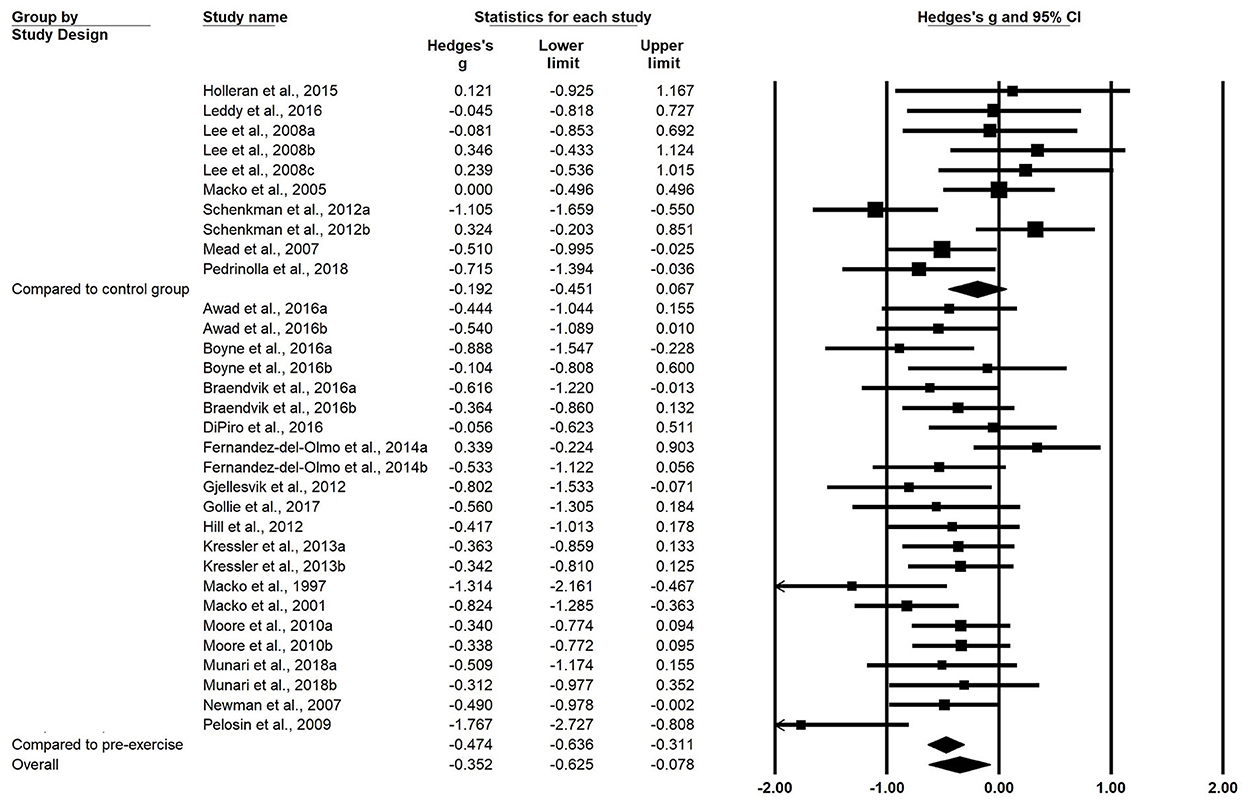
Figure 2. Forest plots of effect sizes and 95% CIs for changes in walking economy after exercise intervention. The superscript numbers refer to different exercise programs assessed in the same study. CI, confidence interval.
