
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 19 December 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1048502
This article is part of the Research Topic Women in Multiple Sclerosis and other demyelinating disorders: a global perspective View all 8 articles
Antibody-mediated central nervous system (CNS) disorders including those associated with aquaporin-4 or myelin oligodendrocyte glycoprotein IgG and autoimmune encephalitis often affect women of childbearing age. Pathogenic antibodies of these diseases can potentially alter reproductive functions and influence fetal development. Hormonal changes occurring during pregnancy may modify the course of autoimmune diseases by influencing relapse risk, attack severity, and affect the delivery and postpartum period. Moreover, balancing treatment related safety issues with the risk of potentially disabling relapses during pregnancy and breastfeeding are major challenges. Intentional prenatal, gestational, and post-partum counseling is paramount to address these issues and mitigate these risks. Fortunately, new insights on risk factors for adverse pregnancy outcomes and possible preventive strategies are emerging. This review aims to summarize the interplay between antibody-mediated CNS disorders and pregnancy during the prenatal, gestational, and postpartum periods, highlight current treatment recommendations, and discuss future areas of research.
The most common Antibody (Ab)-mediated disorders of the central nervous system (CNS) are aquaporin-4 antibody neuromyelitis optica spectrum disorders (AQP4+NMOSD), myelin-oligodendrocyte glycoprotein antibody-associated disease (MOGAD), and autoimmune encephalitis (AE). These diseases are often diagnosed in women of childbearing age (1–3).
Pregnancy-related hormonal fluctuations can influence autoimmune diseases by altering the disease course, while pregnancy itself can significantly impact treatment options in clinical practice. Conversely, pathogenic antibodies of Ab-mediated diseases may alter fertility and reproductive function as well as target the placenta, contributing to adverse pregnancy outcomes and altering fetal development. These factors should be carefully evaluated in women with Ab-mediated disorders to provide comprehensive pre-pregnancy planning, minimize disease activity, and disease burden during gestation, and optimize successful delivery and child and maternal health in the post-partum period.
The aim of this review is to summarize the interplay between Ab-mediated diseases and expectant mothers during the prenatal, gestational, and postpartum periods, highlight current treatment recommendations, and discuss emerging insights and research. As the interactions between the underlying disease and pregnancy can vary between these conditions, we will summarize the key elements separately for AQP4+NMOSD, MOGAD, and AE. For each disease, we will discuss (i) the current state of the art (i.e., “what do we know”) regarding fertility, pregnancy, the postpartum period and treatment strategies, and (ii) future areas of research (i.e., “what should we know”). Table 1 summarizes currently available studies reporting data on pregnancy-related outcomes in AQP4-Ab- and MOG-Ab-mediated disorders; Table 2 provides an overview on the impact of the diseases on pregnancy and vice versa. Figure 1 summarizes the interplay between pregnancy and AE. Box 1 highlights future areas of research.
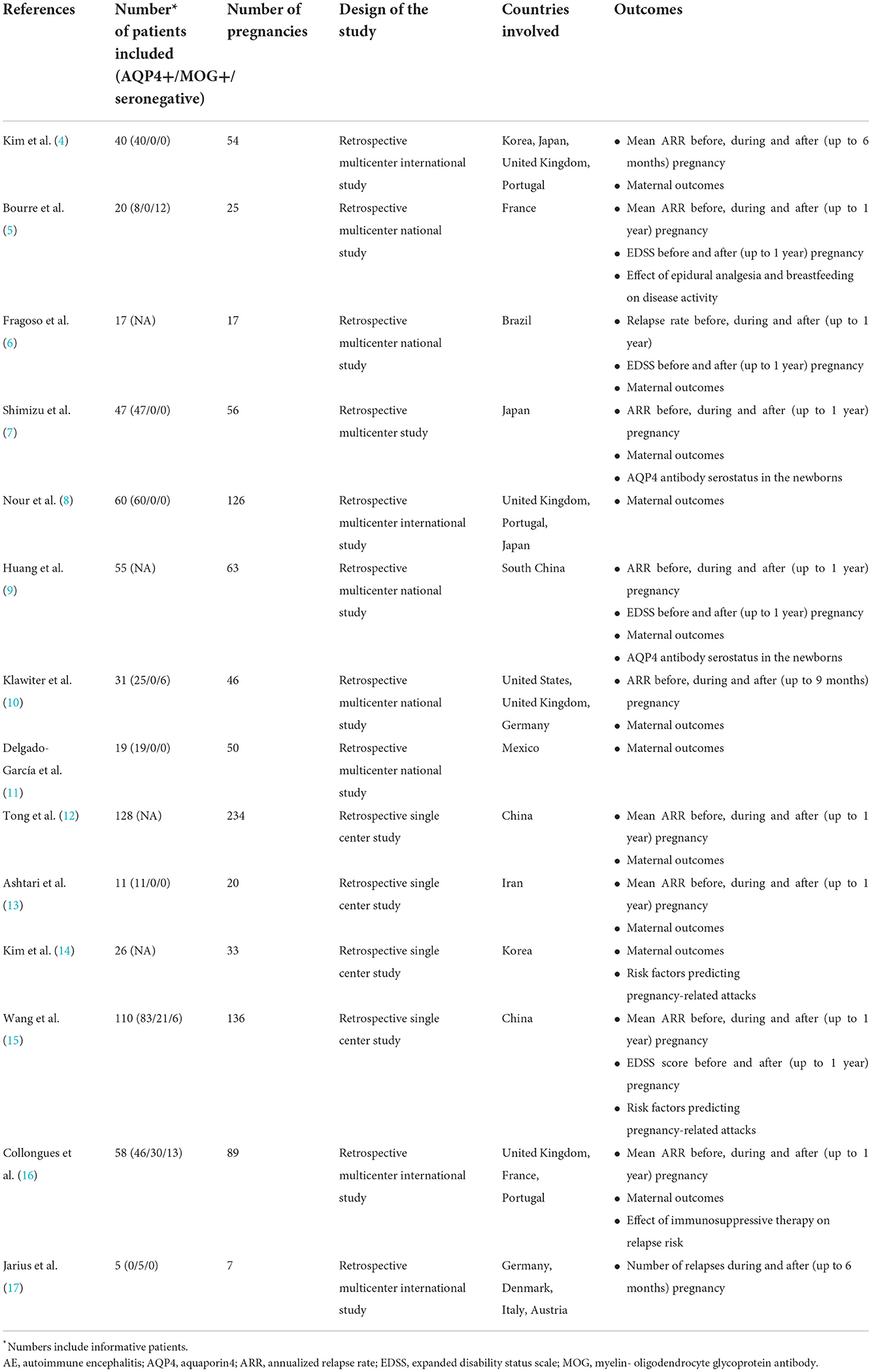
Table 1. Currently available studies assessing the relationship between aquaporin 4 (AQP4) and myelin-oligodendrocyte glycoprotein (MOG) antibody-mediated disorders of the central nervous system (CNS) and pregnancy.
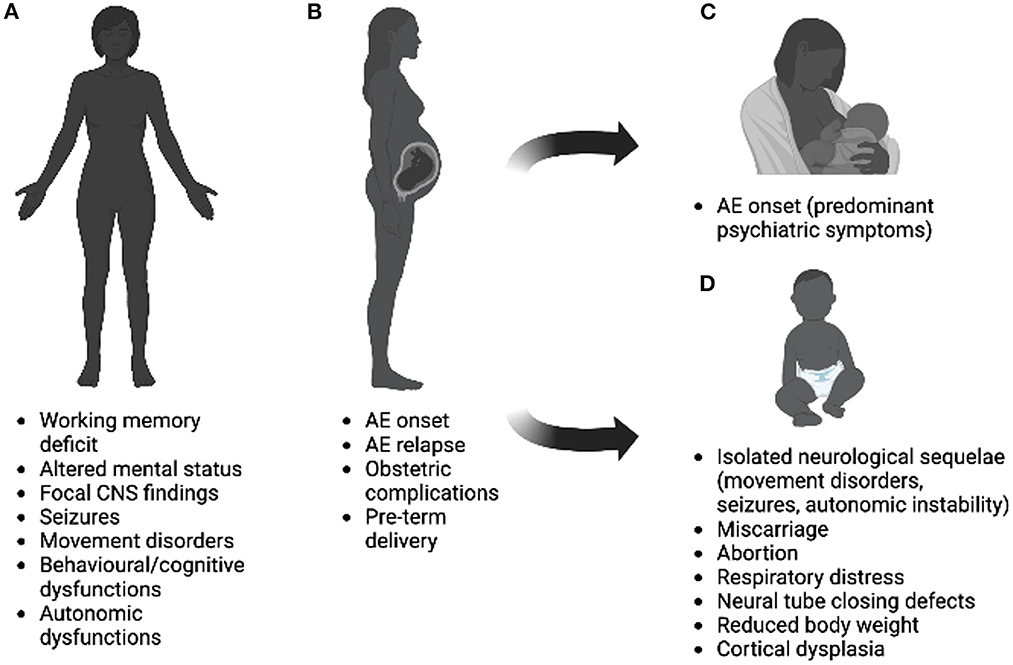
Figure 1. Interplay between pregnancy and autoimmune encephalitis on women before (A), during (B), after (C) pregnancy, and effects on newborns (D). Possible effects illustrated in the figure refer to rare cases, mostly linked to anti-NMDAR encephalitis and can results from the cumulative effect of antibodies, sedatives, and antiepileptic drugs. Image was created using Biorender.com.
