
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 20 October 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1044642
This article is part of the Research TopicNeuroglial antibodies: from clinical associations to pathophysiological investigationsView all 8 articles
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is an inflammatory demyelinating disease that is distinct from multiple sclerosis. Initial manifestations of MOGAD that were reported in the literature included optic neuritis, myelitis, brainstem demyelination and encephalitis, with emphasis placed on acute disseminated encephalomyelitis (ADEM) as the primary encephalitic presentation. In 2017, however, Ogawa et al. described four patients with seizures, unilateral cortical hyperintensities on brain magnetic resonance imaging T2-fluid-attenuated inversion recovery sequences, and anti-MOG positivity, indicating a potentially novel form of encephalitis in MOGAD. In 2019, we systematically reviewed the literature to better characterize this unique syndrome, which we referred to as unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES). Subsequently, anti-MOG positivity in patients with a variety of cortical and meningeal disease presentations has been reported, indicating a broader spectrum of meningo-cortical manifestations in MOGAD that we review herein.
Over the last decade, antibodies against myelin oligodendrocyte glycoprotein (MOG) have emerged as a biomarker of inflammatory demyelinating disease that is distinct from multiple sclerosis (1–7). Classical manifestations of MOG antibody-associated disease (MOGAD) that were initially described in the literature included optic neuritis, myelitis, brainstem demyelination and encephalitis. In particular, mention of encephalitis in MOGAD has historically been in reference to acute disseminated encephalomyelitis (ADEM), which is a multifocal inflammatory demyelinating disease that presents with encephalopathy and large T2-hyperintense lesions predominantly involving the cerebral white matter (8). The focus on ADEM as the primary encephalitic manifestation of MOGAD was highlighted in early recommendations on anti-MOG testing and diagnosis, which did not emphasize any other cerebral disease presentations (9, 10). In 2017, however, Ogawa et al. reported four patients who they described as having MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy, indicating a novel phenotype of MOGAD (11). All four patients had seizures, unilateral cortical hyperintensities on brain magnetic resonance imaging (MRI) T2-fluid-attenuated inversion recovery (T2-FLAIR) sequences, and anti-MOG positivity (11). In 2019, we systematically reviewed the literature to better characterize this unique syndrome and proposed the term unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES; Figure 1), which has since been adopted in the literature (12–18). Although initially reported to be a unilateral cortical encephalitis, we noted the presence of bilateral cortical involvement and possible meningeal inflammation in a subset of cases that was suggestive of a broader disease spectrum. In recent years, this possibility has been supported by the dramatic rise in reports of anti-MOG-positive patients with a variety of cortical and meningeal presentations (19–24). We herein review these meningo-cortical manifestations of MOGAD, with the aim of facilitating their prompt recognition when encountered in clinical practice.
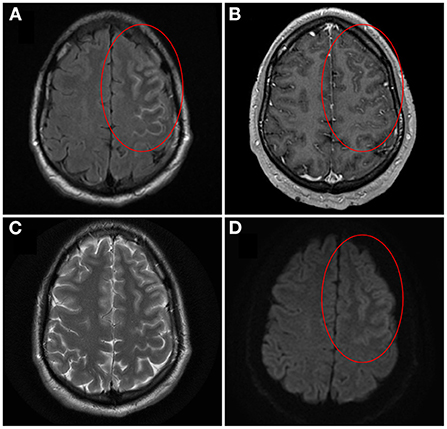
Figure 1. Brain magnetic resonance imaging of unilateral cortical FLAIR-hyperintense Lesion in Anti-MOG-associated Encephalitis with Seizures (FLAMES)/Unilateral cerebral cortical encephalitis (UCCE). On axial T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) image pre-gadolinium, cortical swelling and hyperintensity of both the left frontal cortex and adjacent sulci is seen, with hypointensity of the adjacent juxtacortical white matter [(A) circle]. On axial T1-weighted image post-gadolinium, corresponding leptomeningeal enhancement is also seen [(B) circle]. The cortical hyperintensity is not well visualized on axial T2-weighted image (C). On axial diffusion-weighted image there is brightness of the cortex with sparing of the subarachnoid space [(D) circle]. Corresponding subtle darkness on apparent diffusion coefficient map was seen, compatible with true diffusion restriction (not shown). Image adapted and re-used with permission from Springer Nature: Budhram A, Mirian A, Le C, Hosseini-Moghaddam SM, Sharma M, Nicolle MW. Unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J Neurol. (2019) 266(10):2481–7.