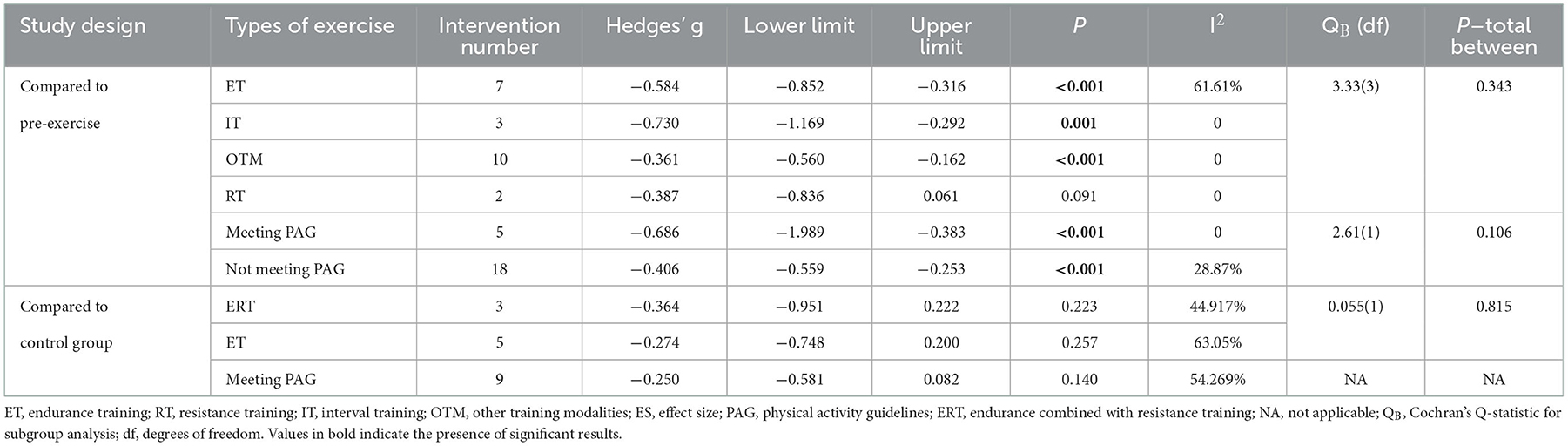
Subgroup analyses based on the training type revealed that among the included N-RCT studies, nine interventions from seven studies investigating ET (n = 7) intervention programs (g = −0.584, 95% CI = −852 to −0.316, P < 0.001) and IT (n = 3) programs (g = −0.730, 95% CI = −1.169 to −0.292, P = 0.001) had moderate effects on WE. OTM (g = −0.361, 95% CI = −0.560 to −0.162, P < 0.001) showed a small but beneficial effect on WE.
Three RCTs used ERT as an intervention and five RCTs used ET, none of which resulted in a significant beneficial effect on WE. Other RCTs with exercise interventions were not analyzed in subgroups because the number of aggregates was less than 2 (Table 3). In addition, studies that achieved PAG showed greater improvement in WE compared with pre-exercise, but there was no significant difference between studies that achieved and did not achieve it. In addition, studies that achieved PAG did not show improvement in WE compared with controls.
3.7. Publication bias and sensitivity analysis
To identify likely publication bias, funnel plots were generated for effect size and standard error. The funnel plots showed that the funnel plots were largely symmetric among the included N-RCT studies. However, studies with disproportionality in RCTs with control groups as a control group were generally located to the right of the variance. One study required adjustments using Duval and Tweedie's trim and fill correction to produce a symmetrical funnel plot around Hedge's g. The correction shifted the overall effect size in the left direction but did not change the main results, although it exhibited a significant trend (Figure 3).
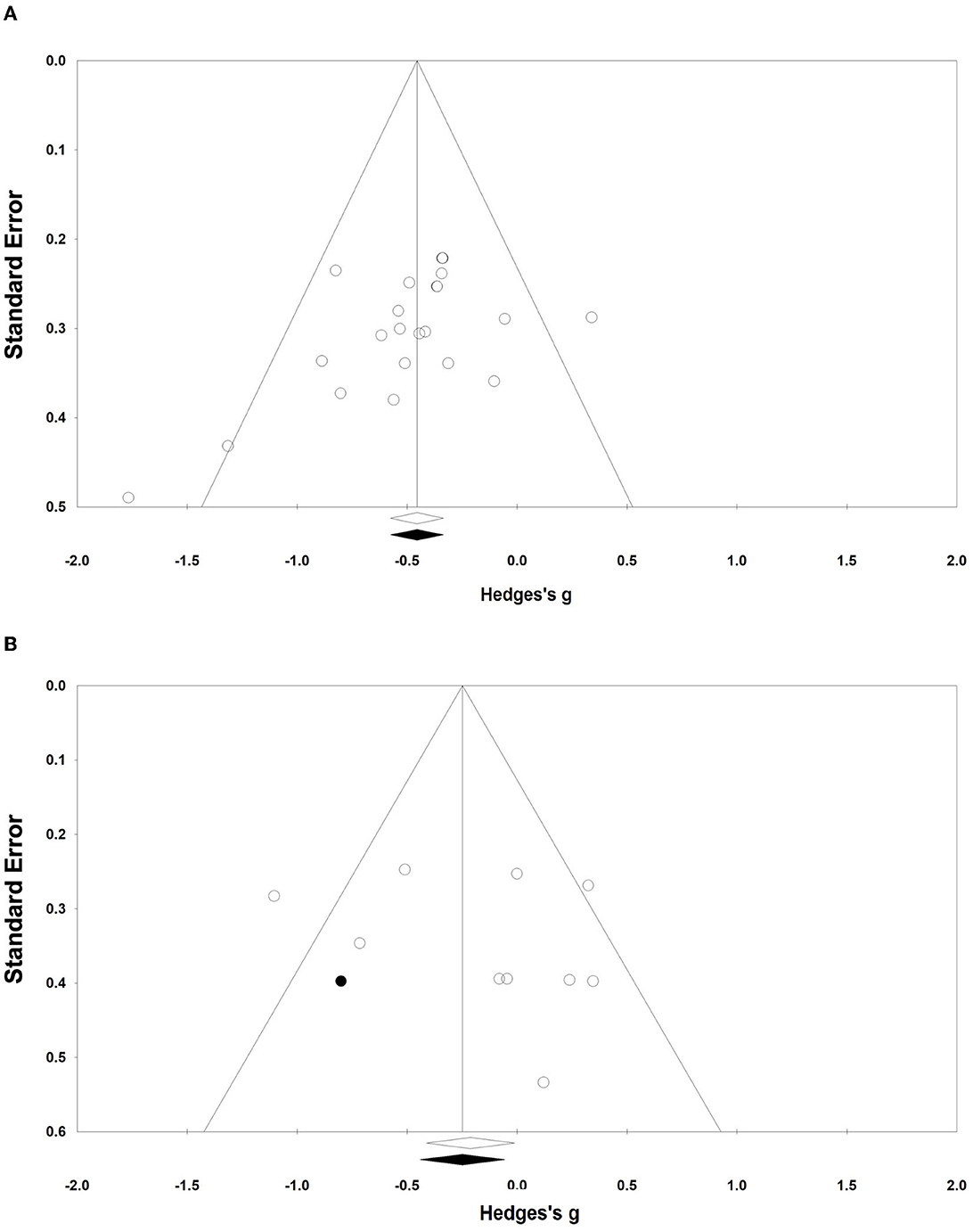
Figure 3. Funnel plots of publication bias. (A, B) Funnel plots (A, B) represent the publication bias of the N-RCT and the RCT, respectively.
Sensitivity analysis conducted by excluding any of all cohorts from the meta-analysis showed that the estimated effects were within the 95% CI of the mean ES in outcomes. This suggested that the results of the meta-analysis did not significantly change after the removal of any one cohort.
4. Discussion
The main objective of this systematic evaluation was to determine the exercise therapies that are used to improve WE in patients with CNCs. We found that exercise improved WE compared with either pre-exercise or non-exercise patients. However, exercise was not more beneficial for WE compared with the control group.
Exercise improves the oxygen cost while walking compared with pre-intervention or no-exercise controls. Several mechanisms may explain the observed results from biomechanics and neuroscience perspectives. In some patients, the improvement in WE is likely related to the biomechanical factors of walking gait because many studies have found an increase in stride length, step length, and a decrease in asymmetrical rows in the affected limb (66). This demonstrates an improvement in the functional capacity of the patient (61). Meanwhile, exercise