Box 1. Summary of areas for future research in the three diseases.
All the Ab-mediated diseases included in this review are rare disorders. Multicenter and possibly registry-based real-word studies are needed to assess the impact of pregnancy on CNS autoimmune mediated disorders and vice versa
Future works should:
• Stratify NMOSD patients by serotype (AQP4-Ab seropositive, seronegative, and double seronegative)
• Evaluate MOGAD separately, as this is now recognized as a distinct disease
• Evaluate if clinical features of NMOSD, MOGAD, or AE (i.e., different phenotypes at onset, number and recovery of relapses, age at conception, treatment) or Ab-characteristics (titers, timing, and persistence over time) may be independent risk factors for pregnancy-related relapses
• Clarify the correlation between mother's serum antibody titer and obstetric or fetal complications to facilitate pre-conceptual planning and postpartum follow-up
• Define guidelines for the treatment of NMOSD, MOGAD, and AE before, during and after pregnancy
• Provide a detailed long-term neuropsychological assessment of children in all Ab-mediated diseases of the CNS to define short and long-term outcomes.
Content for this paper was gathered by PubMed literature review for articles published between 2012 and 2022 with the following search terms: “aquaporin 4,” “neuromyelitis optica spectrum disorder,” “myelin- oligodendrocyte glycoprotein antibody-associated disease,” “autoimmune encephalitis,” “NMDAr,” “LGI1,” “CASPR2,” “fertility,” “partum,” “delivery,” “postpartum,” “pregnancy,” “breastfeeding,” “fetus,” “gestation,” and “treatment.”
Neuromyelitis optica (NMO; previously known as Devic's disease) is a severely disabling Ab-mediated astrocytopathy with secondary demyelination (18, 19). Anti-AQP4 antibodies (AQP4-Ab) have been recognized as the pathogenic hallmark of the disease. The term of NMO spectrum disorders (NMOSD) has been recently adopted to identify conditions in which CNS involvement is not restricted to the optic nerves and spinal cord (1).
The currently established international criteria for NMOSD allow for a minority of patients with no detectable anti-AQP4-Ab to be diagnosed as seronegative NMOSD if specific clinical and magnetic resonance imaging (MRI) requirement are satisfied and alternative diagnoses have been excluded (1). Pathophysiological mechanisms underlying seronegative cases remain unclear, but likely differ from those of seropositive NMOSD (20, 21). Studies report that 10–40% of seronegative NMOSD patients have IgG Ab against myelin oligodendrocyte glycoprotein (MOG) and distinct clinical, epidemiologic and radiological features (17, 22).
NMOSD associated with AQP4-Ab (AQP4+NMOSD) is a rare condition with a prominent female predominance (female-to-male ratio: from 9:1 to 10:1) (20, 23). Genetic, epigenetic or hormonal factors can contribute to female susceptibility as in other autoimmune disorders (24, 25). The typical onset of AQP4+NMOSD occurs between the third and fourth decades of life, when many women are still considering childbearing (23, 26) and highlights the importance of understanding the effects of this disease on fertility, pregnancy, and the postpartum period.
The impact of AQP4+NMOSD on fertility is unclear and poorly investigated. In a multicenter study, Bove et al. evaluated 217 NMOSD women (82% AQP4-Ab seropositive) using a standardized reproductive survey. Six percentage of these patients received infertility treatment, while 13% reported a delay of at least 12 months in achieving pregnancy (27). However, the mean age of NMOSD patients at onset was 40 years and the age at conception was not specified, thus biasing the results for age related infertility which is known to rise with increasing female age (28). Another study measured anti-Mhormone as a marker of ovarian reserve and found that levels were reduced in 14 NMOSD patients (11 AQP4-Ab seropositive, 2 AQP4-Ab seronegative, 1 MOG-Ab seropositive) when compared to 8 healthy controls. However, data were not corrected for factors potentially influencing fertility such as comorbidities or immunotherapies, which limits the generalizability of these findings (29).
AQP4 has variable expression in the female reproductive tracts of adult mammals, having been identified in the uterus and cervix, but not in the ovaries (30). Although AQP4 is thought to be involved in the physiology and pathophysiology of the reproductive system and in pregnancy (30), the exact mechanisms by which AQP4-Ab can affect female fertility remains unknown. In an experimental study, female AQP4-knockout mice showed reduced fertility with defective folliculogenesis, reduced corpora lutea formation, and decreased uterine response to gonadotropins, probably related to a dysregulation of the hypothalamus-pituitary-ovary axis (31). This may be explained by the fact that AQP4 is highly expressed in some brain regions, such as the periventricular area and the paraventricular hypothalamic nucleus, which are involved in the regulation of gonadotropin-releasing hormone neurons and influence the secretion of sexual hormones (32). Alternatively, hypothalamic dysfunctions and secondary endocrinopathies influencing fertility might affect NMOSD patients with diencephalic lesions (33). Finally, some immunosuppressive drugs such as cyclophosphamide or mitoxantrone may be linked to changes in female fertility and ovarian reserve.
Another fertility consideration is the possible effect of AQP4 expression on the placenta and transplacental migration. Autoantibodies, such as the acetylcholine receptor-Ab or Anti-Ro-Ab, can be transmitted to the fetus through transplacental transportation, leading to pregnancy complications and influencing perinatal outcomes. Whether placental or fetal damage may be due to AQP4-Ab exposure is not known. Retrospective studies, including mainly AQP4-Ab cases, showed that NMOSD patients have an increased rate of pregnancy complications, including the risk of miscarriage (8, 10, 11). In particular, one large retrospective study of 60 AQP4+NMOSD women with 126 pregnancies found that pregnancies after NMOSD onset were associated with an increased risk of miscarriage when compared to pregnancies before the onset, independently from the risk associated with an advanced maternal age, particularly in those patients with high disease activity before conception and during pregnancy (8). The exact mechanism underlying miscarriage in NMOSD is also unknown. AQP4-Ab could be a possible causative agent of spontaneous abortion, as AQP4 is expressed in the syncytiotrophoblast of human and mouse placenta, especially during the second trimester. In animal models, the transfer of human AQP4-IgG bound mouse placental AQP4, activated coinjected human complement and led to the induction of placentitis and fetal death (34). In parallel, several regions of necrosis with multiple infarcts were observed in a patient with NMOSD who miscarried in the second trimester, mainly in the maternal part of the placenta (35). AQP4 immunostaining showed a complete loss of immunoreactivity. High titers of circulating antibodies in the context of active disease could favor placental damage and miscarriage. Contrasting results are reported by another recent study including six pregnant AQP4+NMOSD patients, their infants, and three healthy controls. Histological investigation showed no significant difference in the intensity of the immunohistochemical staining for AQP4, and in inflammatory markers in placentae of patients and controls. Four of the six patients were term pregnancy and their infants had a normal development despite showing AQP4-Ab at the time of birth (36). Taking these preliminary data into account, the hypothesis suggesting that AQP4-Ab may cause placentitis with a risk of miscarriage should be further investigated.
The rate of preeclampsia in NMOSD was found to be similar to that of the general population (16), whereas its risk increased in women with at least two other concomitant autoimmune diseases, regardless of NMOSD onset. Additional autoimmune conditions are reported in 20–30% of patients with NMOSD (37), some of which (e.g., systemic lupus erythematosus and antiphospholipid syndrome), are known risk factors for preeclampsia in the general population (38).
AQP4-Ab crosses the placental barrier and can be detected in the blood of newborns at birth. However, infants become seronegative 1–3 months later and usually do not have neurological symptoms (7, 39, 40).
The available evidence on fetal outcomes in NMOSD patients is scarce. In a systematic review and meta-analysis neonatal complications, including low birth weight and stillbirth, were described in 33/619 (5.3%) of the informative pregnancies (41). Among these, immunosuppressive treatment during pregnancy was associated with neonatal complications in 13 events. The possible teratogenic effects of some drugs used in NMOSD during pregnancy need to be specifically evaluated (42).
There is a growing number of retrospective studies evaluating the effect of pregnancy and the postpartum period on NMOSD. While they all report an increased relapse rate postpartum, data on the interplay between the gestation period and the disease are not conclusive (4–7, 9, 10, 12, 15). Differences between study findings may be due to the heterogeneity of the cohorts, including seronegative NMOSD patients as well as those with different treatment history and onset during gestation.
Recently, a retrospective multicenter study assessed the effect of pregnancy on 58 women with NMOSD, stratifying patients by antibody status (AQP4-Ab, MOG-Ab, double-seronegative). Eighty-nine pregnancies were observed. Patients had a reduced risk of relapse during pregnancy in each serostatus group when compared to the pre-pregnancy period, while the annualized relapse rate was higher during the first postpartum trimester only in AQP4-Ab positive women (16). Factors associated with a reduced risk of NMOSD attacks were: being on an immunosuppressive treatment during pregnancy and an older age at conception (7, 41, 43, 44).
The mechanisms underlying the effect of pregnancy on NMOSD have not been sufficiently investigated. It has been suggested that high estrogen levels may influence AQP4-Ab type, titer, and glycosylation pattern, as well as stimulate the differentiation of antibody-producing B-cells. Moreover, a shift toward a Th2-mediated immunity, which occurs during pregnancy, could sustain NMOSD pathogenesis (45).
