- 1Department of Neurology, Veterans Affairs Medical Center, Portland, OR, United States
- 2Department of Neurology, Oregon Health and Sciences University, Portland, OR, United States
- 3Department of Radiology, Neuroradiology Section, Oregon Health & Sciences University, Portland, OR, United States
- 4Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States
- 5Knight Cancer Institute, Oregon Health & Science University, Portland, OR, United States
- 6Department of Human Physiology, University of Oregon, Eugene, OR, United States
While conventional magnetic resonance imaging (MRI) is central to the evaluation of patients with multiple sclerosis, its role in detecting the pathophysiology underlying neurodegeneration is more limited. One of the common outcome measures for progressive multiple sclerosis trials, atrophy on brain MRI, is non-specific and reflects end-stage changes after considerable neurodegeneration has occurred. Identifying biomarkers that identify processes underlying neurodegeneration before it is irreversible and that reflect relevant neurodegenerative pathophysiology is an area of significant need. Accumulating evidence suggests that oxidative stress plays a major role in the pathogenesis of multiple neurodegenerative diseases, including multiple sclerosis. Imaging markers related to inflammation, myelination, and neuronal integrity have been areas of advancement in recent years but oxidative stress has remained an area of unrealized potential. In this article we will begin by reviewing the role of oxidative stress in the pathogenesis of multiple sclerosis. Chronic inflammation appears to be directly related to the increased production of reactive oxygen species and the effects of subsequent oxidative stress appear to be amplified by aging and accumulating disease. We will then discuss techniques in development used in the assessment of MS as well as other models of neurodegenerative disease in which oxidative stress is implicated. Multiple blood and CSF markers of oxidative stress have been evaluated in subjects with MS, but non-invasive imaging offers major upside in that it provides real-time assessment within the brain.
Introduction
Multiple sclerosis (MS) is the most common inflammatory neurologic disease impacting young adults worldwide. The overall annual economic burden of MS in the United States is approximately $85 billion, placing it among the costliest of chronic diseases (1). In the majority of cases, MS begins with a relapsing-remitting (RRMS) course with unpredictable demyelinating events affecting the brain, optic nerves and spinal cord causing episodes of visual disturbance, weakness, sensory loss, coordination problems, bladder and bowel dysfunction, mood and cognitive changes, and other symptoms. Incomplete recovery from relapses leads to accumulated disability which leads to physical dependence, immobility, and inability to maintain employment (2, 3). Most RRMS patients go on to develop progressive clinical worsening in the absence of relapses about 20 years after initial disease onset, termed secondary progressive MS (SPMS) (4). Roughly 15% of MS cases begin with a progressive disease course from onset, termed primary progressive MS (PPMS) (5). RRMS is characterized broadly by an inflammatory process whereas PPMS and SPMS are considered to be driven predominantly by a neurodegenerative process.
Conventional magnetic resonance imaging (MRI) is standard of care for the evaluation of patients with relapsing forms of MS with exquisite sensitivity to changes in lesion burden. Standard T1- and T2-weighted MRI findings are biologically nonspecific and temporally dependent, but, reflect the underlying neuroinflammatory process associated demyelination. None the less, imaging features are all clinically informative and correlate to outcome measures, such as disease disability, prognosis, and response to MS neuroinflammatory disease-modifying therapy (6). Despite the clinical utility of MRI features, they often fail to prospectively capture the sequela of a chronic neuroinflammatory process eventually leading to neurodegeneration changes in both relapsing and progressive forms of MS. Global and regional brain atrophy can be detected early in MS and becomes more prominent in progressive phases of the disease. Thus brain atrophy, while a nonspecific and late biomarker of disease status, has become the most broadly used imaging marker for MS neurodegeneration in progressive MS clinical trials (7).
Identifying biomarkers of neurodegeneration before it becomes irreversible and that reflect relevant MS pathophysiology is an area of unmet need. Indicators of oxidative stress (OS) are promising candidates as a means of assessing a critical aspect of the neurodegenerative process in MS because OS likely occurs early and widely in the cascade leading to the degradation of neurons. In this review, we discuss the role of OS in MS pathophysiology and present imaging techniques with potential for demonstrating OS in vivo in MS.
Oxidative stress in neurodegenerative disease
Fundamentally, OS is generated by an imbalance of reduction and oxidation (redox) reactions wherein highly reactive free radicals seek electrons from other molecules, which then become free radicals themselves, causing a cascade that leads to cellular damage. Agents that induce OS include reactive oxygen species (ROS) such as superoxide radicals and hydrogen peroxide and reactive nitrogen species (RNS) such as nitric oxide radicals and peroxynitrite. Mitochondria are the biggest generators of ROS through oxidative respiration. ROS and RNS in the CNS are also directly produced by microglia and macrophages with the expression of enzymes such as NADPH-oxidases, myeloperoxidase, nitric oxide synthase. Thus, the brain, with its high consumption of oxygen and lipid-rich content is especially susceptible to OS. OS occurs with aging and in multiple neurodegenerative disorders of the CNS in addition to MS including Alzheimer's disease (8, 9), Parkinson disease (10), ALS (11), and Huntington disease (12).
The CNS hosts multiple defenses against OS, which are divided into the antioxidant enzyme system and non-enzymatic low-molecular-weight antioxidants (13). The enzyme defense system includes superoxide dismutases (SODs), glutathione peroxidase, glutathione reductase, and catalase. The non-enzymatic antioxidants most relevant to the CNS include glutathione, ascorbic acid, and melatonin. Both of these defense systems are controlled by the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2). When activated, Nrf2 increases the expression of multiple antioxidants, enzymes, and transporters (14). Under normal conditions, anti-oxidant defense mechanisms mediated by the Nrf2 system and the most abundant anti-oxidant in the CNS, glutathione (GSH), prevent much of the cerebral injury that might occur. However, when these defense mechanisms are outstripped, cellular damage and potential neurodegeneration occurs (15). Once this neurodegeneration surpasses an individual's functional reserve, clinical decline becomes apparent (16).
Oxidative stress in MS
While clinical phenotypes of MS may be distinct, on histopathological investigation there may be a spectrum of pathologies even within a single person. Hallmarks of the early stages of relapsing MS include focal inflammatory plaques characterized by an influx of T cells across the blood brain barrier causing subsequent demyelination, axonal loss, and reactive gliosis. In the later stages of SPMS and in PPMS from onset, microglial activation is diffusely present throughout the brain as well as at lesion edges (smoldering lesions) and meningeal B-cell follicles are characteristic leading to diffuse atrophy (17). Additional mechanisms contributing to MS neurodegeneration include cortical demyelination, meningeal lymphoid follicles, and diffuse microglial activation which are the core histologic features of progressive MS (18–20). In many cases these pathologies do not exist discretely; rather simultaneous inflammation and neurodegeneration may be present, blurring the clinical distinctions between relapsing and progressive MS (21). Chronic inflammation appears to play a role in initiating the neurodegenerative process characteristic of PMS (22–24). Whether inflammation and neurodegeneration are primary or secondary processes and how they might interact is controversial (25, 26).
