
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 23 November 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1020013
 Yi-Bin Zhang1,2†
Yi-Bin Zhang1,2† Bing-Sen Xie1,2†
Bing-Sen Xie1,2† Hao-Jie Wang1†
Hao-Jie Wang1† Sheng-Xuan Huang3†
Sheng-Xuan Huang3† Wen-Jian Fan1†
Wen-Jian Fan1† Mei Zhu4
Mei Zhu4 Guo-Rong Chen1
Guo-Rong Chen1 Deng-Liang Wang1,2
Deng-Liang Wang1,2 Pei-Sen Yao1,2
Pei-Sen Yao1,2 Liang-Hong Yu1
Liang-Hong Yu1 Lin-Sun Dai1*
Lin-Sun Dai1* De-Zhi Kang1,2,5,6,7*
De-Zhi Kang1,2,5,6,7* Shu-Fa Zheng1,2*
Shu-Fa Zheng1,2*Objective: We present our initial experience using the microcatheter-guided compartment packing (MCP) technique for endovascular embolization of acutely ruptured complex intracerebral aneurysms (ARCIAs) and evaluate the safety, feasibility, and efficiency of this technique.
Methods: This retrospective, single-center study included 28 patients who underwent coil embolization using the MCP technique for ARCIAs at our institution between January 2021 and January 2022. The MCP technique was the placement of microcatheters in different compartments within the aneurysm to deploy the coils simultaneously or sequentially. Patient demographics, aneurysm characteristics, procedural parameters, grade of occlusion, complications, and clinical results were analyzed. The clinical outcomes were evaluated with modified Rankin Scale (mRS) scores.
Results: Of the 28 patients successfully treated with the MCP technique, 24 (85.7%) aneurysms were considered as complete occlusions (Raymond I) based on the immediate postembolization angiogram results. Complications occurred in 2/28 treatments, including guidewire perforation with subarachnoid hemorrhage and cerebral vasospasm-related cerebral infarction. An angiography follow-up demonstrated complete occlusion in 25/28 aneurysms. Twenty-six (92.9%) patients had favorable 90-day outcomes (mRS 0-2) after the endovascular coil embolization.
Conclusion: The MCP technique is simple, safe, and effective, achieving good packing density and initial occlusion rate when used to treat ARCIAs.
Complex intracerebral aneurysms (CIAs) are irregular, large or giant, wide-necked or combined with arterial branches, perforating vessels, atheromatous vessels, and partially thrombosed (1–3). The acutely ruptured complex intracerebral aneurysms (ARCIAs) result in devasting consequences, and the treatment of patients with ARCIAs has been cited as the most vexing scientific issue confronting neurosurgeons and neuroradiologists. Endovascular treatment of CIAs has become an effective alternative to standard surgical clipping (4–7). Despite the advances in endovascular technologies, the treatment of ARCIAs is considered more challenging due to the difficulties in positioning the microcatheter, the coil matrix's lack of support, the increased risk of parent vessel compromise, and the inability to pack the aneurysm densely compared with classic aneurysms with narrow necks (1, 3, 5).
Emerging data suggest that coil embolization may result in 50% to 60% recanalization rates in CIAs (8, 9). In endovascular coil embolization of ARCIAs, dense coil packing of the aneurysm sac is crucial in preventing recurrence (10, 11). To achieve the densest possible coil packing, it is essential to correctly select the appropriate embolization strategy. A growing body of literature reports using multi-microcatheter, stent coiling, microcatheter shaping, and balloon assistance for intracerebral aneurysms (IAs) embolization with good clinical follow-up results (12–15). Recent retrospective studies have reported that the dual microcatheter technique is a safe and effective treatment for acutely ruptured aneurysms due to low treatment-related complications and mortality (16–18). Although these studies are informative, most of these studies focus on describing the results of the studies and do not specifically address the selection of microcatheters for IAs embolization and the rationale for the selections. In addition, prior literature demonstrated the use of microcatheters to temporarily alter the configuration of the aneurysm neck and bifurcation branches (also known as microcatheter protection techniques) to assist in aneurysm embolization, rather than direct embolization of the aneurysm using microcatheter coil delivery (15, 19).