has been shown to improve the pull reflex in the hamstrings of the lower limbs, and the adaptive responses resulting from these exercises may enhance locomotion, especially the biomechanical efficiency of gait (62). In previous studies, improvements in neurological function in patients with motor stroke were reported, following exercise intervention. Promoting brain plasticity and compensatory activation through high-intensity step training may be a neural mechanism for improving walking gait by stimulating the activation of the subcortical and cortical networks in post-stroke patients (67, 68). For some patients with AD, there is evidence that exercise enhances neuronal and vascular plasticity and improves their pathophysiology (69).
The results of the meta-analysis also showed that higher-intensity exercise did not significantly improve WE compared with the control group. Through a review, we hypothesize that the non-improved outcome may be related to the exercise pattern and the duration of the intervention. Some studies have used usual care in a control group with an exercise intensity of 30–40% heart rate reserve or walking on a treadmill (46, 61). Two of these studies observed significant temporal changes in WE in both the control and intervention groups that lasted for 10 weeks under supervision, that is, the intervention significantly improved WE in the exercise and control groups (46, 61). This result suggests that treadmill-based gait training may improve WE. The previous meta-analysis showed that low-intensity, prolonged treadmill exercise had the greatest benefit on functional impairment in patients with stroke (70). Shulman et al. compared three different intensities of physical activity in patients with PD and found significant differences in fitness and muscle strengthening between the groups, not in gait function. Even lower intensities were superior to higher intensities in some respects (71). It has been suggested that gait training interventions may preferentially increase the oxygen cost of transport instead of enhancing maximal oxygen consumption or lactate thresholds (18). A study by Macko et al. found significantly higher VO2peak in the intervention group compared with the usual care. This is consistent with a previous review that found that high-intensity exercise improves patients' VO2peak (72). Prioritizing WE improvement is an important approach because patients with CNCs have higher energy costs than healthy individuals. In addition, the duration of the intervention was found to affect outcomes. The only study that yielded beneficial effects administered the intervention for up to 16 months. In conclusion, we hypothesize that both higher-intensity and lower-intensity treadmill training can improve WE, but this would take longer. Notably, relaxation and cognitive interventions were used in the control groups of both studies. The results showed a significant improvement in WE in the exercise group compared with the control group (43, 60). This further strengthens our point.