Unilateral cortical FLAMES, also referred to as unilateral cerebral cortical encephalitis (UCCE), was first highlighted as a potentially novel manifestation of MOGAD by Ogawa et al. in 2017 (11, 12, 22, 25). While patients with compatible disease presentations had been published prior to this seminal work, they were not recognized as potentially harboring a unique MOGAD phenotype. In 2015, Kim et al. described a 34-year-old male with headache, fever, and fluctuating level of consciousness ascribed to seizure activity, along with unilateral cortical T2-FLAIR hyperintensity (26). Antibodies against the N-methyl-D-aspartate receptor (NMDAR) were detected, and he was diagnosed with anti-NMDAR encephalitis. No testing for anti-MOG was reported; however, his neuroimaging findings, clinical presentation, and rapid improvement with only a relatively short course of corticosteroids was highly compatible with unilateral cortical FLAMES/UCCE, which has since been reported to co-exist with anti-NMDAR in a minority of cases (20, 21). Subsequently, in 2016, Numa et al. described a 37-year-old female with a remote history of ADEM who presented with headache, fever and right eye vision loss, followed by abnormal sensation in the right extremities (27). Neuroimaging revealed findings of right optic neuritis as well as the development of left frontal and temporal cortical T2-FLAIR hyperintensities that were suggestive of unilateral cortical FLAMES/UCCE, but that were interpreted at the time as multiphasic ADEM. Fortunately, following its emergence in the literature as a novel manifestation of MOGAD, cases of unilateral cortical FLAMES/UCCE have increasingly been recognized. At present it is the most well-characterized entity within the spectrum of meningo-cortical manifestations that have been observed in MOGAD, with distinct neuroimaging findings, clinical features, cerebrospinal fluid (CSF) abnormalities and treatment considerations that are summarized in Table 1 and discussed below.
Patients with this syndrome have cortical hyperintensity that can affect any lobe of the brain (12, 21, 28, 29). The frontal and parietal lobes are most often involved, while occipital lobe involvement is least common (21). As the name suggests, unilateral involvement is typically observed, although development of bilateral cortical encephalitis with anti-MOG positivity has been described in a small number of patients (discussed later) (12, 20, 21, 23, 30, 31). The cortical hyperintensity is typically most evident on T2-FLAIR sequences, so these should be carefully reviewed for subtle abnormality that may otherwise be missed (11, 12, 32). In contrast, corresponding hyperintensity on T2-weighted imaging (T2WI) may be minimal or absent (11, 12). Classically, the hyperintensity is only cortical at presentation, without involvement of the adjacent juxtacortical white matter (Figure 1) (12, 22). However, T2-FLAIR-hyperintense lesions involving both the cortex and adjacent juxtacortical white matter may be observed with other cerebral presentations of MOGAD including ADEM, and so their presence still warrants consideration of anti-MOG testing in the appropriate clinical context. Attention has recently been drawn to T2-FLAIR hypointensity of the adjacent juxtacortical white matter (Figure 1), and the presence of this finding alongside cortical T2-FLAIR hyperintensity may provide further supportive evidence of unilateral cortical FLAMES/UCCE (12, 22). Hyperperfusion of the cortical lesions can be seen on brain perfusion scans such as single photon emission computed tomography (SPECT), although this testing may not be routinely performed in clinical practice (11, 21). Additional neuroimaging findings that have been observed in a subset of cases include sulcal T2-FLAIR hyperintensity and leptomeningeal enhancement, which are suggestive of leptomeningeal inflammation (12). A small number of patients with leptomeningeal enhancement and symptoms of meningitis (e.g. headache, fever) in the absence of symptoms of encephalitis (e.g. seizures, cortical symptoms, other features of encephalopathy) have been described, indicating a broader spectrum of meningo-cortical manifestations that includes predominantly meningeal disease (discussed later) (33, 34). Similar neuroimaging features can be seen in other disease processes involving the cortex (e.g. Creutzfeldt-Jakob disease, mitochondrial disease, hypoxia, metabolic disturbance, seizure-related change) or the subarachnoid space (e.g. subarachnoid hemorrhage, infectious or carcinomatous meningitis), so alternative diagnoses relevant to the patient presentation should still be thoroughly considered when such imaging abnormalities are encountered (11, 12, 35–39). However, lesions with a typical appearance for unilateral cortical FLAMES/UCCE on MRI should prompt testing for anti-MOG in the appropriate clinical context.
