
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurol., 17 January 2023
Sec. Dementia and Neurodegenerative Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1028448
Introduction: There has been significant development in blood-based biomarkers targeting amyloidopathy of Alzheimer's disease (AD). However, the guidelines for integrating such biomarkers into AD diagnosis are still inadequate. Multimer Detection System-Oligomeric Amyloid-β (MDS-OAβ) as a plasma biomarker detecting oligomerization tendency is available in the clinical practice.
Main text: We suggest how to interpret the results of plasma biomarker for amyloidopathy using MDS-OAβ with neuropsychological test, brain magnetic resonance imaging (MRI), and amyloid PET for AD diagnosis. Combination of each test result differentiates various stages of AD, other neurodegenerative diseases, or cognitive impairment due to the causes other than neurodegeneration.
Discussion: A systematic interpretation strategy could support accurate diagnosis and staging of AD. Moreover, comprehensive use of biomarkers that target amyloidopathy such as amyloid PET on brain amyloid plaque and MDS-OAβ on amyloid-β oligomerization tendency can complement to gain advanced insights on amyloid-β dynamics in AD.
The advances in the development of blood-based biomarkers targeting amyloidopathy of Alzheimer's disease (AD) have been remarkable in showing high performances of predicting clinical AD and central AD pathology (1–3). Although current blood-based biomarkers have the issues to improve, their clinical application is promising based on the strength of low cost, non-invasiveness, and the ease of performance (4). Nevertheless, the guidelines for interpretation of blood-based biomarker with other AD diagnostic tools are scarce, and they would be meaningful in terms of fulfilling the broad interest from primary physicians to AD specialists and from the clinical practice to research field facing patients with progressive cognitive impairment.
Multimer Detection System-Oligomeric Amyloid-β (MDS-OAβ), one of the blood-based biomarkers detecting amyloidopathy, measures the oligomerization tendency of amyloid-β (Aβ) in blood, and its high predicting performances for central amyloidopathy and clinical AD have been reported (5–8). MDS-OAβ was approved by the Ministry of Food and Drug Safety (MFDS) and National Evidence-based healthcare Collaborating Agency (NECA) of Korea and is being used in the clinical practice. At this point, we would like to suggest how to integrate its results with other diagnostic tools including neuropsychological test, brain magnetic resonance imaging (MRI), and amyloid positron emission tomography (PET) for AD diagnosis.
MDS-OAβ, a chemiluminescence immunoassay, detects oligomerization tendency of plasma using epitope-overlapping antibodies specific for N-terminus of Aβ (5, 6). Plasma is spiked with synthetic Aβ and incubated. This pretreated plasma is loaded into the 96-well microplate coated with the capture antibodies during which heterogenous forms of Aβ are captured. After washing, detection antibodies are added and Aβ oligomers, known as the most neurotoxic form, are selectively detected over Aβ monomers. MDS-OAβ could differentiate between AD dementia (ADD) and cognitively normal group with a sensitivity of 100% and specificity of 92% in the tester-blinded study with a MFDS-approved protocol (7).
Previous study showed that MDS-OAβ was higher in subjects with mild cognitive impairment due to AD (AD-MCI) and ADD compared to cognitively normal subjects. MDS-OAβ was lower in advanced stages such as stages in clinical dementia rating of 2–3 than mild dementia or MCI states (6–8). This finding accords with the hypothesis that amyloidopathy progresses in the early stage of AD and reaches plateau state in the advanced stage (9). Namely, whereas the progression rate of amyloidopathy stays stable, the oligomerization tendency of Aβ detected by MDS-OAβ could decrease in the advanced stage. Because MDS-OAβ measures dynamic changes after spiking of synthetic Aβ in plasma, MDS-OAβ reflects plasma milieu of patients and indicates the upstream biomarker of amyloidopathy. Many upstream biomarkers show the dynamic changes of increase in the early stage of disease and decrease in the advanced stages of disease (10). Additionally, high MDS-OAβ was associated with atrophy bilateral temporal, amygdala, parahippocampal, lower parietal lobe, left cingulate, and precuneus area on brain MRI, which corresponds to AD pattern (8).
Patients with positive MDS-OAβ, cognitive impairment with insidious onset and slow progression, and AD-compatible atrophy on brain MRI are most likely to have AD. Rarely, other neurodegenerative diseases with concomitant amyloidopathy could be considered. Dementia with Lewy bodies (DLBs) and Parkinson disease dementia (PDD) have main pathology of α-synucleinopathy and could accompany with amyloidopathy with various extent (11, 12). In frontotemporal dementia (FTD), amyloidopathy could be present with main pathologic protein such as tau or TAR DNA-binding protein 43 (TDP-43) (12, 13). Also, vascular dementia (VD) and normal pressure hydrocephalus (NPH) could show amyloidopathy (12, 14). Limbic-predominant age-related TDP-43 encephalopathy (LATE) could have amyloidopathy along with neurodegeneration by TDP-43 (15).
