- 1Department of Radiology, Affiliated Hospital 4 of Nantong University, Yancheng, China
- 2Department of Radiology, Yancheng First People's Hospital, Yancheng, China
- 3Neurotoxin Research Center of Key Laboratory of Spine and Spinal Cord Injury Repair and Regeneration of Ministry of Education, Department of Neurology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China
- 4Department of Neurology, Jiangsu University Affiliated People's Hospital, Zhenjiang, China
- 5Department of Neurology, Zhenjiang First People's Hospital, Zhenjiang, China
Objectives: This study analyzed the clinical and imaging characteristics of patients with cancer-related cryptogenic stroke (CCS) involving the bilateral anterior and the posterior circulations (three circulations) and evaluate the diagnostic value of clinical and imaging features for patients with cryptogenic stroke involving three circulations (CST).
Methods: Of the 12,580 patients with acute ischemic stroke, 148 patients with CST from May 2017 to November 2021 were divided into the cancer group (n = 81) and the non-cancer group (n = 67). Cardiovascular risk factors, diffusion-weighted imaging patterns of cryptogenic stroke, blood routine, coagulation routine, and biochemical routine were compared between the two groups. Multivariable logistic regression and receiver operating characteristic (ROC) curve analysis were used to determine associations between the two groups.
Results: Compared with the non-cancer group, the cancer group exhibited higher D-dimer levels (P < 0.001), fibrin degradation product (FDP, P < 0.001), international normalized ratio (INR, P = 0.014), neutrophil to lymphocyte ratio (NLR, P < 0.001), platelets to lymphocyte ratio (PLR, P = 0.001), activated partial thromboplastin time (APTT, P = 0.039), more frequent multiple lesions in three circulations (P < 0.001) and lower lymphocytes (P < 0.001), red blood cells (P < 0.001), and thrombin time (TT, P = 0.034). Furthermore, D-dimer [area under the curve (AUC) = 0.915, P < 0.001)], FDP (AUC = 0.923, P < 0.001), INR (AUC = 0.617, P = 0.014), NLR (AUC = 0.700, P < 0.001), PLR (AUC = 0.658, P = 0.001), and multiple lesions in three circulations (AUC = 0.786, P < 0.001) had potential diagnostic value in cryptogenic stroke. When combining these 6 parameters, the predictive power was improved (AUC = 0.949, P < 0.001).
Conclusion: Cryptogenic stroke involving three circulations with cancer has unique clinical features, and these potential diagnostic indicators could help patients identify CCS earlier.
Introduction
Cancer and stroke are the leading causes of death and disability worldwide (1–3). Stroke is the second most common central nervous system complication among cancer patients. A previous study indicated that ~15% of patients with cancer had a stroke at the time of death which was significantly higher than the incidence of stroke in the general population (7.6%) (4). The stroke rate in patients with cancer was twice as high as in the general population (5–7). The incidence of stroke in patients with cancer was also increasing due to the prolonged survival of cancer patients (8). Cancer-related stroke could influence these patients' quality of life seriously.
Previous studies indicated that cancer patients without traditional mechanisms of stroke after thorough examinations were considered to be patients with cancer-related cryptogenic stroke (CCS) (9, 10). Unlike traditional stroke, the occurrence of CCS is associated with hypercoagulable states, non-bacterial thrombotic endocarditis, cancer therapy, etc. (11). A previous study indicated that patients with CCS exhibited high plasma D-dimer levels and multi-vascular ischemic stroke lesions (12). A prospective study demonstrated that these two features might be predictors of occult cancer (13). In addition, patients with cryptogenic stroke had a worse prognosis than those with conventional stroke (14, 15). Previously, ~75% of the patients with three-territory signs (patients with stroke lesions involving the bilateral anterior and the posterior circulations) had a cancer-related stroke. However, the results are waiting to be confirmed due to its small sample size (16). In addition, the clinical and imaging features of CCS involving three circulations (CCST) have not been well studied.
Accordingly, this study aimed (1) to compare the clinical and imaging characteristics of patients with and without CCST and (2) to evaluate the diagnostic value of clinical and imaging characteristics for patients with cryptogenic stroke involving three circulations (CST).