In line with other autoimmune encephalitides, patients with this syndrome typically present subacutely (8, 12). While acute stroke-like presentations have been reported, the history in such cases should be carefully reviewed for symptoms in the preceding days to weeks that may otherwise go unrecognized, such as headache, fever, or intermittent spells suggestive of seizures (12, 40). Seizures are the most prominent clinical manifestation of unilateral cortical FLAMES/UCCE, and occur in the large majority of patients. Our previous review of cases published in the literature found that seizures were reported in 85%, and a recent series of 39 Japanese patients noted that nearly all (97%) presented with seizures (12, 22). They are focal-onset, most often present as motor seizures, ictal aphasia or somatosensory symptoms, and have been reported to progress to bilateral tonic-clonic seizures in as many as 77% (12, 32). Seizure semiology commonly corresponds to lesion location, supporting their epileptogenicity. In addition to seizures, characteristic disease manifestations include headache, fever, cortical symptoms referable to the lesion location, and other features of encephalopathy (12, 21, 28, 29). Headache can be severe, and may have features of high intracranial pressure (12). Cortical symptoms referable to the lesion that are most commonly reported include aphasia and hemiparesis, although depending on the lesion location symptoms such as hemianopsia may occur (28, 41). Other features of encephalopathy that may be observed include altered mental status and behavioral change (11, 12, 28). Importantly, in patients presenting with such symptoms of cerebral dysfunction in the absence of recognized seizures, the possibility that their presentation may represent an ictal/post-ictal phenomenon should be considered, in which case prolonged electroencephalography can be helpful to look for electroencephalographic evidence of seizures that may otherwise go undiagnosed (12, 42). Although the term “epilepsy” was initially used to describe these patients, an enduring predisposition to seizures following treatment does not generally occur. For this reason, use of the term “seizures” rather than “epilepsy” more appropriately reflects their typically acute symptomatic nature (11, 12, 43).
In patients suspected of having this syndrome, a number of CSF abnormalities have been described. As mentioned previously, patients with unilateral cortical FLAMES/UCCE can present with severe headache that has features of high intracranial pressure. In line with this, CSF opening pressure may be elevated and should be measured at time of lumbar puncture (12, 20). CSF white blood cell (WBC) count elevation has been reported in as many as 90% of patients, with pleocytosis that may be in excess of 100 WBC/μl but is usually less than 1000 WBC/μl (12, 20, 22). CSF-specific oligoclonal bands have been reported in less than 20% of patients, which stands in contrast to nearly 90% of patients with MS (12, 20, 44). However, unlike other manifestations of MOGAD such as optic neuritis or myelitis, MS is less likely to be a leading diagnostic consideration in patients with unilateral cortical FLAMES/UCCE because, although cortical presentations of MS with focal-onset seizures have been described, they are rare and lack the characteristic neuroimaging features (45, 46). A neuro-inflammatory disease that may more closely resemble unilateral cortical FLAMES/UCCE is anti-NMDAR encephalitis given its subacute onset, frequent viral prodrome, and propensity to cause seizures (8, 47). Intriguingly, a minority of patients with unilateral cortical FLAMES/UCCE have been reported to harbor co-existent anti-NMDAR; while the uniform detection of anti-MOG in patients with this clinico-radiographic syndrome suggests it is the more relevant disease biomarker, CSF testing of anti-NMDAR should therefore be considered in all cases and may be helpful to inform the potential for phenotypic overlap, role of malignancy screening, treatment considerations, and prognosis in dual-positive patients (20, 21, 48). Although testing for anti-MOG in serum is generally recommended and has overall higher clinical sensitivity for MOGAD compared to CSF, testing of stored CSF may be helpful in cases of unilateral cortical FLAMES/UCCE when serum from the time of attack is not available (40, 49, 50).
In keeping with other manifestations of MOGAD, patients with unilateral cortical FLAMES/UCCE generally have excellent response to corticosteroids (12, 22). Anti-seizure medications are commonly administered once seizures are identified, but seizures typically resolve after corticosteroid treatment. The necessity of anti-seizure medication is thus uncertain, particularly outside the acute symptomatic phase (12). While treatment with corticosteroids is generally recommended, cases of resolution without immunotherapy have also been reported (40, 51). Expectedly, patients can have other attacks compatible with MOGAD prior to, concurrent with, or after unilateral cortical FLAMES/UCCE (12). Maintenance immunotherapy should be considered in patients with MOGAD who have relapsing disease, although the optimal long-term treatment approach remains unclear (52–55).
