- 1Department of Neurology, Yantai Yuhuangding Hospital Affiliated to Qingdao University, Yantai, China
- 2The Second Clinical Medical College, Binzhou Medical University, Yantai, China
- 3Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 4China National Clinical Research Center for Neurological Diseases, Beijing, China
Background: The prominent veins sign (PVS) on susceptibility-weighted imaging (SWI) has been suggested to be related to the prognosis of patients with acute ischemic stroke (AIS). This meta-analysis aims to clarify the association between PVS and the prognosis of patients with AIS.
Methods: This meta-analysis was registered in PROSPERO (no. CRD42022343795). We performed systematic research in PubMed, Web of Science, EMBASE, and Cochrane Library databases for studies investigating the prognostic value of PVS. Based on the enrolled studies, patients were divided into two groups as follows: those with PVS cohort and those without PVS cohort. Outcomes were unfavorable functional outcome, early neurological deterioration (END), and hemorrhagic transformation (HT). The random-effects models were used for the meta-analytical pooled. Heterogeneity was estimated using Cochran's Q-test and I2 value. Subgroup and sensitivity analyses were also performed to explore the potential sources of heterogeneity. Publication bias was assessed with funnel plots and using Begger's and Egger's tests.
Results: A total of 19 studies with 1,867 patients were included. PVS was correlated with an unfavorable functional outcome in patients with AIS (risk ratio [RR] 1.61, 95% CI 1.28–2.02), especially in those receiving recanalization therapy (RR 2.00, 95% CI 1.52–2.63), but not in those treated conservatively (RR 1.33, 95% CI 0.87–2.04). Moreover, PVS was related to END (RR 2.77, 95% CI 2.21–3.46), while without an increased risk of HT (RR 0.97, 95% CI 0.64–1.47).
Conclusion: PVS was associated with an unfavorable prognosis of patients with AIS and increased the risk of END, while not correlated with an increased risk of HT. PVS might be useful for predicting functional outcomes of patients with AIS as a novel imaging maker.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022343795.
Introduction
Acute ischemic stroke (AIS) has been demonstrated to be the leading cause of morbidity and mortality worldwide, accounting for 87% of all strokes (1). It is also the most common cause of death in China due to the huge population, contributing to almost one-third of the total number of deaths due to stroke worldwide (2, 3). Early diagnosis, timely treatment, and improved prognosis in patients with ischemic stroke are vital requirements. Thus, identification of a prognostic imaging marker that could inform treatment decision-making and clinical outcome judging in the early stages of AIS has attracted great research interest.
Susceptibility-weighted imaging (SWI) is a blood oxygen level-dependent magnetic resonance technique developed as a useful tool for evaluating cerebral veins (4, 5). It is sensitive to paramagnetic substances and hence can indirectly reflect oxygen metabolism in tissues using the difference in the deoxygenated hemoglobin content between the hypoperfused region and normal tissues (6). Prominent veins sign (PVS) can be visualized as hypointensity and enlargement of vessels in the acute ischemic cerebral hemisphere with cortical and deep venous drainage (7). The prominence is thought to be an indicator of ischemic penumbra and is correlated with clinical prognosis. To date, 20 studies reported the relationship between PVS and the prognosis of patients with AIS, 15 of which believed that PVS had a worse prognosis than those without PVS (8–22), while the other 5 suggested that the presence of PVS was not related to prognosis (23–27). A previous meta-analysis has indicated that PVS was related to a poor functional prognosis in patients with AIS (28). However, it remains inconclusive whether PVS has an independent effect on prognosis in terms of pathophysiological factors such as large vessel occlusion. These studies generally have different study designs, patient characteristics, treatment administered to patients, small sample sizes, varying assessment of PVS, and heterogeneity of end points across studies. The purpose of this study was to provide a better understanding of the relationship between PVS and prognosis in patients with AIS.
Materials and methods
Search strategy
The study was performed according to the Preferred Reporting Items for Systematic Evaluation and Meta-Analysis guidelines and has been registered in PROSPERO (no. CRD42022343795). Our search strategy was based on a combination of the following keywords: “asymmetric* or prominent or hypointense or cortical or medullary or deep cerebral” and “vessel* or vein*” and “susceptibility-weighted or susceptibility weighted or SWI” and “stroke or cerebral infarction or cerebrovascular disease or brain infarction” (Supplementary Table 1). PubMed, Web of Science, Embase, and Cochrane Library were searched from inception to 1 April 2022. There was no language or other search restrictions. After identifying all potentially relevant studies, we removed duplicate studies using the Endnote X9 reference management software. Moreover, we reviewed the reference lists of the retrieved studies to ensure that no studies were omitted.
Inclusion and exclusion criteria
The following eligibility criteria were used to reduce clinical heterogeneity: (1) patients aged ≥18 years and with an established diagnosis of AIS; (2) a baseline SWI was performed within 7 days after admission; (3) prognostic outcomes of patients with PVS and without PVS reported in eligible studies; and (4) the study design was a prospective or retrospective cohort study. We excluded (1) studies where outcomes were not separate for participants with and without PVS; (2) studies aim to investigate the relationship between PVS and other imaging features such as collateral circulation and perfusion status; and (3) all case series, case reports, letters, editorials, reviews, conference abstracts, guidelines, technical notes, and unrelated studies.
Data extraction
Two authors (MZ and HW) independently reviewed the titles, abstracts, and full text of all the retrieved studies to determine their eligibility for inclusion. Disagreement was resolved by consensus with the corresponding author (ZL). If studies had overlapping samples, only the study with a larger sample size was included. Data extraction for each included study was done independently using a standardized electronic form. The discrepancies were resolved by consulting the third senior coauthor. The data extraction included (1) first author, publication year, study design, sample size, inclusion criteria, and clinical and imaging characteristics of enrolled patients at baseline (age, sex, hypertension, diabetes mellitus [DM], dyslipidemia, atrial fibrillation (AF), organic heart disease, history of stroke, time from stroke onset to SWI); (2) administration of research therapy (recanalization therapy, conservative treatment or mixed treatments); (3) PVS definition or assessment method: (i) visual assessment method, which classified patients with PVS if hypointense vessels were observed in the ipsilateral hemisphere compared with the contralateral side; (ii) patients scored quantitatively according to the Alberta Stroke Program Early CT Score and categorized into the PVS-positive group; (iii) slice assessment method (defining patients with ≥5 slice planes on 10 consecutive slices containing middle cerebral artery [MCA] regions as PVS-positive patients); and (iv) pixel comparison method (classifying PVS-positive patients by differences in venous signal intensity in bilateral cerebral hemispheres); and (5) primary and other outcomes in patients with and without PVS on baseline SWI. In the case of incomplete or unclear data, the authors were contacted using the details given in articles or identified by Internet search.
Outcomes
The modified Rankin Scale (mRS) score will be used to assess the prognosis of patients with AIS. The primary outcome was an unfavorable functional outcome, defined as a score of 2–6 or 3–6 with no limit to the duration of follow-up. Other outcomes were 90-day unfavorable functional outcome, early neurological deterioration (END), and hemorrhagic transformation (HT). END was defined as neurological deterioration with an increase in the National Institutes of Health Stroke Scale (NIHSS) score ≥ 2 points in the first 72 h after admission. HT was determined by CT or MRI within 7 days after admission.
