- 1Department of Neurology, Shanghai Tongji Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Department of Neurology and Neurological Rehabilitation, Shanghai YangZhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center), School of Medicine, Tongji University, Shanghai, China
Being a major component of the midbrain locomotion region, the pedunculopontine nucleus (PPN) is known to have various connections with the basal ganglia, the cerebral cortex, thalamus, and motor regions of the brainstem and spinal cord. Functionally, the PPN is associated with muscle tone control and locomotion modulation, including motor initiation, rhythm and speed. In addition to its motor functions, the PPN also contribute to level of arousal, attention, memory and learning. Recent studies have revealed neuropathologic deficits in the PPN in both patients and animal models of dystonia, and deep brain stimulation of the PPN also showed alleviation of axial dystonia in patients of Parkinson's disease. These findings indicate that the PPN might play an important role in the development of dystonia. Moreover, with increasing preclinical evidences showed presence of dystonia-like behaviors, muscle tone changes, impaired cognitive functions and sleep following lesion or neuromodulation of the PPN, it is assumed that the pathological changes of the PPN might contribute to both motor and non-motor manifestations of dystonia. In this review, we aim to summarize the involvement of the PPN in dystonia based on the current preclinical and clinical evidences. Moreover, potential mechanisms for its contributions to the manifestation of dystonia is also discussed base on the dystonia-related basal ganglia-cerebello-thalamo-cortical circuit, providing fundamental insight into the targeting of the PPN for the treatment of dystonia in the future.
Introduction
The pedunculopontine nucleus (PPN) is located in the dorsal pontomesencephalic tegmentum, and it has wide anatomical connections with the central nervous system (1). Together with the cuneiform nucleus, the PPN is involved in the control of locomotion, including motor initiation, rhythm, and speed (2–4). In addition, the neural activity of the PPN has been reported closely associated with muscle tone control, along with its contributions to the level of arousal, attention, memory and learning (5, 6). Dystonia is a movement disorder characterized by abnormal involuntary movements or postures caused by sustained or intermittent muscle contractions, which are often repetitive (7). Pathologic changes of the PPN have been reported in patients of different types of dystonia, and the manifestation of dystonia could be alleviated through DBS of the PPN, reflecting a crucial role of the PPN in the development of dystonia (8–11). So far, increasing preclinical evidences also showed alterations of the muscle tone, locomotion, cognitive functions and sleep following lesion or neuromodulation of the PPN, which mimicked the motor and non-motor manifestations of dystonia (2, 5, 12–14). Moreover, from the anatomical aspects, the PPN has dense connections with the dystonia-related basal ganglia-cerebello-thalamo-cortical circuit, which lays the foundation of the PPN involved in dystonia (15).
Anatomical and functional characteristics of the PPN
The PPN has been identified to be a major component of the mesencephalic locomotion region located in the brainstem. It has been divided into the rostral and caudal part which contain cholinergic, glutamatergic and GABAergic neurons (16). The cholinergic neurons are intermingled with numerous glutamatergic and GABAergic neurons, which are distributed throughout the rostro-caudal extent of the PPN. The GABAergic neurons are mainly located in the rostral part, whereas the glutamatergic neurons are abundantly situated in the caudal part (17). In addition, less than 5% of cholinergic neurons co-release either glutamate or GABA and may possess more complex motor functions (18).
The PPN is surrounded by the superior cerebellar peduncle (medially), the medial lemniscus (laterally), the red nucleus (rostrally), and the laterodorsal tegmental nucleus (LDT, caudally) (1). It has ascending and descending connections with multiple motor areas, including the motor and premotor cortex, basal ganglia, reticular formation, superior colliculus and cranial nerve nuclei (V, VII and XII) (6). The ascending network is involved in motor planning, selection, and sensory motor integration, whereas the descending network functions to directly modulate locomotion and muscle tone (19, 20). In the ascending motor connectome, the PPN has complex anatomical connections with the basal ganglia, which is known to play a crucial role in the central selection of competing alternative actions through direct, indirect, and hyperdirect pathways (21). In particular, GABAergic input from the internal part of the globus pallidus internus (GPi) and the substantia nigra pars reticulata (SNpr) and glutamatergic input from the subthalamic nucleus (STN) primarily project to the PPN (22, 23). Reciprocally, The SNpc and STN are densely innervated by the PPN. Cholinergic and glutamatergic neurons of the PPN form synaptic connections with dopaminergic neurons in the SNpc, which thereafter modulate the dopamine release in the striatum (24, 25). And both glutamatergic and GABAergic fibers project to the STN from the PPN to influence the indirect pathway of excessive movement inhibition (26). Additionally, a recent study reported that the cholinergic and glutamatergic neurons in the PPN could directly project to the striatum and form synaptic connections with medium-sized spiny neurons, cholinergic interneurons, and GABAergic interneurons and modulate their neural activity (19, 27). Of note, the PPN and LDT has been determined as the only external source of cholinergic projection of the striatum (28). Along with the basal ganglia, the PPN could also send cholinergic projection to the cerebellum and activate the deep cerebellar nuclei, while the cerebellum sends reciprocal projections to both cholinergic and glutamatergic neurons in the PPN (2, 29, 30). Since the cerebellum has a vital role in flexible modification of behavior and error-based learning, the PPN acts as an important interface between the cerebellum and basal ganglia which participates in the motor control and cognitive functions (31).
Multiple cortex areas including the motor cortex, pre-motor cortex, somatosensory motor cortex and frontal eye fields send excitatory outputs to the PPN, and the PPN densely innervates the motor cortex, pre-motor cortex and frontal lope, respectively. The PPN might affect the cortical functions of movements, cognitive and sleep through these connections (32). With regard to the thalamus, the PPN can send strong cholinergic projections to the thalamic nuclei, and the ascending reticular activating system connects to the cortex via these cholinergic projections of the PPN to the thalamus, which is involved in the arousal and rapid eye movement sleep (32). In addition, the PPN can send cholinergic output to the thalamic centromedian-parafascicular complex, which in turn connects to multiple cortex areas and allows the PPN participating in modulation of action selection, attention, memory, learning, spatial perception, impulsivity control and decision making (6). Other brain areas, such as the superior and inferior colliculi and periaqueductal gray, also have reciprocal projections with the PPN (4). Meanwhile, descending fibers from the PPN directly feed to the pontine and medullary reticular formation and spinal cord, including the pontine reticular nuclei oralis and caudalis and the gigantocellular reticular nucleus, which might participate in the locomotion and muscle tone control (5, 6) (Figure 1A).