In the study by Lee et al., passive leg cycling resistance training did not significantly improve either VO2peak or WE (58). Therefore, differences in exercise modality were considered. Previous studies have found no significant difference in VO2peak between cycling and treadmill exercise in patients with stroke (73). Moreover, they reported that the choice of exercise modality depended on individual ability and preference. However, the application of cycling to improve gait should be applied with caution because gait is a complex sensorimotor function, and walking and strength-oriented lower extremity therapies are more beneficial to walking ability than cycling (74). A meta-regression based on walking ability in patients with stroke also showed that traditional seated aerobic exercise was unlikely to cause meaningful improvements in walking function (75).
In further analyses, we performed a subgroup analysis to determine the effect of different training types on WE. For ET, pooled results from the N-RCT trials showed a moderate effect on WE. However, it did not have significant benefits compared with conventional rehabilitation (46, 61, 64). About the reviewed results, it does not appear that these two types of training had better effects on WE compared with conventional rehabilitation. Therefore, the effect of ET on WE should be viewed with caution. Compared with other types of exercises, high-intensity IT intervention had the most significant improvement on WE. Unfortunately, there are no higher-quality randomized controlled trials to validate this result. One study found that high-intensity IT had a greater effect on patients' cardiorespiratory fitness than high-intensity exercise alone and sustained aerobic training (10, 12, 76). Due to the increased demand for oxygen during exercise training, the reserves are increased VO2, allowing patients to reach higher intensities or greater VO2 after training (44). A meta-analysis showed that high-intensity IT induced good adaptations in older adults in terms of cardiorespiratory fitness, body mass, muscle strength, cardiac contractility, mitochondrial citrate, enzyme activity, and lower blood triglyceride and glucose levels (31). Currently, it is not known which type of exercise is more effective in improving WE. However, high-intensity IT has been recognized in many reviews for promoting other physical functions in patients with CNCs (10, 12, 31, 32, 77).
The ERT and OTM interventions showed a small-to-moderate effect on WE. In comparison, previous reviews found that ERT could be the most effective among these interventions in improving cardiopulmonary function in patients with stroke (14). The ASCM guidelines also state that aerobic and resistance exercise is more effective than either form of training in counteracting the adverse effects of a sedentary lifestyle on a healthy cardiovascular system and skeletal muscle function (78). However, the pooled results of the only two RCTs do not confirm that ERT is best for improving WE. Previous systematic evaluations and meta-analyses reported that resistance exercise training had positive effects on overall muscle strength, fatigue, balance, and quality of life in patients with CNCs (11, 79, 80). However, our pooled results only showed a trend of improvement in WE with RT. The confounding effects of our outcome variables make the interpretation difficult. The current evidence only weakly supports the benefit of exercise on WE. In future, continued high-quality randomized controlled trials should be performed to provide more compelling evidence.
Overall, most previous investigations used walking speed, distance, and VO2peak as indicators of cardiorespiratory fitness. This is the first systematic evaluation and meta-analysis that focused on WE and provides valuable ideas for strengthening the cardiorespiratory capacity of patients with CNCs.
4.1. Limitations
Some limitations exist in this study. First, a few high-quality RCTs were included. Moreover, most of them were single-arm studies based on before-and-after controls, which may lead to type II errors (e.g., false-negative results) in subgroup analyses. There was some publication bias in the included RCTs, with the pooled results moving in the direction of being more beneficial to WE, although still not constituting significance, which also suggests that there may still be better interventions than traditional rehabilitation. Second, several exploratory preliminary studies with small sample sizes (some below 10 cases) were included in the analysis. Third, while the evidence base was overall of good quality with most studies being of moderate-to-low risk of bias, future research should seek to improve certain points. Of the included studies, most did not use blinding of participants and experimenters based on limitations of the study design. Blinded assignment and assessment of outcomes could limit bias associated with self-report measures in exercise interventions. Finally, the presence of confounding variables in the studies prevented more detailed subgroup analyses, and some results with high heterogeneity could not be interpreted. Therefore, the results of our study should be referred to with caution.
5. Conclusion
This systematic review and meta-analysis showed that exercise training improved WE. Notably, the effect of exercise interventions on WE may be the same as usual care appeared to be. Gait-based low-intensity usual care also improved WE. However, it remains to be determined whether there is a more effective means of training that will result in a higher improvement in WE. The prevailing limited evidence suggests that high-intensity IT may be more beneficial for WE compared with other forms of exercise. There is an urgent need for future larger and high-quality studies to find an optimal training modality to improve the cost of walking ability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BL, QF, and ZR designed the research. BL and JY conducted the searches and screening process. BL and FH completed the full-text screening. BL, JY, and FH assessed methodological quality. BL, FY, and FH extracted the data, which were checked by JW. BL performed the statistical analysis and interpreted it. BL wrote the manuscript with critical input from ZR and QF. All authors contributed to the article and approved the submitted version.