In the context of MS, oxidative injury is demonstrated by histopathology throughout the duration of the disease with evidence of OS found in actively enhancing lesions from relapsing MS lesions as well as non-enhancing (non-active) lesions in progressive MS (27, 28). Within MS lesions, enzymes related to free radical production are increased (27). Oxygen free radicals are generated directly by inflammation, particularly due to microglia. This innate immune system activation is present in the relapsing stage but may also be present in the progressive phases of MS, though blood-brain-barrier damage may be beneath the sensitivity of gadolinium-enhanced MRI (23).
Mitochondrial dysfunction is present in MS and along with causing axonal dysfunction produces excessive ROS (23, 29). In fact, exposing healthy rat neurons to CSF from patients with progressive MS induced mitochondrial dysfunction and axonal damage, and caused bioenergetic failure. Importantly, this study also identified the role of ceramide as the mediator of this mitochondrial failure (30). Short-chain ceramides have been shown to induce mitochondrial alterations such as ROS production and permeabilization of the mitochondrial membrane leading to neuronal death (31). A functional screening study that performed live-imaging of rat neurons treated with the CSF of progressive MS patients demonstrated a morphological change (elongation) of mitochondria with decreased activity of complexes I, III, IV that correlated with axonal damage.
Iron concentration within the parenchyma increases with aging but is more pronounced in the context of progressive MS. Based on in vitro studies, iron appears to accentuate ROS release by NADPH oxidase in microglia (32). The presence of divalent iron ions generated by oligodendrocytes damaged by MS during demyelination may amplify free radical formation (33). Iron is primarily stored in oligodendrocytes, and high intracytoplasmic accumulation of iron in oligodendrocytes may explain preferential susceptibility of these cells to degeneration in response to OS (23, 34). Excess iron in the extracellular space, a consequence of oligodendrocyte destruction, may be taken up by activated microglia which alters them to become dystrophic and degenerative, creating a cyclical process causing further oxidative damage. Since iron increases in the brain normally with age, this damaging cycle is more common in those with secondary progressive MS (23). While iron does play a role in OS, many regions accumulate iron that do not exhibit excessive free radical generation. This makes iron-sensitive techniques such as susceptibility weighted imaging, insufficient biomarkers for assessing oxidative stress.