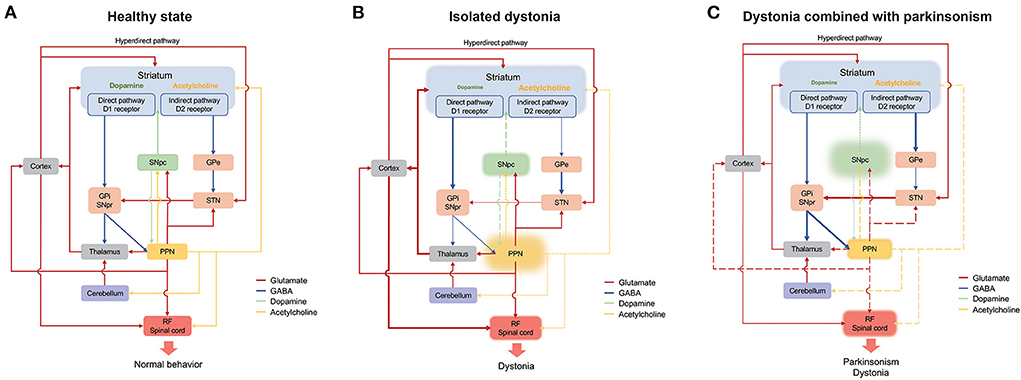
Figure 1. Schematic representation of the connection of the PPN in the basal ganglia-cerebello-thalamo-cortical circuit under normal (A), isolated dystonia (B), and dystonia combined with parkinsonism (C). Thickened and thinned lines represent enhanced and decreased activity, respectively. Dashed lines show primarily loss whereas long dashed lines indicate secondarily loss. Cortico-striatum inputs modulate central selection of competing alternative actions through direct, indirect and hyperdirect pathways. And the inhibitory GABA outputs from GPi and SNpr project to the cortex through the thalamus and eventually participate in motor execution. The PPN has connections with the basal ganglia, the thalamus, cerebellum, and RF/spinal cord, with its cholinergic outputs mainly project to the striatum, the SNpc and the RF/spinal cord. In normal state (A), adequate motor selection of the basal ganglia and the balance of the dopamine and the acetylcholine result in normal behavior. In isolated dystonia (B), the PPN is primarily affected, which mainly involves the loss of cholinergic neurons. The cholinergic deficit of the PPN directly influences the neural activity of the striatum and the RF/spinal cord and indirectly affects the dopamine release in the striatum though SNpc, which result in the disturbance of central selection and the presence of dystonia. In dystonia combined with parkinsonism (C), the dopaminergic system of the SNpc is primarily affected. The deficit of the dopamine release in the striatum results in the overactivity of the indirect pathway, which leads to the presence of parkinsonism. On the other hand, the pathology of the SNpc contributes to the dysfunction of both glutamatergic and cholinergic systems in the PPN, which thereafter directly induces the disinhibition of the RF/spinal cord or indirectly affects the function of the striatum, thalamus and cortex. GABA, γ-aminobutyric acid; GPe, globus pallidus externus; GPi, globus pallidus internus; PPN, pedunculopontine tegmental nucleus; RF, reticular formation; SNpc, substantia nigra pars compact; SNpc, substantia nigra pars reticulata (SNpr); STN, subthalamic nucleus.
Preclinical findings of the involvement of the PPN in dystonia
In preclinical experiments, the relationship between the PPN and dystonia was primarily revealed through the neurochemical and pathological findings in dystonia animal models. Additionally, stereotaxic modulation of the PPN in normal animals also provided evidence of its effect on muscle tone and dystonia manifestation.
Pathologic changes of the PPN in animal models of dystonia
The cholinergic pathology of the PPN was observed in genotypic animal models of dystonia (33, 34). As for mouse models of Dyt1/Tor1a gene mutation, including the Dyt1 knock-in, knockdown, and knockout model, most studies found that there was no overt dystonia manifestation (35). However, in an animal study examining four independent lines of transgenic mice overexpressing the abnormal human torsinA, approximately 40% of the mice presented with abnormal dystonia-like behaviors such as self-clasping and head deviation. Of note, ubiquitin-positive and torsinA-positive perinuclear inclusions were prominently aggregated in the cholinergic neurons of the PPN, whereas no pathologic changes were found in other brain areas, such as the cerebral cortex, hippocampus, striatum, SNpc, and cerebellum (34). Another study has explored the behavior and neurochemical lesions in a dystonia musculorum mutant mouse model. The animal model had severe dystonic movements, motor coordination deficits, and spastic ataxia, which correlated with an increase in acetylcholinesterase activity in the PPN, along with major regions of the basal ganglia (33).
Dystonia and the change in muscle tone under the intervention of the PPN
Dystonia and the change in muscle tone were observed when the PPN underwent pharmacological lesions and neuromodulation. It is assumed that the cholinergic integrity of the PPN might closely correlate with muscle tone and the development of dystonia. Rats that have underwent bilateral non-specific lesions of the PPN exhibited increased muscle tone and abnormal flexion of the spine and limbs while suspended (36). In another study, monkeys that received unilateral non-specific lesions of the PPN also showed flexed posture, hypokinesia, and rigidity of the limbs contralateral to the lesioned side, and these symptoms gradually improved and became stationary in 2–3 weeks after surgery (37). When the cholinergic cell-specific lesion was performed on the PPN, the primates showed imbalanced muscle tone between the hindlimbs of the lesioned and non-lesioned sides. Additionally, the rigidity of the tail and proximal part of the limb contralateral to the lesioned side also appeared along with the back curvature (12). Moreover, when wild-type human tau was specifically expressed in the cholinergic neurons of the PPN, rats showed dystonia-like behavior of the hindlimb (hindlimb retracted toward body, crossing, and immobility) while suspended (38).
Neuromodulation studies examining PPN have indicated that low-frequency electrical stimulation of the PPN could induce a change in muscle tone, which presented as atonia and hypotonia; this may be due to the change in the neural activity of the cholinergic neurons in the PPN. In de-cerebrated cats, low-frequency electrical stimulation of the PPN suppressed the muscle tone of hindlimbs, and this effect was also elicited through either GABA antagonists or glutamatergic agonist injection into the PPN. Moreover, when the cholinergic muscarinic receptor antagonist was injected into the downstream reticular formation of the PPN, the suppression effect of the PPN stimulation was abolished, which indicates that the cholinergic output from the PPN could modulate the muscle tone through the reticular formation (20). Meanwhile, electrical stimulation of the PPN could also suppress the muscle tone of the neck and forelimb in decerebrated cats, and the suppressive effect was noted to be frequency- and intensity-dependent (39). Furthermore, when topographically analyzing the stimulation sites that induced atonia and hypotonia in the PPN, they were found to agree well-with the dorsoventral distribution of the cholinergic neurons in the PPN (5). However, an early study demonstrated that electrical stimulation of the PPN could produce an excitatory effect on bilateral cervical muscles, and the effect of excitation decreased while the frequency of stimulation increased (40). This finding might be explained by the fact that the PPN could both innervate the excitatory and inhibitory zones of reticular formation, although the specific mechanism still needs to be explored.