Herein, we presented our initial experience with a microcatheter-guided compartment packing (MCP) technique for ARCIAs. The MCP technique was that the distal tips of the microcatheter were cast into different shapes based on the aneurysm type, shape, and dome orientation, and the tips of microcatheters were placed in a different compartment within the aneurysm to deploy the coils simultaneously or sequentially. The intraoperative microcatheter was adjusted according to the embolization strategy, and the microcatheters might be adjusted according to the embolization area of the expected aneurysm, even with the single microcatheter technique. Additionally, more 3-dimensional (3-D) coils were used intraoperatively to be woven into a basket based on preset microcatheter varying with the size and shape of the aneurysm since coil selection is an essential factor in successful embolization and postoperative recurrence (16). The MCP technique enables neurointerventionalists to effectively lock the coil to form a stable frame, reduce the likelihood of coil protrusion, and achieve a high-volume embolization ratio for ARCIAs.
The Institutional Review Board approved this retrospective analysis of the First Affiliated Hospital of Fujian Medical University. The medical records of the patients with acutely ruptured complex intracerebral aneurysms (ARCIAs) were treated with the MCP technique at our institution between January 2021 and January 2022. Patients were eligible when (i) diagnosis of the ruptured intracerebral aneurysm (IA) by computed tomography angiography (CTA) or/and digital subtraction angiography (DSA). CTA or/and DSA were conducted before the endovascular procedure to determine the size and location of IA. (ii) Patients who met the diagnostic criteria of CIAs. According to the published literature, CIAs were defined as irregular, large or giant, wide neck or combined arterial branches, perforating vessels, atherosclerotic vessels, and partial thrombosis (1, 3, 20). Wide-necked aneurysms were those with a diameter greater than 4 mm or a dome-to-neck ratio less than 2 (20). (iii) ARCIAs were treated with the MCP technique upon admission or within 72 h of hospital admission. Exclusion criteria were as follows: (i) patients with multiple aneurysms; (ii) patients who died during follow-up; (iii) those who could not undergo another DSA or magnetic resonance angiography (MRA) because of other diseases; (iv) those who refused to undergo a DSA or MRA review; (v) patients with pre-treatment modified Rankin Scale (mRS) score >2. In this study, Hunt-Hess grade 4-5 was defined as a severe clinical condition and mFisher grade 3-4 was considered severe aneurysmal subarachnoid hemorrhage (aSAH) (21). Neurointerventionalists and neurosurgeons immediately accessed all patients after initial CTA or/and DSA, and the mode of therapy was chosen consensually.
All patients were treated as a priority with endovascular coil embolization. Figure 1 illustrates the decision flow chart for treating ARCIAs. Immediate radiological outcomes were analyzed. Procedure-associated complications were defined as any outcomes related to the procedure, such as hemorrhage, infarction, or dissection, which was life-threatening or resulted in permanent morbidity or mortality or any unexpected event that caused persistent or significant disability (22). Neurological outcome was assessed using the mRS score before the procedure and at discharge. The mRS score was categorized into favorable (mRS 0-2) or unfavorable (mRS 3-6) (21).