While histopathological evidence demonstrates the presence of OS, laboratory biomarkers of OS have been limited by the inability to directly access the CNS. However, a recent meta-analysis indicated increased blood and CSF malondialdehyde and decreased blood albumin levels which strongly suggests a link between OS and MS (35). Additionally, CSF and urinary 8-iso-PGF2α levels, which reflect the degree of lipid peroxidation due to ongoing oxidative stress, were found to be significantly higher in MS subjects than in controls (36, 37). GSH and redox values of GSH/GSSH may be measured in both peripheral blood and CSF. Challenges to measuring glutathione include that the quantity of intracerebral GSH is much higher intracellularly (10 ×) than extracellularly, and the resulting CSF concentrations are so low in CSF as to be difficult to detect and may not accurately reflect intracerebral GSH concentration (38). Studies of erythrocyte glutathione levels as well as glutathione peroxidase activity have suggested decreases of this critical antioxidant in MS (38). While taken as a whole these lab markers do suggest cellular redox pathway changes in MS, due to the heterogeneity of techniques, imaging markers may offer a superior means of real-time in vivo quantification and provide more anatomically precise assessments of OS-related pathology.
Imaging oxidative stress
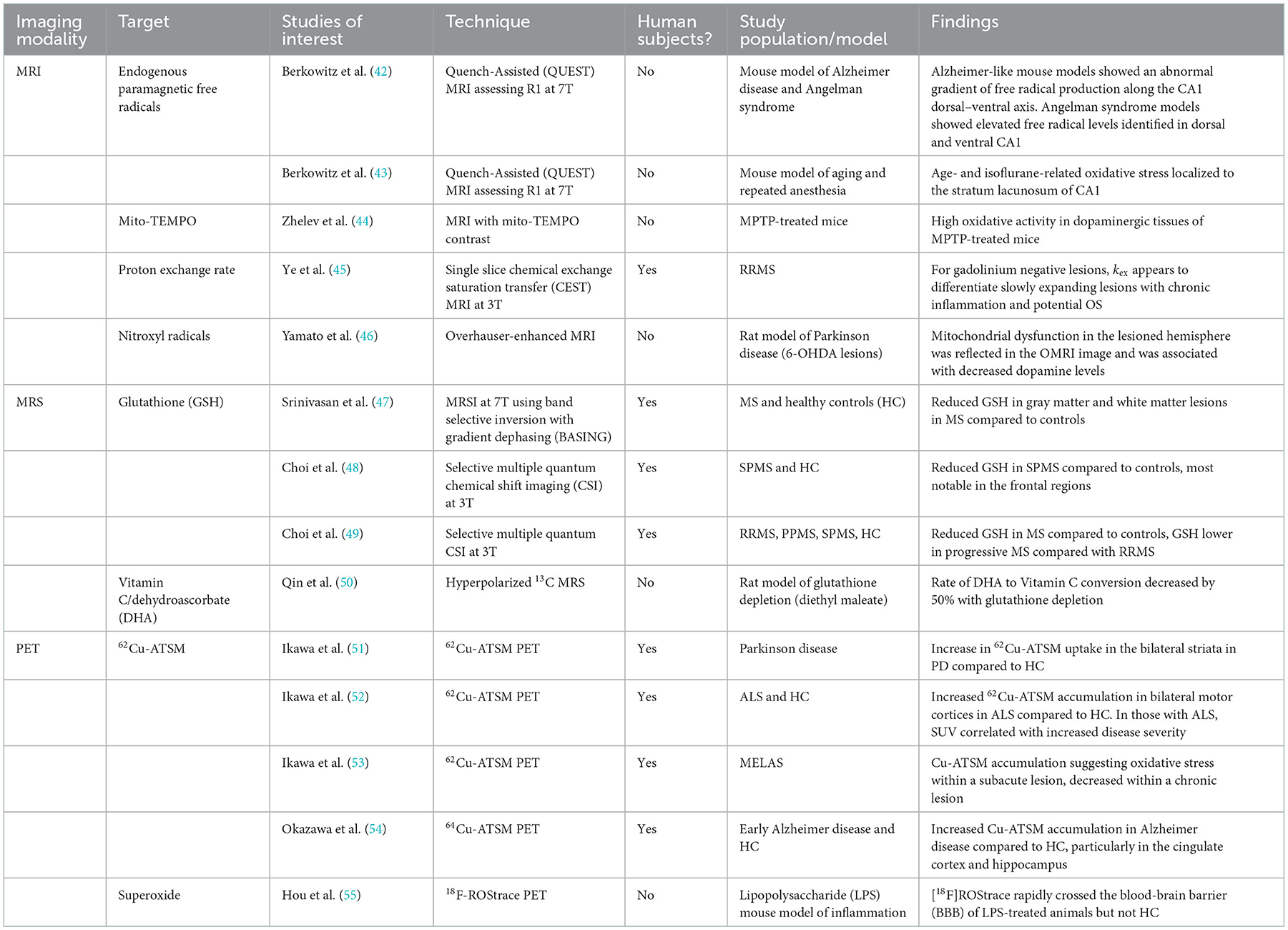
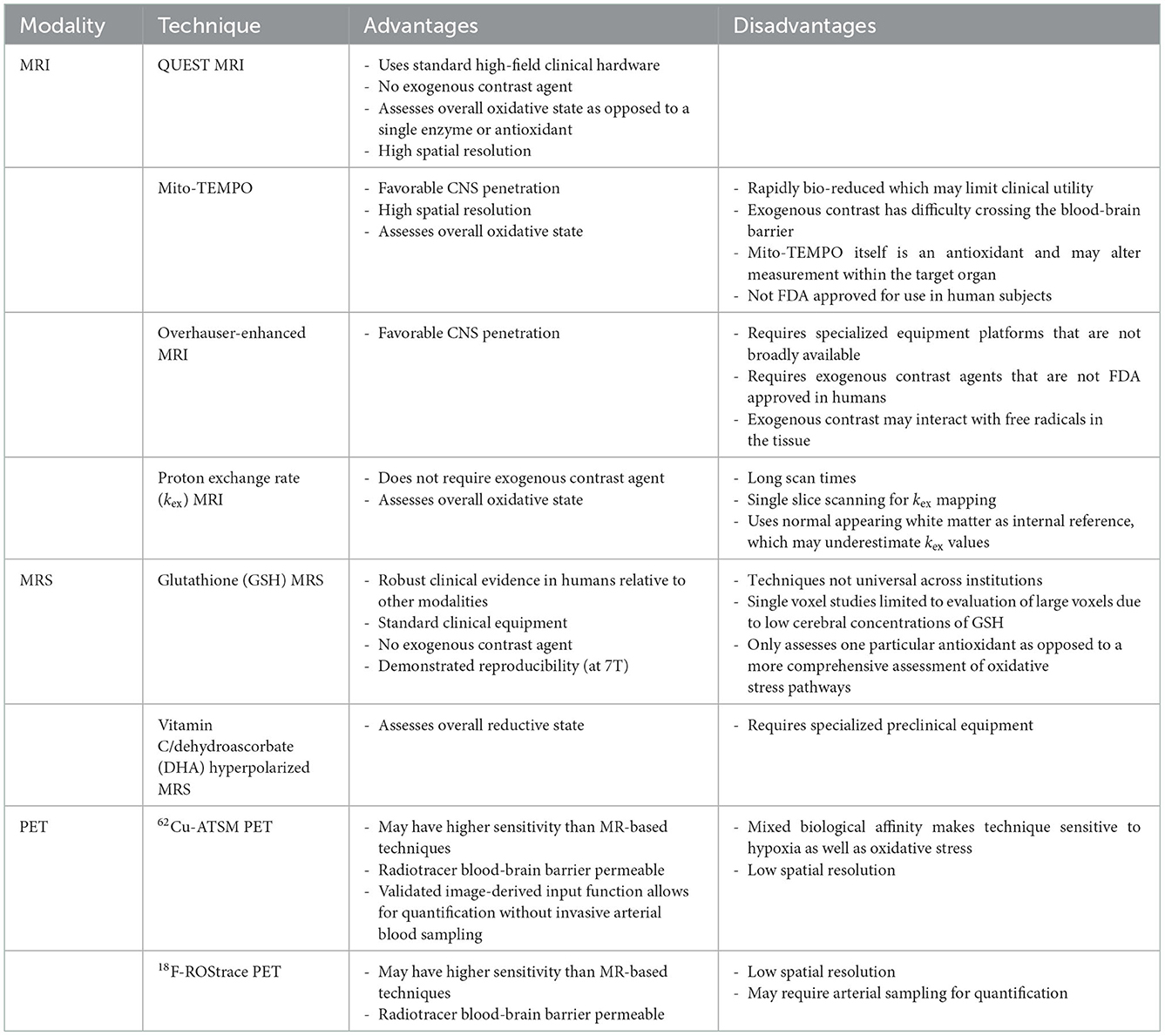
Development of OS imaging techniques has emerged in the field of neurodegenerative diseases due to altered mitochondrial oxidative metabolism (39–41). More recently, imaging with magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and positron emission tomography (PET) may allow clinically-relevant quantification of OS non-invasively in vivo. These techniques have varied approaches in assessing OS pathophysiology. Some directly interrogate for the presence of ROS, while others target downstream consequences or related processes. A summary of these imaging modalities, targets, and key studies are presented in Table 1. Some of the specific advantages and disadvantages of these techniques are identified in Table 2. While few of these techniques have been studied in MS, these techniques do aim to target OS pathways relevant to MS. There is significant interest in OS imaging as a potential means of developing earlier, more effective biomarkers of neurodegeneration. While histopathological evidence strongly points to the role of OS in MS neurodegeneration, assessments of OS throughout the course of MS in vivo alongside assessments of downstream neurodegenerative pathophysiology would provide critical information regarding the link between OS and neurodegeneration. Finding techniques that assess localized OS within the brain prior to colocalized degeneration would be an important step in this direction. Such a technique should be able to be feasibly applied to demonstrate efficacy of interventions in preventing both OS and the development of neurodegeneration. This will require sensitive, high-resolution imaging techniques performed alongside established neurodegenerative biomarkers.