Methods
Patients and stroke etiology
This retrospective study enrolled 12,580 patients with acute ischemic stroke from the Fourth Affiliated Hospital of Nantong University between May 2017 and November 2021. Cryptogenic stroke was defined as having no specific attributable cause after a comprehensive evaluation of common causes (17). Cancer patients with unconventional stroke mechanisms could be considered patients with CCS (9). Active cancer was defined as newly diagnosed, metastatic, progressive, or recurrent within 6 months prior to this enrollment. In addition, patients with active cancer, squamous skin cell carcinoma, or patients with localized basal cell carcinoma need to be excluded (18, 19). Inclusion criteria of cancer patients with CST were as follows: (1) age > 18 years; (2) diagnosed as acute ischemic stroke (within 72 h of onset symptoms) involving three circulations; (3) classified as cryptogenic stroke; (4) with active cancer; and (5) with complete clinical information. Exclusion criteria of cancer patients with CST were as follows: (1) recently with myocardial infarction, anticoagulant therapy, chronic kidney disease, or other diseases that might affect the coagulation index; (2) with inactive cancer, a primary brain tumor, or an undiagnosed tumor; and (3) with incomplete medical records. Inclusion criteria of non-cancer patients with CST were as follows: (1) age > 18 years; (2) diagnosed as acute ischemic stroke (within 72 h of onset symptoms) involving three circulations; (3) classified as cryptogenic stroke; (4) without cancer; and (5) with complete clinical information. Exclusion criteria of non-cancer patients with CST were as follows: (1) patients with myocardial infarction, anticoagulant therapy, chronic kidney disease, or other diseases that might affect the coagulation index; (2) with a primary brain tumor; and (3) with incomplete medical records. This study was approved by the Ethical Committee of the institution [(2021) - (k-104)], and all informed consents were obtained.
Ischemic stroke pattern
The cerebral vascular (including a total of 23 vascular territories) distribution is divided into the bilateral anterior and the posterior circulations (20, 21). Based on observed diffusion-weighted imaging (DWI) patterns, ischemic stroke lesions were classified into (1) a single lesion in a single one vascular territory, (2) scattered lesions in one vascular territory, (3) multiple lesions in multiple vascular territories, and (4) multiple lesions in three circulations (it is defined as all three circulations with multiple lesions). All imaging assessments are carried out independently by two experienced physicians.
Clinical information
This study collected data on patients during their hospitalization, including baseline characteristics (including age, gender, hypertension, diabetes, hyperlipidemia, and smoking), medical history (including recurrence, metastasis, and treatment of cancer patients), and medical examination (including echocardiography, ECG, extracranial vascular examination, and pathological reports of cancer). In addition, this study collected complete blood count [including white blood cell count, red blood cells, neutrophils, lymphocytes, platelet count, serum lactate dehydrogenase (LDH)], coagulation routine during admission without anticoagulant treatment [including D-dimer, international normalized ratio (INR), fibrinogen (FIB), fibrin degradation product (FDP), thrombin time (TT), activated partial thromboplastin time (APTT), antithrombin-III (AT-III)], and biochemical routine (including total protein, globulin, albumin, triglyceride, and total cholesterol levels).
Statistical analysis
Quantitative data were expressed as mean ± standard deviation or median (interquartile range) as appropriate. All categorical data were presented as a percentage. Values were compared using the Mann–Whitney U test or the independent t-test for quantitative variables as appropriate. Categorical variables were analyzed for the chi-square test or Fisher's exact test as appropriate. The independent non-collinear clinical features that were significantly correlated with CST at the univariate level (P < 0.01) were then included in the multivariate logistic regression model. Receiver operating characteristic (ROC) curve analysis and Youden's index were performed to determine the best cutoff value for some factors that differentiated cryptogenic stroke with or without cancer. SPSS software version 26 (IBM Corp, Armonk, NY, United States) was used for data analyses. Statistical significance was considered to be P < 0.05.
Results
Of all 12,580 patients diagnosed with ischemic stroke on MRI, 2.92% of patients (367/12,580) had ischemic stroke involving three circulations. According to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria (22), 367 patients were classified into the subtypes that incorporated large-artery atherosclerosis, small-vessel occlusion, cardioembolism, stroke of other determined cause, and cryptogenic stroke (Figure 1). Notably, 148 patients with cryptogenic stroke were selected from 367 patients with ischemic stroke involving three circulations. A total of 148 patients with CST were divided into the cancer group (81, 54.37%) and the non-cancer group (67, 45.27%) based on whether they had active cancer or not (Figure 1). The incidence of cryptogenic stroke in all patients with active cancer was 71.05% (81/114), and the incidence of cryptogenic stroke in all patients with non-cancer was 26.48% (67/253) (P < 0.001).

Figure 1. Flowchart of patient selection. CE, cardioembolism; LAA, large-artery atherosclerosis; SVO, small-vessel occlusion.
The most common cancer in patients with CCST was lung cancer (29/81, 35.80%), followed by pancreatic cancer (9/81, 11.11%), gastroduodenal cancer (9/81, 11.11%), esophageal cancer (8/81, 9.99%), other cancers (5/81, 6.17%), prostate cancer (4/81, 4.94%), cholangiocarcinoma (3/81, 3.70%), breast cancer (3/81, 3.70%), ovarian cancer (3/81, 3.70%), bladder cancer (2/81, 2.47%), gallbladder cancer (2/81, 2.47%), cervical cancer (2/81, 2.47%), colon cancer (1/81, 1.23%), and kidney cancer (1/81, 1.23%). Of note, 45.68% (37/81) of patients had lymph node metastases, and 60.49% (49/81) of patients had distant metastases. Notably, 20.99% (17/81) of patients were treated with surgery, and 28.40% (23/81) of patients were treated with chemotherapy or radiotherapy.