Quality assessment
The Newcastle–Ottawa scale (NOS) was used to assess the quality of the included cohort studies, which included three factors, namely, selection, comparability, and exposure to assess the quality of each enrolled study. The NOS scores of 1–3, 4–6, and 7–9 indicate low, moderate, and high quality of studies, respectively. Two investigators (YM and HZ) independently conducted the quality assessment, and the differences were settled via consensus.
Statistical analyses
All meta-analyses were performed using Stata software (v.16.0). A random-effects model was applied to calculate the pooled proportion meta-analysis. Pooled risk ratios (RRs) with 95% CI were estimated using the DerSimonian and Laird random-effects model. The data are presented as forest plots, with a two-sided p-value of <0.05 indicating statistical significance. We assessed statistical heterogeneity between studies using Cochran's Q-test (p < 0.1 indicating heterogeneity) and I2 statistic (I2 value of 25, 50, and 75% were considered as low, moderate, and high heterogeneity, respectively). The subgroup analysis of the primary outcome was based on the patients' treatment modalities, the types of PVS defined by location, and the stenosis rate of the intracranial artery. In addition, a random-effects meta-analysis was performed to show the association of clinical and imaging factors with the presence of PVS on baseline SWI. The publication bias was assessed using Begger's and Egger's tests of funnel plot symmetry in addition to visual inspection of the funnel plots.
Results
Literature research
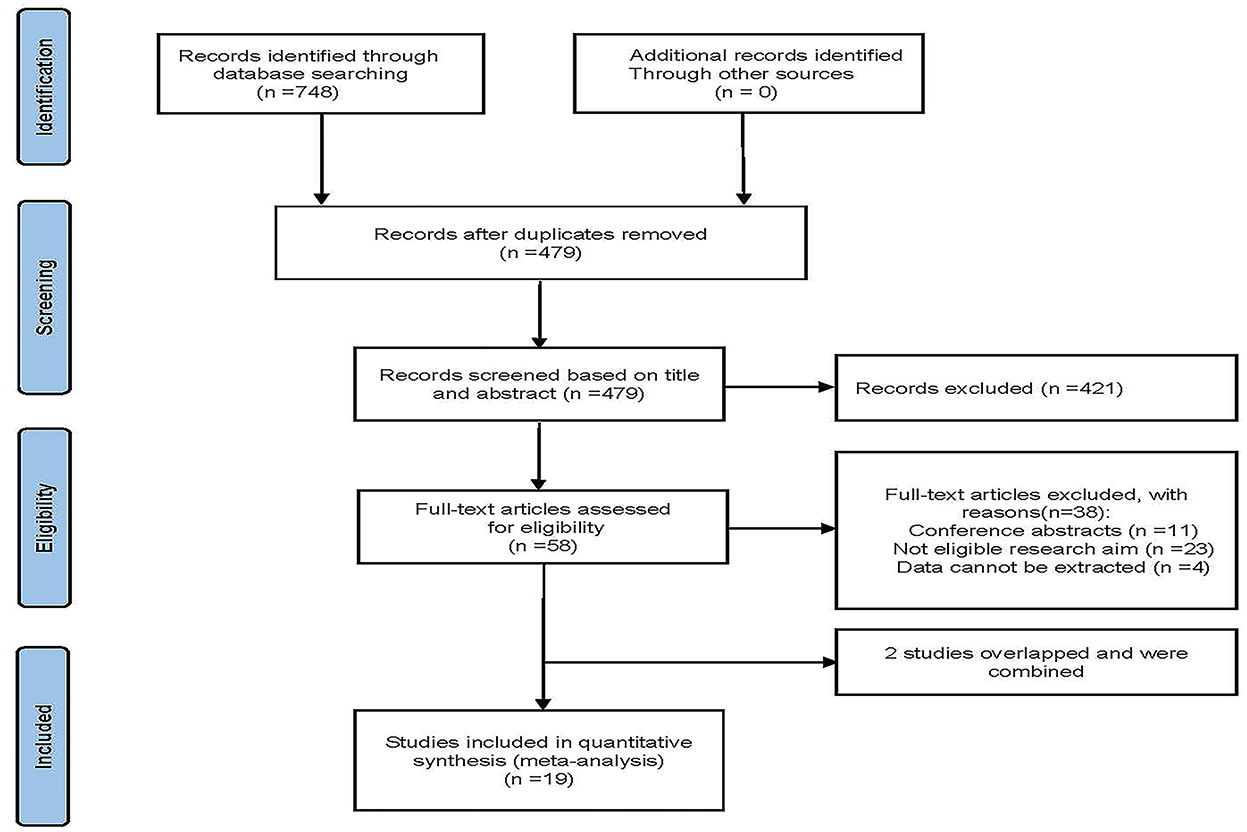
Figure 1 shows the research flow diagram. The initial literature search provided a total of 748 relevant studies, of which 269 were excluded due to repetition. A total of 479 studies were reviewed by titles and abstracts, and 58 studies were included for full-text review. During the full-text assessment, 38 studies were excluded because they did not meet the eligibility criteria (n = 23), data could not be extracted from the study (n = 4), or studies were conference abstracts (n = 11). In the case of overlap in patient cohorts of the two studies, only the largest study was included in this systematic review (10, 11). Eventually, 19 cohort studies with 1,867 patients were included in our analysis (8–10, 12–27).