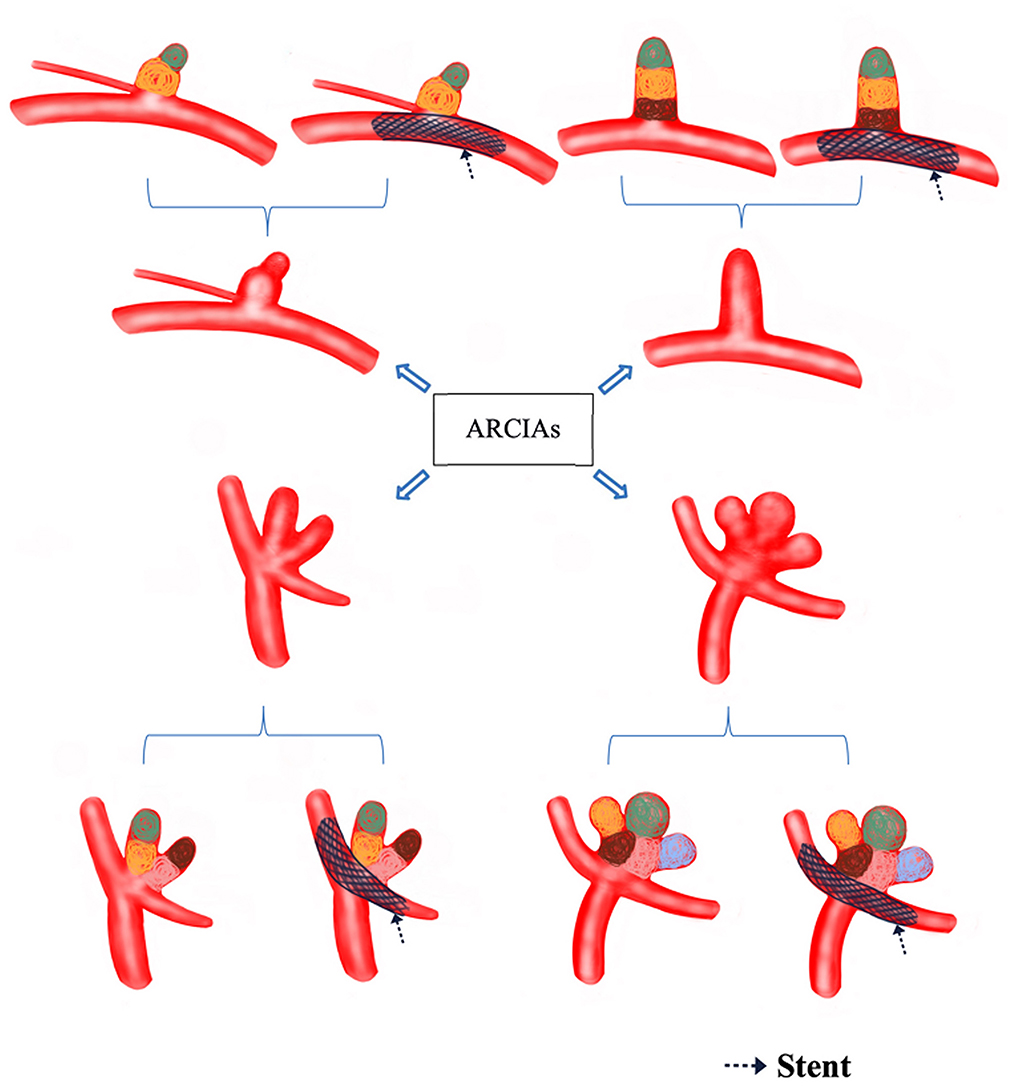
Figure 1. Decision flow chart for treating ARCIAs. Black arrows indicate the stents (which can also be balloons) that assist in compartment packing. The different colored circles represent compartment packing in different compartments. ARCIAs, acutely ruptured complex intracranial aneurysms.
Angiography was graded according to the Raymond scale (23). The degree of occlusion following embolization was classified into three classes: Raymond I (complete occlusion, including the neck), Raymond II (residual neck), and Raymond III (residual aneurysm sac) (23). Follow-up angiographic examinations were performed at 6 months post-embolization with DSA or MRA.