To date, pathologic data remains sparse among patients with this syndrome. In 2018, Ikeda et al. described a 29-year-old female who presented with unilateral cortical FLAMES/UCCE and underwent brain biopsy of the involved right parietal gyrus while symptomatic, prior to immunotherapy (56). The subarachnoid space and brain parenchyma revealed mild lymphocytic infiltration and perivascular lymphocyte cuffing without distinct demyelination, although the authors questioned whether the biopsy findings could reflect a very early stage of demyelinating disease. In 2019, Patterson et al. described a 39-year-old female with unilateral cortical FLAMES/UCCE who underwent brain biopsy of the involved left parietal lobe and dura while symptomatic, prior to immunotherapy (57). This showed interstitial and perivascular lymphocytic infiltrates, without clear evidence of demyelination. In 2020, Takai et al. reviewed 11 brain biopsies of patients with MOGAD, two of whom were classified as having cortical encephalitis (58). One was previously described (56), while the other was a 15-year-old female who underwent biopsy of a left temporal lobe lesion after initiation of corticosteroids. Of note, this patient had T2-FLAIR hyperintensity involving both the cortex and adjacent juxta-cortical white matter. In addition to perivascular lymphocytic infiltrates, subpial and perivenous demyelination with cortical encephalitis was observed. A similar pattern of demyelination was observed in one patient with ADEM, leading the authors to hypothesize that ADEM and cortical presentations of MOGAD may have a shared underlying pathology (58).
Differing reports regarding demyelination in unilateral cortical FLAMES/UCCE may relate to one or more factors. It is possible that patchy demyelination could be missed depending on the biopsy location, leading to sampling variability. Variable reports of demyelination could also reflect different stages of disease, depending on timing of biopsy. Finally, it bears emphasis that typically, neuroimaging of unilateral cortical FLAMES/UCCE at presentation shows cortical T2-FLAIR hyperintensity without hyperintensity of the adjacent juxtacortical white matter, rather than T2-FLAIR hyperintensity of both (12, 22). Labeling patients with strictly cortical T2-FLAIR hyperintense lesions and patients with T2-FLAIR hyperintense lesions involving the cortex and adjacent juxtacortical white matter as both having “cortical encephalitis” could hinder the discovery of pathologic findings that may potentially distinguish between meningo-cortical manifestations of MOGAD and other manifestations such as ADEM, a possibility that should be considered in future pathologic analyses.
Although unilateral cortical FLAMES/UCCE is classically an affliction of one hemisphere, progression to bilateral cortical involvement has been reported in a subset of cases (12). Among patients with anti-MOG positivity and bilateral cortical involvement, the frontal lobes are most commonly involved (21). An exemplary case of this was provided by Fujimori et al. who in 2017 described a 46-year-old male with cortical T2-FLAIR hyperintensity of the left medial frontal cortex and a focal motor seizure of the right leg that secondarily generalized (30). While disease was initially limited to one hemisphere, the patient went on to develop more extensive bilateral medial frontal cortical T2-FLAIR hyperintensities as well as paraparesis. He was treated empirically with high-dose corticosteroids for suspected autoimmune encephalitis and had good response. Biopsy of the right cingulate gyrus showed lymphocytic infiltrates without demyelination, with the caveat that it was performed after approximately 1 month of corticosteroids. The patient did well until he developed optic neuritis nearly 2 years later, and subsequent testing of stored serum from the time of his initial encephalitic presentation revealed positivity for anti-MOG. Fujimori et al. later reviewed the literature for similar cases of what they referred to as MOG antibody-associated bilateral medial frontal cerebral cortical encephalitis (BFCCE), and identified six patients (23). Similar to unilateral cortical FLAMES/UCCE, a subset of those with BFCCE were noted to have overlying leptomeningeal enhancement, which has also been referred to in the literature as bilateral parafalcine cortical and leptomeningeal impairment (BPCLI) (31). Other disease features of MOG antibody-associated BFCCE/BPCLI (headache, fever, seizures, cortical symptoms referable to the lesion location in the form of paraparesis, CSF pleocytosis and steroid-responsiveness) closely resemble those observed in unilateral cortical FLAMES/UCCE, suggesting that the two entities exist on a continuum of meningo-cortical manifestations in MOGAD (12, 23, 31). To examine differences in topography, Fujimori et al. superimposed lesions in unilateral cortical FLAMES/UCCE and BFCCE/BPCLI and found that lesions in the former were mostly located in the territories of the middle cerebral arteries, while lesions in the latter were mostly located in the territories of the anterior cerebral arteries (23). Taken together with reports of vessel dilation (e.g. on magnetic resonance angiography) and lesion hyperperfusion (e.g. on SPECT) in patients with cortical presentations of MOGAD, the authors suggested that vascular involvement may influence the location of lesion development, which is a hypothesis that would benefit from further study (14, 21, 23, 59).