Electrical stimulation studies could not fully elucidate the function of the PPN because they were mainly based on the transected animals that lack the descending inhibitory control over the PPN (21). In normal animals, a recent optogenetic study (41) found that short-term photoactivation of the glutamatergic neurons of the PPN could evoke strong and fast motor responses in the bilateral flexor muscles of hindlimbs, whereas the photoactivation of the cholinergic neurons might induce strong and slower motor changes in bilateral extensor muscles. Additionally, long-term photoinhibition of both the glutamatergic and cholinergic neurons increased the extensor burst. In another study (2), the photoactivation of glutamatergic neurons of the PPN was shown to induce long-lasting increases in the muscle tone of flexors such as the biceps motor activity and gait performance. Combined with the former findings, the cholinergic neuropathy of the PPN might cause dystonia and be closely related to flexor muscle hyperexcitation.
With regard to the pathophysiology of dystonia, loss of inhibition at multiple levels of nervous system including the cortex, brainstem and spinal cord and impaired sensory-motor integration have been noted in certain patients (42–44). In particular, the surround inhibition (the decrease of corticospinal excitability of the surround muscles nearby the active muscles) was absent in patients with focal hand dystonia (45). And deficits of the afferent inhibition (the decrease of the motor-evoked potential under a peripheral electrical stimulation to the hand prior to the contralateral motor cortex stimulation) have also been found in patients of dystonia (44, 46). Based on the evidence of dystonia and muscle tone change after the PPN intervention, it is assumed that the cholinergic neuropathy of the PPN might induce the hyperexcitation of flexor muscles through its disinhibition on the reticular formation or its effect on the basal ganglia-cerebello-thalamo-cortical circuit, which in turn contribute to the impairments of surround inhibition and sensory-motor integration.
Role of the PPN in the modulation of different locomotion patterns
The PPN has also been determined to be involved in the modulation of different patterns of locomotion, and the abnormal locomotion caused by the PPN intervention may be due to dystonia (47). Several studies have explored the effect of PPN on locomotion through in vivo electrophysiological recording, neuromodulation, and pharmacological lesions, wherein it was found that the PPN could modulate the motor initiation, duration, rhythm, and speed (2, 4, 41). Through sing-unit activity recording, the change in neuronal activity in the PPN preceded the onset of bilateral limb movements in primates, indicating its role in motor initiation (48). When the motor processes were further specified and correlated with the neural activity of the PPN, it was found that neurons in the PPN were preferentially related to the onset and termination of motor episodes (3).
Interventional experiments have also elucidated that electrical stimulation of the PPN could elicit machine-like coordinated locomotion in decerebrated animals (5, 49). Recent optogenetic studies have shown that activation of the cholinergic neurons of the PPN could slow or terminate the ongoing locomotion, while inhibition might increase the step cycle (4, 41). However, it remains unclear whether photoactivation of the glutamatergic neurons could elicit low-speed locomotion and explorative behavior (2, 4). There is also evidence that activation of the glutamatergic neurons of the PPN might rescue the severe locomotor deficit and produce high-quality locomotion in mouse models of parkinsonism (50). Moreover, non-specific pharmacologic studies on the PPN showed that the spontaneous or drug-induced locomotion was unaffected (51–53). Nevertheless, excitotoxic lesions of the PPN might impair fine motor control and increase the incidence of oral motor behavior under intra-putamen injection of amphetamine in rats (54, 55).
Pathologic change of the PPN in patients with dystonia
According to associated features, dystonia can be classified into isolated dystonia (dystonia is the only motor feature) and combined dystonia (dystonia combined with other movement disorders) (7). Both types of dystonia are featured by the deficits of network involving the basal ganglia-cerebello-thalamo-cortical circuit (56). And pathologic changes in the PPN are found in both types of dystonia.
Pathologic changes of the PPN in isolated dystonia
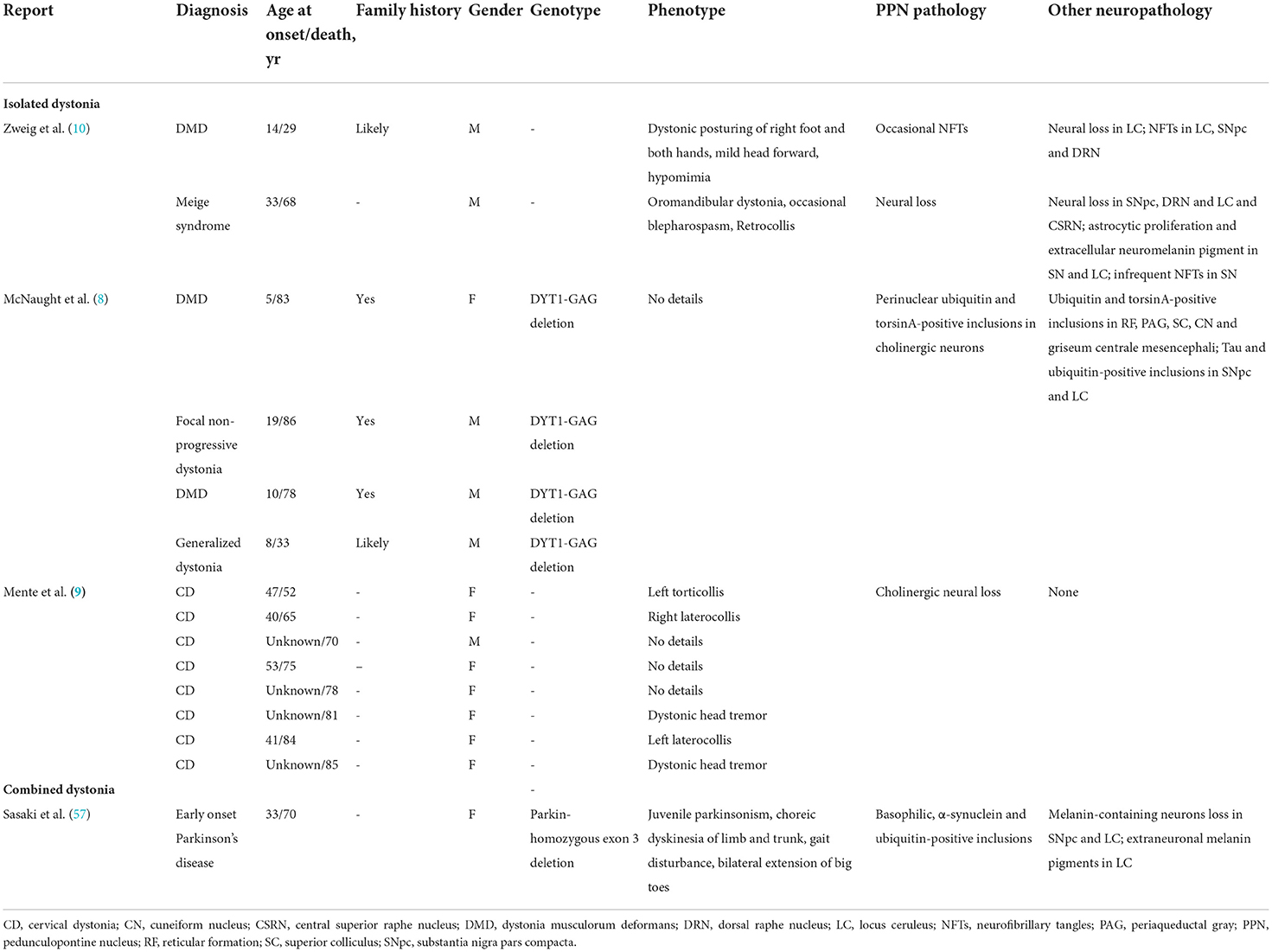
At present, no consistent or specific neuropathologic changes have been found in postmortem studies of patients with isolated dystonia (58). Several autopsy reports of patients with isolated dystonia showed that the pathologic changes of the PPN include the presence of neurofibrillary tangles (NFTs), cholinergic neural loss, and perinuclear inclusions (Table 1). In the autopsy report of a 29-year-old male patient with dystonia musculorum deformans, NFTs were found in the locus coeruleus (LC), SNpc, PPN, and dorsal raphe nucleus. The patient presented with early-onset dystonia, mainly affecting the right foot, both hands and head (10). In another 68-year-old male patient with Meige syndrome (blepharospasm combined with oromandibular dystonia), the significant neural loss was observed in the SNpc, dorsal raphe, PPN, and LC along with astrocytic proliferation (10). Besides NFTs and neural loss found in the PPN, the cholinergic pathology of the PPN has also been found correlated with dystonia in some studies (8, 9). In a study that enrolled four patients with a GAG deletion of the dystonia gene DYT1, ubiquitin-positive and torsinA-positive perinuclear inclusions were found to be aggregated in cholinergic and other neurons of the PPN, cuneiform nucleus, and periaqueductal gray (8). Although these results were not confirmed by a recent study (58), absent or reduced choline acetyltransferase staining was confirmed in all six available PPN samples in another recent study examining eight patients with cervical dystonia (9); this, together with the finding that the diffusion tensor imaging of the PPN was identical between patients and controls, suggests that a functional cholinergic deficit might be involved in cervical dystonia (9).