The interventional procedures were performed under general anesthesia. After a successful puncture through the right femoral arteries using the Seldinger technique, a 6F or 8F arterial catheter sheath was placed on the right side, and the patients received intravenous systemic heparinization. A 6 or 8-Fr guiding catheter was connected to a Y-valve with one or two double-head Y-valves in the petrous internal carotid artery for anterior circulation aneurysms or vertebral artery at the C2 spine level for posterior circulation aneurysms. First, cerebral angiography was performed to evaluate interventional treatment pathways, arteriosclerosis, stenosis, and collateral circulation. Aneurysm height, transverse diameter, neck sizes, the number of substances or lobules, and the positional relationship between the parent artery and the aneurysm, as well as proximal and distal parent artery diameter, were obtained upon review of procedural images, which included DSA and 3-dimensional (3D) rotational angiography reconstructions. These were the basis for implementing MCP technology. Aneurysm dimensions were measured by 3D images derived from rotational angiography. The optimal working angle was determined by analyzing the 3D reconstruction results and the embolization strategies, including the shape and angle of the microcatheter and the need for stent assistance. Second, according to the shape and size of the aneurysm, the morphology of the true aneurysm and the sac, the relationship between the aneurysm and the parent artery, and the order of compartment packing were determined. When necessary, precise shaping of the microcatheter tip with steam was performed to improve navigability and stability. The principle of shaping the tip of the microcatheter was that it could enter the first partition smoothly and then select the second microcatheter for shaping to enter the second partition. Multiple microcatheters may be required if ARCIAs have multiple compartments, which can not be satisfied by a single or dual microcatheter. In addition, individual microcatheters should be able to enter the second or even the third partition smoothly. The microcatheters would occupy different parts of the aneurysm. Care must be taken when positioning the second microcatheter, as it can cause the first microcatheter to migrate forward. Under the 3D Roadmap, the microcatheters were slowly advanced into the daughter sac of the target partition via a 0.014-inch micro guidewire to perform the MCP.
Under “roadmap” conditions, coils were deployed into the aneurysm. The first 3D coil that matched the target sac size was woven into a basket on a preset microcatheter varying the size and shape of the aneurysm, and the size of the coils was successively decreased to perform embolization of the target partition until it was compactly embolized. Coil-assisted embolization can be used for aneurysms with multiple daughter sacs and wide necks. The rims in the second partition must climb several times within the first partition to increase the stability of the rims in the second partition. If stent-assisted embolization of IA is required, the Jailing, semi-Jailing, or Mesh technique can be used depending on the situation (24–27). If a stent-assisted embolization was necessary for patients with ARCIAs, tirofiban was administered intravenously before the stent was released. After the interventional operation, continuous drainage of the lumbar cistern or lumbar puncture was performed to release bloody cerebrospinal fluid (CSF) according to the amount of bleeding on brain CT and the Hunt-Hess grade. All surgical procedures and perioperative management followed the institutional protocols which were based on current guidelines (28).
Here, examples of clinical application of the MCP technique for ARCIAs embolization were illustrated in Figures 2–5.