A subset of patients with both unilateral and bilateral cortical manifestations of MOGAD have been reported to have overlying leptomeningeal enhancement, indicating possible meningeal inflammation and the potential for broader disease involvement beyond the cortex (12, 23, 33). Intriguingly, at the time of our initial literature review of unilateral cortical FLAMES in 2019, we identified one patient who been reported to have unilateral sulcal T2-FLAIR hyperintensity and leptomeningeal enhancement, without significant cortical T2-FLAIR hyperintensity (12, 60). This patient had no symptoms referable to this finding, which was incidentally identified during evaluation of optic neuritis (60). Subsequently, a review of the Mayo Clinic anti-MOG-positive database identified two patients with unilateral leptomeningeal enhancement and minimal-to-no cortical T2-FLAIR hyperintensity, which was referred to as FLAIR-variable Unilateral Enhancement of the Leptomeninges (FUEL) (34, 61). At the time of leptomeningeal enhancement, both of these patients had symptoms suggestive of meningeal irritation (e.g. headache, fever) without additional features to indicate encephalitis (e.g. seizures, focal cortical symptoms, other features of encephalopathy). These reports indicate that predominantly meningeal involvement falls on the continuum of meningo-cortical manifestations observed in MOGAD (34, 60, 61). This was emphasized in a review of twelve patients with MOG antibody positivity and aseptic meningitis in the absence of brain parenchymal lesions, which the authors referred to as MOG antibody-associated aseptic meningitis (MOGAM) (24). In keeping with meningeal inflammation all patients had CSF pleocytosis, and elevated CSF opening pressure was documented in the four who had this measured. Clinically, all had headache and/or fever, while only one-third had seizures; the clinical symptoms reported in MOGAM were therefore similar to those observed in cortical presentations of MOGAD, but with expected differences in relative frequencies that likely relate to whether involvement of the meninges or cortex predominates (12, 24). Excellent response to corticosteroid was generally observed in patients with MOGAM, further supporting the notion that it exists on a broader spectrum of meningo-cortical manifestations in MOGAD.
Over the last 5 years, dramatic advancements have been made in the characterization of anti-MOG-positive cortical and meningeal presentations. Variations in these presentations, such as whether neuroimaging abnormalities are unilateral or bilateral, or whether cortical or meningeal involvement predominates, have been described by different groups and led to a plethora of nomenclature that includes unilateral cortical FLAMES, UCCE, BFCCE, BPCLI, FUEL and MOGAM. However, commonalities across their neuroimaging findings, clinical features, CSF parameters and responses to treatment suggest that these entities lie on a continuum of meningo-cortical manifestations in MOGAD. Increased recognition of this novel clinico-radiographic spectrum is essential for accurate patient diagnosis, as well as future studies dedicated to uncovering potentially unique pathophysiologic mechanisms.
AB planned and drafted the manuscript. AM and MS revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
AB reports that he holds the London Health Sciences Centre and London Health Sciences Foundation Chair in Neural Antibody Testing for Neuro-Inflammatory Diseases, and is supported by the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario (AMOSO).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. O'Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, Xu C, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. (2007) 13:211–7. doi: 10.1038/nm1488
2. Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. (2014) 71:276–83. doi: 10.1001/jamaneurol.2013.5857
3. Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. (2012) 79:1273–7. doi: 10.1212/WNL.0b013e31826aac4e
4. Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. (2017) 140:3128–38. doi: 10.1093/brain/awx276
5. Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. (2018) 90:e1858–69. doi: 10.1212/WNL.0000000000005560
6. Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a review of clinical and mri features, diagnosis, and management. Front Neurol. (2022) 13:885218. doi: 10.3389/fneur.2022.885218
7. Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. (2021) 20:762–72. doi: 10.1016/S1474-4422(21)00218-0
8. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
9. Jarius S, Paul F, Aktas O, Asgari N, Dale RC, Seze DE, et al. (2018). MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 15:134. doi: 10.1186/s12974-018-1144-2
10. López-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. (2018) 75:1355–63. doi: 10.1001/jamaneurol.2018.1814
11. Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e322. doi: 10.1212/NXI.0000000000000322
12. Budhram A, Mirian A, Le C, Hosseini-Moghaddam SM, Sharma M, Nicolle MW, et al. Unilateral cortical FLAIR-hyperintense lesions in anti-MOG-associated Encephalitis with seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J Neurol. (2019) 266:2481–7. doi: 10.1007/s00415-019-09440-8
13. Stamenova S, Redha I, Schmierer K, Garcia ME. FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES) unmasked by withdrawal of immunosuppression for Crohn's disease? Mult Scler Relat Disord. (2021) 48:102729. doi: 10.1016/j.msard.2020.102729
14. Jain K, Cherian A, Thomas BJN. FLAMES: a novel burning entity in MOG IgG associated disease. Mult Scler Relat Disord. (2021) 49:102759. doi: 10.1016/j.msard.2021.102759
15. Cabezudo-García P, Vidal Denis M, Ciano-Petersen NL, Irigoyen-Oyarzábal MV, Serrano-Castro PJ. FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES) in a patient with Crohn's disease on anti-TNF treatment. Neurologia. (2021) 30:304–6. doi: 10.1016/j.nrl.2021.06.002
16. Hokama H, Sakamoto Y, Hayashi T, Hatake S, Takahashi M, Kodera H, et al. A case report of FLAMES with elevated myelin basic protein followed by myelitis. Intern Med. (2022) 9439-22. doi: 10.2169/internalmedicine.9439-22. [Epub ahead of print].