Patients with positive MDS-OAβ, cognitive impairment, and normal brain MRI could be in the early stage of AD during which structural changes on brain MRI are not clear. Rarely, other causes including depression, vitamin B12/folate deficiency, electrolyte imbalance, and poor general medical condition could cause cognitive impairment and amyloidopathy to co-exist.
Patients with positive MDS-OAβ, normal cognition, and AD-compatible atrophy on MRI could have preclinical AD with progressive amyloidopathy without clinical manifestation. Rarely, other neurodegenerative diseases such as DLB, FTD, NPH, VD, PDD, or LATE mixed with amyloidopathy could be considered.
Patients with positive MDS-OAβ, normal cognition, and normal brain MRI could have preclinical amyloidopathy or they are at a high risk of AD. In case of negative MDS-OAβ, cognitive impairment, and AD-compatible atrophy on MRI, other neurodegenerative diseases such as DLB, FTD, NPH, VD, PDD, or LATE could be considered.
Patients with negative MDS-OAβ, cognitive impairment, and normal brain MRI could attribute the cognitive impairment to other causes including depression, vitamin B12/folate deficiency, electrolyte imbalance, or poor general medical condition.
Patients with negative MDS-OAβ, normal cognition, and AD-compatible atrophy on MRI could have other preclinical neurodegenerative diseases such as DLB, FTD, NPH, VD, PDD, or LATE.
Patients with negative MDS-OAβ, normal cognition, and normal brain MRI could be the conditions without AD-suspicious pathological evidence (Table 1).
Previous study reported that MDS-OAβ could predict amyloid PET positivity with area under the receiver operating characteristic curve value of 0.855 (16). MDS-OAβ and amyloid PET detect amyloidopathy with different biomarkers. MDS-OAβ measures oligomerization tendency instead of measuring concentration of each Aβ species or related peptides, but amyloid PET ligands react to insoluble amyloid fibril incorporated in plaque. Since both biomarkers have different characteristics and dynamics, their interpretation requires caution.
Patients with positive MDS-OAβ and positive amyloid PET could be most likely to have AD.
Patients with positive MDS-OAβ and negative amyloid PET could present amyloidopathy in progress without manifestation of amyloid plaques. These unmatched cases require further observation and studies regarding their different characteristics of patients and biomarkers itself. Issues to think are that ligands of amyloid PET are reactive to fibrillary form Aβ and low binding affinity to diffuse plaques. Moreover, although visual assessment of amyloid PET dichotomizes the patients into positive or negative, from the perspective of continuous spectrum, borderline negative cases near the cut-off values and definite negative cases far from the cut-off values should be differentiated based on the degree of amyloidopathy.
Patients with negative MDS-OAβ and positive amyloid PET could indicate advanced AD in case of cognitive impairment, where amyloidopathy is in plateau state (17); otherwise, preclinical AD is also possible in case of no cognitive impairment (18). Researches showed that the prevalence rate of cognitively normal elderly with positive amyloid PET reaches 10–30%, and each individual has different starting points of amyloid deposition (19, 20). Therefore, positive amyloid PET does not always indicate advanced stage or longer duration of disease (17). Patients with negative MDS-OAβ and negative amyloid PET are not likely to have amyloidopathy. In case of cognitive impairment, causes other than AD should be considered.
Empirically, some chemotherapeutic agents, immunotherapeutic agents, or passive immunization could lower the value of MDS-OAβ.
Even though the advanced diagnostic tools may present high performance, practical use in the clinical field requires proper interpretation to be of real value. This systematic interpretation suggests that MDS-OAβ could support more accurate diagnosis and staging of AD when combined with other biomarkers and provide helpful clues in diverse matches of clinical manifestation and test results, even in atypical presentation due to mixed pathologies. MDS-OAβ as a unique technique detecting oligomerization tendency measures the key neurotoxic process of AD, and therefore, MDS-OAβ would be suitable for diagnosis and disease monitoring with multiple re-tests possible due to low cost and invasiveness. Additionally, understanding factors affecting values of MDS-OAβ could provide clues about plasma milieu of patients with AD and might be useful in disease intervention in the future.