Magentic resonance imaging
Most conventional MRI methods are insensitive to OS at the cellular level. However, a recently developed technique, QUEnch-assiSTed (QUEST) MRI, may have utility in the identification of OS in vivo. As opposed to assessing a single antioxidant or free radical, QUEST-MRI assesses the overall redox state. Free radicals are paramagnetic, and during times of OS they cause shortening of spin-lattice relaxation time (T1) and thus prolongation of the spin-lattice relaxation rate (R1). By assessing a reduction in R1 after a quench with anti-oxidant treatment during the scan (e.g., administration of alpha-lipoic acid) this weights the specific contribution of ROS to the increased R1 (56). However, relaxation rates may be impacted by factors that are not related to ROS—such as water and oxygen content (57, 58). Several QUEST-MRI studies show agreement with free radical measurements assessed ex vivo (43, 59). QUEST MRI was also used in a small study of AD-like and Angelman Syndrome mouse model hippocampi which suggested a gradient of excessive free radical production similar to that found in ex vivo experiments (42). Whether QUEST-MRI is practical for in vivo human studies remains to be seen as studies of this technique to date have all been performed in animal models. Advantages of this technique include the lack of contrast agent and the use of standard clinical MRI hardware. Further assessment of this technique would be of interest in the context of multiple human neurodegenerative diseases.
While quench-assisted MRI techniques utilize an exogenous contrast agent to assess the relationship of ROS production to T1 shortening, the in vivo proton exchange rate (kex), aims to be an endogenous contrast-based ROS MRI method capable of assessing oxidative stress. Proton exchange occurs between bulk water and metabolites primarily through endogenous metabolites such as amine, amide, hydroxyl, and hydrosulfuric groups. Using chemical exchange saturation transfer (CEST), protons are excited by a pre-saturation pulse and then magnetization is transferred to the bulk water, which can then be detected by MRI. The resulting proton exchange rate is significantly altered by the presence of ROS and less so by temperature, metabolite concentrations, and pH of the tissue (60). In a study of 16 subjects with RRMS, kex was elevated in gadolinium enhancing lesions, but also in gadolinium-negative, slowly expanding MS lesions which suggests it may be a feasible technique to detect “smoldering” chronically inflamed gadolinium-negative lesions with higher levels of oxidative stress (45). However, this technique may be limited by long scan duration requirements which limits acquisition to single slices.
Another technique utilizes paramagnetic nitroxide contrast agents aimed at detecting ROS. Nitroxides are stable free radicals with a single unpaired electron, capable of shortening the longitudinal relaxation time (T1) and thus providing MRI contrast. Nitroxides have much lower relaxivity compared to conventional gadolinium-based contrast agents, though they do have higher volume of distribution due to improved cell permeability. One drawback is that they are rapidly bio-reduced which has limited their suitability as an MRI-contrast agent (61–63). Mito-TEMPO is a blood-brain barrier- (BBB) and mitochondria-penetrating nitroxide derivative. It has a relatively high contrast on T1 images and specifically scavenges superoxide, accumulating in areas of high superoxide concentration. Studies in MPTP-treated mice, a model of Parkinson's disease, showed high mito-TEMPO concentrations in the dopaminergic areas of the brain—indicating high oxidative activity due to abnormal production of mitochondrial superoxide, concordant with what is found in autopsy studies (44). The ability of mito-TEMPO to penetrate the BBB and mitochondria is advantageous to studying CNS-specific diseases. However, using exogenous MRI redox contrast agents does have limitations. For instance, mito-TEMPO itself acts as an antioxidant that may alter the tissue under evaluation. Additionally, much remains unknown in regards to their pharmacokinetics, safety, and suitability for use in the evaluation of the human brain. Studies of these agents to date has largely been performed in animal models of tumors; Development of these agents for human subjects and neurodegenerative disease is an area of limited evaluation to this point (64).
Overhauser-enhanced MRI (OMRI), builds on electron paramagnetic resonance (EPR) techniques and is another imaging method utilizing nitroxide radicals to assess oxidative stress. Direct EPR has very limited resolution due to the short half-life of nitroxides which are used as contrast agents and is less promising for use in vivo in human subjects. To improve the spatial resolution, OMRI indirectly assesses the activity of the nitroxide radical by using the Overhauser effect which transfers large spin polarizations from nitroxide radicals to dipolar-coupled protons, the enhanced spin polriazations of these protons are then imaged to assess free radical ditribution. The adoption of OMRI has been limited due to low sensitivity and the necessity that it is performed at ultra-low magnetic fields. The permeability of TEMPOL across the blood-brain barrier increases in the setting of oxidative stress (65). Dynamic tracking of oxidative stress by monitoring a nitroxide radical antioxidant such as 4-hydroxy-TEMPO (TEMPOL) may be more feasible with new techniques allowing improved speed, sensitivity, and temporal resolution (66). OMRI has been used in in vivo studies of animal models of Parkinson disease using the nitroxide radical 3-methoxycarbonyl-2,2,5,5,-tetramethylprrolidine-1-oxyl (methoxy-carbonyl-PROXYL). In this study, OMRI of 6-OHDA-lesioned rats suggested that 6-OHDA induced an environment of oxidative stress and subseuqnet ex-vivo spin-trapping demonstrated the direct induction of hydroxyl radical generation associated with mitochondrial dysfunction (46). Although there have been developments in very-low-field MRI systems for human studies, this remains a limitation to this technique at this point.
Magnetic resonance spectroscopy
Spectroscopy is a powerful, non-invasive method of quantifying metabolites within a region of interest. Magnetic resonance spectroscopic imaging (MRSI) comprises a class of methods, including chemical shift imaging, echo planar spectroscopic imaging, and IDEAL spectral decomposition. MRSI is performed either dynamically, or at a single time-point that is often chosen to coincide with the peak MR signal of the metabolic product of interest. Most MRS studies are based on proton (1H) nuclear magnetic resonance (NMR), though other nuclei such as 13C or 31P can be utilized. MRS offers the advantage of detecting its target in real-time, and it can be performed with standard clinical equipment and without exogenous contrast agents or substantial cost. However, metabolites associated with OS, such as GSH, exist in relatively low concentrations in comparison to other cerebral metabolites, and often large voxels must be examined to account for the low concentration of target molecules, which limits anatomical specificity. Additionally, metabolites with overlapping resonances pose a challenge to resolving separate contributions to spectra, and a reference metabolite, e.g., water, is typically chosen to calculate relative metabolite ratios. Several MRS techniques have been developed to overcome these disadvantages. Likewise, there is a push toward the utilization of universal sequences across institutions and manufacturers which may help minimize technical barriers, develop normative databases, and facilitate inter-institutional collaboration (67).
GSH is one of the best-studied metabolites associated with OS in MS. It is the most abundant antioxidant of the CNS, and the GSH oxidation status (GSH/GSSG) is considered one of the best markers of cellular redox equilibrium (68). Detection of GSH using MRS is technically challenging at clinical field strengths of 1.5 or 3 T due to its low concentration in the human brain coupled with the fact that conventional single-echo acquisitions, typically used for MRS acquisitions, cannot be used to resolve GSH given its overlap with other metabolite resonances. Srinivasan et al. evaluated GSH concentrations in people with MS with single voxel MR spectroscopy at 7T and identified differences in white and gray matter GSH concentrations (47). While they did not identify significant GSH differences in the WM between controls and people with MS, they did observe reduced GSH in MS white matter lesions (47). Since publication of this preliminary and promising investigation, advancement of spectral-edited MRS techniques such as MEGA-PRESS show improved capability and reliability such that assessments of GSH are now possible even at lower field strengths (69–71). Additional work using J-difference edited MRS of GSH at 7T exhibited good reliability in controls and established feasibility in a single MS patient (69).