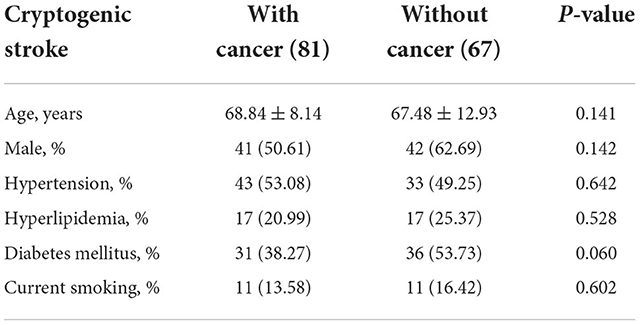
Among the cancer group, 41 (50.61%) were men, and the mean age was 68.84 ± 8.14 years. Among the non-cancer group, 42 (62.69%) were men, and the mean age was 67.48 ± 12.93 years. Baseline characteristics were compared between cancer and non-cancer groups. There were no statistical differences between the two groups in any of the baseline characteristics (Table 1).
For clinical characteristics (Table 2), the cancer group exhibited higher neutrophil to lymphocyte ratio (NLR, P < 0.001), platelets to lymphocyte ratio (PLR, P = 0.001), D-dimer (P < 0.001), FDP (P < 0.001), INR (P = 0.014), and APTT (P = 0.039) than the non-cancer group (Figure 2). In addition, the cancer group had lower lymphocytes (P < 0.001), red blood cells (P < 0.001), and TT (P = 0.034) than the non-cancer group.
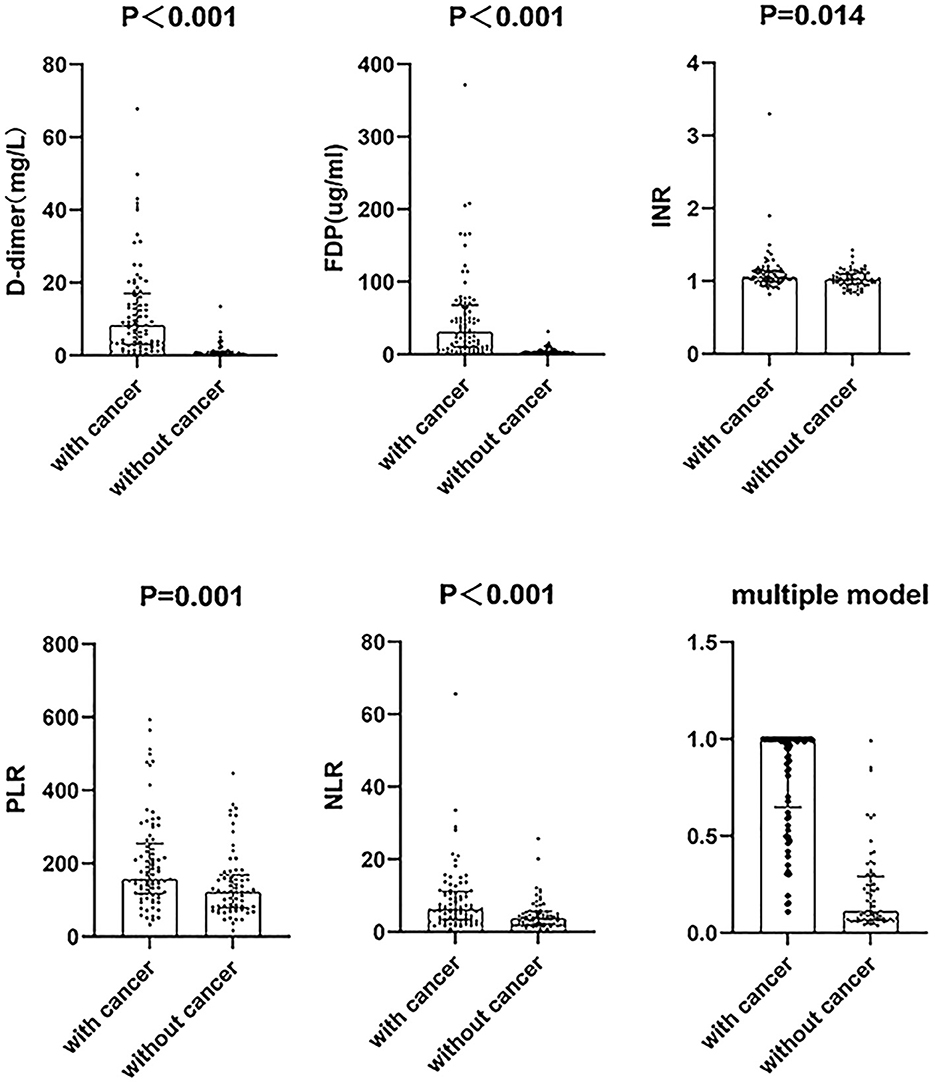
Figure 2. The difference between cancer and non-cancer groups in six parameters of cryptogenic stroke involving three circulations patients. The distribution of the data points including the median with an interquartile range. NLR, neutrophil to lymphocyte ratio; PLR, platelets to lymphocyte ratio; FDP, fibrin degradation product; INR, international normalized ratio.