Study characteristics
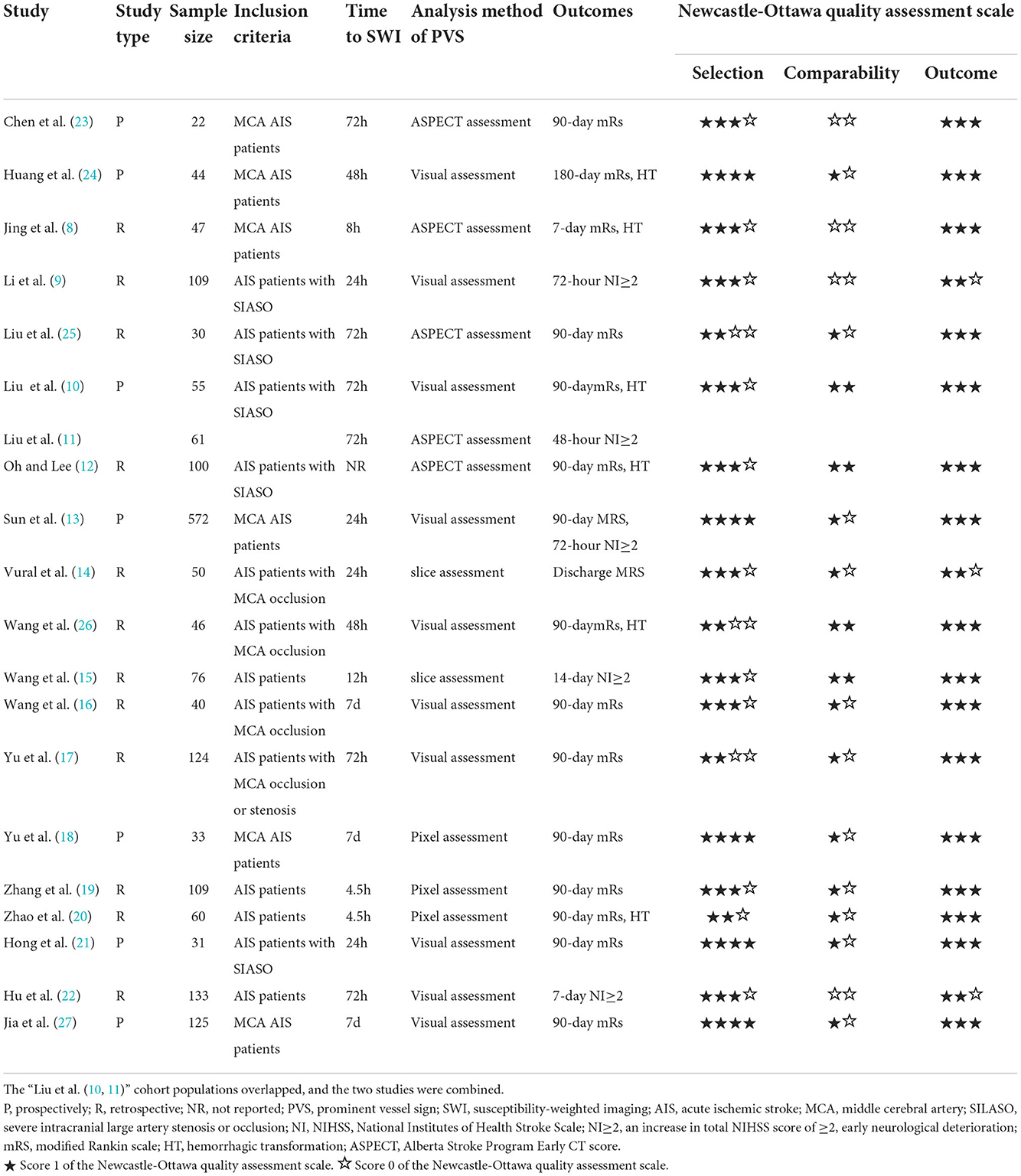
The included studies and relevant characteristics are provided in Table 1. The included studies were published between 2012 and 2022, and the sample sizes ranged from 22 to 572, with anterior circulation cerebral artery stenosis and infarction being the predominant type. A total of 7 studies were prospective in design, while 13 studies were retrospective. A total of 16 studies involving 1,488 patients (8, 10, 12–14, 16–21, 23–27) were pooled for an assessment of functional outcome, in which 13 studies involving 1,347 patients had this reported at 90 days (10, 12, 13, 16–21, 23, 25–27). Four studies (n = 324) (10, 12, 19, 20) were performed on recanalization therapy, three (10, 19, 20) of which were intravenous thrombolysis (IVT), and one (12) of which combined endovascular treatment (EVT) with IVT. Five studies (n = 951) reported END (9, 11, 13, 15, 22), while six studies (n = 352) reported the risk of HT (8, 10, 20, 24, 26). Data from 15 studies were pooled for influencing factors with the presence of PVS (8, 10, 12, 13, 15, 17–20, 22–27). The NOS scores ranged from 5 to 8, indicating a moderate-to-high quality of the included studies.
Association of baseline factors with the presence of PVS
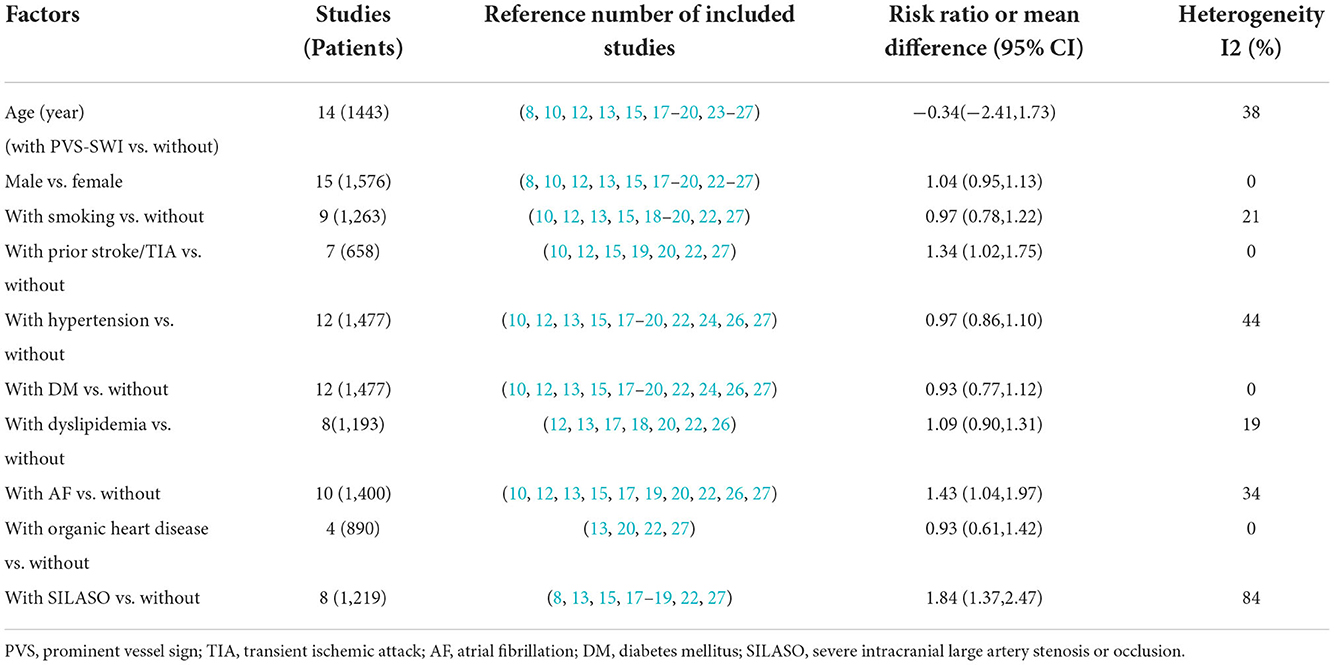
The factors related to the presence of PVS are shown in Table 2. Prior stroke/transient ischemic attack (TIA) (RR 1.34, 95% CI 1.02–1.75, p = 0.034) and AF (RR 1.43, 95% CI 1.04–1.97, p = 0.026) had an independent relationship with the presence of PVS. Furthermore, severe intracranial large-artery stenosis/occlusion (SILASO) was significantly associated with the presence of PVS (RR 1.84, 95% CI 1.37–2.47, p < 0.001).