In more recent studies of GSH in MS, Choi and Lee (49) and Choi et al. (72, 73) used a specially designed selective multiple quantum chemical shift imaging (CSI) technique to assess glutathione concentration throughout a 3 cm thick axial brain slice. GSH concentrations were lower in MS subjects than controls, and lower still in people with progressive MS compared to RRMS (72). Follow-up over a 3–5-year period showed that decreased GSH concentrations persisted in patients with SPMS compared to controls and that participants judged to have clinical worsening had sharper declines in GSH concentrations than clinically stable patients (73). While MRS of glutathione is promising, it is important to note that assessments of GSH reflect only a portion of a tissue's anti-oxidative stress defenses, and that it is not well suited to directly measure the production of excessive ROS from sources such as mitochondria.
Another novel spectroscopic technique uses 13C, a nuclear magnetic resonance (NMR)-sensitive isotope of carbon. Thanks to its extensive chemical shift range 13C NMR is an attractive method for interrogation of redox states in vivo and hyperpolarization (HP) offers a means of improving sensitivity. Although there are many 13C metabolic tracers under development, only a few have been evaluated as detectors of redox state. The most studied HP 13C-labeled tracers for assessment of redox state in vivo are the reduced and oxidized forms of vitamin C: HP[1–13C] dehydroascorbic acid (DHA) and [1–13C] ascorbic acid (AA). These reflect intracellular and extracellular redox states respectively. The reduction of DHA to AA is mediated by glutathione- or NADPH-dependent mechanisms. Increases in HP[1–13C] DHA in tumors appear to reflect increases in glutathione (74). In the cuprizone model of MS, hyperpolarized 13C-MRS has been used to characterize inflammatory lesions that attract proinflammatory mononuclear phagocytes. An elevated lactate signal was demonstrated within MS lesions that were histologically confirmed to be rich in mononuclear phagocytes. Moreover, these activated phagocytes had elevated pyruvate dehydrogenase kinase 1 (PDK1), responsible for inhibition of the oxidation of pyruvate by mitochondrial PDH, leading to increased metabolism to lactate in the cytosol. While this technique was aimed at detecting inflammation, there could be overlap in the ability of this technique to detect concomitant OS in areas outside of active lesions (75). Similar techniques have been utilized in only a few in vivo studies of the human brain metabolism and none yet in MS or neurodegenerative disease (76). Barriers to adoption of 13C NMR MRS include the need for specialized equipment such as a polarizer, isotope-specific 13C head coils, and MRI machines capable of multi-nuclear scans which most clinical machines are not equipped to perform.
Positron emission tomography
Molecular imaging with positron emission tomography (PET) can directly evaluate subtle biological changes, including redox status. The 62Cu-diacetyl-bis(N4-methylthiosemicarbazone) (62Cu-ATSM) radioligand has been evaluated in neurodegenerative disorders including Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke Like Episodes (MELAS), Parkinson disease, and amyotrophic lateral sclerosis (ALS) (51–53). 62Cu-ATSM is a PET radioligand that is a chelate complex containing a radioactive divalent copper. Because of its divalence (Cu2+) it is reduced to the monovalent form (Cu1+) in tissues with excess electrons, indicating states of increased oxidative stress. This reduced Cu1+ dissociates from the ATSM and is retained in the reductive tissues which allows for its detection via PET. While it was initially designed to identify areas of hypoxia, it has also been shown to identify tissues with over-reductive states with elevated NADH concentrations in a normoxic state (77). This is believed to be due to the reduced respiratory capacity of local mitochondria.
In regards to studies in other neurodegenerative diseases—62Cu-ATSM was evaluated in patients with idiopathic Parkinson's disease which demonstrated increased uptake in the bilateral striata compared to healthy controls, and increased uptake was correlated with increased disease severity (51). Similarly, OS and related mitochondrial dysfunction appears to have a role in the motor neuron degeneration in ALS based on post-mortem studies and the roles of gene mutations (such as SOD1 and TDP-43) which are implicated both in the development of ALS as well as disruption of common oxidative defense pathways. A study using 62Cu-ATSM was performed in patients with sporadic ALS—which demonstrated significantly greater accumulation of 62Cu-ATSM in the bilateral motor cortices in ALS as compared to healthy controls (52). Another investigation of early Alzheimer disease also suggested increased accumulation of 62Cu-ATSM compared to controls (54). As of now this radioligand has not been evaluated in the context of MS, though such investigations may be a useful complement to the MRSI studies described above. Other PET radiotracers are being investigated in the context of OS such as 18F-FASu and 18F-ROStrace in other organ systems or targeted for the assessment of neoplasms (78–80). Limited human data is available in neurodegenerative disease using these radioligands and the specificity of these techniques in assessing oxidative stress is an area still under investigation. While PET offers the potential of relatively high sensitivity, it continues to be limited by low spatial resolution relative to MR based techniques.
Conclusions
OS is a key pathophysiological mechanism underlying neurodegeneration in MS. Because of the complexity of the OS pathways, multiple potential targets to capture OS using in vivo imaging techniques have emerged. High spatial resolution methods using MRI offer advantages in the evaluation of MS such as assessing regional or lesional differences in OS. Contrast agents like nitroxides and mito-TEMPO are T1 contrast agents with anti-oxidant properties that penetrate the brain and mitochondria to distribute to tissues where ongoing OS occurs, thus highlighting regional areas with an abundance of ROS. Techniques with high spatial resolution offer the advantage of obtaining more granular regional or lesional variation in OS. A direct measure of overall ROS burden would also be advantageous in the context of MS as opposed to a technique that measures a single ROS, such as glutathione in the case of MRS. In this respect, 62Cu-ATSM may offer a sensitive marker that measures OS comprehensively but with a sacrifice in spatial resolution. At present, MRS investigations of glutathione have the most robust literature in the context of MS, but it does have low spatial resolution and requires specific expertise in analysis which has slowed broad uptake of this method. 62Cu-ATSM PET appears to be primed for investigations of MS with multiple other studies in neurodegenerative disease, however PET has limitations in spatial resolution that are not ideal. The potential advantages offered by QUEST MRI should encourage further development of this technique for early studies on human subjects with MS. Ultimately performing multimodal studies, for example combining QUEST MRI with susceptibility weighted imaging (SWI) sequences for the detection or iron (81) or PET markers of neuroinflammation (82) may help us obtain multiple layers of information regarding neurodegeneration and the relationship of multiple degenerative processes in MS. Regardless of modality, further investigations of MS using in vivo imaging techniques alongside markers of localized neurodegeneration is an important next step in our understanding of the pathophysiology of this still poorly understood aspect of the disease.