Pathologic change of the PPN in dystonia combined with parkinsonism
Dystonia combined with parkinsonism has been determined as the most common type of combined dystonia. Within PD, the prevalence of dystonia could be more than 30% if the onset of parkinsonism is before the age of 40 (59). Various types of dystonia can co-occur with parkinsonism, including blepharospasm, camptocormia, Pisa syndrome, anterocollis, and limb dystonia. Among them, lower limb dystonia is relatively common (60). Patients with lower limb dystonia mainly present with ankle inversion, hyperextension of the big toe, and flexion of the other toes, which is also known as “striatal foot.” The striatal foot might appear due to dystonia, rigidity, and inappropriate contraction of the foot muscle, and the dystonic, disinhibited foot grasp might also induce the freezing of gait (47). At present, DBS of the PPN showed beneficial effect on improving gait freezing (61), which may be attributed to its effect on the locomotion along with foot dystonia.
Only a few studies mention the relationship between the presentation of dystonia combined with parkinsonism and the pathological changes in the brain. The available studies show that among the patients with dystonia combined with parkinsonism, there are NFTs and inclusions in the PPN, and the pathological changes in the PPN are more related to the lower limb (57). Patients of PD with parkin gene mutation commonly have dystonia as their initial symptom, which is known to often affect the lower limb (59). In the autopsy report of a 33-year-old female patient with PD with a homozygous exon 3 deletion in the parkin gene, basophilic inclusions were occasionally found in the PPN, and ubiquitin-positive and α-synuclein-positive inclusions were also observed (Table 1). The dystonia of the patients mainly presented as a bilateral extension of large toes (57). Apart from the autopsy studies, DBS of the PPN also showed marked efficacy in alleviating axial dystonia in patients with PD, which might reflect the relationship between PPN and dystonia from the therapeutic aspect. Shih et al. (11) reported that DBS of the contralateral PPN to the bending side could improve not only gait but also dystonia in a 62-year-old female patient with PD combined with Pisa syndrome. Additionally, Ricciardi et al. (62) found that DBS on the ipsilateral PPN to the bending side could also provide short-term benefits on PD associated with Pisa syndrome. Although patients with PD often develop Pisa syndrome away from the side with predominant symptoms, the efficacy of either side of the PPN DBS might be due to the bilateral modulative effect of the PPN on axial muscle tone (63).
Potential mechanisms underlying PPN pathology on the development of dystonia
Dystonia is known to be a network disorder characterized by the involvement of the basal ganglia-cerebello-thalamo-cortical circuit. The pathology in the basal ganglia and cerebellum has often been observed in patient and animal models of dystonia (56, 64). In addition, the imbalance between the striatal dopamine and acetylcholine systems has commonly been regarded as the key mechanism for the development of dystonia (65). Primates and humans could develop dystonia under the treatment of acetylcholine agonists and dopaminergic antagonists (66, 67). Most types of dystonia could be effectively treated with anticholinergic therapy (68). Moreover, in the striatum of the Dyt1 mouse model, the dopamine D2 receptor agonist could induce paradoxical excitation of the cholinergic interneurons, rather than the physiological inhibition, leading to the over release of acetylcholine and dysfunction of the striatum (69). As mentioned above, the PPN has various connections with both the basal ganglia and cerebellum. The PPN might be primarily affected in isolated dystonia whereas secondarily affected in PD combined with dystonia. The primary deficit of the PPN has been determined in patient and animal models of the Dyt1 mutation, which mainly presented as cholinergic pathology and was involved in the integration and output of the motor information (8, 34). On the basis of the directly modulative effect the PPN on the medium-sized spiny neurons and interneurons in the striatum mentioned above, the cholinergic loss of the PPN might ascendingly contribute to the dysfunction of the central selection, the neural plasticity, and the imbalance of the dopamine and acetylcholine in the striatum (19). In addition, the cholinergic lesion of the PPN were significantly associated with loss and morphologic changes of nigral dopaminergic neurons, which indirectly affects the dopamine release in the striatum through the nigra-striatum pathway (70). Meanwhile, patients with dystonia have been reported as hypoactivity at the cerebellar cholinergic terminals at lobules VI and VII that response for the cerebellar sensorimotor projections to the cortex and working memory, which reflects that the abnormal cholinergic input form the PPN to the cerebellum might be related to the impaired sensorimotor integration and cognitive functions in patients of dystonia (15). Moreover, the cholinergic lesion of the PPN could also descendingly contribute to the disinhibition of the motor neurons and interneurons in the spinal cord, leading to muscle hyperexcitaion and loss of surround inhibition (21) (Figure 1B).