Association of PVS with functional outcome
In general, PVS was associated with an unfavorable functional outcome (RR 1.61, 95% CI 1.28–2.02, p < 0.001), especially in patients who underwent recanalization treatment (RR 2.00, 95% CI 1.52–2.63, p < 0.001), but not in patients treated conservatively (RR 1.33, 95% CI 0.87–2.04, p = 0.186) (Figure 2). There was moderate heterogeneity in results pooled for functional outcome (I2 = 66.2%). Comparable results were obtained in a pooled analysis of the 90-day functional outcome (Figure 3). The subgroup was stratified by the location of the PVS. As shown in Figure 4A, we found that the differences in the cortex or medulla were related to a poorer functional prognosis (RR 1.79, 95% CI 1.36–2.37, p<0.001, I2 = 62.4%/RR 2.38, 95% CI 1.82–3.11, p < 0.001, I2 = 11.1%). There were similar associations in subgroup analyses by stenosis rate of the intracranial artery and functional outcome: being associated with an unfavorable outcome for patients with SILASO (RR 1.74, 95% CI 1.17–2.59, p =0.006) and without SILASO (RR 1.53, 95% CI 1.14–2.05, p =0.005) (Figure 4B). Heterogeneity for associations was generally moderate across studies (I2 = 69.4/I2 = 67.1).
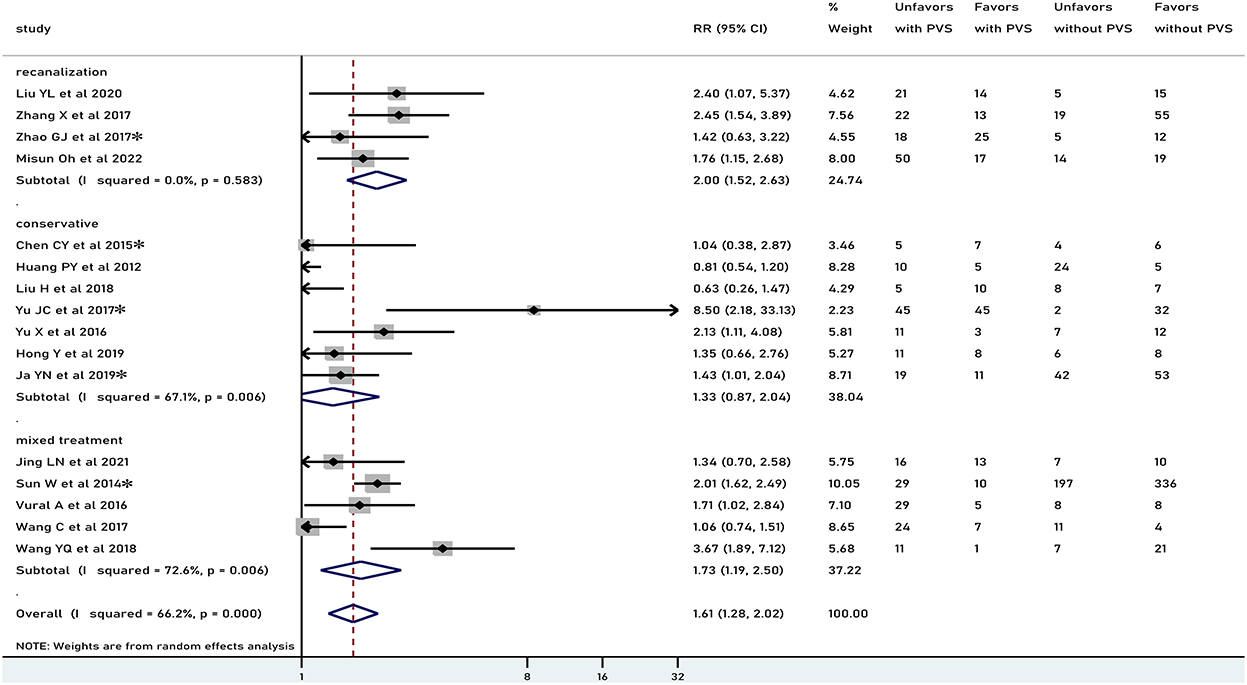
Figure 2. Meta-analysis of associations between PVS and unfavorable functional outcome by modality of treatment to the patients. PVS, prominent vessel sign; Unfavors, unfavorable functional outcome; Favors, favorable functional outcome; *mRS scores 2–6 were regarded as unfavorable outcome (mRS scores 3–6 for other studies).
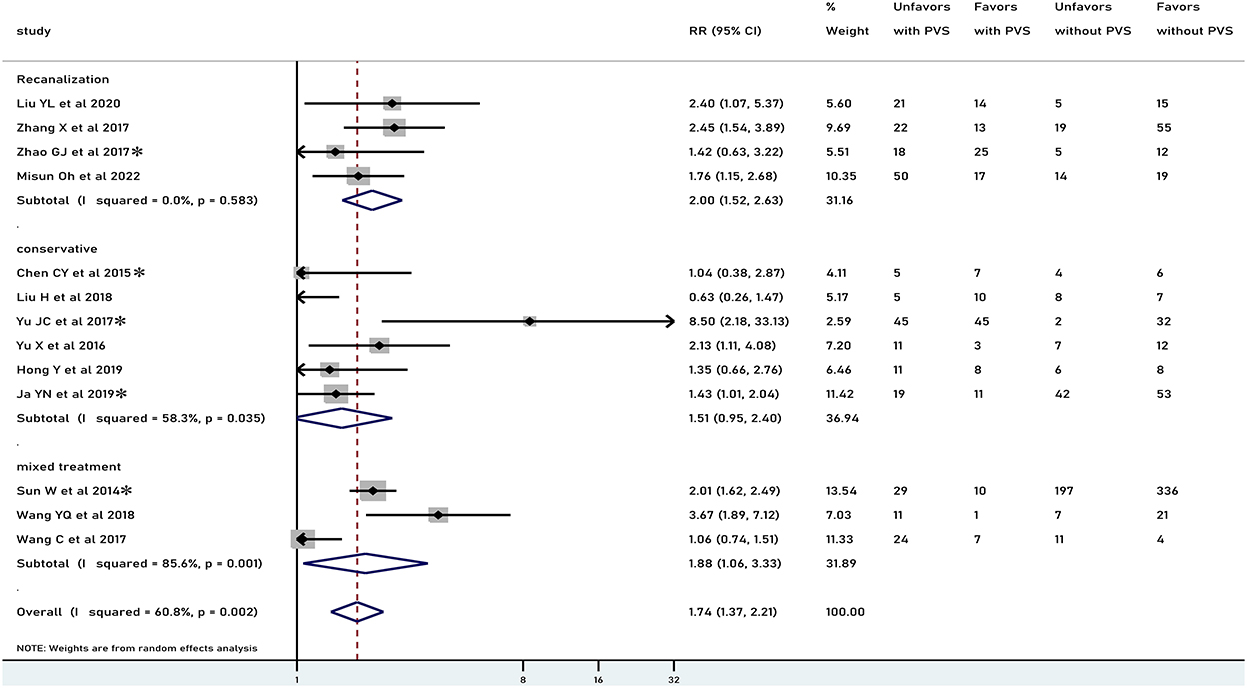
Figure 3. Meta-analysis of associations between PVS and 90-day functional outcome by modality of treatment to the patients. PVS, prominent vessel sign; Unfavors, unfavorable functional outcome; Favors, favorable functional outcome; *mRS scores 2–6 were regarded as unfavorable outcome (mRS scores 3–6 for other studies).