To date, there are no studies specifically investigating the effect of obstetrical analgesia or type of delivery on disease activity in NMOSD. However, retrospective and observational data suggest that cesarean delivery, spinal, or epidural anesthesia do not affect the disease (4, 5, 9, 10). Likewise, breastfeeding does not appear to influence on the disease course (4, 5). Given the paucity of data, the management of patients with NMOSD could be guided by extensive experience in multiple sclerosis (MS). In MS, the type of delivery and anesthetic options are not influenced by the disease unless there is significant disability and is based on obstetric criteria (46).
In conclusion, current evidence indicates that pregnancy is associated with a risk of relapse in women with AQP4-Ab NMOSD, especially in the postpartum period and in young women with no previous immunosuppressive treatment. Due to the rarity of the disease, prospective and large cohort studies are scarce.
Relapses can be devastating in NMOSD resulting in permanent disability. Thus, prevention and timely treatment of relapses is of primary importance. During pregnancy and breastfeeding, specific considerations and a careful risk-benefit analysis should be performed, considering the possible effects of drugs on the fetus and infant. To date, specific treatment guidelines are not available. However, a detailed review with recommendations based on available evidence and expert opinion on the therapeutic management of NMOSD during pregnancy have recently been published (42).
Acute treatment of relapses with methylprednisolone, plasma exchange and immunoadsorption is possible during pregnancy and breastfeeding. Corticosteroids may cross into the fetal circulation, depending on the type of steroids administered, with low risk when non-fluorinated corticosteroids such as prednisone, prednisolone, and methylprednisolone are used after the first trimester. Since corticosteroid levels in the breast milk are low, lactation is also feasible. Nevertheless, it would be advisable to delay breastfeeding for at least 4 h from treatment if high doses of methylprednisolone are administered (42).
Plasma exchange has been used in pregnant women with other Ab-mediated conditions, such as antiphospholipid Ab-syndrome and thrombotic thrombocytopenic purpura, with no evidence of increased risk of adverse effects. This treatment is considered relatively safe for acute NMOSD relapse, but an accurate risk-benefit evaluation is advised, with the same indications applied for immunoadsorption (42).
Considering the effect of pregnancy on the course of NMOSD, especially in the postpartum period, the immunosuppressive treatment choice before conception and the decision to stop or continue these drugs during pregnancy and after delivery requires careful evaluation. Immunosuppressant treatment discontinuation or insufficient immunosuppression have been proposed as risk factors for NMOSD attacks during or soon after pregnancy (7, 41, 44). Yet, NMOSD treatments may be harmful to the fetus and many lack adequate safety data in this population.
Cyclophosphamide and mitoxantrone are contraindicated during pregnancy and breastfeeding. Due to their potential ovarian toxicity, they should also be avoided in women of childbearing age.
Mycophenolate mofetil and methotrexate carry a high risk of miscarriage and congenital malformations. If necessary, an appropriate wash-out period before attempted conception must be ensured, while breastfeeding should be avoided in women on these treatments (42, 47).
Azathioprine has been deemed a relatively safe therapy during pregnancy and lactation based on a large number of exposed pregnant women with several other autoimmune conditions. After a risk-benefit evaluation, especially in active NMOSD patients, continuing azathioprine may be considered (42, 47).
Rituximab readily crosses the placenta from the second trimester and depletes fetal B-cells, an effect that reverts within 6 months from birth (48). Recently, it has been demonstrated that rituximab, administered within 6 months of conception or during pregnancy in more than 100 women with MS or NMOSD, was not associated with an increased risk of adverse outcomes (49). Considering the growing evidence on the use of anti-CD20 therapies in women of childbearing age, the use of rituximab in selected cases might be considered, ensuring a period of at least 3 months between the last infusion and conception (49, 50), or less as suggested by some experts (42). Moreover, IgG1-based monoclonal antibodies are minimally transferred into breastmilk. In particular, the “relative infant dose” for rituximab is < 0.4% and significantly less than the acceptable threshold of 10%, alluding to its probably safety during breastfeeding (51).
Data on the safety of tocilizumab use during pregnancy come primarily from patients with rheumatological diseases. In an analysis from a global safety database, prospective, and retrospective data on pregnancies during tocilizumab treatment showed a slightly increased risk of miscarriage, preterm birth, and malformations without a distinct pattern. However, one third of these patients with adverse outcomes were concomitantly treated with methotrexate and leflunomide (52). Of the few reports concerning breastfeeding in tocilizumab treated patients, none have reported negative effects on the infant. Therefore, the American College of Rheumatology concluded that treatment until conception and breastfeeding during treatment are supported by conditional evidence (53). To date, a single report described a double-seronegative NMOSD woman with a highly active disease course who continued tocilizumab until the 28th week of gestation and resumed infusions 4 days after delivery. Her pregnancy course was clinically unremarkable and no congenital malformation nor hematological alterations were detected in the infant up to 1 year of age (54). Based on this data, NMOSD experts suggest tocilizumab can be used during pregnancy in patients with severe disease and during breastfeeding, after a careful risk-benefit analysis has been conducted.
To date, there is scant data concerning pregnancy and breastfeeding in women with NMOSD treated with new monoclonal antibodies (mAbs). However, clues from other indications and drugs with similar mechanisms of action are available. Satralizumab, a IL-6 receptor mAb, is expected to have no specific teratogenic effects in humans, due to its similar mechanism of action with tocilizumab. Similarly, inebilizumab, an anti-CD19 mAb, may have a comparable safety profile in pregnancy and on the newborn to other B-cell-depleting. Data from a ten-year real world hematological registry of eculizumab, a mAbs against the C5 fraction of complement, suggest that the rate of live births without fetal/maternal complications associated with eculizumab-exposure during pregnancy and the postpartum period were consistent with that of the general population (55). Moreover, eculizumab is not detected in breast milk samples (56), making it a potential option in pregnant and breastfeeding women with NMOSD.
To summarize, severe relapses may be treated during pregnancy in NMOSD, and due to the high risk of relapses in the postpartum period, when the immunosuppressant treatment has been stopped, an early resumption of the drug should be carefully evaluated.
Despite the growing interest on the effect of pregnancy on NMOSD and vice versa, available studies are mainly retrospective and include a relatively small number of cases. Given the rarity of this condition, international and collaborative prospective registries are needed. Moreover, rigorous inclusion criteria should be shared among studies to include more homogenous groups. Separating AQP4 seropositive from seronegative NMOSD patients would also be advantageous acknowledging the limited sample sizes of this approach. In the future, pregnant women should be further stratified according to their ethnicity and clinical factors (i.e., number and severity of previous attacks, use of immunosuppressive treatments) to assess whether these factors may influence the disease course.
Immunological changes occurring during pregnancy and the role of pregnancy-related hormones should be investigated to better understand their possible effect on NMOSD immunopathogenesis. For example, any variation in immune regulatory cells and complement, as well as in IL-6 and neurofilament light chain levels, evaluated during pregnancy may be informative. Speculations on this topic have all focused on AQP4 autoimmunity. The role of the placenta as a possible source of AQP4 in disease onset and in triggering relapses should be elucidated, as well as the relationship between the transfer of AQP4-Ab into the placenta and complications of pregnancy.
Finally, the decision to stop or continue immunosuppressive treatment during pregnancy and breastfeeding remains controversial. Considering the efficacy and the effect on specific targets of immunopathogenesis of AQP4+NMOSD, guidelines on the use of the new approved mAbs during pregnancy are necessary.
Myelin-oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a distinct autoimmune demyelinating disorder of the CNS characterized by the presence of a pathogenic autoantibody against a CNS-specific protein located in the outer layers of the myelin sheath (2). The immune attack of MOG-Ab is associated with myelin and oligodendrocyte damage, leading to wide and heterogeneous clinical manifestations in both pediatric and adult patients (22, 57). MOGAD can be either monophasic or relapsing, and its typical clinical phenotypes include acute disseminated encephalomyelitis (ADEM), isolated transverse myelitis, isolated optic neuritis (ON), and unilateral cerebral cortical encephalitis with epilepsy (22, 58, 59). Due to its rarity and relatively recent identification as a distinct disease, our knowledge on the epidemiology of MOGAD is still evolving. In general, MOGAD occurs in greater frequency in younger people compared with AQP4+NMOSD (60). Unlike AQP4+NMOSD, which has a significant female predominance, most studies have shown that MOGAD equally affects males and females in young children (age < 10 years), with a slight female predominance in older post-pubertal children and adults (61). MOGAD patients have been identified in the cohorts of seronegative NMOSD in previous works, which did not separately analyze results by antibody type. Therefore, studies assessing the effect of MOG-Ab on pregnancy and of pregnancy on MOGAD disease course are both limited and conflicting.
To date, there is almost no data concerning the effect of MOG-Ab on fertility and pregnancy. As for AQP4, MOG antigens can be also found in the placenta, and MOG-Ab crosses the placental barriers during the second and third trimesters. MOG-Ab are likely to be transferred to the fetus and found in the blood of the newborn (42). However, their role in the pathophysiology of pregnancy is still unknown.
MOG-specific B-cells in the peripheral immune system produce MOG-Ab, which cross the blood-brain barrier (BBB) and enter the CNS where they bind MOG on oligodendrocytes, leading to myelin injury and demyelination. T-cells are also involved in the pathogenesis of MOGAD. Indeed, T helper cells are needed for the differentiation of B-cells into specific plasma cells, as human MOG-IgG are mainly of the IgG1 phenotype. Moreover, MOG-specific CD4+ T-cells or myelin basic protein-specific T effector cells and macrophages in the CNS are increasingly activated and cytokines and chemokines levels enhanced further propagating the immune reaction. During pregnancy, hormonal fluctuations can change B- and T-cells ratios potentially inducing inflammation and increasing pregnancy complications (42). The role of hormonal-related changes in pregnant MOGAD patients requires further evaluation.