Author contributions
CH and RS proposed the review. CH prepared the tables, and drafted the manuscript for intellectual content. LN, IG, and RB critically reviewed the manuscript with major contributions to intellectual content. All authors read and approved the submitted version.
Funding
This work was supported in part by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veteran Affairs and the VA Parkinson's Disease Research, Education and Clinical Centers (PADRECC).
Acknowledgments
The authors would like to thank Dr. Kathy Hoang, MD for insightful commentary and critique during the writing process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bebo B, Cintina I, Larocca N, Ritter L, Talente B, Hartung D, et al. The economic burden of multiple sclerosis in the United States: estimate of direct and indirect costs. Neurology. (2022) 98:e1810–7. doi: 10.1212/WNL.0000000000200150
2. Iezzoni LI, Ngo L. Health, disability, and life insurance experiences of working-age persons with multiple sclerosis. Mult Scler. (2007) 13:534–46. doi: 10.1177/1352458506071356
3. Dehghani A, Khoramkish M, Shahsavari Isfahani S. Challenges in the daily living activities of patients with multiple sclerosis: a qualitative content analysis. Int J Community Based Nurs Midwifery. (2019) 7:201–10. doi: 10.30476/IJCBNM.2019.44995
4. Cree BAC, Arnold DL, Chataway J, Chitnis T, Fox RJ, Pozo Ramajo A, et al. Secondary progressive multiple sclerosis: new insights. Neurology. (2021) 97:378–88. doi: 10.1212/WNL.0000000000012323
5. Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. (2017) 389:1357–66. doi: 10.1016/S0140-6736(16)31320-4
6. Klawiter EC. Current and new directions in MRI in multiple sclerosis. Continuum. (2013) 19:1058–73. doi: 10.1212/01.CON.0000433283.00221.37
7. Andravizou A, Dardiotis E, Artemiadis A, Sokratous M, Siokas V, Tsouris Z, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Auto Immun Highlights. (2019) 10:7. doi: 10.1186/s13317-019-0117-5
8. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. (2006) 443:787–95. doi: 10.1038/nature05292
9. Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, et al. Oxidative stress in blood in Alzheimer's disease and mild cognitive impairment: a meta-analysis. Neurobiol Dis. (2013) 59:100–10. doi: 10.1016/j.nbd.2013.07.005
10. Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative stress in Parkinson's disease: a systematic review and meta-analysis. Front Mol Neurosci. (2018) 11:236. doi: 10.3389/fnmol.2018.00236
11. Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. (2010) 48:629–41. doi: 10.1016/j.freeradbiomed.2009.11.018
12. Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E, et al. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med. (2008) 45:667–78. doi: 10.1016/j.freeradbiomed.2008.05.014
13. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. (2017) 360:201–5. doi: 10.1124/jpet.116.237503
14. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. (2007) 47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046
15. Licht-Mayer S, Wimmer I, Traffehn S, Metz I, Bruck W, Bauer J, et al. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol. (2015) 130:263–77. doi: 10.1007/s00401-015-1452-x
16. Krieger SC, Cook K, De Nino S, Fletcher M. The topographical model of multiple sclerosis: a dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e279. doi: 10.1212/NXI.0000000000000279
17. Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med. (2018) 8:a028936. doi: 10.1101/cshperspect.a028936
18. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. (2000) 343:1430–8. doi: 10.1056/NEJM200011163432001
19. Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. (2005) 128:2705–12. doi: 10.1093/brain/awh641
20. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. (2006) 129:584–94. doi: 10.1093/brain/awh721
21. Ontaneda D, Fox RJ. Progressive multiple sclerosis. Curr Opin Neurol. (2015) 28:237–43. doi: 10.1097/WCO.0000000000000195
22. Bruck W, Lucchinetti C, Lassmann H. The pathology of primary progressive multiple sclerosis. Mult Scler. (2002) 8:93–7. doi: 10.1191/1352458502ms785rr
23. Lassmann H, van Horssen J, MAhad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. (2012) 8:647–56. doi: 10.1038/nrneurol.2012.168
24. Matthews PM. Chronic inflammation in multiple sclerosis - seeing what was always there. Nat Rev Neurol. (2019) 15:582–93. doi: 10.1038/s41582-019-0240-y
25. Hutchinson M. Neurodegeneration in multiple sclerosis is a process separate from inflammation: no. Mult Scler. (2015) 21:1628–31. doi: 10.1177/1352458515612244
26. Louapre C, Lubetzki C. Neurodegeneration in multiple sclerosis is a process separate from inflammation: yes. Mult Scler. (2015) 21:1626–8. doi: 10.1177/1352458515587598
27. Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, et al. Oxidative damage in multiple sclerosis lesions. Brain. (2011) 134:1914–24. doi: 10.1093/brain/awr128
28. Adamczyk B, Niedziela N, Adamczyk-Sowa M. Novel approaches of oxidative stress mechanisms in the multiple sclerosis pathophysiology and therapy. In:Zagon IS, Mclaughlin PJ, , editors. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Brisbane: Codon Publications (2017), p. 155–71. doi: 10.15586/codon.multiplesclerosis.2017.ch10
29. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. (2009) 417:1–13. doi: 10.1042/BJ20081386
30. Vidaurre OG, Haines JD, Katz Sand I, Adula KP, Huynh JL, Mcgraw CA, et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain. (2014) 137:2271–86. doi: 10.1093/brain/awu139
31. Darios F, Lambeng N, Troadec JD, Michel PP, Ruberg M. Ceramide increases mitochondrial free calcium levels via caspase 8 and Bid: role in initiation of cell death. J Neurochem. (2003) 84:643–54. doi: 10.1046/j.1471-4159.2003.01590.x