17. Maniscalco GT, Allegorico L, Alfieri G, Napolitano M, Ranieri A, Renna R, et al. Anti-MOG-associated demyelinating disorders: two sides of the same coin. Neurol Sci. (2021) 42:1531–4. doi: 10.1007/s10072-020-04892-7
18. Lopez Chiriboga S, Flanagan EP. Myelitis and other autoimmune myelopathies. Continuum. (2021) 27:62–92. doi: 10.1212/CON.0000000000000900
19. Armangue T, Olivé-Cirera G, Martínez-Hernandez E, Sepulveda M, Ruiz-Garcia R, Muñoz-Batista M, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. (2020) 19:234–46. doi: 10.1016/S1474-4422(19)30488-0
20. Yao T, Zeng Q, Xie Y, Bi F, Zhang L, Xiao B, et al. Clinical analysis of adult MOG antibody-associated cortical encephalitis. Mult Scler Relat Disord. (2022) 60:103727. doi: 10.1016/j.msard.2022.103727
21. Shu H, Ding M, Shang P, Song J, Lang Y, Cui L, et al. Myelin oligodendrocyte glycoprotein antibody associated cerebral cortical encephalitis: case reports and review of literature. Front Hum Neurosci. (2021) 15:782490. doi: 10.3389/fnhum.2021.782490
22. Fujimori J, Ogawa R, Murata T, Takai Y, Misu T, Nakashima I, et al. Decreased subcortical T2 FLAIR signal with cortical T2 FLAIR hyperintense lesions in unilateral cerebral cortical encephalitis with myelin oligodendrocyte glycoprotein antibody. Neuroimmunol Rep. (2022) 2:100096. doi: 10.1016/j.nerep.2022.100096
23. Fujimori J, Nakamura M, Yagihashi T, Nakashima I. Clinical and radiological features of adult onset bilateral medial frontal cerebral cortical encephalitis with anti-myelin oligodendrocyte glycoprotein antibody. Front Neurol. (2020) 11:600169. doi: 10.3389/fneur.2020.600169
24. Lin S, Long W, Wen J, Su Q, Liao J, Hu Z, et al. Myelin oligodendrocyte glycoprotein antibody-associated aseptic meningitis without neurological parenchymal lesions: a novel phenotype. Multiple Sclerosis Related Disorders. (2022) 68:104126. doi: 10.1016/j.msard.2022.104126
25. Budhram A, Mirian A, Flanagan EP. Unilateral cortical fluid-attenuated inversion recovery-hyperintense lesions in anti-myelin oligodendrocyte glycoprotein-associated encephalitis with seizures (FLAMES): an under-recognized entity. Pediatr Neurol. (2020) 110:99–100. doi: 10.1016/j.pediatrneurol.2020.05.003
26. Kim H, Ryu H, Kang JK. Anti-NMDA receptor antibody encephalitis presenting with unilateral non-convulsive status epilepticus in a male patient. J Epilepsy Res. (2015) 5:17–9. doi: 10.14581/jer.15004
27. Numa S, Kasai T, Kondo T, Kushimura Y, Kimura A, Takahashi H, et al. An adult case of anti-myelin oligodendrocyte glycoprotein (mog) antibody-associated multiphasic acute disseminated encephalomyelitis at 33-year intervals. Intern Med. (2016) 55:699–702. doi: 10.2169/internalmedicine.55.5727
28. Wang YF, Liu XW, Lin JM, Liang JY, Zhao XH, Wang SJ, et al. The clinical features of FLAIR-hyperintense lesions in anti-mog antibody associated cerebral cortical encephalitis with seizures: case reports and literature review. Front Immunol. (2021) 12:582768. doi: 10.3389/fimmu.2021.582768
29. Tian F, Liu X, Yang C, Wang B, Song Z, Zhang YMOG, et al. Antibody-positive cerebral cortical encephalitis: two case reports and literature review. Int J Dev Neurosci. (2021) 81:342–51. doi: 10.1002/jdn.10106
30. Fujimori J, Takai Y, Nakashima I, Sato DK, Takahashi T, Kaneko K, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry. (2017) 88:534–6. doi: 10.1136/jnnp-2016-315094