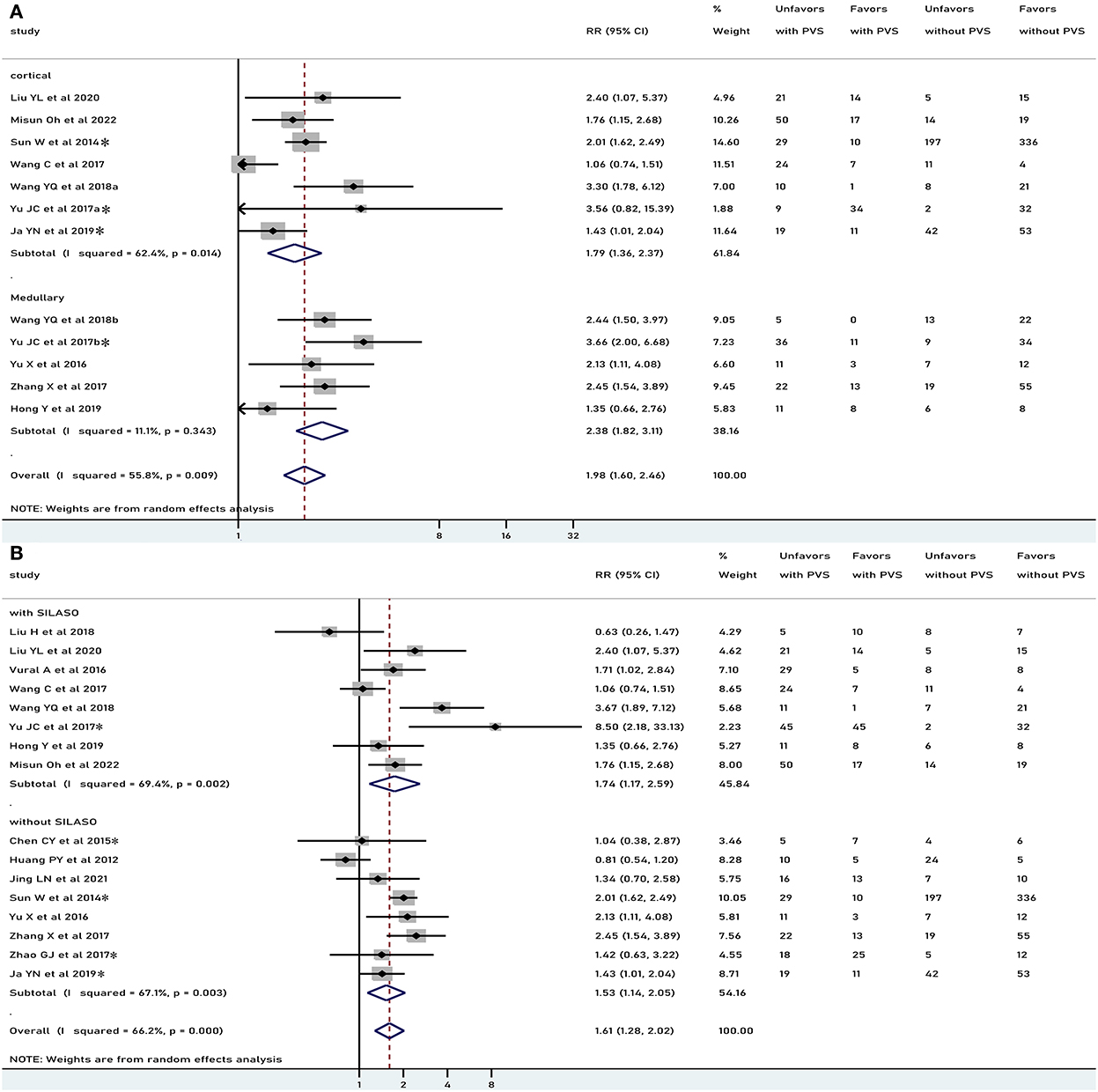
Figure 4. Meta-analysis of associations between location of PVS and unfavorable functional outcome (A) and meta-analysis of association between PVS and unfavorable functional outcome, by different stenosis of intracranial artery to the study patients (B). PVS, prominent vessel sign; Unfavors, unfavorable functional outcome; Favors, favorable functional outcome; *mRS scores 2–6 were regarded as unfavorable outcome (mRS scores 3–6 for other studies).
Association of PVS with other outcomes
Five studies with 951 patients reported the relationship between PVS and END. Two studies were excluded from the meta-analysis due to the different criteria time of END. PVS was significantly associated with increased risk of END (RR 2.77, 95% CI 2.21–3.46, p<0.001, I2 = 0%) (Figure 5A). Six studies (n = 352) explored the relationship between PVS and HT. Among them, one study was excluded from the meta-analysis due to the definition time of HT was unclear. There was no significant association with HT (RR 0.97, 95% CI 0.64–1.47, p = 0.889, I2 = 0%) (Figure 5B).