In dystonia combined with parkinsonism, the PPN might be affected secondary to the pathological change of the basal ganglia-cerebello-thalamo-cortical circuit. PD is featured by the neurodegeneration of the dopaminergic neurons in the SNpc. The deficit of the dopamine release in the striatum results in the overactivity of the indirect pathway. The overactivity of the GPi and SNpr could suppress the neural activity of the thalamus, leading to decreased activity of the cortex and presence of parkinsonism (71). On the other hand, it could decrease the neural activity of cholinergic and glutamatergic neurons in the PPN, which directly induces the disinhibition of the reticular formation and spinal cord or indirectly affects the function of the striatum, thalamus and cortex (1). Moreover, in a PD rat model under intra-nigral lesion with lactacystin, alpha-synuclein aggregation might trigger both PPN cholinergic and non-cholinergic neuron loss via neuronophagia (72). The PPN neuron loss triggered by the pathological change of dopaminergic neurons in the SNpc might further contribute to the dystonia manifestations in PD patients such as striatal foot and Pisa syndrome as mentioned above. Together with the neurodegeneration of dopaminergic neurons in the SNpc, the striatal cholinergic impairment might also affect the PPN and induce dystonia. Striatal cholinergic dysregulation was noted after neonatal decrease in X-Linked dystonia parkinsonism-related TAF1 isoforms in mice (73). And loss of striatal interneurons was also found in rapid-onset dystonia-parkinsonism mice (74). In summary, the role of the PPN in the dystonia combined with parkinsonism is complicated and its effects on the basal ganglia, cerebellum and spinal cord are under further exploration (Figure 1C).
Regarding the non-motor manifestations of dystonia, patients of primary dystonia had deficits on a broad range of cognitive domains including global cognitive function, attention, memory and conceptualization (75). In more detail, dystonia patients presented cognitive impairments related to the executive dysfunctions such as set-shifting, category fluency and verbal learning, and impaired time-based prospective memory was also found in patients of cervical dystonia, reflecting the potential dysfunctions in the prefrontal cortex and basal ganglia (76, 77). The PPN might be involved in the cognitive functions through its connections with the cortex, thalamus (especially the thalamic centromedian-parafascicular complex), basal ganglia and cerebellum as mentioned above, and cognitive impairments mimicked non-motor manifestations of dystonia could appeared following the PPN lesion. In detail, different types of learning (mnemonic motor task related learning, reversal learning and stimulus-reward related learning) and attentional performance were impaired following the PPN lesion in rats (13, 52, 78, 79). In addition, dystonia patients have been reported poor sleep quality and disturbed sleep architecture, and these impairments of sleep might not due to the persistence of muscle activity (80). In particular, certain dystonia patients showed increased latency of rapid eye movement (REM) sleep and reduced REM duration (81). It is reported that the PPTg had cholinergic projections to the gigantocellular tegmental field, which might participate in the induction and maintenance of normal REM sleep and cortical activity (6). And rat studies showed that lesion of the PPN could disturb sleep/wake state transitions and reduce the REM sleep duration under hypoxic conditions, which might be in favor of the involvement of the PPN in the sleep disturbance of dystonia (14, 82). Moreover, from the therapeutic aspect, bilateral low-frequency DBS of the PPN improved simple reaction times without a warning cue in PD patients, indicating its beneficial effect on the attentional processing (83). And improved executive functions and working memory were also noted after low-frequency DBS of bilateral PPN for PD patients (84, 85). Furthermore, bilateral low-frequency PPN DBS also produced marked cognitive improvement associated with a significant increase in cortical metabolism in both prefrontal areas and mono-lateral ventral striatum (86). Regarding the sleep disturbance, unilateral low-frequency DBS of the PPN significantly doubled the duration of REM sleep in PD patients through increasing REM sleep episodes, indicating that the PPN DBS might affect the transitioning between sleep states (87). While no improvement of cognitive, psychiatric or emotional functions for PD patients underwent PPN DBS were also found in other studies (88, 89), theses above clinical evidences are in favor of the PPN involving in the non-motor manifestations of movement disorders, and whether DBS of the PPN could contribute to the improvement of non-motor manifestations in the dystonia still need further exploration.
Conclusion
Preclinical and clinical evidences have revealed the involvement of the PPN in the development of dystonia. The dense anatomical connectome between the PPN and dystonia-related basal ganglia-cerebello-thalamo-cortical circuit lays the foundation for the contributions of the PPN to both motor and non-motor manifestations of dystonia. Of note, the cholinergic pathology of the PPN might affect the balance of striatal dopamine and acetylcholine systems and mimic the pathophysiological processing of dystonia. Different neural populations of the PPN might have distinct effects on motor and non-motor functions, and their roles in the development and treatment of dystonia requires further exploration to provide evidence for targeting the PPN for the treatment of dystonia in the future.
Author contributions
J-hS: design and writing. Y-wH: visualization. YY: resources. R-yL and L-xL: revising. FT: design and revising. L-jJ: reviewing, supervision, and editing of final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Medical Innovation Project of Shanghai Science and Technology Commission (20Y11906000), Outstanding academic leader of Shanghai Science and Technology Commission (20XD1403400), National Natural Science Foundation of China (81971074), and Clinical Science and Technology Innovation project of Shanghai Shen-kang Hospital Development Center (SHDC12020119).