31. Jiang W, Sun X, Huang H, Sun H, Zhang S, He M, et al. Bilateral parafalcine cortical and leptomeningeal impairment: a characteristic pattern of MOG antibody disease and AQP4 neuromyelitis optica spectrum disorders? J Neuroimmunol. (2022) 369:577898. doi: 10.1016/j.jneuroim.2022.577898
32. Tokumoto K, Nishida T, Kawaguchi N, Kaneko K, Takahashi T, Takahashi Y, et al. Electroclinical features of seizures in myelin oligodendrocyte glycoprotein antibody-associated cerebral cortical encephalitis: a case report and literature review. Seizure. (2022) 98:13–8. doi: 10.1016/j.seizure.2022.04.001
33. Gombolay GY, Gadde JA. Aseptic meningitis and leptomeningeal enhancement associated with anti-MOG antibodies: a review. J Neuroimmunol. (2021) 358:577653. doi: 10.1016/j.jneuroim.2021.577653
34. Budhram A, Kunchok AC, Flanagan EP. Unilateral leptomeningeal enhancement in myelin oligodendrocyte glycoprotein immunoglobulin G-associated disease. JAMA Neurol. (2020) 77:648–9. doi: 10.1001/jamaneurol.2020.0001
35. Ng YS, Bindoff LA, Gorman GS, Klopstock T, Kornblum C, Mancuso M, et al. Mitochondrial disease in adults: recent advances and future promise. Lancet Neurol. (2021) 20:573–84. doi: 10.1016/S1474-4422(21)00098-3
36. Renard D, Castelnovo G, Bouly S, Le Floch A, Waconge A, Verdal DE, et al. Cortical abnormalities on MRI: what a neurologist should know. Pract Neurol. (2015) 15:257–65. doi: 10.1136/practneurol-2015-001113
37. Fragoso DC, Gonçalves Filho AL, Pacheco FT, Barros BR, Aguiar Littig I, Nunes RH, et al. Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics. (2017) 37:234–57. doi: 10.1148/rg.2017160075
38. Rosenbloom MH, Tartaglia MC, Forner SA, Wong KK, Kuo A, Johnson DY, et al. Metabolic disorders with clinical and radiologic features of sporadic Creutzfeldt-Jakob disease. Neurol Clin Pract. (2015) 5:108–15. doi: 10.1212/CPJ.0000000000000114
39. Sheerin F, Pretorius PM, Briley D, Meagher T. Differential diagnosis of restricted diffusion confined to the cerebral cortex. Clin Radiol. (2008) 63:1245–53. doi: 10.1016/j.crad.2007.12.018
40. Mirian A, Yu YJ, Casserly CS, Budhram A. FLAIR-hyperintense Lesion in anti-MOG-associated encephalitis with seizures (FLAMES). Can J Neurol Sci. (2022) 49:420–1. doi: 10.1017/cjn.2021.134
41. Chang YC, Sharma M. Budhram, A. Unilateral cortical FLAIR-hyperintense Lesion in Anti-MOG-associated Encephalitis with Seizures (FLAMES) on TNF inhibitor therapy. J Neurol Neurosurg Psychiatry. (2021) 92:678–9. doi: 10.1136/jnnp-2021-326401
42. Ericson EJ, Gerard EE, Macken MP, Schuele SU. Aphasic status epilepticus: electroclinical correlation. Epilepsia. (2011) 52:1452–8. doi: 10.1111/j.1528-1167.2011.03084.x
43. Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: Conceptual definitions. Epilepsia. (2020) 61:1341–51. doi: 10.1111/epi.16571
44. Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry. (2013) 84:909–14. doi: 10.1136/jnnp-2012-304695
45. Koch M, Uyttenboogaart M, Polman S, Keyser DE. Seizures in multiple sclerosis. Epilepsia. (2008) 49:948–53. doi: 10.1111/j.1528-1167.2008.01565.x
46. Jain J, Son M, Debicki DB, Jog M, Casserly CS, Burneo JG, et al. Epilepsia partialis continua in relapsing-remitting multiple sclerosis: a possible distinct relapse phenotype. Clin Neurol Neurosurg. (2022) 213:107099. doi: 10.1016/j.clineuro.2021.107099
47. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
48. Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. (2014) 13:167–77. doi: 10.1016/S1474-4422(13)70282-5