Collective interpretation of plasma biomarkers of AD should be avoided because each plasma biomarker of AD has different mechanisms and targets. Additionally, further autopsy studies and longitudinal data can strengthen evidence base for the interpretation. However, strategies of their clinical integration shall be essential in the era of immediate application of plasma biomarker of AD. Moreover, comprehensive use of various biomarker tools related to amyloidopathy such as amyloid plaque using PET, oligomerization tendency using MDS-OAβ, plasma, and CSF Aβ concentration could complement to gain advanced insights on Aβ dynamics in AD (Figure 1).
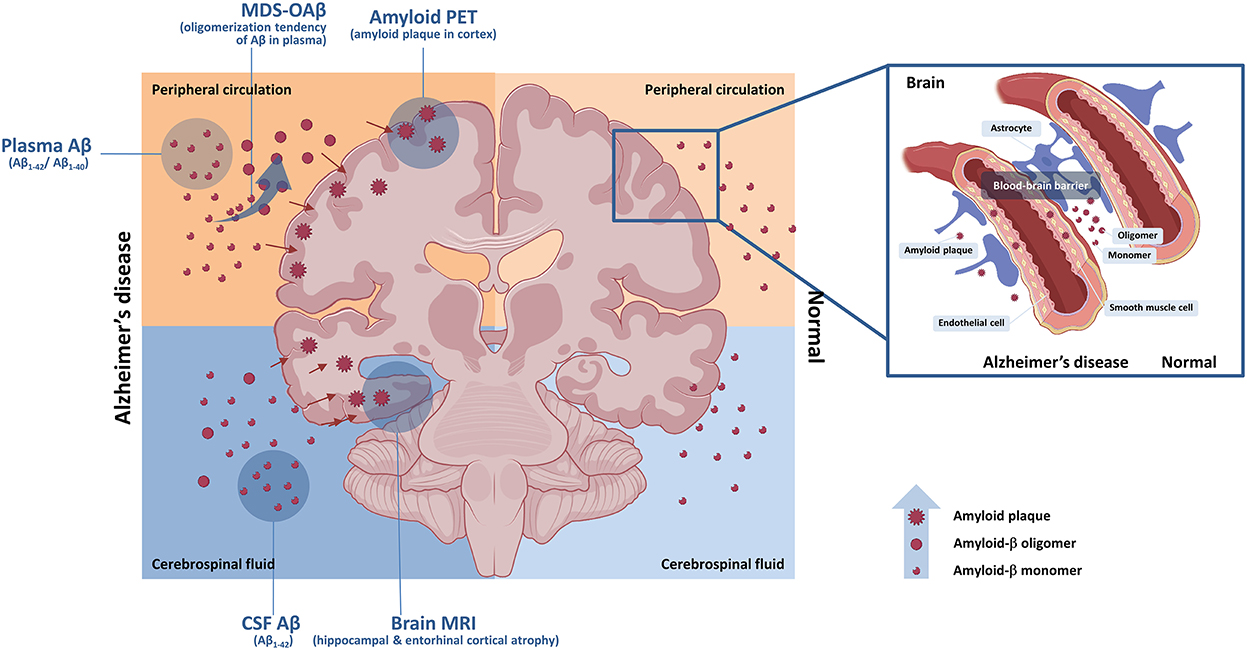
Figure 1. Central and peripheral amyloid-β and biomarkers. Central and peripheral Aβ can be detected by various biomarkers. Aβ oligomerization tendency in plasma can be detected by MDS-OAβ. Concentration of Aβ in CSF can be measured by CSF study with spinal tapping. Accumulation of Aβ plaque in brain can be evaluated by amyloid PET. Impaired blood–brain barrier can contribute to acceleration of amyloid pathology. Aβ, amyloid-β; CSF, cerebrospinal fluid; MDS-OAβ, Multimer Detection System-Oligomeric Amyloid-β; MRI, brain magnetic resonance imaging; PET, positron emission tomography.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
J-MP was a major contributor in writing the manuscript. YY and YP revised the manuscript. SK designed the work and revised the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aβ, amyloid-β; AD, Alzheimer's disease; ADD, Alzheimer's disease dementia; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; LATE, limbic-predominant age-related TDP-43 encephalopathy; MCI, mild cognitive impairment; MDS-OAβ, multimer detection system-oligomeric amyloid-β; MRI, brain magnetic resonance imaging; NDD, neurodegenerative disease; NPH, normal pressure hydrocephalus; NPT, neuropsychological test; PET, positron emission tomography; PDD, Parkinson disease dementia; TDP-43, TAR DNA-binding protein 43; VD, vascular dementia.