In MOGAD, disease phenotype, relapse risk, relapse severity, and degree of recovery are all age dependent. Children more frequently experience brain involvement, a monophasic disease course, worse severity, and faster recovery than adults. In women of childbearing age, relapses preferentially involve the optic nerve and spinal cord, particularly the lower cord, and conus medullaris (2, 62). In a retrospective study assessing the clinical outcomes of transverse myelitis, Mariano et al. showed that the overall mobility recovery was better in patients with MOGAD than AQP4+NMOSD, but sphincter dysfunction remained a significant characteristic in MOGAD (63). This residual dysfunction may increase the risk of infections, with potential complications in pregnancy.
MOGAD patients are rarely treated with aggressive immunosuppressive treatments, such as cyclophosphamide, which may reduce ovarian reserve and fertility. However, this should be taken into consideration in clinical practice, and when immunosuppressants are needed, alternative therapeutic options should be preferred in young women patients.
In summary, the effect of MOG-Ab on fertility, pregnancy, and the newborn need to be further elucidated. In MOGAD women, disease characteristics as well as residual symptoms from previous cord relapses may complicate pregnancy.
Currently, there are only a few reports and two systematic studies on the effect of pregnancy and the postpartum period on MOGAD.
An association between assisted reproductive technology with frozen embryo transfer (FET) and the first manifestation of MOG optic neuritis in a previously healthy patient with unexplained infertility was recently proposed (64). This patient experienced bilateral optic neuritis after a single FET, which recovered completely after intravenous steroids and plasma exchange. However, further studies are needed to confirm this potential association.
A retrospective multicenter study of 30 patients with MOGAD reported one or more relapses in about half of MOGAD women with a documented pregnancy (5/10) most of them in the postpartum period within 8 months after delivery. Interestingly, while the cases that occurred during pregnancy were in patients with an already diagnosed relapsing MOGAD disease course, the disease started postpartum in three other patients (17). This may suggest that immunological changes related to pregnancy and delivery may play a role in triggering relapses and inducing the disease.
Recently, the effect of pregnancy on MOGAD was systematically evaluated in two independent cohorts. One study involving a Caucasian cohort from France, the United Kingdom, and Portugal, found that the annualized relapse rate (ARR) was lower during pregnancy than pre-pregnancy in all NMOSD serostatus groups, including 30 MOGAD patients, but rebounded during the first postpartum trimester. Unlike AQP4+NMOSD, immunosuppressant treatment during pregnancy or postpartum did not reduce the risk of relapses in MOGAD, though the majority of patients in this cohort were untreated (16).
Similarly, a Chinese study including 21 patients with MOGAD, showed that the first postpartum trimester was the highest risk period for relapse, and that the relapse risk during the first year postpartum was 1.5 time higher compared to 1 year pre-pregnancy. The pregnancy-related relapses in this MOGAD cohort were characterized by more episodes of optic neuritis, but fewer episodes of acute myelitis than the AQP4+NMOSD cohort. While the disability level during the pregnancy-related relapses did not differ between the two disease cohorts, EDSS scores were lower in the remission phase in MOGAD patients, suggesting a better recovery than AQP4+NMOSD even during pregnancy. In this study, only one premature delivery was observed with no spontaneous abortion, neonatal malformations, or pre-eclampsia reported (15).
Recently, an isolated case of MOGAD presenting 3 weeks postpartum with bilateral optic neuritis and a history of SARS-COVID19 infection 1 week before the delivery has been described (65). In another recently reported case, MOG-Abs were found in a patient with systemic lupus erythematosus and thoracic longitudinally extensive transverse myelitis 1 month postpartum. This case illustrates that the two diseases can coexist and the postpartum state may have facilitated the onset of the both autoimmune conditions (66).
A severe postpartum rhombencephalitis presenting 6 months after delivery in an undiagnosed patient with a history of recurrent LETM was found to be MOG-Ab positive. Symptoms improved after plasmapheresis with complete resolution of the infratentorial lesion and no relapses after 1 year on long-term immunosuppression with azathioprine (67). A Japanese patient diagnosed with MOGAD following cortical encephalitis, experienced increased seizure frequency in the 2 months postpartum of two pregnancies, despite treatment with levetiracetam. The umbilical cord blood of the second child was positive for MOG-Ab (68).
To date, little is known about MOGAD and breastfeeding, with no systematic study assessing the relationship between the two conditions.
To summarize, a rebound of disease activity during the first postpartum trimester and potentially up to 6 months postpartum is frequent in MOGAD, but further studies are needed to explore the role of other pregnancy-related factors in this Ab-mediated disorder.
Currently, there are no clinical trials for the treatment of MOGAD. Management strategies are primarily based on repurposing medications from other autoimmune diseases of the CNS.
Like AQP4+NMOSD, acute relapses should be promptly treated in MOGAD with intravenous methylprednisolone followed by escalation therapies, such as plasma exchange in patients with severe attacks or incomplete recovery (2).
Conversely, while all patients with AQP4+NMOSD require long-term immunotherapy because of the high rate of relapse and poor recovery, the same is not true for all patients with MOGAD. Approximately 50% of patients with MOGAD will be monophasic and recovery from relapses is superior than AQP4-Ab positive relapses. Thus, long-term immunotherapy for MOGAD is typically reserved for patients with relapsing disease or in patients with significant disability from a prior relapse (17, 60). Maintenance infusions of intravenous immunoglobulins, azathioprine and rituximab are the most commonly used agents, despite the latter being less effective in MOGAD than AQP4+NMOSD. Recommendations on the use of these treatments during pregnancy reflect those of the more studied autoimmune diseases. IL-6 targeting treatments (e.g., tocilizumab) look promising in a small number of relapsing MOGAD patients not responding to other immunosuppressant drugs (69, 70). A 3 months washout period per pharmacokinetic/pharmacodynamic placental transfer and potential risks is recommended (71).
An alternative future option may be rozanolixizumab. In a phase 2 trial in myasthenia gravis, this anti-neonatal Fc receptor humanized monoclonal antibody showed clinical improvement, suggesting a potential beneficial effect in MOGAD, which shares some pathological mechanisms. However, data on pregnancy and washout period are not currently available (72).
Limited by the relative rarity of MOG-Ab positivity, the data currently available to guide treatment decisions during pregnancy derive primarily from real-world clinical experience. Whether in the acute or non-acute phase, treatment should be personalized with consideration given to the age at conception, the severity of relapses and the disease course. Similarly, recommendations regarding resuming treatments while breastfeeding should be evaluated on a case-by-case basis.
MOGAD is a newly defined disease requiring further characterization and investigation. Improving our knowledge of MOGAD pathological mechanisms is of primary importance to understand how these pathways may influence pregnancy and pregnancy-related outcomes. Likewise, further elucidation of the immunological changes in pregnancy in MOGAD patients may give us clues to the immunopathogenesis of MOGAD itself.
A significant challenge in MOGAD research is the rarity of the disease. Small sample size is the most common limitation of studies aiming at better understanding this condition. Future international, multicenter studies will be a key to collecting the number of patients necessary to systematically assess the interplay between pregnancy, the postpartum period and breastfeeding with different MOGAD phenotypes. Including women in international pregnancy registries would be of additional value. Meanwhile, sharing and publishing real-word data of experiences across centers will continue to guide pregnancy-related MOGAD issues in clinical practice.
In the future, it would be useful to identify factors associated with pregnancy-related relapses in MOGAD, as has been done for AQP4+NMOSD (41). Prospective studies with large sample size are needed to evaluate if clinical features (e.g., different phenotypes at onset, number and recovery of relapses, age at conception, treatment) or Ab-characteristics (titers, timing and persistence over time) may be independent risk factors for pregnancy-related relapses. The discovery of risk factors would facilitate earlier and more comprehensive care, including planning of laboratory studies, neuroimaging, clinical evaluations, and rehabilitation programs to reduce the impact of disease burden on MOGAD mothers, especially in the postpartum period.
Finally, there is an urgent need for evidence-based guidelines for the treatment of MOGAD. Specifically in pregnancy, the key questions that need to be addressed are (i) how do we best manage clinical relapses, (ii) do we stop and when do we stop disease modifying treatments, (iii) what pregnancy related factors may influence the decision regarding continuous immunotherapy, as the postpartum period may represent a particularly high risk for relapse, and (iv) how long do we delay restarting immunotherapy in those who are breastfeeding?