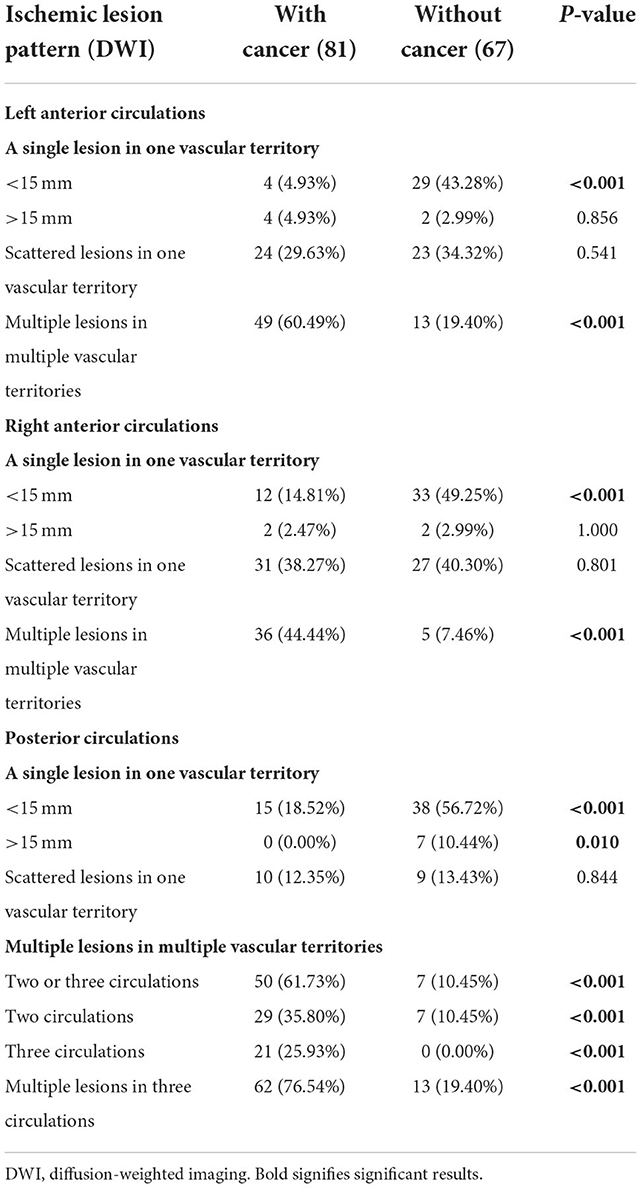
When evaluating the pattern of cryptogenic stroke lesions on DWI studies, cryptogenic stroke as a single lesion in one vascular territory of left anterior circulations was significantly less often observed in patients with CCST than in patients with CST without cancer. Similar results were also observed in the right anterior and posterior circulations (Table 3). The incidence of multiple lesions in multiple vascular territories in multiple circulations (two or three circulations) (Figure 3) of the cancer group was significantly higher (50/81, 61.71%) than that of the non-cancer group (7/67, 10.54%) (P < 0.001). The incidence of multiple lesions in three circulations of the cancer group (62/81, 76.54%) was higher than that of the non-cancer group (13/67, 19.40%) (P < 0.001) (Table 3).
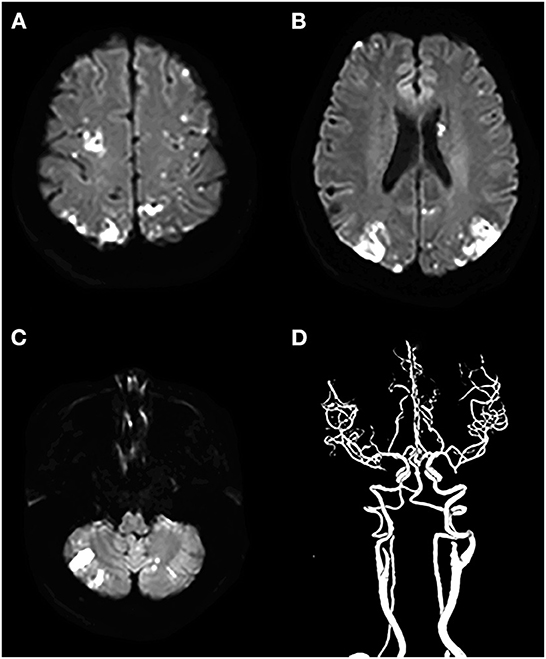
Figure 3. A cryptogenic stroke involving three circulations in a 72-year-old woman with a malignant tumor of the abdomen. The patient had no traditional risk factors for ischemic stroke such as atrial fibrillation, hypertension, diabetes mellitus, etc. (A–C) DWI images show multiple acute lesions in multiple vascular territories (in the bilateral anterior and the posterior circulations), and (D) shows no meaningful stenosis on CTA.