Acknowledgments
We thank all the members of the Department of Neurology, Shanghai Tongji Hospital, and Tongji University School of Medicine for their help in this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. (2013) 80:1148–55. doi: 10.1212/WNL.0b013e3182886a76
2. Dauta D, Kovacs A, Bayasgalan T, Diaz-Acevedo MA, Pal B, Mena-Segovia J. Modulation of motor behavior by the mesencephalic locomotor region. Cell Rep. (2021) 36:109594. doi: 10.1016/j.celrep.2021.109594
3. Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neuroscience. (1988) 27:639–54. doi: 10.1016/0306-4522(88)90295-3
4. Caggiano V, Leiras R, Goni-Erro H, Masini D, Bellardita C, Bouvier J, et al. Midbrain circuits that set locomotor speed and gait selection. Nature. (2018) 553:455–60. doi: 10.1038/nature25448
5. Takakusaki K, Chiba R, Nozu T, Okumura T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J Neural Transm. (2016) 123:695–729. doi: 10.1007/s00702-015-1475-4
6. Vitale F, Capozzo A, Mazzone P, Scarnati E. Neurophysiology of the pedunculopontine tegmental nucleus. Neurobiol Dis. (2019) 128:19–30. doi: 10.1016/j.nbd.2018.03.004
7. Albanese A, Bhatia K, Bressman SB, DeLong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. (2013) 28:863–73. doi: 10.1002/mds.25475
8. McNaught KS, Kapustin A, Jackson T, Jengelley TA, JnoBaptiste R, Shashidharan P, Olanow C. W., et al Brainstem pathology in DYT1 primary torsion dystonia. Ann Neurol. (2004) 56:540–7. doi: 10.1002/ana.20225
9. Mente K, Edwards NA, Urbano D, Ray-Chaudhury A, Iacono D, Alho AT, et al. Pedunculopontine nucleus cholinergic deficiency in cervical dystonia. Mov Disord. (2018) 33:827–834. doi: 10.1002/mds.27358
10. Zweig RM, Hedreen JC, Jankel WR, Casanova MF, Whitehouse PJ, Price DL. Pathology in brainstem regions of individuals with primary dystonia. Neurology. (1988) 38:702–6. doi: 10.1212/WNL.38.5.702
11. Shih LC, Vanderhorst VG, Lozano AM, Hamani C, Moro E. Improvement of pisa syndrome with contralateral pedunculopontine stimulation. Mov Disord. (2013) 28:555–6. doi: 10.1002/mds.25301
12. Gay M, Belaid H, Rogers A, Pérez-García F, Roustan M, Bardinet E, et al. Anatomo-functional mapping of the primate mesencephalic locomotor region using stereotactic lesions. Mov Disord. (2020) 35:789–99. doi: 10.1002/mds.27983
13. Cyr M, Parent MJ, Mechawar N, Rosa-Neto P, Soucy JP, Clark SD, et al. Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post-mortem immunocytochemistry and in vivo PET imaging with [(1)(8)F]fluoroethoxybenzovesamicol. Behav Brain Res. (2015) 278:107–14. doi: 10.1016/j.bbr.2014.09.021
14. Petrovic J, Ciric J, Lazic K, Kalauzi A, Saponjic J. Lesion of the pedunculopontine tegmental nucleus in rat augments cortical activation and disturbs sleep/wake state transitions structure. Exp Neurol. (2013) 247:562–71. doi: 10.1016/j.expneurol.2013.02.007
15. Sival DA, van Noort SA, Tijssen MA, de Koning TJ, Verbeek DS. Developmental neurobiology of cerebellar and Basal Ganglia connections. Eur J Paediatr Neurol. (2022) 36:123–9. doi: 10.1016/j.ejpn.2021.12.001
16. Chambers NE, Lanza K, Bishop C. Pedunculopontine nucleus degeneration contributes to both motor and non-motor symptoms of Parkinson's disease. Front Pharmacol. (2019). 10:1494. doi: 10.3389/fphar.2019.01494
17. Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. (2011) 5:22. doi: 10.3389/fnana.2011.00022
18. Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. (2009) 29:340–58. doi: 10.1111/j.1460-9568.2008.06576.x
19. Dautan D, Huerta-Ocampo I, Gut NK, Valencia M, Kondabolu K, Kim Y, et al. Cholinergic midbrain afferents modulate striatal circuits and shape encoding of action strategies. Nat Commun. (2020) 11:1739. doi: 10.1038/s41467-020-15514-3
20. Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. (2003) 119:293–308. doi: 10.1016/S0306-4522(03)00095-2
21. Gut NK, Winn P. The pedunculopontine tegmental nucleus-A functional hypothesis from the comparative literature. Mov Disord. (2016) 31:615–24. doi: 10.1002/mds.26556
22. Shink E, Sidibe M, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol. (1997) 382:48–63. doi: 10.1002/(SICI)1096-9861(19970609)382:3<348::AID-CNE4>3.0.CO;2-3
23. Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. (1987) 260:435–52. doi: 10.1002/cne.902600309
24. Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. (1995) 21:331–42. doi: 10.1016/0168-0102(94)00869-H
25. Xiao C, Cho JR, Zhou C, Treweek JB, Chan K, McKinney SL, et al. Cholinergic mesopontine signals govern locomotion and reward through dissociable midbrain pathways. Neuron. (2016) 90:333–47. doi: 10.1016/j.neuron.2016.03.028
26. Bevan MD, Bolam JP. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci. (1995) 15:7105–20. doi: 10.1523/JNEUROSCI.15-11-07105.1995
27. Assous M, Dautan D, Tepper JM, Mena-Segovia J. Pedunculopontine glutamatergic neurons provide a novel source of feedforward inhibition in the striatum by selectively targeting interneurons. J Neurosci. (2019) 39:4727–37. doi: 10.1523/JNEUROSCI.2913-18.2019
28. Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci. (2014) 34:4509–18. doi: 10.1523/JNEUROSCI.5071-13.2014
29. Vitale F, Mattei C, Capozzo A, Pietrantoni I, Mazzone P, Scarnati E. Cholinergic excitation from the pedunculopontine tegmental nucleus to the dentate nucleus in the rat. Neuroscience. (2016) 317:12–22. doi: 10.1016/j.neuroscience.2015.12.055
30. Henrich MT, Geibl FF, Lakshminarasimhan H, Stegmann A, Giasson BI, Mao X, et al. Determinants of seeding and spreading of alpha-synuclein pathology in the brain. Sci Adv. (2020) 6:2487. doi: 10.1126/sciadv.abc2487
31. Mori F, Okada KI, Nomura T, Kobayashi Y. The pedunculopontine tegmental nucleus as a motor and cognitive interface between the cerebellum and Basal Ganglia. Front Neuroanat. (2016) 10:109. doi: 10.3389/fnana.2016.00109
32. French IT, Muthusamy KA. A Review of the Pedunculopontine Nucleus in Parkinson's Disease. Front Aging Neurosci. (2018) 10:99. doi: 10.3389/fnagi.2018.00099