32. Yauger YJ, Bermudez S, Moritz KE, Glaser E, Stoica B, Byrnes KR. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J Neuroinflammation. (2019) 16:41. doi: 10.1186/s12974-019-1430-7
33. Kamma E, Lasisi W, Libner C, Ng HS, Plemel JR. Central nervous system macrophages in progressive multiple sclerosis: relationship to neurodegeneration and therapeutics. J Neuroinflammation. (2022) 19:45. doi: 10.1186/s12974-022-02408-y
34. Zhang X, Haaf M, Todorich B, Grosstephan E, Schieremberg H, Surguladze N, et al. Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia. (2005) 52:199–208. doi: 10.1002/glia.20235
35. Zhang SY, Gui LN, Liu YY, Shi S, Cheng Y. Oxidative stress marker aberrations in multiple sclerosis: a meta-analysis study. Front Neurosci. (2020) 14:823. doi: 10.3389/fnins.2020.00823
36. Mir F, Lee D, Ray H, Sadiq SA. CSF isoprostane levels are a biomarker of oxidative stress in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. (2014) 1:e21. doi: 10.1212/NXI.0000000000000021
37. Guan JZ, Guan WP, Maeda T, Guoqing X, Guangzhi W, Makino N, et al. Patients with multiple sclerosis show increased oxidative stress markers and somatic telomere length shortening. Mol Cell Biochem. (2015) 400:183–7. doi: 10.1007/s11010-014-2274-1
38. Carvalho AN, Lim JL, Nijland PG, Witte ME, Van Horssen J. Glutathione in multiple sclerosis: more than just an antioxidant? Mult Scler. (2014) 20:1425–31. doi: 10.1177/1352458514533400
39. Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. (2014) 16:193–217. doi: 10.3390/ijms16010193
40. Ikawa M, Okazawa H, Nakamoto Y, Yoneda M. PET imaging for oxidative stress in neurodegenerative disorders associated with mitochondrial dysfunction. Antioxidants. (2020) 9:861. doi: 10.3390/antiox9090861
41. Greenwood HE, Witney TH. Latest advances in imaging oxidative stress in cancer. J Nucl Med. (2021) 62:1506–10. doi: 10.2967/jnumed.120.256974
42. Berkowitz BA, Lenning J, Khetarpal N, Tran C, Wu JY, Berri AM, et al. In vivo imaging of prodromal hippocampus CA1 subfield oxidative stress in models of Alzheimer disease and Angelman syndrome. FASEB J. (2017) 31:4179–86. doi: 10.1096/fj.201700229R
43. Berkowitz BA, Podolsky RH, Childers KL, Gow A, Schneider BL, Lloyd SC, et al. Age-related murine hippocampal CA1 laminae oxidative stress measured in vivo by QUEnch-assiSTed (QUEST) MRI: impact of isoflurane anesthesia. Geroscience. (2020) 42:563–74. doi: 10.1007/s11357-020-00162-8
44. Zhelev Z, Bakalova R, Aoki I, Lazarova D, Saga T. Imaging of superoxide generation in the dopaminergic area of the brain in Parkinson's disease, using mito-TEMPO. ACS Chem Neurosci. (2013) 4:1439–45. doi: 10.1021/cn400159h
45. Ye H, Shaghaghi M, Chen Q, Zhang Y, Lutz SE, Chen W, et al. In vivo proton exchange rate (kex) MRI for the characterization of multiple sclerosis lesions in patients. J Magn Reson Imaging. (2021) 53:408–15. doi: 10.1002/jmri.27363
46. Yamato M, Shiba T, Naganuma T, Ichikawa K, Utsumi H, Yamada K, et al. Overhauser-enhanced magnetic resonance imaging characterization of mitochondria functional changes in the 6-hydroxydopamine rat model. Neurochem Int. (2011) 59:804–11. doi: 10.1016/j.neuint.2011.08.010
47. Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging. (2010) 28:163–70. doi: 10.1016/j.mri.2009.06.008
48. Choi IY, Lee SP, Denney DR, Lynch SG. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult Scler. (2011) 17:289–96. doi: 10.1177/1352458510384010
49. Choi IY, Lee P. Doubly selective multiple quantum chemical shift imaging and T(1) relaxation time measurement of glutathione (GSH) in the human brain in vivo. NMR Biomed. (2013) 26:28–34. doi: 10.1002/nbm.2815
50. Qin H, Carroll VN, Sriram R, Villanueva-Meyer JE, Von Morze C, Wang ZJ, et al. Imaging glutathione depletion in the rat brain using ascorbate-derived hyperpolarized MR and PET probes. Sci Rep. (2018) 8:7928. doi: 10.1038/s41598-018-26296-6
51. Ikawa M, Okazawa H, Kudo T, Kuriyama M, Fujibayashi Y, Yoneda M, et al. Evaluation of striatal oxidative stress in patients with Parkinson's disease using [62Cu]ATSM PET. Nucl Med Biol. (2011) 38:945–51. doi: 10.1016/j.nucmedbio.2011.02.016
52. Ikawa M, Okazawa H, Tsujikawa T, Matsunaga A, Yamamura O, Mori T, et al. Increased oxidative stress is related to disease severity in the ALS motor cortex: a PET study. Neurology. (2015) 84:2033–9. doi: 10.1212/WNL.0000000000001588
53. Ikawa M, Okazawa H, Arakawa K, Kudo T, Kimura H, Fujibayashi Y, et al. PET imaging of redox and energy states in stroke-like episodes of MELAS. Mitochondrion. (2009) 9:144–8. doi: 10.1016/j.mito.2009.01.011
54. Okazawa H, Ikawa M, Tsujikawa T, Mori T, Makino A, Kiyono Y, et al. Cerebral oxidative stress in early Alzheimer's disease evaluated by (64)Cu-ATSM PET/MRI: a preliminary study. Antioxidants. (2022) 11:1022. doi: 10.3390/antiox11051022
55. Hou C, Hsieh CJ, Li S, Lee H, Graham TJ, Xu K, et al. Development of a positron emission tomography radiotracer for imaging elevated levels of superoxide in neuroinflammation. ACS Chem Neurosci. (2018) 9:578–86. doi: 10.1021/acschemneuro.7b00385
56. Kuhl A, Dixon A, Hali M, Apawu AK, Muca A, Sinan M, et al. Novel QUEST MRI in vivo measurement of noise-induced oxidative stress in the cochlea. Sci Rep. (2019) 9:16265. doi: 10.1038/s41598-019-52439-4
57. Fatouros PP, Marmarou A, Kraft KA, Inao S, Schwarz FP. In vivo brain water determination by T1 measurements: effect of total water content, hydration fraction, and field strength. Magn Reson Med. (1991) 17:402–13. doi: 10.1002/mrm.1910170212
58. Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. (1992) 89:212–6. doi: 10.1073/pnas.89.1.212
59. Berkowitz BA, Podolsky RH, Lenning J, Khetarpal N, Tran C, Wu JY, et al. Sodium iodate produces a strain-dependent retinal oxidative stress response measured in vivo using QUEST MRI. Invest Ophthalmol Vis Sci. (2017) 58:3286–93. doi: 10.1167/iovs.17-21850