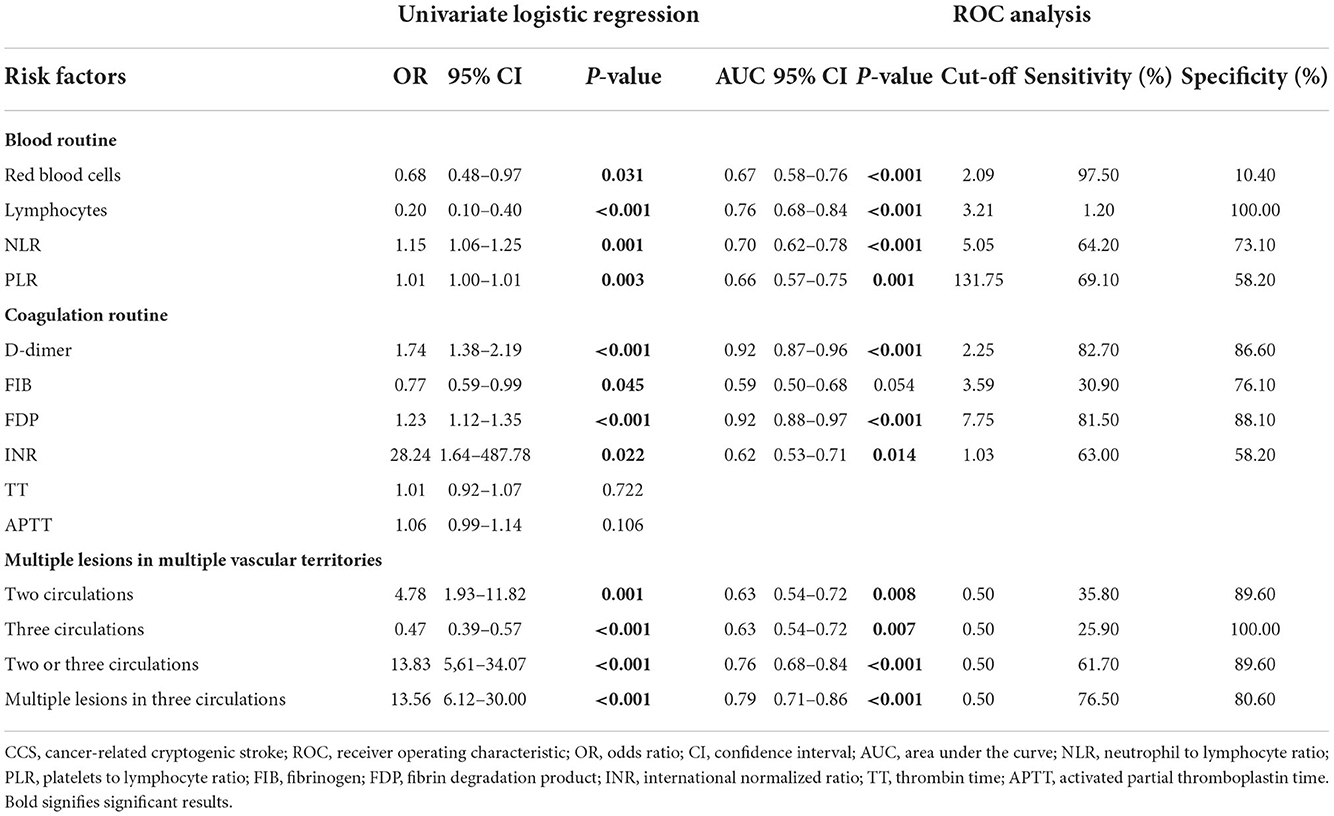
A total of 12 potential markers of CCST were selected based on the univariate logistic regression (Table 4). We used the ROC curve to evaluate the value of clinical and imaging parameters in distinguishing cancer and non-cancer groups. We found 11 parameters with potential diagnostic values (Table 4). The optimal cut-off for ROC curves analysis of the D-dimer level was 2.25, with sensitivity and specificity of 82.70 and 86.60%, respectively. The optimal FDP cut-off was 7.75, with sensitivity and specificity of 81.50 and 88.10%, respectively. The optimal INR cut-off was 1.03, with sensitivity and specificity of 63.00 and 58.20%, respectively. The optimal PLR cut-off was 131.75, with sensitivity and specificity of 69.10 and 58.20%, respectively. The optimal NLR cut-off was 5.05, with sensitivity and specificity of 64.20 and 73.10%, respectively. The optimal cut-off for multiple lesions in three circulations is 0.5, with sensitivity and specificity of 76.50 and 80.60%, respectively (Table 4).