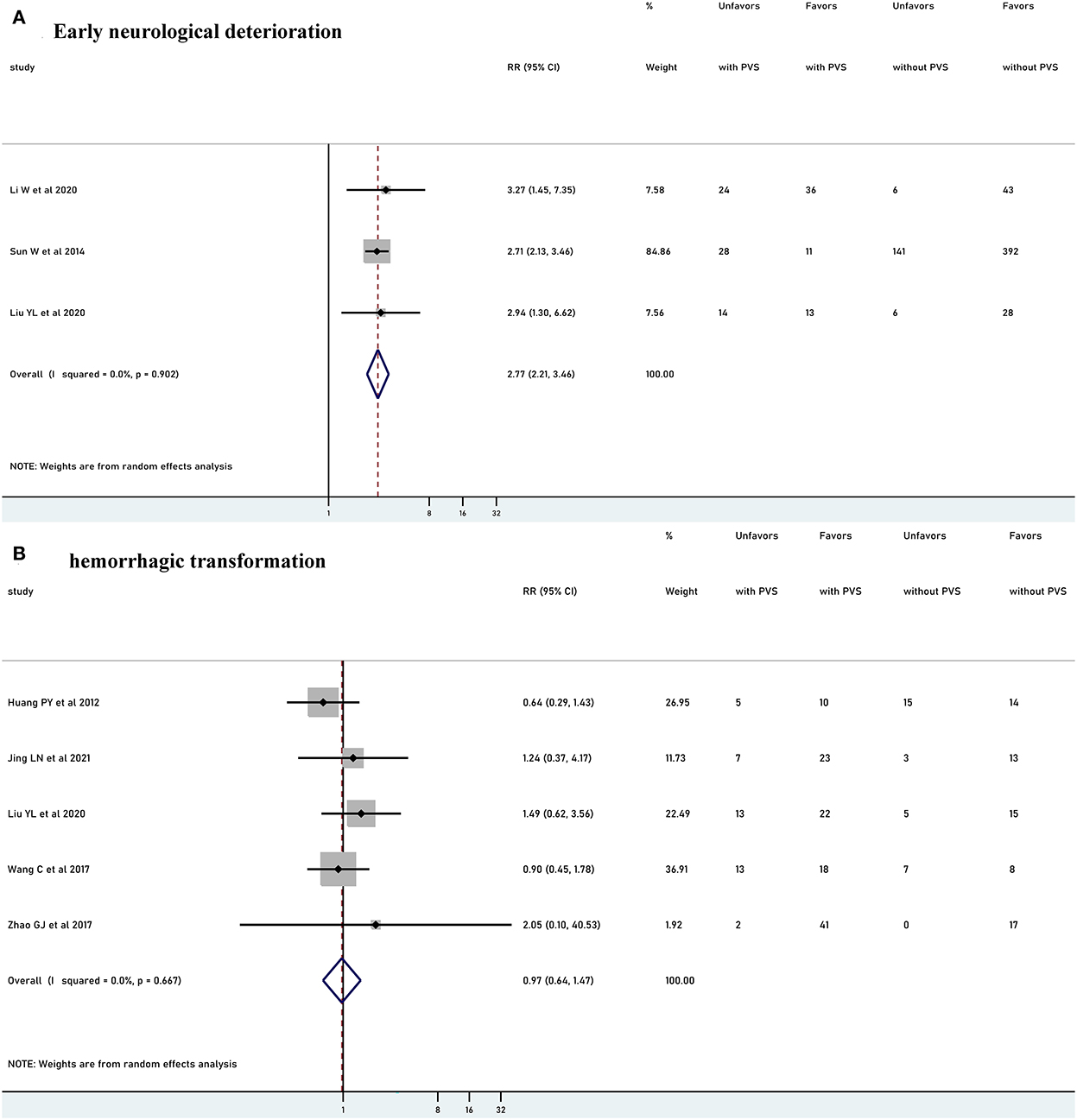
Figure 5. Meta-analysis of relationships between PVS and early neurological deterioration (A) and meta-analysis of relationships between PVS and hemorrhagic transformation (B). PVS, prominent vessel sign; Unfavors, unfavorable functional outcome; Favors, favorable functional outcome.
Publication bias
The funnel plot was symmetrical, indicating no publication bias in this meta-analysis. The Begg's and Egger's tests were insignificant, indicating no publication bias (Supplementary Figures 1–4).
Discussion
In this systematic review and meta-analysis, we have identified the key factors determining the presence of PVS that were previous stroke/TIA, AF, and SILASO. While overall PVS was associated with an unfavorable outcome especially when recanalization treatment was performed, but not in patients treated conservatively. PVS was also associated with END but without an increased risk of HT. Those results may be helpful for clinicians to identify the high-risk populations with poor prognosis after AIS, thus providing timely and appropriate interventions.
The incidence of PVS has been reported to be ranging from 6.8 to 64% (16, 23, 29). Patients with cerebral artery stenosis or occlusion were more likely to present with PVS (30–32). Our results suggest that PVS was significantly related to SILASO, which was consistent with the published findings. The underlying mechanism of PVS was an imbalance that occurred between cerebral oxygen supply and demand in the infarct area after AIS, which caused an increased level of deoxyhemoglobin in the vessels (33). Severe stenosis/occlusion of the cerebral arteries led to a more significant decrease in cerebral blood flow to the distal tissues, resulting in a more pronounced increase in deoxyhemoglobin. Furthermore, our analysis showed that the presence of PVS was also associated with a history of AF. One explanation may be that embolization could cause stenosis of large arteries in patients with AF (34). Association between PVS and previous stroke/TIA may be supported by the fact that patients with a previous stroke were more likely to have stenosis in the large cerebral arteries.
Our research demonstrated that PVS was significantly associated with poor prognosis in those who received recanalization therapy. Early recanalization has been proven to be the first line of therapy to improve the outcome of patients with AIS (35). However, there was a significant number of patients with poor outcomes despite successful recanalization (36, 37). A growing body of evidence suggests that the perfusion status of ischemic brain regions was closely related to the prognosis after recanalization (38, 39). To some extent, PVS indicated a state of perfusion in AIS that reduces the efficacy of recanalization and increases the risk of poor prognosis (20). Bilk et al. (40) reported that all 10 patients with hyperacute ischemic stroke complicated by large-artery occlusion showed notable PVS before recanalization therapy, while there were no longer observed in SWI after recanalization. PVS can be reversed or disappear by timely reperfusion, and hence can be used as an indicator to evaluate the effect of IVT or EVT to guide individualized treatment (41). Therefore, PVS still existing after treatment may be a sign of poor patient prognosis (42, 43). Notably, it is not recanalization therapy itself that leads to poor prognosis as our study showed that PVS did not increase the risk of HT. Conversely, recanalization led to more obvious differences in poor outcomes between PVS-positive and PVS-negative patients. Thus, recanalization should not be delayed owing to the appearance of PVS. Given the narrow time window constraints (<4.5 h for IVT or <6 h for EVT), the time of SWI examination was not uniform in the included studies, which limited the robustness of these results. Future stratified research is warranted with larger sample sizes.
Interestingly, the pooled results for PVS and poor prognosis in the conservative treatment group were not significant. On the one hand, it was believed that if the hypoperfusion state was not improved in time in patients with PVS, the structural integrity of brain cells was irreversibly damaged with the prolongation of the disease. Eventually, the cells lost their oxygen uptake capacity and died. At the same time, vasodilatory regulation gradually weakened, and the venous structures collapsed (44). Both these changes were manifested as the disappearance of PVS on SWI, decreasing the difference in the poor prognosis between patients with and without PVS. In contrast, it appeared that patients were treated conservatively because they were beyond the time window for recanalization and had a higher incidence of contraindications. Compared with the recanalization group, the aforementioned populations had more underlying diseases and a worse overall prognosis.
The subgroup analysis based on location revealed that both cortical and medullary veins were related to an unfavorable outcome. Medullary veins are better than cortical veins as predictors of poor prognosis. A possible pathophysiological factor may be that compared with cortical PVS, the presence of medullary PVS reflects the increase in the deoxyhemoglobin level in the draining deep medullary veins above a limit of detection, suggesting the decompensation of oxygen capacity, which is more likely to be reached in severe strokes (45, 46). Subsequent studies indicated that compared with patients with cortical PVS alone patients with both cortical and medullary PVS had larger infarct sizes and more severe neurological impairments, which was also consistent with our findings (8). Medullary PVS may be more sensitive in predicting early poor prognosis. Hence, successful reperfusion therapy may be more urgent for patients with medullary PVS. Many studies have shown that PVS occurred more in patients with SILASO, which suggests that SILASO should be taken into account in the analysis of patients with AIS. In the subgroup analysis of stenosis rate, we found that the PVS is associated with a poor functional prognosis regardless of whether the patient combined with SILASO. Moreover, patients with SILASO may be at higher risk of a poor outcome compared with without SILASO, which is consistent with prior studies reporting patients with AIS due to intracranial atherosclerosis stenosis or occlusion have a higher risk of unfavorable outcome (47).