Autoimmune encephalitis (AE) includes a group of disorders, sometimes of paraneoplastic origin, characterized by subacute onset of neurological and psychiatric manifestations associated with brain inflammation. These syndromes can be associated with antibodies targeting intracellular, synaptic or neuronal cell-surface antigens, although seronegative cases can also occur. Suggestive neurological symptoms include working memory deficits, altered mental status, focal CNS findings, seizures, movement disorders, and behavior/cognitive/autonomic dysfunctions (3). The presence of specific antibodies defines the association and frequency of cancer, age, and gender ratio (3, 73). The most common form of AE is that associated with anti-NMDAR antibodies (40% of all seropositive cases), with a median onset age of 21 years and a strong (9:1) female preponderance. This predominance is more pronounced when the onset is between puberty and menopause, when the association with tumors, in particular ovarian teratoma, is also more common. Anti-GAD-associated encephalitis also effects predominantly young women (median age 26 years-old, 9:1 female to male ratio). Anti-GABAaR or anti-mGluR5 AE also affect women of childbearing age, but are less common and show a 1:1 female to male ratio (73). As a consequence, almost all studies which have analyzed the correlation between AE and pregnancy have focused on anti-NMDAR encephalitis, with rare single cases describing AE onset during pregnancy in association with other autoantibodies (e.g., anti-AMPAR) (74).
There is no available data on fertility in women during or after AE. Although the autoimmune/inflammatory process per se should not directly influence fertility, residual symptoms, immunotherapy, and socioeconomic factors might have an indirect effect. Disease course (i.e., monophasic vs. relapsing or chronic disease, the latter being more common in GAD rather than NMDAR-associated encephalitis) may play a significant role in decisions regarding pregnancy (73). Of note, 94% of tumors associated with anti-NMDAR encephalitis are ovarian teratomas (75) where surgical resection is recommended. Although teratoma resection is the first treatment choice, sometimes unilateral, or bilateral oophorectomy is required, directly influencing future fertility and the need for reproductive technologies (76, 77).
Maternofetal transfer of anti-NMDAR antibodies can occur during pregnancy in both symptomatic and asymptomatic women, as supported by animal models showing reversible pathogenic effects (78). However, obstetric complications are mainly related to the severity of neurological symptoms (e.g., seizures or autonomic dysfunctions), which often require admission to an intensive care unit. Data suggest that fetal exposure to maternal antibodies rarely causes neurological complications in the developing fetus or newborn (79).
The effect of antibodies on fertility and pregnancy should be analyzed in future studies addressing this specific topic.
There are limited case reports and case series of patients who developed anti-NMDAR encephalitis during or after pregnancy, but of those available the disease course was similar to that observed in non-pregnant women. Few cases who have relapsed during or after pregnancy or had other concomitant autoimmune conditions have been also reported (80–83).
The possible proposed triggering factors include: changes in hormonal status (in particular the effect of estrogen and progesterone on antibody production), B cell maturation, IL10 secretion, Th1/Th2 shift, and the modification of immune tolerance induced by the embryo or placenta (84). In most cases, a good clinical outcome of the newborn and mother is reported after the administration of first line (i.e., steroids, plasma exchange, intravenous immunoglobulins—IVIG)—or second line (rituximab and in one case cyclophosphamide) treatment with no obstetric complications or fetal distress (79, 85–91). These outcomes are also in line with the authors' experience (unpublished data).
Anti-NMDAR encephalitis can occur after delivery in some patients and can be misdiagnosed as postpartum psychosis. Onset is usually reported within 3 months from delivery, regardless of multiparity, and usually occurs after normal vaginal delivery. Psychiatric symptoms (psychomotor excitement, confusion, depression, anxiety, delusions, bizarre behavior, insomnia, agitation, irritability, catatonic features, and hallucinations) are usually predominant, although this clinical phenotype and the tumor association are similar to that reported in non-pregnant women (92–95). Experts recommend systematic screening of serum anti-NMDAR antibodies in patients with acute psychosis during the postpartum period (96), in particular in those with additional neurological symptoms, EEG abnormalities or MRI signs of mesial temporal lobe involvement that support the diagnosis.
A single case of a non-paraneoplastic, treatment-responsive CASPR-2-associated encephalitis presenting with postpartum psychosis has been also described (97), suggesting that other Ab-mediated AE might occur in the postpartum period and extensive screening should be performed in these conditions.
Fetal outcome is better in patients with anti-NMDAR encephalitis in mid- to late-pregnancy, when the fetal blood-brain-barrier begins to function (84, 98) preventing the transfer of IgG1/IgG3, which are able to bind to the Fc neonatal receptor of the syncytiotrophoblasts at ~14–16 weeks of gestation. Most reports described healthy infants, but isolated neurological sequel or miscarriage/abortion have also been reported (84, 99). Despite most data not supporting an increased teratogenicity rate or fetal development delay, a higher rate of preterm delivery has been reported, mainly linked with neurological symptoms displayed by the mother (73, 79). Pre-term delivery can also be planned to reduce fetal antibody exposure and improve maternal and fetal outcomes (98). Isolated cases with respiratory distress, neural tube closing defects, and reduced body weight have been described, possibly correlated with the administration of antiepileptic and sedative treatments (79, 100). Among cases with poor fetal outcomes, a symptomatic woman who developed an anti-NMDAR encephalitis relapse in the context of a new ovarian teratoma at 37-week's gestation with a fatal fetal outcome has been described. After Cesarean section, the infant displayed hypotonia, respiratory insufficiency, and seizures followed by progressive worsening of neurological function unresponsive to IVIG. Both mother and newborn showed anti-NMDAR antibodies positivity (101).
In another case, a woman developed anti-NMDAR encephalitis at 9-weeks gestational age, delivering at 34-weeks. Despite transient improvement, she died soon after delivery and the serum anti-NMDAR positive newborn displayed movement disorders, cortical dysplasia and development delay associated with seizures at 2 years follow-up (102). In addition, an asymptomatic woman with a previous history of relapsing anti-NMDAR encephalitis at 37-weeks gestational age delivered an NMDAR positive newborn with low responsiveness and respiratory insufficiency. The infant improved spontaneously and was asymptomatic with negative anti-NMDAR serology after 1 year (103). Another anti-NMDAR positive neonate with autonomic instability has been described, but he was healthy at 1 year of age (104). Although data on the effect of NMDAR antibodies is partially discordant and the outcomes in these cases could result from a combination of factors, including the side effects of sedatives and antiepileptics drugs, anti-NMDAR testing is recommended in pregnant women with an antecedent or recent history of anti-NMDAR encephalitis.
To summarize, the onset or relapse of autoimmune encephalitis during/after pregnancy is rare and usually not associated with fetal complications.
Although a consensus on treatment strategies for AE in pregnancy does not exist, experts agree that timely, and prompt treatment, including tumor resection and immunotherapy, is recommended in pregnant women with AE and is related to mother and fetal outcome. Among acute therapy, IVIG, corticosteroids, and plasma exchange are usually well-tolerated as first line therapies and may reduce mother and fetus circulating antibodies (105). Among additional second line therapies azathioprine and rituximab have been administered but might retain a teratogenic and premature delivery risk, although their use is supported by studies in different autoimmune conditions during pregnancy (47, 49). Therefore, the potential benefits for the mother and the risk to the fetus should be carefully considered (73, 84). Psychotropic drugs such as haloperidol, olanzapine, lithium, and lorazepam can also improve behavioral symptoms (96). Antiepileptic drugs are also recommended, although seizures are more responsive to immunotherapy.
In patients with teratoma, laparoscopic surgery is usually the treatment of choice as it is considered curative and is not associated with fetal complications. Close fetal monitoring during this operative procedure is additionally recommended. Of note, teratoma identification might be difficult in mid- to late-pregnancy, due to the obstruction of the ultrasound field by the enlarged uterus. In these cases MRI or targeted reduced-dose computed tomography scan may be necessary to identify the teratoma (98, 106). Of note, similarly to the non-pregnant cases, cerebrospinal fluid (CSF)-restricted antibodies can be detected in pregnant/postpartum women, so that testing CSF in suspected seronegative cases is strongly recommended (88, 94, 95).
To summarize, available evidence supports prompt surgical and immunotherapy treatments in patients who develop autoimmune encephalitis during/after pregnancy, considering fetus effects in the choice of second line drugs.
Literature related to pregnancy and delivery in patients with AE is scarce, usually limited to case reports, and mainly to women with anti-NMDAR encephalitis. Further multicenter studies incorporating epidemiological surveys with prolonged follow-up including detailed neuropsychological assessments of the children and mothers are paramount to defining short and long-term outcomes, treatment strategies, and clarifying the immunopathogenesis of the disease. In particular, the correlation between a mother's antibody titer and obstetric or fetal complications requires clarification in order to tailor pre-conceptual planning and postpartum follow-up. In addition, the optimal timing and type of delivery should be clarified. Finally, literature should be expanded to patients with other forms of AE.
Pregnancy may influence the course of Ab-mediated disorders of the CNS. And while the manner in which this influence occurs may differ in AQP4+NMOSD, MOGAD, and AE, similarities between the three diseases can also be identified.
First, while the ability of CNS specific Ab to affect fertility is still debated due to the lack of high quality studies, disease activity and course, recovery and treatments certainly indirectly affect pregnancy (73, 107).
Second, hormonal fluctuations, particularly estrogens can potentially induce inflammation and increase pregnancy complications (42). Nonetheless, data about the role of pregnancy on all the described diseases are scarce and contrasting. On one side, a possible protective role of pregnancy on AQP4+NMOSD has been hypothesized, with a trend of reduced ARR compared to the pre-partum period. On the other side, few cases with acute events have been described in MOGAD and AE, being often the first attack ever in the former, while usually associated with other concomitant autoimmune diseases in the latter disease.
By contrast, the first postpartum trimester represents a risk for women with Ab-mediated disorders of the CNS. A rebound in the ARR was reported in NMOSD, which was higher than the pre-pregnancy period in AQP4-Ab positive patients. The onset of AE encephalitis (i.e., anti-NMDAR, or anti-CASPR-2) can occur with psychiatric symptoms soon after the delivery and can be misdiagnosed as postpartum psychosis (16, 83).