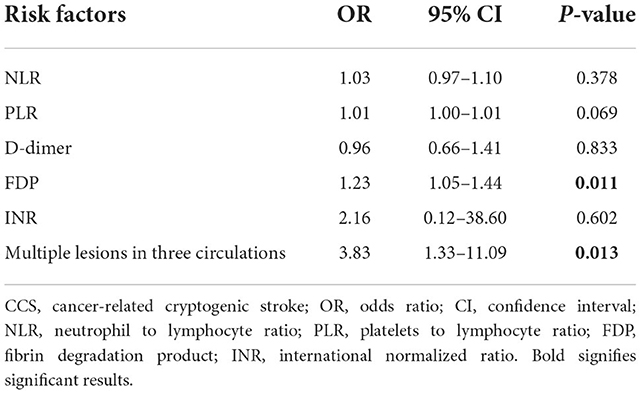
Then, in multivariate logistic regression analysis, variables were statistically significant (P < 0.01) at the univariate analysis level as shown in Table 5. Moreover, INR is considered meaningful in clinical practice, and it was also enrolled in multivariate logistic regression analysis. The model had a better diagnostic value with an area under the curve (AUC) of 0.95 (95% CI: 0.92–0.98). At a cut-off value of 0.42, the sensitivity and specificity of the multiple models were 88.9 and 89.5%, respectively (Figure 4).
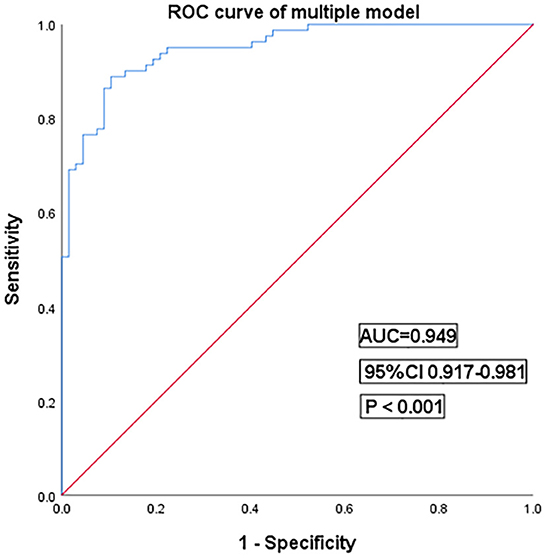
Figure 4. Receiver operating characteristic curves for the multiple models of cryptogenic stroke involving three circulations patients. AUC, area under the curve; CI, confidence interval.
Discussion
The main purpose of this study was to analyze the clinical and imaging characteristics of patients with CCST and evaluate the diagnostic value of clinical and imaging features for patients with CST. The DWI patterns of cryptogenic stroke, blood routine, and coagulation routine of cancer patients with CST are significantly different from non-cancer patients with CST, and these parameters can aid in diagnosing patients with CCST.
As yet, there is still no consensus on how to identify cancer risk in patients with acute stroke (23). In terms of the incidence of cancer in ischemic stroke, a study carried out in the United States revealed that approximately 10% of ischemic stroke inpatients had cancer (24). Another research showed that the rate of ischemic stroke patients with cancer was ~12% (25), which was smaller than the results of our research. Our study reported that 31.06% of ischemic strokes involving three circulations patients have comorbid cancer. Additionally, 54.37% of patients with CST have comorbid cancer, which was significantly higher than the incidence of stroke patients with cancer. Previous studies proposed that three territory signs indicated a possible hypercoagulable stroke due to cancer when no other cause is apparent (16). These studies indicated that there was a strong correlation between cancer and CST. Accordingly, we need to pay more attention to patients with CST.
Our study showed that patients with CCST had unique clinical and imaging features with higher D-dimer, FDP, INR, PLR, and NLR and more frequent multiple lesions in three circulations compared to patients with non-cancer CST. The possible reasons may be as follows. First, in the OASIS-Cancer study, they reported that cancer cell-derived extracellular vesicle levels correlated with D-dimer levels, a non-specific marker of hypercoagulability, and that cancer cell-derived extracellular vesicle levels were highest among patients with cryptogenic stroke (26). These mechanisms may lead to elevated D-dimer in patients with CCST. In addition, the D-dimer level is also associated with the severity and prognosis of cancer (13, 14, 27). These could also explain the high proportion of patients with CCST in advanced stages in this study. Second, Kono et al. (28) showed a correlation between increased FDP and cancer-related stroke, which indicated that FDP could be used as a potential biomarker to identify CCST from CST. Third, our study illustrated a higher INR in patients with CCS than in the non-cancer group. Previous studies showed that the INR of the cancer-related stroke group was higher than the INR of the non-cancer group (29, 30), which was similar to our study. Furthermore, the PLR is an easily applied blood test based on platelet and lymphocyte counts. Platelet aggregation and degranulation and the subsequent release of platelet granules and extracellular vesicles are thought to be important determinants of tumor growth. In turn, platelets promote tumor growth through proliferative signaling, anti-apoptotic effects, and angiogenic factors (31). Moreover, the release of immune mediators could cause a significant immunosuppressive effect and lymphocyte counts reduce. These reasons lead to an increased PLR in patients with cancer (32). Studies had reported a correlation between increased PLR and thromboembolism in patients with cancer (33, 34). The above theory could explain why PLR levels were significantly higher in patients with CCST than PLR in CST patients without cancer in our study. At the same time, NLR and PLR are easy to calculate from blood routine, and they are used as new predictors of thrombotic events (33). A retrospective analysis study showed a strong correlation between NLR and CCS (35), which could also explain why NLR was significantly higher in the cancer group than in the non-cancer group. Therefore, we included NLR in this study as an indicator to differentiate CCST from CST. Finally, several studies had shown that cancer-related stroke was associated with stroke in multiple vascular territories (36, 37). However, the above studies did not have a relationship between multi-vascular stroke and cancer. The results of this study showed for the first time that patients with CST had a significantly higher rate of active cancer than non-cancerous patients (76.54 vs. 19.40%). This finding could have a high diagnostic value in clinical practice. Our study expanded previous studies and demonstrated that these risk factors can be used to discriminate patients with CCST from patients with CST.