This study had several limitations. First, the included studies were observational studies, leading to a selection bias. Second, due to the low number of studies and sample size related to END and HT, further meta-analyses with larger samples are needed.
Conclusion
Our study suggests that PVS was closely related to the poor prognosis of patients with AIS treated with recanalization. Among the PVS-positive population, the efficacy of aggressive reperfusion therapy was not significant. However, we believe that reperfusion therapy should not be postponed because of the presence of PVS. PVS is valuable as a noninvasive imaging marker for predicting prognosis and warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
ZL and WX conceived the idea and designed the research. MZ and HW searched databases and performed initial screening. YM and HZha performed data extraction and interpretation. ZS and YL performed the statistical analysis. WX wrote the first draft of the manuscript. ZL, WL, and HZhe revised the manuscript. All the authors read and approved the final version of the manuscript for publication.
Acknowledgments
We thank all the authors related to the study of PVS-SWI.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1052035/full#supplementary-material
Abbreviations
PVS, Prominent veins sign; SWI, Susceptibility-weighted imaging; AIS, Acute ischemic stroke; END, Early neurological deterioration; HT, Hemorrhagic transformation; IVT, Intravenous thrombolysis; EVT, Endovascular thrombectomy.
References
1. Global R. and National burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. (2019) 18:394–405. doi: 10.1016/S1474-4422(18)30500-3
3. Chao BH, Yan F, Hua Y, Liu JM, Yang Y, Ji XM, et al. Stroke prevention and control system in China: Csppc-stroke program. Int J Stroke. (2021) 16:265–72. doi: 10.1177/1747493020913557
4. Mittal P, Kalia V, Dua S. Pictorial essay: susceptibility-weighted imaging in cerebral ischemia. Indian J Radiol Imaging. (2010) 20:250–3. doi: 10.4103/0971-3026.73530
5. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. (2009) 30:19–30. doi: 10.3174/ajnr.A1400
6. Zaitsu Y, Kudo K, Terae S, Yazu R, Ishizaka K, Fujima N, et al. Mapping of cerebral oxygen extraction fraction changes with susceptibility-weighted phase imaging. Radiology. (2011) 261:930–6. doi: 10.1148/radiol.11102416
7. Morita N, Harada M, Uno M, Matsubara S, Matsuda T, Nagahiro S, et al. Ischemic findings of T2*-weighted 3-Tesla Mri in acute stroke patients. Cerebrovasc Dis (Basel, Switzerland). (2008) 26:367–75. doi: 10.1159/000151640
8. Jing LN, Sui BB, Shen M, Qin HQ, Gao PY. Are prominent medullary veins better than prominent cortical veins as predictors of early clinical outcome in patients with acute ischemic stroke? Diagnos Interven Radiol. (2021) 27:285–92. doi: 10.5152/dir.2021.19644
9. Li W, Xiao WM, Luo GP, Liu YL, Qu JF, Fang XW, et al. Asymmetrical cortical vein sign predicts early neurological deterioration in acute ischemic stroke patients with severe intracranial arterial stenosis or occlusion. BMC Neurol. (2020) 20:331. doi: 10.1186/s12883-020-01907-w
10. Liu YL, Xiao WM, Lu JK, Wang YZ, Lu ZH, Zhong HH, et al. Asymmetrical cortical vessel sign predicts prognosis after acute ischemic stroke. Brain Behav. (2020) 10:e01657. doi: 10.1002/brb3.1657
11. Liu YL, Yin HP, Qiu DH, Qu JF, Zhong HH, Lu ZH, et al. Multiple hypointense vessels on susceptibility-weighted imaging predict early neurological deterioration in acute ischaemic stroke patients with severe intracranial large artery stenosis or occlusion receiving intravenous thrombolysis. Stroke Vasc Neurol. (2020) 5:361–7. doi: 10.1136/svn-2020-000343
12. Oh M, Lee M. Clinical implications of prominent cortical vessels on susceptibility-weighted imaging in acute ischemic stroke patients treated with recanalization therapy. Brain Sci. (2022) 12:184. doi: 10.3390/brainsci12020184
13. Sun W, Liu W, Zhang Z, Xiao L, Duan Z, Liu D, et al. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: a novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur J Neurol. (2014) 21:1411–8. doi: 10.1111/ene.12510
14. Vural A, Gocmen R, Oguz KK, Topcuoglu MA, Arsava EM. Bright and dark vessels on stroke imaging: different sides of the same coin? Diagnos Interv Radiol. (2016) 22:284–90. doi: 10.5152/dir.2015.15271
15. Wang Y, Xiao JJ, Zhao L, Wang SS, Wang MM, Luo Y, et al. The frequency and associated factors of asymmetrical prominent veins: a predictor of unfavorable outcomes in patients with acute ischemic stroke. Neural plasticity. (2021) 2021:9733926. doi: 10.1155/2021/9733926
16. Wang YQ, Shi TM, Chen B, Lin GP, Xu YY, Geng Y. Prominent hypointense vessel sign on susceptibility-weighted imaging is associated with clinical outcome in acute ischaemic stroke. Eur Neurol. (2018) 79:231–9. doi: 10.1159/000488587
17. Yu JC, Wang LM Li ZZ, Wang SS, Wang GB. Related factors of asymmetrical vein sign in acute middle cerebral artery stroke and correlation with clinical outcome. J Stroke Cerebrovasc Dis. (2017) 26:2346–53. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.023
18. Yu X, Yuan L, Jackson A, Sun J, Huang P, Xu X, et al. Prominence of medullary veins on susceptibility-weighted images provides prognostic information in patients with subacute stroke. Am J Neuroradiol. (2016) 37:423–9. doi: 10.3174/ajnr.A4541
19. Zhang X, Zhang S, Chen Q, Ding W, Campbell BCV, Lou M. Ipsilaterat prominent thalamostriate vein on susceptibility-weighted imaging predicts poor outcome after intravenous thrombolysis in acute ischemic stroke. Am J Neuroradiol. (2017) 38:875–81. doi: 10.3174/ajnr.A5135
20. Zhao GJ, Sun L, Wang ZR, Wang LQ, Cheng ZR, Lei HY, et al. Evaluation of the role of susceptibility-weighted imaging in thrombolytic therapy for acute ischemic stroke. J Clin Neurosci. (2017) 40:175–9. doi: 10.1016/j.jocn.2017.01.001
21. Hong Y, Zhu YL, Huang ZF, Zhai DY, Zhang ZH, Wang SL. Prognostic value of deep medullary vein signs on magnetic sensitive weighted imaging in acute anterior circulation cerebral infarction. Chin J Neuromed. (2019) 18:464–9. doi: 10.3760/cma.j.issn.1671-8925.2019.05.006
22. Hu ZJ, Tan Q, Liu L, Huang RX, Li Z, Zhu GM, et al. Correlation between asymmetrically prominent cortical veins on susceptibility-weighted imaging and early neurological deterioration in patients with acute ischemic stroke. Int J Cerebrovasc Dis. (2020) 28:87–92. doi: 10.3760/cma.j.issn.1673-4165.2020.02.002