In addition, timing and type of delivery are not generally affected in AQP4+NMOSD and MOGAD, while delivery can be planned in AE to reduce fetal antibodies exposure and improve maternal and fetal outcome, as cases of fetal complications have been reported.
Finally, there are currently no evidence-based guidelines for the management of Ab-mediated disease of the CNS during pregnancy. Treatment of intrapartum relapses should be recommended, particularly when women may incur accumulation of neurological disability and functional limitations. High-dose steroids are usually well-tolerated, but close clinical surveillance should be performed to identify complications (i.e., deficit in fetal organogenesis in the first trimester, hypertension, pre-eclampsia, intrauterine growth restriction in the second, and third trimesters). Data currently available on the use of intravenous immunoglobulins during pregnancy are more reassuring than plasma exchange due to the lower risks of circulatory instability (71). Tumor resection is recommended in pregnant women with AE, while immunotherapy during pregnancy should be evaluated on a case by case, as some therapies may be dangerous for the woman and fetus.
Overall, most of the evidence related to the interplay between pregnancy and these disorders is limited to case reports. Further multicenter studies should be undertaken to clarify unresolved questions and define monitoring and treatment strategies to be used in a clinical setting.
In conclusion, in women at childbearing age with an Ab-mediated disease of the CNS, pregnancy should be carefully planned, patients and fetus routinely checked intrapartum, and a postpartum care plan organized before delivery to allow comprehensive support and reduced the burden of complications on mothers and newborns.
RC designed the project and wrote the manuscript. SM and CM wrote and revised the manuscript. CT supervised the project and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors thank Dr. Lukas B. Kipp, Clinical Assistant Professor at Division of MS/Neuroimmunology—Department of Neurology & Neurological Sciences, Stanford University, School of Medicine for his thoughtful comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
2. Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. (2021) 20:762–72. doi: 10.1016/S1474-4422(21)00218-0
3. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
4. Kim W, Kim SH, Nakashima I, Takai Y, Fujihara K, Leite MI, et al. Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology. (2012) 78:1264–7. doi: 10.1212/WNL.0b013e318250d812
5. Bourre B, Marignier R, Zephir H, Papeix C, Brassat D, Castelnovo G, et al. Neuromyelitis optica and pregnancy. Neurology. (2012) 78:875–9. doi: 10.1212/WNL.0b013e31824c466f
6. Fragoso YD, Adoni T, Bichuetti DB, Brooks JB, Ferreira ML, Oliveira EM, et al. Neuromyelitis optica and pregnancy. J Neurol. (2013) 260:2614–9. doi: 10.1007/s00415-013-7031-y
7. Shimizu Y, Fujihara K, Ohashi T, Nakashima I, Yokoyama K, Ikeguch R, et al. Pregnancy-related relapse risk factors in women with anti-AQP4 antibody positivity and neuromyelitis optica spectrum disorder. Mult Scler J. (2016) 22:1413–20. doi: 10.1177/1352458515583376
8. Nour MM, Nakashima I, Coutinho E, Woodhall M, Sousa F, Revis J, et al. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology. (2016) 86:79–87. doi: 10.1212/WNL.0000000000002208
9. Huang Y, Wang Y, Zhou Y, Huang Q, Sun X, Chen C, et al. Pregnancy in neuromyelitis optica spectrum disorder: a multicenter study from South China. J Neurol Sci. (2017) 372:152–6. doi: 10.1016/j.jns.2016.11.054
10. Klawiter EC, Bove R, Elsone L, Alvarez E, Borisow N, Cortez M, et al. High risk of postpartum relapses in neuromyelitis optica spectrum disorder. Neurology. (2017) 89:2238–44. doi: 10.1212/WNL.0000000000004681
11. Delgado-García G, Chávez Z, Rivas-Alonso V, Corona T, Flores-Rivera J. Obstetric outcomes in a Mexican cohort of patients with AQP4-antibody-seropositive neuromyelitis optica. Mult Scler Relat Disord. (2018) 25:268–70. doi: 10.1016/j.msard.2018.08.015
12. Tong Y, Liu J, Yang T, Kang Y, Wang J, Zhao T, et al. Influences of pregnancy on neuromyelitis optica spectrum disorders and multiple sclerosis. Mult Scler Relat Disord. (2018) 25:61–5. doi: 10.1016/j.msard.2018.07.006
13. Ashtari F, Mehdipour R, Shaygannejad V, Asgari N. Pre-pregnancy, obstetric and delivery status in women with neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. (2020) 44:102252. doi: 10.1016/j.msard.2020.102252
14. Kim SH, Huh SY, Jang H, Park NY, Kim Y, Jung JY, et al. Outcome of pregnancies after onset of the neuromyelitis optica spectrum disorder. Eur J Neurol. (2020) 27:1546–55. doi: 10.1111/ene.14274
15. Wang L, Zhou L, ZhangBao J, Huang W, Chang X, Lu C, et al. Neuromyelitis optica spectrum disorder: pregnancy-related attack and predictive risk factors. J Neurol Neurosurg Psychiatry. (2021) 92:53–61. doi: 10.1136/jnnp-2020-323982
16. Collongues N, Do Rego CA, Bourre B, Biotti D, Marignier R, da Silva AM, et al. Pregnancy in patients with AQP4-Ab, MOG-Ab, or double-negative neuromyelitis optica disorder. Neurology. (2021) 96:e2006–15. doi: 10.1212/WNL.0000000000011744
17. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. (2016) 13:280. doi: 10.1186/s12974-016-0718-0
18. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. (2004) 364:2106–12. doi: 10.1016/S0140-6736(04)17551-X
19. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. (2005) 202:473–7. doi: 10.1084/jem.20050304
20. Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. (2012) 9:1–17. doi: 10.1186/1742-2094-9-14
21. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. (2018) 15:89–102. doi: 10.1038/s41582-018-0112-x
22. Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. (2018) 90:e1858–69. doi: 10.1212/WNL.0000000000005560
23. Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler. (2015) 21:845–53. doi: 10.1177/1352458515572406
24. Borisow N, Kleiter I, Gahlen A, Fischer K, Wernecke KD, Pache F, et al. Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult Scler. (2017) 23:1092–103. doi: 10.1177/1352458516671203
25. Selmi C, Gershwin ME. Sex and autoimmunity: proposed mechanisms of disease onset and severity. Expert Rev Clin Immunol. (2019) 15:607–15. doi: 10.1080/1744666X.2019.1606714
26. Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. (2012) 69:1176–80. doi: 10.1001/archneurol.2012.314
27. Bove R, Elsone L, Alvarez E, Borisow N, Cortez MM, Mateen FJ, et al. Female hormonal exposures and neuromyelitis optica symptom onset in a multicenter study. Neurol Neuroimmunol Neuroinflammation. (2017) 4:e339. doi: 10.1212/NXI.0000000000000339
28. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life- years for infertility in 195 countries and territories,1990-2017:results from a global burden of disease study, 2017. Aging. (2019) 11:1990–2017. doi: 10.18632/aging.102497
29. Thöne J, Lichtenberg S, Stahl A, Pache F, Kleiter I, Ruprecht K, et al. Ovarian reserve in women with neuromyelitis optica spectrum disorder. Front Neurol. (2018) 9:446. doi: 10.3389/fneur.2018.00446
30. Huang HF, He RH, Sun CC, Zhang Y, Meng QX, Ma YY. Function of aquaporins in female and male reproductive systems. Hum Reprod Update. (2006) 12:785–95. doi: 10.1093/humupd/dml035
31. Sun XL, Zhang J, Fan Y, Ding JH, Sha JH, Hu G. Aquaporin-4 deficiency induces subfertility in female mice. Fertil Steril. (2009) 92:1736–43. doi: 10.1016/j.fertnstert.2008.07.1785
32. Venero JL, Vizuete ML, Machado A, Cano J. Aquaporins in the central nervous system. Prog Neurobiol. (2001) 63:321–36. doi: 10.1016/S0301-0082(00)00035-6
33. Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci. (2016) 1366:20–39. doi: 10.1111/nyas.12794
34. Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol. (2013) 191:2999–3005. doi: 10.4049/jimmunol.1301483