1. Pyun J-M, Kang MJ, Ryoo N, Suh J, Youn YC, Park YH, et al. Amyloid metabolism and amyloid-targeting blood-based biomarkers of Alzheimer's disease. J Alzheimer's Dis. (2020) 75:685–96. doi: 10.3233/JAD-200104
2. Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer' s disease. Mol Brain. (2019) 1:1–7. doi: 10.1186/s13041-019-0448-1
3. Leuzy A, Mattsson-Carlgren N, Palmqvist S, Janelidze S, Dage JL, Hansson O. Blood-based biomarkers for Alzheimer's disease. EMBO Mol Med. (2022) 14:1–15. doi: 10.15252/emmm.202114408
4. Chong JR, Ashton NJ, Karikari TK, Tanaka T, Schöll M, Zetterberg H, et al. Blood-based high sensitivity measurements of beta-amyloid and phosphorylated tau as biomarkers of Alzheimer's disease: a focused review on recent advances. J Neurol Neurosurg Psychiatry. (2021) 92:1231–41. doi: 10.1136/jnnp-2021-327370
5. An SSA, Lee BS Yu JS, Lim K, Kim GJ, Lee R, et al. Dynamic changes of oligomeric amyloid β levels in plasma induced by spiked synthetic Aβ42. Alzheimer's Res Ther. (2017) 9:86. doi: 10.1186/s13195-017-0310-6
6. Wang MJ Yi S, Han JY, Park SY, Jang JW, Chun IK, et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer's disease. Alzheimer's Res Ther. (2017) 9:98. doi: 10.1186/s13195-017-0324-0
7. Youn YC, Lee BS, Kim GJ, Ryu JS, Lim K, Lee R, et al. Blood amyloid-β oligomerization as a biomarker of Alzheimer's disease: a blinded validation study. J Alzheimer's Dis. (2020)75:493–9. doi: 10.3233/JAD-200061
8. Youn YC, Kang S, Suh J, Park YH, Kang MJ, Pyun JM, et al. Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer's disease. Alzheimer's Res Ther. (2019) 11:1–8. doi: 10.1186/s13195-019-0499-7
9. Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. (2013) 12:207–16. doi: 10.1016/S1474-4422(12)70291-0
10. Youn YC, Park KW, Han SH, Kim S. Urine neural thread protein measurements in Alzheimer disease. J Am Med Dir Assoc. (2011) 12:372–6. doi: 10.1016/j.jamda.2010.03.004
11. Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, et al. Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. (2015) 129:729–48. doi: 10.1007/s00401-015-1406-3
12. Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, Van Der Flier WM, Van Berckel BNM, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. (2015) 313:1939–49. doi: 10.1001/jama.2015.4669
13. Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. (2014) 85:865–70. doi: 10.1136/jnnp-2013-306948
14. Kang K, Yoon U, Hong J, Jeong SY, Ko PW, Lee SW, et al. Amyloid deposits and idiopathic normal-pressure hydrocephalus: an 18F-florbetaben study. Eur Neurol. (2018) 79:192–9. doi: 10.1159/000487133
15. Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. (2019) 142:1503–27. doi: 10.1093/brain/awz186
16. Pyun JM Ryu JS, Lee R, Shim KH, Youn YC, Ryoo N, et al. Plasma amyloid-β oligomerization tendency predicts amyloid pet positivity. Clin Interv Aging. (2021) 16:749–55. doi: 10.2147/CIA.S312473
17. Palmqvist S, Insel PS, Stomrud E, Janelidze S, Zetterberg H, Brix B, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. (2019) 11:e11170. doi: 10.15252/emmm.201911170
18. Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. (2016) 12:292–323. doi: 10.1016/j.jalz.2016.02.002
19. Roberts RO, Aakre JA, Kremers WK, Vassilaki M, Knopman DS, Mielke MM, et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. (2018) 75:970–9. doi: 10.1001/jamaneurol.2018.0629
Keywords: Alzheimer's disease, blood-based biomarker, amyloid-β, oligomerization tendency, diagnosis, neuropsychological test, brain MRI
Citation: Pyun J-M, Youn YC, Park YH and Kim S (2023) Integration of amyloid-β oligomerization tendency as a plasma biomarker in Alzheimer's disease diagnosis. Front. Neurol. 13:1028448. doi: 10.3389/fneur.2022.1028448
Received: 26 August 2022; Accepted: 20 December 2022;
Published: 17 January 2023.
Edited by:
Fanny M. Elahi, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Min Ju Kang, VHS Medical Center, Republic of KoreaCopyright © 2023 Pyun, Youn, Park and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: SangYun Kim,  bmV1cm9rc3lAc251LmFjLmty
bmV1cm9rc3lAc251LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.