Funding
The article was supported by the National Natural Science Foundation of China (NSFC) [Grant No.: 11002036]; the Research Foundation for Young Teachers of Shenzhen University [Grant No.: QNJS0274]; the High-level Scientific Research Foundation for the Introduction of Talent of Shenzhen University [Grant No.: RC00228]; the Natural Science Featured Innovation Projects in Ordinary Universities in Guangdong Province [Grant No.: 2021KTSCX297]; and the Scientific Research Platform and Project of Colleges and Universities of the Education Department of Guangdong Province (2022ZDZX2087).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Organization WH. The World Health Report 2007: A Safer Future: Global Public Health Security in the 21st century. World Health Organization. (2007).
2. Bhatt JM. The Epidemiology of Neuromuscular Diseases. Neurol Clin. (2016) 34:999–1021. doi: 10.1016/j.ncl.2016.06.017
3. Mulligan HF, Hale LA, Whitehead L, Baxter GD. Barriers to physical activity for people with long-term neurological conditions: a review study. Adapt Phys Act Q. (2016) 29:243–65. doi: 10.1123/apaq.29.3.243
4. Cahn DA, Sullivan EV, Shear PK, Pfefferbaum A, Heit G, Silverberg G. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson's disease. Arch Clin Neuropsychol. (1998) 13:575–83. doi: 10.1016/S0887-6177(98)00024-9
5. Månsson E, Lexell J. Performance of activities of daily living in multiple sclerosis. Disabil Rehabil. (2004) 26:576–85. doi: 10.1080/09638280410001684587
6. Mercier L, Audet T, Hebert R, Rochette A, Dubois MF. Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke. (2007) 32:2602–8. doi: 10.1161/hs1101.098154
7. Mang CS, Peters S. Advancing motor rehabilitation for adults with chronic neurological conditions through increased involvement of kinesiologists: a perspective review. BMC Sports Sci Med Rehabil. (2021) 13:132. doi: 10.1186/s13102-021-00361-6
8. Nijkrake MJ, Keus SHJ, Kalf JG, Sturkenboom IHWM, Munneke M, Kappelle AC, et al. Allied health care interventions and complementary therapies in Parkinson's disease. Parkinsonism Relat Disord. (2008) 13:S488–94. doi: 10.1016/S1353-8020(08)70054-3
9. Salari N, Hayati A, Kazeminia M, Rahmani A, Mohammadi M, Fatahian R, et al. The effect of exercise on balance in patients with stroke, Parkinson, and multiple sclerosis: a systematic review and meta-analysis of clinical trials. Neurol Sci. (2022) 43:167–85. doi: 10.1007/s10072-021-05689-y
10. Anjos JM, Neto MG, dos Santos FS, Almeida K de O, Bocchi EA, Lima Bitar Y de S, et al. The impact Of high-intensity interval training On functioning And health-related quality Of life In post-stroke patients: A systematic review With meta-analysis. Clin Rehabil. (2022) 36:726–39. doi: 10.1177/02692155221087082
11. Farrell JW, Merkas J, Pilutti LA. The effect of exercise training on gait, balance, and physical fitness asymmetries in persons with chronic neurological conditions: a systematic review of randomized controlled trials. Front Physiol. (2020) 11:585765. doi: 10.3389/fphys.2020.585765
12. Luo L, Meng H, Wang Z, Zhu S, Yuan S, Wang Y, et al. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: A systematic review and meta-analysis. Ann Phys Rehabil Med. (2019) 63:59–68. doi: 10.1016/j.rehab.2019.07.006
13. Eng JJ, Reime B. Exercise for depressive symptoms in stroke patients: a systematic review and meta-analysis. Clin Rehabil. (2014) 28:731–9. doi: 10.1177/0269215514523631
14. Marsden DL, Dunn A, Callister R, Levi CR, Spratt NJ. Characteristics of exercise training interventions to improve cardiorespiratory fitness after stroke: a systematic review with meta-analysis. Neurorehabil Neural Repair. (2013) 27:775–88. doi: 10.1177/1545968313496329
15. Edwards T, Michelsen AS, Fakolade AO, Dalgas U, Pilutti LA. Exercise training improves participation in persons with multiple sclerosis: A systematic review and meta-analysis. J Sport Health Sci. (2021) 11:393–402. doi: 10.1016/j.jshs.2021.07.007
16. Christiansen CL, Schenkman ML, McFann K, Wolfe P, Kohrt WM. Walking economy in people with Parkinson's disease. Mov Disord. (2009) 24:1481–7. doi: 10.1002/mds.22621