33. Clément C, Lalonde R, Strazielle C. Acetylcholinesterase activity in the brain of dystonia musculorum (Dst(dt-J)) mutant mice. Neurosci Res. (2012) 72:79–86. doi: 10.1016/j.neures.2011.09.005
34. Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, et al. Transgenic mouse model of early-onset DYT1 dystonia. Hum Mol Genet. (2005) 14:125–33. doi: 10.1093/hmg/ddi012
35. Wilson BK, Hess EJ. Animal Models For Dystonia. Mov Disord. (2013) 28:982–9. doi: 10.1002/mds.25526
36. Olmstead MC, Franklin KB. Lesions of the pedunculopontine tegmental nucleus abolish catalepsy and locomotor depression induced by morphine. Brain Res. (1994) 662:134–40. doi: 10.1016/0006-8993(94)90805-2
37. Matsumura M, Kojima J. The role of the pedunculopontine tegmental nucleus in experimental parkinsonism in primates. Stereotact Funct Neurosurg. (2001) 77:108–15. doi: 10.1159/000064614
38. King G, Veros KM, MacLaren DA, Leigh MP, Spernyak JA, Clark SD. Human wildtype tau expression in cholinergic pedunculopontine tegmental neurons is sufficient to produce PSP-like behavioural deficits and neuropathology. Eur J Neurosci. (2021) 54:7688–709. doi: 10.1111/ejn.15496
39. Lai YY, Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci. (1990) 10:2727–34. doi: 10.1523/JNEUROSCI.10-08-02727.1990
40. Kelland MD, Asdourian D. Pedunculopontine tegmental nucleus-induced inhibition of muscle activity in the rat. Behav Brain Res. (1989) 34:213–34. doi: 10.1016/S0166-4328(89)80103-2
41. Josset N, Roussel M, Lemieux M, Lafrance-Zoubga D, Rastqar A, Bretzner F. Distinct contributions of mesencephalic locomotor region nuclei to locomotor control in the freely behaving mouse. Curr Biol. (2018) 28:884–901. doi: 10.1016/j.cub.2018.02.007
42. Tisch S, Limousin P, Rothwell JC, Asselman P, Zrinzo L, Jahanshahi M, et al. Changes in forearm reciprocal inhibition following pallidal stimulation for dystonia. Neurology. (2018) 66:1091–3. doi: 10.1212/01.wnl.0000204649.36458.8f
43. Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. (2008) 28:10363–9. doi: 10.1523/JNEUROSCI.3564-08.2008
44. Richardson SP, Bliem B, Lomarev M, Shamim E, Dang N, Hallett M. Changes in short afferent inhibition during phasic movement in focal dystonia. Muscle Nerve. (2008) 37:358–63. doi: 10.1002/mus.20943
45. Beck S, Schubert M, Richardson SP, Hallett M. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol. (2009). (1985) 107:1513–8. doi: 10.1152/japplphysiol.91580.2008
46. Pirio Richardson S, Bliem B, Voller B, Dang N, Hallett M. Long-latency afferent inhibition during phasic finger movement in focal hand dystonia. Exp Brain Res. (2009) 193:173–9. doi: 10.1007/s00221-008-1605-4
47. Jankovic J, Tintner R. Dystonia and parkinsonism. Parkinsonism Relat Disord. (2001) 8:109–21. doi: 10.1016/S1353-8020(01)00025-6
48. Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res. (1997) 28:155–65. doi: 10.1016/S0168-0102(97)00039-4
49. Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. (1987) 18:731–8. doi: 10.1016/0361-9230(87)90208-5
50. Masini D, Kiehn O. Targeted activation of midbrain neurons restores locomotor function in mouse models of parkinsonism. Nat Commun. (2022) 13:504. doi: 10.1038/s41467-022-28075-4
51. Alderson HL, Faulconbridge LF, Gregory LP, Latimer MP, Winn P. Behavioural sensitisation to repeated d-amphetamine: effects of excitotoxic lesions of the pedunculopontine tegmental nucleus. Neuroscience. (2003) 118:311–5. doi: 10.1016/S0306-4522(03)00152-0
52. Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. Learning disturbances following excitotoxic lesion of cholinergic pedunculo-pontine nucleus in the rat. Brain Res. (1991) 544:126–32. doi: 10.1016/0006-8993(91)90893-Z
53. Inglis WL, Dunbar JS, Winn P. Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience. (1994) 62:51–64. doi: 10.1016/0306-4522(94)90314-X
54. MacLaren DA, Santini JA, Russell AL, Markovic T, Clark SD. Deficits in motor performance after pedunculopontine lesions in rats–impairment depends on demands of task. Eur J Neurosci. (2014) 40:3224–36. doi: 10.1111/ejn.12666
55. Allen LF, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus disinhibit orofacial behaviours stimulated by microinjections of d-amphetamine into rat ventrolateral caudate-putamen. Exp Brain Res. (1995) 104:262–74. doi: 10.1007/BF00242012
56. Balint B, Mencacci NE, Valente EM, Pisani A, Rothwell J, Jankovic J, et al. Dystonia. Nat Rev Dis Primers. (2018) 4:25. doi: 10.1038/s41572-018-0023-6
57. Sasaki S, Shirata A, Yamane K, Iwata M. Parkin-positive autosomal recessive juvenile Parkinsonism with alpha-synuclein-positive inclusions. Neurology. (2004) 63:678–82. doi: 10.1212/01.WNL.0000134657.25904.0B
58. Pratt D, Mente K, Rahimpour S, Edwards NA, Tinaz S, Berman BD, et al. Diminishing evidence for torsinA-positive neuronal inclusions in DYT1 dystonia. Acta Neuropathol Commun. (2016) 4:85. doi: 10.1186/s40478-016-0362-z
59. Shetty AS, Bhatia KP, Lang AE. Dystonia and Parkinson's disease: what is the relationship? Neurobiol Dis. (2019) 132:104462. doi: 10.1016/j.nbd.2019.05.001
60. Jankovic J. Dystonia and other deformities in Parkinson's disease. J Neurol Sci. (2005) 239:1–3. doi: 10.1016/j.jns.2005.08.004
61. Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson's disease: a clinical review. Mov Disord. (2018) 33:10–20. doi: 10.1002/mds.27098
62. Ricciardi L, Piano C, Bentivoglio AR, Fasano A. Long-term effects of pedunculopontine nucleus stimulation for Pisa syndrome. Parkinsonism Relat Disord. (2014) 20:1445–6. doi: 10.1016/j.parkreldis.2014.10.006
63. Wijemanne S, Jankovic J. Hand, foot, and spine deformities in parkinsonian disorders. J Neural Transm. (2019) 126:253–64. doi: 10.1007/s00702-019-01986-1
64. Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. (2011) 42:185–201. doi: 10.1016/j.nbd.2011.01.026
65. Jaunarajs KE, Bonsi P, Chesselet MF, Standaert DG, Pisani A. Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia. Prog Neurobiol. (2015) 127:91–107. doi: 10.1016/j.pneurobio.2015.02.002