49. Alkabie S, Budhram A. Testing for antibodies against aquaporin-4 and myelin oligodendrocyte glycoprotein in the diagnosis of patients with suspected autoimmune myelopathy. Front Neurol. (2022) 13:912050. doi: 10.3389/fneur.2022.912050
50. Mariotto S, Gajofatto A, Batzu L, Delogu R, Sechi G, Leoni S, et al. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology. (2019) 93:e1867–e72. doi: 10.1212/WNL.0000000000008479
51. Budhram A, Sechi E, Nguyen A, Lopez-Chiriboga AS, Flanagan EP. FLAIR-hyperintense lesions in Anti-MOG-associated encephalitis with seizures (FLAMES): is immunotherapy always needed to put out the fire? Mult Scler Relat Disord. (2020) 44:102283. doi: 10.1016/j.msard.2020.102283
52. Chen JJ, Huda S, Hacohen Y, Levy M, Lotan I, Wilf-Yarkoni A, et al. Association of maintenance intravenous immunoglobulin with prevention of relapse in adult myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. (2022) 79:518–25. doi: 10.1001/jamaneurol.2022.0489
53. Cobo-Calvo A, Sepúlveda M, Rollot F, Armangué T, Ruiz A, Maillart E, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflamm. (2019) 16:134. doi: 10.1186/s12974-019-1525-1
54. Hacohen Y, Wong YY, Lechner C, Jurynczyk M, Wright S, Konuskan B, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. (2018) 75:478–87. doi: 10.1001/jamaneurol.2017.4601
55. Satukijchai C, Mariano R, Messina S, Sa M, Woodhall MR, Robertson NP, et al. Factors associated with relapse and treatment of myelin oligodendrocyte glycoprotein antibody-associated disease in the United Kingdom. JAMA Netw Open. (2022) 5:e2142780. doi: 10.1001/jamanetworkopen.2021.42780
56. Ikeda T, Yamada K, Ogawa R, Takai Y, Kaneko K, Misu T, et al. The pathological features of MOG antibody-positive cerebral cortical encephalitis as a new spectrum associated with MOG antibodies: a case report. J Neurol Sci. (2018) 392:113–5. doi: 10.1016/j.jns.2018.06.028
57. Patterson K, Iglesias E, Nasrallah M, González-Álvarez V, Suñol M, Anton J, et al. Anti-MOG encephalitis mimicking small vessel CNS vasculitis. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e538. doi: 10.1212/NXI.0000000000000538
58. Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. (2020) 143:1431–46. doi: 10.1093/brain/awaa102
59. Fujimori J, Ogawa R, Murata T, Jin K, Yazawa Y, Nakashima I, et al. Unilateral chronic pulsatile headache as the single manifestation of anti-MOG antibody-associated unilateral cerebral cortical encephalitis. J Neuroimmunol. (2020) 346:577322. doi: 10.1016/j.jneuroim.2020.577322
60. Salama S, Khan M, Levy M, Izbudak I. Radiological characteristics of myelin oligodendrocyte glycoprotein antibody disease. Mult Scler Relat Disord. (2019) 29:15–22. doi: 10.1016/j.msard.2019.01.021
Keywords: MOG, FLAMES, cerebral cortical encephalitis, meningitis, autoimmune neurology, neuroimmunology, MOGAD
Citation: Budhram A, Mirian A and Sharma M (2022) Meningo-cortical manifestations of myelin oligodendrocyte glycoprotein antibody-associated disease: Review of a novel clinico-radiographic spectrum. Front. Neurol. 13:1044642. doi: 10.3389/fneur.2022.1044642
Received: 14 September 2022; Accepted: 29 September 2022;
Published: 20 October 2022.
Edited by:
Sergio Muñiz-Castrillo, Stanford Center for Sleep Sciences and Medicine, United StatesReviewed by:
Giulia Fadda, McGill University, CanadaCopyright © 2022 Budhram, Mirian and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adrian Budhram, QWRyaWFuLmJ1ZGhyYW1AbGhzYy5vbi5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.