35. Reuss R, Rommer PS, Brück W, Paul F, Bolz M, Jarius S, et al. Interactive case report - a woman with acute myelopathy in pregnancy: case presentation. BMJ. (2009) 339:1143. doi: 10.1136/bmj.b3862
36. Chang Y, Shu Y, Sun X, Lu T, Chen C, Fang L, et al. Study of the placentae of patients with neuromyelitis optica spectrum disorder. J Neurol Sci. (2018) 387:119–23. doi: 10.1016/j.jns.2018.01.040
37. Iyer A, Elsone L, Appleton R, Jacob A. A review of the current literature and a guide to the early diagnosis of autoimmune disorders associated with neuromyelitis optica. Autoimmunity. (2014) 47:154–61. doi: 10.3109/08916934.2014.883501
38. Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. (2010) 341:499–501. doi: 10.1136/bmj.c2207
39. Ringelstein M, Harmel J, Distelmaier F, Ingwersen J, Menge T, Hellwig K, et al. Neuromyelitis optica and pregnancy during therapeutic B cell depletion: infant exposure to anti-AQP4 antibody and prevention of rebound relapses with low-dose rituximab postpartum. Mult Scler J. (2013) 19:1544–7. doi: 10.1177/1352458513498125
40. Asgari N, Henriksen TB, Petersen T, Lillevang ST, Weinshenker BG. Clinical/scientific notes: pregnancy outcomes in a woman with neuromyelitis optica. Neurology. (2014) 83:1576–7. doi: 10.1212/WNL.0000000000000911
41. Wang L, Su M, Zhou Z, Zhou L, ZhangBao J, Tan H, et al. Analysis of pregnancy-related attacks in neuromyelitis optica spectrum disorder. JAMA Netw Open. (2022) 5:e2225438. doi: 10.1001/jamanetworkopen.2022.25438
42. Mao-Draayer Y, Thiel S, Mills EA, Chitnis T, Fabian M, Katz Sand I, et al. Neuromyelitis optica spectrum disorders and pregnancy: therapeutic considerations. Nat Rev Neurol. (2020) 16:154–70. doi: 10.1038/s41582-020-0313-y
43. Wang J, Tian Y, Shao Y, Feng H, Qin L, Xu W, et al. Comparison of spontaneous brain activity revealed by regional homogeneity in AQP4-IgG neuromyelitis optica-optic neuritis versus MOG-IgG optic neuritis patients: a resting-state functional MRI study. Neuropsychiatr Dis Treat. (2017) 13:2669–79. doi: 10.2147/NDT.S145183
44. Deng S, Lei Q, Lu W. Pregnancy-Related attack in neuromyelitis optica spectrum disorder with AQP4-IgG: a single-center study and meta-analysis. Front Immunol. (2022) 12:800666. doi: 10.3389/fimmu.2021.800666
45. Davoudi V, Keyhanian K, Bove RM, Chitnis T. Immunology of neuromyelitis optica during pregnancy. Neurol Neuroimmunol NeuroInflammation. (2016) 3:e288. doi: 10.1212/NXI.0000000000000288
46. Dobson R, Dassan P, Roberts M, Giovannoni G, Nelson-Piercy C, Brex PA. UK consensus on pregnancy in multiple sclerosis: ‘association of British neurologists' guidelines. Pract Neurol. (2019) 19:106–14. doi: 10.1136/practneurol-2018-002060
47. Skorpen CG, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. (2016) 75:795–810. doi: 10.1136/annrheumdis-2015-208840
48. Pentšuk N, Van Der Laan JW. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res Part B Dev Reprod Toxicol. (2009) 86:328–44. doi: 10.1002/bdrb.20201
49. Das G, Damotte V, Gelfand JM, Bevan C, Cree BAC, Do L, et al. Rituximab before and during pregnancy. Neurol Neuroimmunol Neuroinflammation. (2018) 5:e453. doi: 10.1212/NXI.0000000000000453
50. Galati A, McElrath T, Bove R. Use of B-cell–depleting therapy in women of childbearing potential with multiple sclerosis and neuromyelitis optica spectrum disorder. Neurol Clin Pract. (2022) 12:154–63. doi: 10.1212/CPJ.0000000000001147
51. Krysko KM, LaHue SC, Anderson A, Rutatangwa A, Rowles W, Schubert RD, et al. Minimal breast milk transfer of rituximab, a monoclonal antibody used in neurological conditions. Neurol Neuroimmunol neuroinflammation. (2020) 7:1–10. doi: 10.1212/NXI.0000000000000637
52. Hoeltzenbein M, Beck E, Rajwanshi R, Skorpen CG, Berber E, Schaefer C, et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin Arthritis Rheum. (2016) 46:238–45. doi: 10.1016/j.semarthrit.2016.05.004
53. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse ME, Lockshin MD, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. (2020) 72:529–56. doi: 10.1002/art.41191
54. Capobianco M, Marozio L, Malucchi S, Malentacchi M, Re ML, Coscia A, et al. Successful pregnancy and disease outcomes in a NMOSD patient treated with tocilizumab. Neuroimmunol Rep. (2021) 1:100014. doi: 10.1016/j.nerep.2021.100014
55. Socié G, Caby-Tosi MP, Marantz JL, Cole A, Bedrosian CL, Gasteyger C, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. (2019) 185:297–310. doi: 10.1111/bjh.15790
56. Kelly RJ, Höchsmann B, Szer J, Kulasekararaj A, de Guibert S, Röth A, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. (2015) 373:1032–9. doi: 10.1056/NEJMoa1502950
57. Armangue T, Olivé-Cirera G, Martínez-Hernandez E, Sepulveda M, Ruiz-Garcia R, Muñoz-Batista M, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. (2020) 19:234–46. doi: 10.1016/S1474-4422(19)30488-0
58. Tao R, Qin C, Chen M, Yu HH, Wu LJ, Bu BT, et al. Unilateral cerebral cortical encephalitis with epilepsy: a possible special phenotype of MOG antibody-associated disorders. Int J Neurosci. (2020) 130:1161–5. doi: 10.1080/00207454.2020.1720676
59. Chang YC, Sharma M, Budhram A. Unilateral cortical FLAIR-hyperintense lesion in anti-MOG-associated encephalitis with seizures (FLAMES) on TNF inhibitor therapy. J Neurol Neurosurg Psychiatry. (2021) 92:678–9. doi: 10.1136/jnnp-2021-326401
60. Tajfirouz DA, Bhatti MT, Chen JJ. Clinical characteristics and treatment of MOG-IgG–associated optic neuritis. Curr Neurol Neurosci Rep. (2019) 19:100. doi: 10.1007/s11910-019-1014-z
61. Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. (2017) 140:617–27. doi: 10.1093/brain/aww350
62. Hacohen Y, Rossor T, Mankad K, Chong WK, Lux A, Wassmer E, et al. ‘Leukodystrophy-like' phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev Med Child Neurol. (2018) 60:417–23. doi: 10.1111/dmcn.13649
63. Mariano R, Messina S, Roca-Fernandez A, Leite MI, Kong Y, Palace JA. Quantitative spinal cord MRI in MOG-antibody disease, neuromyelitis optica and multiple sclerosis. Brain. (2020) 144:198–212. doi: 10.1093/brain/awaa347
64. Siegel D, Luu TH, Skaznik-Wikiel M. Primary myelin oligodendrocyte glycoprotein-immunoglobulin G-associated optic neuritis presenting after a frozen embryo transfer. J Hum Reprod Sci. (2021) 14:203–5. doi: 10.4103/jhrs.jhrs_30_21
65. Lotlikar R, Menon RN, Thomas B, Poyuran R, Narasimhaiah D, Ponnambath DK. MOG-antibody mediated clinically isolated syndrome after COVID-19 in a post partum woman—a veritable “double hit”! Neurol India. (2022) 70:1281. doi: 10.4103/0028-3886.349651
66. Bilodeau PA, Kumar V, Rodriguez AE, Li CT, Sanchez-Alvarez C, Thanarajasingam U, et al. MOG-IgG myelitis coexisting with systemic lupus erythematosus in the post-partum setting. Mult Scler J. (2020) 26:997–1000. doi: 10.1177/1352458519872895
67. Vecchio D, Virgilio E, Naldi P, Comi C, Cantello R. MOG-antibody demyelinating diseases: a case of post-partum severe rhombencephalitis and transverse myelitis. Mult Scler Relat Disord. (2018) 21:9–10. doi: 10.1016/j.msard.2018.02.006
68. Yamamoto S, Yano M, Miyamoto Y, Hanaoka T, Nishida Y, Kawano Y. The postpartum period can worsen myelin oligodendrocyte glycoprotein antibody-associated encephalomyelitis: a case report. Internal Med. (2022). doi: 10.2169/internalmedicine.0170-22 [Epub ahead of print].
69. Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): a case-series and review. Mult Scler Relat Disord. (2021) 48:102696. doi: 10.1016/j.msard.2020.102696
70. Ringelstein M, Ayzenberg I, Lindenblatt G, Fischer K, Gahlen A, Novi G, et al. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG–associated disease and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflammation. (2022) 9. doi: 10.1212/NXI.0000000000001100 [Epub ahead of print].