This study still had some limitations. First, our study was a retrospective analysis with a relatively small sample size, which might lead to selection bias. Second, patients with cryptogenic stroke involving a single circulations were not included in this study. In addition, ischemic stroke patients without MRI scanning due to the severity of their disease were not enrolled in this study. This might result in selection bias. Third, the mechanism of cancer-related hypercoagulable state was not fully understood, and there were large differences between individual cancer patients.
Conclusion
There was a high incidence of cancer in patients with stroke involving three circulations and a high incidence of cancer in patients with cryptogenic stroke involving three circulations. Blood routine, coagulation routine, and DWI patterns of cryptogenic stroke are potential biomarkers to identify CCST.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Affiliated Hospital 4 of Nantong University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YX contributed to the design of this study, helped in drafting the manuscript for intellectual content, had a major role in the acquisition of data, and contributed to the analysis and interpretation of the data. ZW and HX contributed to the design of the study and helped in drafting the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. (2020) 21:e342–9. doi: 10.1016/S1470-2045(20)30073-5
2. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, Mcbee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. (2019) 393:1021–32. doi: 10.1016/S0140-6736(19)30195-3
3. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
4. Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, et al. Association between incident cancer and subsequent stroke. Ann Neurol. (2015) 77:291–300. doi: 10.1002/ana.24325
5. Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. (2011) 42:3034–9. doi: 10.1161/STROKEAHA.111.615534
6. De La Aleja JG, Martinez-Salio A, Benito-Leon J. Association between incident cancer and subsequent stroke. Ann Neurol. (2015) 78:660. doi: 10.1002/ana.24476
7. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. (2017) 70:926–38. doi: 10.1016/j.jacc.2017.06.047
8. Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. (2018) 83:873–83. doi: 10.1002/ana.25227
9. Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. (2010) 41:798–801. doi: 10.1161/STROKEAHA.109.571356
10. Yamaura G, Ito T, Miyaji Y, Ueda N, Nakae Y, Momoo T, et al. Therapeutic efficacy of heparin and direct factor Xa inhibitors in cancer-associated cryptogenic ischemic stroke with venous thromboembolism. Thromb Res. (2021) 206:99–103. doi: 10.1016/j.thromres.2021.08.016
11. Bang OY, Chung JW, Lee MJ, Seo WK, Kim GM, Ahn MJ, et al. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke. (2020) 22:1–10. doi: 10.5853/jos.2019.02278
12. Gon Y, Okazaki S, Terasaki Y, Sasaki T, Yoshimine T, Sakaguchi M, et al. Characteristics of cryptogenic stroke in cancer patients. Ann Clin Transl Neurol. (2016) 3:280–7. doi: 10.1002/acn3.291
13. Gon Y, Sakaguchi M, Takasugi J, Kawano T, Kanki H, Watanabe A, et al. Plasma D-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke. Eur J Neurol. (2017) 24:503–8. doi: 10.1111/ene.13234
14. Nezu T, Mukai T, Uemura J, Yamashita M, Kitano T, Wada Y, et al. Multiple infarcts are associated with long-term stroke recurrence and all-cause mortality in cryptogenic stroke patients. Stroke. (2016) 47:2209–15. doi: 10.1161/STROKEAHA.116.014178
15. Nezu T, Kitano T, Kubo S, Uemura J, Yamashita S, Iwanaga T, et al. Impact of D-dimer levels for short-term or long-term outcomes in cryptogenic stroke patients. J Neurol. (2018) 265:628–36. doi: 10.1007/s00415-018-8742-x
16. Finelli PF, Nouh A. Three-territory DWI acute infarcts: diagnostic value in cancer-associated hypercoagulation stroke (trousseau syndrome). AJNR Am J Neuroradiol. (2016) 37:2033–6. doi: 10.3174/ajnr.A4846
17. Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke. (2014) 45:1186–94. doi: 10.1161/STROKEAHA.113.003720
18. Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. (2003) 349:146–53. doi: 10.1056/NEJMoa025313
19. Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. (2010) 68:213–9. doi: 10.1002/ana.22050
20. Darby DG, Parsons MW, Barber PA, Davis SM. Significance of acute multiple brain infarction on diffusion-weighted imaging. Stroke. (2000) 31:2270–1. doi: 10.1161/01.STR.31.9.2266-e
21. Wen HM, Lam WW, Rainer T, Fan YH, Leung TW, Chan YL, et al. Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology. (2004) 63:1317–9. doi: 10.1212/01.WNL.0000140490.22251.B6
22. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. trial of Org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
23. Salazar-Camelo RA, Moreno-Vargas EA, Cardona AF, Bayona-Ortiz HF. Ischemic stroke: a paradoxical manifestation of cancer. Crit Rev Oncol Hematol. (2021) 157:103181. doi: 10.1016/j.critrevonc.2020.103181
24. Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis. (2013) 22:1146–50. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.016
25. Dardiotis E, Aloizou AM, Markoula S, Siokas V, Tsarouhas K, Tzanakakis G, et al. Cancer-associated stroke: pathophysiology, detection and management (Review). Int J Oncol. (2019) 54:779–96. doi: 10.3892/ijo.2019.4669
26. Bang OY, Chung JW, Lee MJ, Kim SJ, Cho YH, Kim GM, et al. Cancer cell-derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor-independent way: the OASIS-CANCER study. PLoS ONE. (2016) 11:e0159170. doi: 10.1371/journal.pone.0159170
27. Nam KW, Kim CK, Kim TJ, An SJ, Demchuk AM, Kim Y, et al. D-dimer as a predictor of early neurologic deterioration in cryptogenic stroke with active cancer. Eur J Neurol. (2017) 24:205–11. doi: 10.1111/ene.13184
28. Kono T, Ohtsuki T, Hosomi N, Takeda I, Aoki S, Sueda Y, et al. Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int. (2012) 12:468–74. doi: 10.1111/j.1447-0594.2011.00796.x
29. Wu S, Xing Z, Lin J, Cui F, Liu H. Tumor metastasis has a significant relationship with the development of acute ischemic stroke in Chinese cancer patients: a retrospective study. J Int Med Res. (2021) 49:300060520986298. doi: 10.1177/0300060520986298
30. Sun B, Fan S, Li Z, Guo W, Liu L, Zhou Y, et al. Clinical and neuroimaging features of acute ischemic stroke in cancer patients. Eur Neurol. (2016) 75:292–9. doi: 10.1159/000447126
31. Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. (2018) 131:1777–89. doi: 10.1182/blood-2017-05-743187
32. Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, GERGER A, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. (2014) 110:2524–30. doi: 10.1038/bjc.2014.163
33. Ferroni P, Riondino S, Formica V, Cereda V, Tosetto L, La Farina F, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. Int J Cancer. (2015) 136:1234–40. doi: 10.1002/ijc.29076
34. Cheng X, Qin Q, Lu L, Chen C, Wei Y, Wang D, et al. The independent risks and specific biomarker of breast cancer-related ischemic stroke. Int J Neurosci. (2021) 131:135–43. doi: 10.1080/00207454.2020.1733562
35. Nam KW, Kim TJ, Kim CK, Mo H, Jeong HY, Kang MK, et al. Temporal changes in the neutrophil to lymphocyte ratio and the neurological progression in cryptogenic stroke with active cancer. PLoS ONE. (2018) 13:e0194286. doi: 10.1371/journal.pone.0194286
36. Lee EJ, Nah HW, Kwon JY, Kang DW, Kwon SU, Kim JS. Ischemic stroke in patients with cancer: is it different from usual strokes? Int J Stroke. (2014) 9:406–12. doi: 10.1111/ijs.12124
Keywords: cancer-related stroke, brain circulations, cryptogenic stroke, DWI, cancer
Citation: Xu Y, Wu Z and Xu H (2022) Cancer-related cryptogenic stroke involving the bilateral anterior and the posterior circulations: Diagnostic value of clinical and imaging characteristics. Front. Neurol. 13:1032984. doi: 10.3389/fneur.2022.1032984
Received: 31 August 2022; Accepted: 17 November 2022;
Published: 12 December 2022.
Edited by:
Sonu M. M. Bhaskar, Liverpool Hospital and South West Sydney Local Health District (SWSLHD), AustraliaReviewed by:
Asaf Honig, Faculty of Medicine, University of British Columbia, CanadaHenrik Gensicke, University Hospital of Basel, Switzerland
Copyright © 2022 Xu, Wu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hang Xu, bmV1cm8teHVoYW5nQGhvdG1haWwuY29t; Zhuang Wu, bmV1cm9fd3V6aHVhbmdAMTYzLmNvbQ==
 Yifan Xu
Yifan Xu Zhuang Wu
Zhuang Wu Hang Xu
Hang Xu