17. Reisman DS, Rudolph KS, Farquhar WB. Influence of speed on walking economy poststroke. Neurorehabil Neural Repair. (2009) 23:529–34. doi: 10.1177/1545968308328732
18. Reisman DS, Binder-MacLeod S, Farquhar WB. Changes in metabolic cost of transport following locomotor training poststroke. Top Stroke Rehabil. (2013) 20:161–70. doi: 10.1310/tsr2002-161
19. Das Gupta S, Bobbert MF, Kistemaker DA. The metabolic cost of walking in healthy young and older adults – a systematic review and meta analysis. Sci Rep. (2019) 9:9956. doi: 10.1038/s41598-019-45602-4
20. Bernardi M, Macaluso A, Sproviero E, Castellano V, Coratella D, Felici F, et al. Cost of walking and locomotor impairment. J Electromyogr Kinesiol. (2002) 9:149–57. doi: 10.1016/S1050-6411(98)00046-7
21. Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. (2002) 9:207–31. doi: 10.1016/S0966-6362(99)00009-0
22. Platts MM, Rafferty D, Paul L. Metabolic cost of over ground gait in younger stroke patients and healthy controls. Med Sci Sports Exerc. (2006) 38:1041–6. doi: 10.1249/01.mss.0000222829.34111.9c
23. Dougherty RJ, Ramachandran J, Liu F, An Y, Wanigatunga AA, Tian Q, et al. Association of walking energetics with amyloid beta status: Findings from the baltimore longitudinal study of aging. Alzheimers Dement Diagn Assess Amp Dis Monit. (2021) 13:e12228. doi: 10.1002/dad2.12228
24. Chung LH, Angelo J, van Emmerik REA, Kent JA. Energy cost of walking, symptomatic fatigue and perceived exertion in persons with multiple sclerosis. Gait Posture. (2016) 48:215–9. doi: 10.1016/j.gaitpost.2016.05.005
25. Dearwater SR, LaPorte RE, Robertson RJ, Brenes G, Adams LL, Becker D. Activity in the spinal cord-injured patient: an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc. (1986) 18:541–4. doi: 10.1249/00005768-198610000-00008
26. Finley JM, Bastian AJ. Associations between foot placement asymmetries and metabolic cost of transport in hemiparetic gait. Neurorehabil Neural Repair. (2016) 31:168–77. doi: 10.1177/1545968316675428
27. Reitz C, Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. (2014) 88:640–51. doi: 10.1016/j.bcp.2013.12.024
28. Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, et al. Physiotherapy intervention in Parkinson's disease: systematic review and meta-analysis. BMJ. (2012) 345:e5004. doi: 10.1136/bmj.e5004
29. Kwok T, Lo RS, Wong E, Wai-Kwong T, Mok V, Kai-Sing W. Quality of life of stroke survivors: a 1-year follow-up study. Arch Phys Med Rehabil. (2006) 87:1177–82. doi: 10.1016/j.apmr.2006.05.015
30. Brola W, Sobolewski P, Fudala M, Flaga S, Jantarski K, Ryglewicz D, et al. Self-reported quality of life in multiple sclerosis patients: preliminary results based on the Polish MS registry. Patient Prefer Adherence. (2016) 10:1647–56. doi: 10.2147/PPA.S109520
31. Wu ZJ, Wang ZY, Gao HE, Zhou XF Li FH. Impact of high-intensity interval training on cardiorespiratory fitness, body composition, physical fitness, and metabolic parameters in older adults: A meta-analysis of randomized controlled trials. Exp Gerontol. (2021) 150:111345. doi: 10.1016/j.exger.2021.111345
32. Poon ETC, Wongpipit W, Ho RST, Wong SHS. Interval training versus moderate-intensity continuous training for cardiorespiratory fitness improvements in middle-aged and older adults: a systematic review and meta-analysis. J Sports Sci. (2021) 39:1996–2005. doi: 10.1080/02640414.2021.1912453
33. Aburub A, Ledger SJ, Sim J, Hunter SM. Cardiopulmonary function and aerobic exercise in Parkinson's: a systematic review of the literature. Mov Disord Clin Pract. (2020) 7:599–606. doi: 10.1002/mdc3.13011
34. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83:713–21. doi: 10.1093/ptj/83.8.713
35. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) 55:129–33. doi: 10.1016/S0004-9514(09)70043-1
36. Ferguson B. ACSM's guidelines for exercise testing and prescription 9th Ed 2014. J Can Chiropr Assoc. (2014) 58:328.
37. MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. (2016) 595:2915–30. doi: 10.1113/JP273196
38. Physical Activity Guidelines for Americans | health.gov. Available online at: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/previous-guidelines/2008-physical-activity-guidelines (accessed September 29, 2022).