23. Chen CY, Chen CI, Tsai FY, Tsai PH, Chan WP. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: prediction of infarct growth and clinical outcome. PloS ONE. (2015) 10:e0131118. doi: 10.1371/journal.pone.0131118
24. Huang PY, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol. (2012) 259:1426–32. doi: 10.1007/s00415-011-6369-2
25. Liu H, Mei W, Huang Y, Li Y, Liu H, Zhang J. The association between asymmetrically hypointense veins on susceptibility-weighted imaging and collateral circulation. Chin J Neurol. (2018) 51:21–7. doi: 10.3760/cma.j.issn.1006-7876.2018.01.005
26. Wang C, Wu S, Gong X. Asymmetrical hypointense cortical veins on susceptibility weighted imaging in patients with acute cerebral infarction. Chin J Neurol. (2017) 50:452–6. doi: 10.3760/cma.j.issn.1006-7876.2017.06.010
27. Jia YN, Liu CC, Liu JY. Predictive value of asymmetrical cortical vein sign based on susceptibility weighted imaging for prognosis of patients with acute ischemic stroke. Chin J Stroke. (2019) 14:639–44. doi: 10.3969/j.issn.1673-5765.2019.07.003
28. Lu P, Cui L, Zhao X. The prognostic impact of susceptibility-weighted imaging prominent veins in acute ischemic stroke: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. (2021) 17:3069–79. doi: 10.2147/NDT.S331874
29. Mundiyanapurath S, Ringleb PA, Diatschuk S, Burth S, Möhlenbruch M, Floca RO, et al. Cortical vessel sign on susceptibility weighted imaging reveals clinically relevant hypoperfusion in internal carotid artery stenosis. Eur J Radiol. (2016) 85:534–9. doi: 10.1016/j.ejrad.2015.12.020
30. Liu Z, Li Y. Cortical cerebral blood flow, oxygen extraction fraction, and metabolic rate in patients with middle cerebral artery stenosis or acute stroke. AJNR Am J Neuroradiol. (2016) 37:607–14. doi: 10.3174/ajnr.A4624
31. Jiang HF, Zhang YQ, Pang JX, Shao PN, Qiu HC, Liu AF, et al. Factors associated with prominent vessel sign on susceptibility-weighted imaging in acute ischemic stroke. Sci Rep. (2021) 11:1–8. doi: 10.1038/s41598-021-84269-8
32. Payabvash S, Benson JC, Taleb S, Rykken JB, Hoffman B, Oswood MC, et al. Prominent cortical and medullary veins on susceptibility-weighted images of acute ischaemic stroke. Br J Radiol. (2016) 89:20160714. doi: 10.1259/bjr.20160714
33. Rauscher A, Sedlacik J, Barth M, Haacke EM, Reichenbach JR. Nonnvasive assessment of vascular architecture and function during modulated blood oxygenation using susceptibility weighted magnetic resonance imaging. Mag Reson Med. (2005) 54:87–95. doi: 10.1002/mrm.20520
34. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. (2016) 47:895–900. doi: 10.1161/STROKEAHA.115.012004
35. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D. et al. Thrombolysis with alteplase 3 to 45 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
36. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England). (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
37. Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of Ecass, Atlantis, Ninds, and Epithet Trials. Lancet (London, England). (2010) 375:1695–703. doi: 10.1016/S0140-6736(10)60491-6
38. Bivard A, Levi C, Krishnamurthy V, McElduff P, Miteff F, Spratt NJ, et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. (2015) 138:1919–31. doi: 10.1093/brain/awv071
39. Li Z, Bai X, Gao P, Lin Y, Ju Y, Sui B. Changes of prominent vessel sign and susceptibility vessel sign in acute ischemic stroke patients with and without successful recanalization: a study based on susceptibility weighted images. Neurol Res. (2022) 44:583–90. doi: 10.1080/01616412.2021.2024729
40. Baik SK, Choi W, Oh SJ, Park KP, Park MG, Yang TI, et al. Change in cortical vessel signs on susceptibility-weighted images after full recanalization in hyperacute ischemic stroke. Cerebrovasc Dis. (2012) 34:206–12. doi: 10.1159/000342148
41. Luo S, Yang L, Wang L. Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol. (2015) 42:255–60. doi: 10.1016/j.neurad.2014.07.002
42. Meoded A, Poretti A, Benson JE, Tekes A, Huisman TAGM. Evaluation of the ischemic penumbra focusing on the venous drainage: the role of susceptibility weighted imaging (Swi) in pediatric ischemic cerebral stroke. J Neuroradiol. (2014) 41:108–16. doi: 10.1016/j.neurad.2013.04.002
43. Park MG, Yang TI, Oh SJ, Baik SK, Kang YH, Park KP. Multiple hypointense vessels on susceptibility-weighted imaging in acute ischemic stroke: surrogate marker of oxygen extraction fraction in penumbra? Cerebrovasc Dis. (2014) 38:254–61. doi: 10.1159/000367709
44. Xia H, Sun H, He S, Zhao M, Huang W, Zhang Z, et al. Absent cortical venous filling is associated with aggravated brain edema in acute ischemic stroke. AJNR Am J Neuroradiol. (2021) 42:1023–9. doi: 10.3174/ajnr.A7039
45. Yan S, Wan J, Zhang X, Tong L, Zhao S, Sun J, et al. Increased visibility of deep medullary veins in leukoaraiosis: A 3-T Mri study. Front Aging Neurosci. (2014) 6:144. doi: 10.3389/fnagi.2014.00144
46. Mucke J, Möhlenbruch M, Kickingereder P, Kieslich PJ, Bäumer P, Gumbinger C, et al. Asymmetry of deep medullary veins on susceptibility weighted Mri in patients with acute Mca stroke is associated with poor outcome. PloS ONE. (2015) 10:e0120801. doi: 10.1371/journal.pone.0120801
Keywords: acute ischemic stroke, meta-analysis, prognosis, prominent veins, susceptibility-weighted imaging (SWI)
Citation: Xiang W, Liang Z, Zhang M, Wei H, Sun Z, Lv Y, Meng Y, Li W, Zheng H and Zhang H (2022) Prognostic value of susceptibility-weighted imaging of prominent veins in acute ischemic stroke: A systematic review and meta-analysis. Front. Neurol. 13:1052035. doi: 10.3389/fneur.2022.1052035
Received: 23 September 2022; Accepted: 25 October 2022;
Published: 01 December 2022.
Edited by:
Shinichiro Uchiyama, Sanno Medical Center, JapanReviewed by:
Sohei Yoshimura, National Cerebral and Cardiovascular Center, JapanZhu Zhengyu, The Second Hospital of Shandong University, China
Copyright © 2022 Xiang, Liang, Zhang, Wei, Sun, Lv, Meng, Li, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Liang, emdsaWFuZyYjeDAwMDQwO2hvdG1haWwuY29t
 Wei Xiang
Wei Xiang Zhigang Liang
Zhigang Liang Manman Zhang1,2
Manman Zhang1,2 Hongchun Wei
Hongchun Wei Wei Li
Wei Li Huaguang Zheng
Huaguang Zheng