66. Cools AR, Hendriks G, Korten J. The acetylcholine-dopamine balance in the basal ganglia of rhesus monkeys and its role in dynamic, dystonic, dyskinetic, and epileptoid motor activities. J Neural Transm. (1975) 36:91–105. doi: 10.1007/BF01256757
67. Shafrir Y, Levy Y, Beharab A, Nitzam M, Steinherz R. Acute dystonic reaction to bethanechol–a direct acetylcholine receptor agonist. Dev Med Child Neurol. (1986) 28:646–8. doi: 10.1111/j.1469-8749.1986.tb03909.x
68. Jankovic J. Medical treatment of dystonia. Mov Disord. (2013) 28:1001–12. doi: 10.1002/mds.25552
69. Sciamanna G, Hollis R, Ball C, Martella G, Tassone A, Marshall A, et al. Cholinergic dysregulation produced by selective inactivation of the dystonia-associated protein torsinA. Neurobiol Dis. (2012) 47:416–27. doi: 10.1016/j.nbd.2012.04.015
70. Bensaid M, Michel PP, Clark SD, Hirsch EC, François C. Role of pedunculopontine cholinergic neurons in the vulnerability of nigral dopaminergic neurons in Parkinson's disease. Exp Neurol. (2016) 275(Pt 1):209–19. doi: 10.1016/j.expneurol.2015.11.004
71. Blesa J, Foffani G, Dehay B, Bezard E, Obeso JA. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci. (2022) 23:115–28. doi: 10.1038/s41583-021-00542-9
72. Elson JL, Yates A, Pienaar IS. Pedunculopontine cell loss and protein aggregation direct microglia activation in parkinsonian rats. Brain Struct Funct. (2016) 221:2319–41. doi: 10.1007/s00429-015-1045-4
73. Cirnaru MD, Creus-Muncunill J, Nelson S, Lewis TB, Watson J, Ellerby LM, et al. Striatal cholinergic dysregulation after neonatal decrease in X-linked dystonia parkinsonism-related TAF1 isoforms. Mov Disord. (2021) 36:2780–94. doi: 10.1002/mds.28750
74. Rauschenberger L, Knorr S, Al-Zuraiqi Y, Tovote P, Volkmann J, Ip CW. Striatal dopaminergic dysregulation and dystonia-like movements induced by sensorimotor stress in a pharmacological mouse model of rapid-onset dystonia-parkinsonism. Exp Neurol. (2020) 323:113109. doi: 10.1016/j.expneurol.2019.113109
75. Niccolai L, Aita SL, Walker HC, Martin RC, Clay OJ, Crowe M, et al. An examination of the neurocognitive profile and base rate of performance impairment in primary dystonia. J Clin Neurosci. (2020) 74:1–5. doi: 10.1016/j.jocn.2019.12.050
76. Maggi G, D'Iorio A, Mautone G, Peluso S, Manganelli F, Dubbioso R, et al. Cognitive correlates of prospective memory in dystonia. Parkinsonism Relat Disord. (2019) 66:51–5. doi: 10.1016/j.parkreldis.2019.06.027
77. Gilbertson T, Steele JD. Cognitive correlates of prospective memory in dystonia. Parkinsonism Relat Disord. (2019) 68:91.
78. Inglis WL, Olmstead MC, Robbins TW. Pedunculopontine tegmental nucleus lesions impair stimulus–reward learning in autoshaping and conditioned reinforcement paradigms. Behav Neurosci. (2000) 114:285–94. doi: 10.1037/0735-7044.114.2.285
79. Syed A, Baker PM, Ragozzino ME. Pedunculopontine tegmental nucleus lesions impair probabilistic reversal learning by reducing sensitivity to positive reward feedback. Neurobiol Learn Mem. (2016) 131:1–8. doi: 10.1016/j.nlm.2016.03.010
80. Antelmi E, Ferri R, Provini F, Scaglione CM, Mignani F, Rundo F, et al. Modulation of the muscle activity during sleep in cervical dystonia. Sleep. (2017) 40:88. doi: 10.1093/sleep/zsx088
81. Hertenstein E, Tang NK, Bernstein CJ, Nissen C, Underwood MR, Sandhu HK. Sleep in patients with primary dystonia: a systematic review on the state of research and perspectives. Sleep Med Rev. (2016) 26:95–107. doi: 10.1016/j.smrv.2015.04.004
82. Fink AM, Burke LA, Sharma K. Lesioning of the pedunculopontine nucleus reduces rapid eye movement sleep, but does not alter cardiorespiratory activities during sleep, under hypoxic conditions in rats. Respir Physiol Neurobiol. (2021) 288:103653. doi: 10.1016/j.resp.2021.103653
83. Fischer J, Schwiecker K, Bittner V, Heinze HJ, Voges J, Galazky I, et al. Modulation of attentional processing by deep brain stimulation of the pedunculopontine nucleus region in patients with parkinsonian disorders. Neuropsychology. (2015) 29:632–7. doi: 10.1037/neu0000179
84. Costa A, Carlesimo GA, Caltagirone C, Mazzone P, Pierantozzi M, Stefani A, et al. Effects of deep brain stimulation of the peduncolopontine area on working memory tasks in patients with Parkinson's disease. Parkinsonism Relat Disord. (2010) 16:64–7. doi: 10.1016/j.parkreldis.2009.05.009
85. Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. (2010) 289:44–8. doi: 10.1016/j.jns.2009.08.017
86. Ceravolo R, Brusa L, Galati S, Volterrani D, Peppe A, Siciliano G, et al. Low frequency stimulation of the nucleus tegmenti pedunculopontini increases cortical metabolism in parkinsonian patients. Eur J Neurol. (2011) 18:842–9. doi: 10.1111/j.1468-1331.2010.03254.x
87. Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, Hutchison WD, et al. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol. (2009) 66:110–4. doi: 10.1002/ana.21631
88. Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. (2010) 133:205–14. doi: 10.1093/brain/awp229
Keywords: pedunculopontine nucleus, dystonia, neuropathology, muscle tone, deep brain stimulation
Citation: Su J-h, Hu Y-w, Yang Y, Li R-y, Teng F, Li L-x and Jin L-j (2022) Dystonia and the pedunculopontine nucleus: Current evidences and potential mechanisms. Front. Neurol. 13:1065163. doi: 10.3389/fneur.2022.1065163
Received: 09 October 2022; Accepted: 08 November 2022;
Published: 23 November 2022.
Edited by:
Abhishek Lenka, Baylor College of Medicine, United StatesReviewed by:
Sergio A. Castillo-Torres, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaChi-Ying Lin, Baylor College of Medicine, United States
Copyright © 2022 Su, Hu, Yang, Li, Teng, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-jing Jin, bGluZ2ppbmdqaW5AMTYzLmNvbQ==
 Jun-hui Su
Jun-hui Su Yao-wen Hu
Yao-wen Hu Yi Yang1
Yi Yang1 Fei Teng
Fei Teng Ling-jing Jin
Ling-jing Jin