60. Tain RW, Scotti AM, Cai K. Improving the detection specificity of endogenous MRI for reactive oxygen species (ROS). J Magn Reson Imaging. (2019) 50:583–91. doi: 10.1002/jmri.26629
61. Hyodo F, Chuang KH, Goloshevsky AG, Sulima A, Griffiths GL, Mitchell JB, et al. Brain redox imaging using blood-brain barrier-permeable nitroxide MRI contrast agent. J Cereb Blood Flow Metab. (2008) 28:1165–74. doi: 10.1038/jcbfm.2008.5
62. Davis RM, Sowers AL, Degraff W, Bernardo M, Thetford A, Krishna MC, et al. A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radic Biol Med. (2011) 51:780–90. doi: 10.1016/j.freeradbiomed.2011.05.019
63. Bacic G, Pavicevic A, Peyrot F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. (2016) 8:226–42. doi: 10.1016/j.redox.2015.10.007
64. Nguyen HV, Chen Q, Paletta JT, Harvey P, Jiang Y, Zhang H, et al. Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of tumors. ACS Cent Sci. (2017) 3:800–11. doi: 10.1021/acscentsci.7b00253
65. Lochhead JJ, Mccaffrey G, Quigley CE, Finch J, Demarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. (2010) 30:1625–36. doi: 10.1038/jcbfm.2010.29
66. Waddington DEJ, Sarracanie M, Salameh N, Herisson F, Ayata C, Rosen MS, et al. An overhauser-enhanced-MRI platform for dynamic free radical imaging in vivo. NMR Biomed. (2018) 31:e3896. doi: 10.1002/nbm.3896
67. Saleh MG, Rimbault D, Mikkelsen M, Oeltzschner G, Wang AM, Jiang D, et al. Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. Neuroimage. (2019) 189:425–31. doi: 10.1016/j.neuroimage.2019.01.056
68. Rae CD, Williams SR. Glutathione in the human brain: review of its roles and measurement by magnetic resonance spectroscopy. Anal Biochem. (2017) 529:127–43. doi: 10.1016/j.ab.2016.12.022
69. Prinsen H, de Graaf, RA, Mason GF, Pelletier D, Juchem C. Reproducibility measurement of glutathione, GABA, and glutamate: towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T. J Magn Reson Imaging. (2017) 45:187–98. doi: 10.1002/jmri.25356
70. Bottino F, Lucignani M, Napolitano A, Dellepiane F, Visconti E, Rossi Espagnet MC, et al. In vivo brain GSH: MRS methods and clinical applications. Antioxidants. (2021) 10:1407. doi: 10.3390/antiox10091407
71. Anton A, Mead RJ, Shaw PJ, Edden RAE, Bigley J, Jenkins TM, et al. Assessment of the precision in measuring glutathione at 3 T with a MEGA-PRESS sequence in primary motor cortex and occipital cortex. J Magn Reson Imaging. (2022) 55:435–42. doi: 10.1002/jmri.27842
72. Choi IY, Lee P, Adany P, Hughes AJ, Belliston S, Denney DR, et al. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult Scler. (2018) 24:1029–38. doi: 10.1177/1352458517711568
73. Choi IY, Lee P, Hughes AJ, Denney DR, Lynch SG. Longitudinal changes of cerebral glutathione (GSH) levels associated with the clinical course of disease progression in patients with secondary progressive multiple sclerosis. Mult Scler. (2017) 23:956–62. doi: 10.1177/1352458516669441
74. Keshari KR, Kurhanewicz J, Bok R, Larson PE, Vigneron DB, Wilson DM, et al. Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proc Natl Acad Sci U S A. (2011) 108:18606–11. doi: 10.1073/pnas.1106920108
75. Guglielmetti C, Najac C, Didonna A, Van der Linden A, Ronen SM, Chaumeil MM, et al. Hyperpolarized (13)C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model. Proc Natl Acad Sci U S A. (2017) 114:E6982–91. doi: 10.1073/pnas.1613345114
76. Grist JT, Mclean MA, Riemer F, Schulte RF, Deen SS, Zaccagna F, et al. Quantifying normal human brain metabolism using hyperpolarized [1-(13)C]pyruvate and magnetic resonance imaging. Neuroimage. (2019) 189:171–9. doi: 10.1016/j.neuroimage.2019.01.027
77. Lapi SE, Lewis JS, Dehdashti F. Evaluation of hypoxia with copper-labeled diacetyl-bis(N-methylthiosemicarbazone). Semin Nucl Med. (2015) 45:177–85. doi: 10.1053/j.semnuclmed.2014.10.003
78. Webster JM, Morton CA, Johnson BF, Yang H, Rishel MJ, Lee BD, et al. Functional imaging of oxidative stress with a novel PET imaging agent, 18F-5-fluoro-L-aminosuberic acid. J Nucl Med. (2014) 55:657–64. doi: 10.2967/jnumed.113.126664
79. Qi Y, Liu X, Li J, Yao H, Yuan S. Fluorine-18 labeled amino acids for tumor PET/CT imaging. Oncotarget. (2017) 8:60581–8. doi: 10.18632/oncotarget.19943
80. Yang H, Jenni S, Colovic M, Merkens H, Poleschuk C, Rodrigo I, et al. (18)F-5-Fluoroaminosuberic acid as a potential tracer to gauge oxidative stress in breast cancer models. J Nucl Med. (2017) 58:367–73. doi: 10.2967/jnumed.116.180661
81. Dal-Bianco A, Schranzer R, Grabner G, Lanzinger M, Kolbrink S, Pusswald G, et al. Iron rims in patients with multiple sclerosis as neurodegenerative marker? A 7-Tesla magnetic resonance study. Front Neurol. (2021) 12:632749. doi: 10.3389/fneur.2021.632749
Keywords: progressive multiple sclerosis, oxidative stress, magnetic resonance spectroscopy, glutathione, positron emission tomography, magnetic resonance imaging
Citation: Hollen C, Neilson LE, Barajas RF Jr, Greenhouse I and Spain RI (2023) Oxidative stress in multiple sclerosis—Emerging imaging techniques. Front. Neurol. 13:1025659. doi: 10.3389/fneur.2022.1025659
Received: 24 August 2022; Accepted: 23 December 2022;
Published: 12 January 2023.
Edited by:
Chase R. Figley, University of Manitoba, CanadaReviewed by:
Bruce Berkowitz, Wayne State University School of Medicine, United StatesKejia Cai, University of Illinois at Chicago, United States
Copyright © 2023 Hollen, Neilson, Barajas, Greenhouse and Spain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Hollen,  aG9sbGVuQG9oc3UuZWR1
aG9sbGVuQG9oc3UuZWR1
 Christopher Hollen
Christopher Hollen Lee E. Neilson
Lee E. Neilson Ramon F. Barajas Jr.3,4,5
Ramon F. Barajas Jr.3,4,5 Ian Greenhouse
Ian Greenhouse