71. Bove RM, Houtchens MK. Pregnancy management in multiple sclerosis and other demyelinating diseases. Contin Lifelong Learn Neurol. (2022) 28:12–33. doi: 10.1212/CON.0000000000001108
72. Bril V, Benatar M, Andersen H, Vissing J, Brock M, Greve B, et al. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: a phase 2 randomized control trial. Neurology. (2021) 96:e853–65. doi: 10.1212/WNL.0000000000011108
73. Altintas A, Dargvainiene J, Schneider-Gold C, Asgari N, Ayzenberg I, Ciplea AI, et al. Gender issues of antibody-mediated diseases in neurology: (NMOSD/autoimmune encephalitis/MG). Ther Adv Neurol Disord. (2020) 13:1756286420949808. doi: 10.1177/1756286420949808
74. Wei YC, Liu CH, Lin JJ, Lin KJ, Huang KL, Lee TH, et al. Rapid progression and brain atrophy in anti-AMPA receptor encephalitis. J Neuroimmunol. (2013) 261:129–33. doi: 10.1016/j.jneuroim.2013.05.011
75. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
76. Terada A, Tasaki S, Tachibana T, Sakamoto Y, Yokomine M, Shimomura T, et al. Two cases of acute limbic encephalitis in which symptoms improved as a result of laparoscopic salpingo-oophorectomy. Gynecol Minim Invasive Ther. (2017) 6:34. doi: 10.1016/j.gmit.2016.06.004
77. Li W, Jia D, Tong L, Lun Z, Li H. Anti- N -methyl- d -aspartate receptor encephalitis induced by bilateral ovarian teratomas with distinct histopathologic types: a case report and brief literature review. Medicine. (2019) 98:e18148. doi: 10.1097/MD.0000000000018148
78. García-Serra A, Radosevic M, Pupak A, Brito V, Ríos J, Aguilar E, et al. Placental transfer of NMDAR antibodies causes reversible alterations in mice. Neurol Neuroimmunol Neuroinflammation. (2021) 8:e915. doi: 10.1212/NXI.0000000000000915
79. Joubert B, García-Serra A, Planagumà J, Martínez-Hernandez E, Kraft A, Palm F, et al. Pregnancy outcomes in anti-NMDA receptor encephalitis: case series. Neurol Neuroimmunol neuroinflammation. (2020) 7:e668. doi: 10.1212/NXI.0000000000000668
80. Liu H, Chen X. Recurrent anti-NMDAR encephalitis during pregnancy combined with two antibodies positive. Arch Womens Ment Health. (2021) 24:1045–50. doi: 10.1007/s00737-021-01124-5
81. Lu J, Samson S, Kass J, Ram N. Case report: acute psychosis in a pregnant patient with graves' hyperthyroidism and anti-NMDA receptor encephalitis. BMJ Case Rep. (2015) 2015:bcr2014208052. doi: 10.1136/bcr-2014-208052
82. Magley J, Towner D, Taché V, Apperson ML. Pregnancy outcome in anti-N-methyl-D-aspartate receptor encephalitis. Obstet Gynecol. (2012) 120:480–3. doi: 10.1097/AOG.0b013e31825935d4
83. Zhang S, Yang Y, Long T, Li Z. Systemic lupus erythematosus associated with recurrent anti-NMDA receptor encephalitis during pregnancy. Arch Womens Ment Health. (2021) 24:525–8. doi: 10.1007/s00737-020-01088-y
84. Huang Q, Xie Y, Hu Z, Tang X. Anti-N-methyl-D-aspartate receptor encephalitis: a review of pathogenic mechanisms, treatment, prognosis. Brain Res. (2020) 1727:146549. doi: 10.1016/j.brainres.2019.146549
85. Dono F, Evangelista G, Consoli S, Scarrano G, Russo M, di Pietro M, et al. Anti N-methyl-D-aspartate receptor (NMDAr) encephalitis during pregnancy: a case report. Epilepsy Behav Rep. (2022) 19:100535. doi: 10.1016/j.ebr.2022.100535
86. Jung KO, Moon HJ. A case of NMDAR encephalitis treated in the third trimester - novel arterial spin labeling findings and a review of literature. J Neuroimmunol. (2020) 343:577235. doi: 10.1016/j.jneuroim.2020.577235
87. Kalam S, Baheerathan A, McNamara C, Singh-Curry V. Anti-NMDAR encephalitis complicating pregnancy. Pract Neurol. (2019) 19:131–5. doi: 10.1136/practneurol-2018-002042
88. Kumar MA, Jain A, Dechant VE, Saito T, Rafael T, Aizawa H, et al. Anti-N-methyl-D-aspartate receptor encephalitis during pregnancy. Arch Neurol. (2010) 67:884–7. doi: 10.1001/archneurol.2010.133
89. Shi YC, Chen XJ, Zhang HM, Wang Z, Du DY. Anti-N-Methyl-d-Aspartate receptor (NMDAR) encephalitis during pregnancy: clinical analysis of reported cases. Taiwan J Obstet Gynecol. (2017) 56:315–9. doi: 10.1016/j.tjog.2017.04.009
90. Shahani L. Steroid unresponsive anti-NMDA receptor encephalitis during pregnancy successfully treated with plasmapheresis. Case Rep. (2015) 2015:bcr2014208823. doi: 10.1136/bcr-2014-208823
91. Xiao X, Gui S, Bai P, Bai Y, Shan D, Hu Y, et al. Anti-NMDA-receptor encephalitis during pregnancy: a case report and literature review. J Obstet Gynaecol Res. (2017) 43:768–74. doi: 10.1111/jog.13262
92. Mariotto S, Tamburin S, Salviati A, Ferrari S, Zoccarato M, Giometto B, et al. Anti-N-methyl-d-aspartate receptor encephalitis causing a prolonged depressive disorder evolving to inflammatory brain disease. Case Rep Neurol. (2014) 6:38–43. doi: 10.1159/000358820
93. Doden T, Sekijima Y, Ikeda J, Ozawa K, Ohashi N, Kodaira M, et al. Postpartum anti-N-methyl-D-aspartate receptor encephalitis: a case report and literature review. Intern Med. (2017) 56:357. doi: 10.2169/internalmedicine.56.7442
94. Koksal A, Baybas S, Mutluay B, Altunkaynak Y, Keskek A. A case of NMDAR encephalitis misdiagnosed as postpartum psychosis and neuroleptic malignant syndrome. Neurol Sci. (2015) 36:1257–8. doi: 10.1007/s10072-014-1966-3
95. Reddy MS, Thippeswamy H, Ganjekar S, Nagappa M, Mahadevan A, Arvinda HR, et al. Anti-NMDA receptor encephalitis presenting as postpartum psychosis-a clinical description and review. Arch Womens Ment Health. (2018) 21:465–9. doi: 10.1007/s00737-018-0816-3
96. Bergink V, Armangue T, Titulaer MJ, Markx S, Dalmau J, Kushner SA. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. (2015) 172:901–8. doi: 10.1176/appi.ajp.2015.14101332
97. Warren N, Theodoros T, Gunawardana R, Gillinder L. CASPR2 encephalitis presenting as post-partum psychosis. Aust N Z J Psychiatry. (2019) 53:174–5. doi: 10.1177/0004867418812687
98. Gu J, Chen Q, Gu H, Duan R. Research progress in teratoma-associated anti-N-methyl-D-aspartate receptor encephalitis: the gynecological perspective. J Obstet Gynaecol Res. (2021) 47:3749–57. doi: 10.1111/jog.14984
99. Kim J, Park SH, Jung YR, Park SW, Jung DS. Anti-NMDA receptor encephalitis in a pregnant woman. J Epilepsy Res. (2015) 5:29. doi: 10.14581/jer.15008
100. Scorrano G, Dono F, Evangelista G, Chiarelli F, Anzellotti F. Fetal outcome in anti-NMDAR encephalitis during pregnancy: a case report. Acta Neurol Belg. (2022). doi: 10.1007/s13760-022-02020-0 [Epub ahead of print].
101. Chourasia N, Watkins MW, Lankford JE, Kass JS, Kamdar A. An infant born to a mother with anti-N-methyl-d-aspartate receptor encephalitis. Pediatr Neurol. (2018) 79:65–8. doi: 10.1016/j.pediatrneurol.2017.11.010
102. Jagota P, Vincent A, Bhidayasiri R. Transplacental transfer of NMDA receptor antibodies in an infant with cortical dysplasia. Neurology. (2014) 82:1662. doi: 10.1212/WNL.0000000000000384
103. Hilderink M, Titulaer MJ, Schreurs MWJ, Keizer K, Bunt JEH. Transient anti-NMDAR encephalitis in a newborn infant due to transplacental transmission. Neurol Neuroimmunol neuroinflammation. (2015) 2:e126. doi: 10.1212/NXI.0000000000000126
104. Lamale-Smith LM, Moore GS, Guntupalli SR, Scott JB. Maternal-fetal transfer of anti-N-methyl-D-aspartate receptor antibodies. Obstet Gynecol. (2015) 125:1056–8. doi: 10.1097/AOG.0000000000000548
105. Ueda A, Nagao R, Maeda T, Kikuchi K, Murate K, Niimi Y, et al. Absence of serum anti-NMDAR antibodies in anti-NMDAR encephalitis mother predicts having healthy newborn. Clin Neurol Neurosurg. (2017) 161:14–6. doi: 10.1016/j.clineuro.2017.07.012
106. Mizutamari E, Matsuo Y, Namimoto T, Ohba T, Yamashita Y, Katabuchi H. Successful outcome following detection and removal of a very small ovarian teratoma associated with anti-NMDA receptor encephalitis during pregnancy. Clin Case Rep. (2016) 4:223–5. doi: 10.1002/ccr3.475
Keywords: neuromyelitis optica spectrum disorders (not in MeSH), autoimmune encephalitis, myelin oligodendrocyte (MOG) antibody associated disease, pregnancy, postpartum, breastfeeding
Citation: Cortese R, Mariotto S, Mancinelli CR and Tortorella C (2022) Pregnancy and antibody-mediated CNS disorders: What do we know and what should we know? Front. Neurol. 13:1048502. doi: 10.3389/fneur.2022.1048502
Received: 19 September 2022; Accepted: 21 November 2022;
Published: 19 December 2022.
Edited by:
Antonia Ceccarelli, Centre Hospitalier EpiCURA/Free University of Brussels (UZ Brussel) VUB, BelgiumReviewed by:
Rana Khalil Zabad, University of Nebraska Medical Center, United StatesCopyright © 2022 Cortese, Mariotto, Mancinelli and Tortorella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosa Cortese, cm9zYS5jb3J0ZXNlQHVuaXNpLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.