39. Adamson BC, Ensari I, Motl RW. Effect of Exercise on Depressive Symptoms in Adults With Neurologic Disorders: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. (2015) 96:1329–38. doi: 10.1016/j.apmr.2015.01.005
40. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge. (2013). doi: 10.4324/9780203771587
41. Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis. In: Publication bias in meta-analysis: Prevention, assessment and adjustments. John Wiley & Sons, Ltd. (2005) p. 1–7. doi: 10.1002/0470870168.ch1
42. da Cunha IT, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: A randomized controlled pilot study. Arch Phys Med Rehabil. (2002) 83:1258–65. doi: 10.1053/apmr.2002.34267
43. Pedrinolla A, Venturelli M, Fonte C, Munari D, Benetti MV, Rudi D, et al. Exercise training on locomotion in patients with alzheimer's disease: a feasibility study. J Alzheimers Dis. (2018) 61:1599–608. doi: 10.3233/JAD-170625
44. Munari D, Pedrinolla A, Smania N, Picelli A, Gandolfi M, Saltuari L, et al. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med. (2018) 54:408–18. doi: 10.23736/S1973-9087.16.04224-6
45. Gollie JM, Guccione AA, Panza GS, Jo PY, Herrick JE. Effects of overground locomotor training on walking performance in chronic cervical motor incomplete spinal cord injury: a pilot study. Arch Phys Med Rehabil. (2017) 98:1119–25. doi: 10.1016/j.apmr.2016.10.022
46. Leddy AL, Connolly M, Holleran CL, Hennessy PW, Woodward J, Arena RA, et al. Alterations in aerobic exercise performance and gait economy following high-intensity dynamic stepping training in persons with subacute stroke. J Neurol Phys Ther. (2016) 40:239–48. doi: 10.1097/NPT.0000000000000147
47. DiPiro ND, Embry AE, Fritz SL, Middleton A, Krause JS, Gregory CM. Effects of aerobic exercise training on fitness and walking-related outcomes in ambulatory individuals with chronic incomplete spinal cord injury. Spinal Cord. (2016) 54:675–81. doi: 10.1038/sc.2015.212
48. Braendvik SM, Koret T, Helbostad JL, Lorås H, Bråthen G, Hovdal HO, et al. Treadmill training or progressive strength training to improve walking in people with multiple sclerosis? A randomized parallel group trial. Physiother Res Int. (2016) 21:228–36. doi: 10.1002/pri.1636
49. Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Rockwell B, et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther. (2016) 96:1533–44. doi: 10.2522/ptj.20150277
50. Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing the cost of transport and increasing walking distance after stroke. Neurorehabil Neural Repair. (2016) 30:661–70. doi: 10.1177/1545968315619696
51. Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther. (2015) 39:95–102. doi: 10.1097/NPT.0000000000000077
52. Fernández-Del-Olmo MA, Sanchez JA, Bello O, Lopez-Alonso V, Márquez G, Morenilla L, et al. Treadmill training improves overground walking economy in Parkinson's disease: a randomized, controlled pilot study. Front Neurol. (2014) 5:191. doi: 10.3389/fneur.2014.00191
53. Kressler J, Nash MS, Burns PA, Field-Fote EC. Metabolic Responses to 4 Different Body Weight-Supported Locomotor Training Approaches in Persons With Incomplete Spinal Cord Injury. Arch Phys Med Rehabil. (2013) 94:1436–42. doi: 10.1016/j.apmr.2013.02.018
54. Ivar Gjellesvik T, Brurok B, Hoff J, TørhT, Helgerud J. Effect of high aerobic intensity interval treadmill walking in people with chronic stroke: a pilot study with one year follow-up top. Stroke Rehabil. (2012) 19:353–60. doi: 10.1310/tsr1904-353
55. Hill TR, Gjellesvik TI, Moen PMR, TørhT, Fimland MS, Helgerud J, et al. Maximal strength training enhances strength and functional performance in chronic stroke survivors. Am J Phys Med Rehabil. (2012) 91:393–400. doi: 10.1097/PHM.0b013e31824ad5b8
56. Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. (2010) 41:129–35. doi: 10.1161/STROKEAHA.109.563247
57. Pelosin E, Faelli E, Lofrano F, Avanzino L, Marinelli L, Bove M, et al. Effects of treadmill training on walking economy in Parkinson's disease: a pilot study. Neurol Sci. (2009) 30:499–504. doi: 10.1007/s10072-009-0141-8
58. Lee MJ, Kilbreath SL, Singh MF, Zeman B, Lord SR, Raymond J, et al. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise–controlled study. J Am Geriatr Soc. (2008) 56:976–85. doi: 10.1111/j.1532-5415.2008.01707.x
59. Newman MA, Dawes H, van den Berg M, Wade DT, Burridge J, Izadi H. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: A pilot study. Mult Scler J. (2007) 13:113–9. doi: 10.1177/1352458506071169
60. Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: A randomized trial of exercise or relaxation. J Am Geriatr Soc. (2007) 55:892–9. doi: 10.1111/j.1532-5415.2007.01185.x
61. Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke. Stroke. (2005) 36:2206–11. doi: 10.1161/01.STR.0000181076.91805.89
62. Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. (2001) 82:879–84. doi: 10.1053/apmr.2001.23853
63. Macko RF, DeSouza CA, Tretter LD, Silver KH, Smith GV, Anderson PA, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. Stroke. (1997) 28:326–30. doi: 10.1161/01.STR.28.2.326
64. Schenkman M, Hall DA, Barón AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage parkinson disease: a 16-month randomized controlled trial. Phys Ther. (2012) 92:1395–410. doi: 10.2522/ptj.20110472
65. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-−2019 update: a report from the American heart association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
66. Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. (2014) 29:416–23. doi: 10.1177/1545968314552528
67. Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke. Stroke. (2008) 39:3341–50. doi: 10.1161/STROKEAHA.108.527531
68. Enzinger C, Dawes H, Johansen-Berg H, Wade D, Bogdanovic M, Collett J, et al. Brain activity changes associated with treadmill training after stroke. Stroke. (2009) 40:2460–7. doi: 10.1161/STROKEAHA.109.550053
69. Lange-Asschenfeldt C, Kojda G. Alzheimer's disease, cerebrovascular dysfunction and the benefits of exercise: From vessels to neurons. Exp Gerontol. (2008) 43:499–504. doi: 10.1016/j.exger.2008.04.002
70. Abbasian S, Rastegar Mm M. Is the intensity or duration of treadmill training important for stroke patients? A meta-analysis. J Stroke Cerebrovasc Dis. (2018) 27:32–43. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.061
71. Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. (2012) 70:183. doi: 10.1001/jamaneurol.2013.646
72. Saunders DH, Sanderson M, Hayes S, Kilrane M, Greig CA, Brazzelli M, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. (2016) 2016:CD003316. doi: 10.1002/14651858.CD003316.pub6
73. Pang MYC, Charlesworth SA, Lau RWK, Chung RCK. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis. (2013) 35:7–22. doi: 10.1159/000346075
74. Veldema J, Jansen P. Ergometer training in stroke rehabilitation: systematic review and meta-analysis. Arch Phys Med Rehabil. (2020) 101:674–89. doi: 10.1016/j.apmr.2019.09.017
75. Boyne P, Welge J, Kissela B, Dunning K. Factors influencing the efficacy of aerobic exercise for improving fitness and walking capacity after stroke. Arch Phys Med Rehabil. (2016) 98:581–95. doi: 10.1016/j.apmr.2016.08.484
76. Schootemeijer S, van der Kolk NM, Bloem BR, de Vries NM. Current perspectives on aerobic exercise in people with Parkinson's disease. Neurotherapeutics. (2020) 17:1418–33. doi: 10.1007/s13311-020-00904-8
77. Fernández-Rodríguez R, Álvarez-Bueno C, Martínez-Ortega IA, Martínez-Vizcaíno V, Mesas AE, Notario-Pacheco B. Immediate effect of high-intensity exercise on brain-derived neurotrophic factor in healthy young adults: A systematic review and meta-analysis. J Sport Health Sci. (2021) 11:367–75. doi: 10.1016/j.jshs.2021.08.004
78. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. Exercise and Physical Activity for Older Adults. Med Sci Sports Exerc. (2009) 41:1510. doi: 10.1249/MSS.0b013e3181a0c95c
79. Cruickshank TM, Reyes AR, Ziman MR. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine. (2015) 94:e411. doi: 10.1097/MD.0000000000000411
Keywords: exercise, walking, energy cost, chronic neurological conditions, rehabilitation
Citation: Liu B, Yu J, Fan Q, Hao F, Wu J, Xiao W, Yu F and Ren Z (2023) The effect of exercise on walking economy in patients with chronic neurological conditions: A systematic review and meta-analysis. Front. Neurol. 13:1074521. doi: 10.3389/fneur.2022.1074521
Received: 20 October 2022; Accepted: 15 December 2022;
Published: 11 January 2023.
Edited by:
Brian M. Sandroff, Kessler Foundation, United StatesReviewed by:
Xiaoyu Chen, Shanghai University of Medicine and Health Sciences, ChinaFatih Özden, Mugla University, Turkey
Copyright © 2023 Liu, Yu, Fan, Hao, Wu, Xiao, Yu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanbing Ren,  cnpiQHN6dS5lZHUuY24=
cnpiQHN6dS5lZHUuY24=
 Bowen Liu
Bowen Liu Jingxuan Yu1
Jingxuan Yu1 Zhanbing Ren
Zhanbing Ren