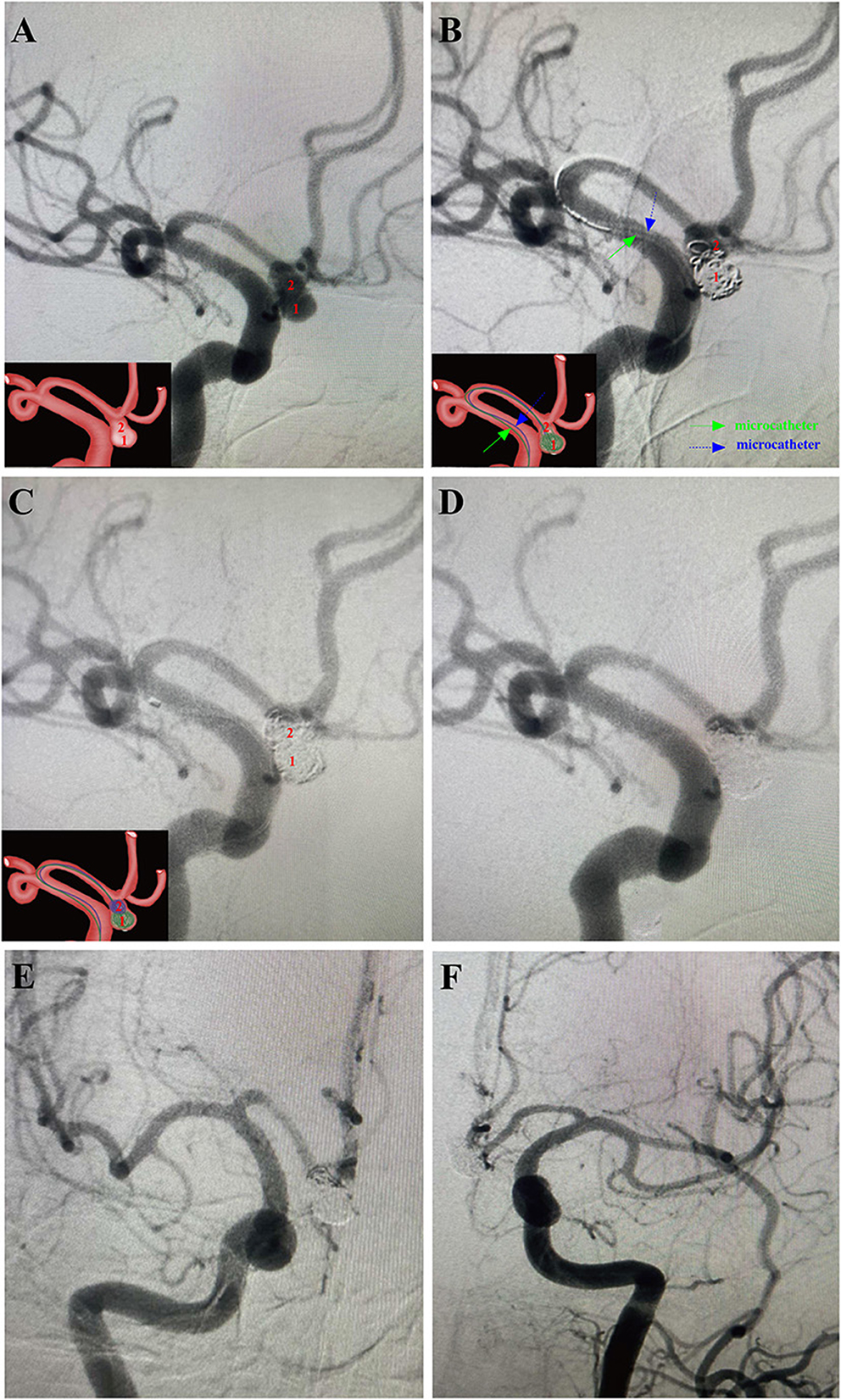
Figure 2. A 54-year-old male patient with a sudden headache for 7 h was admitted into our department with the diagnosis of subarachnoid hemorrhage (Hunt-Hess grade II) arising from ruptured irregular AComA. Then, this aneurysm was successfully treated using the MCP technique in the acute stage. Arabic numerals indicate the ascus of an IA. Right ICA DSA (working angle) demonstrated a gourd-like irregular aneurysm with a daughter sac at the AComA. Double microcatheters were introduced in the IA sac. Embolization was performed with a microcatheter (green arrow) navigating into the lower daughter sac and coils packing. Embolization with coils of appropriate size alternatively through the tips of the two microcatheters until embolism was satisfactory. After packing the lower sac, the upper daughter sac was coiled via the other microcatheter (blue arrow). (D–F) Post-embolization digital subtraction angiograms exhibited occlusion at Raymond grade II. (D) The working angle view of postoperative embolization. (E) The anteroposterior view of the right ICA after postoperative embolization. (F) The anteroposterior view of the left ICA after postoperative embolization. The inserts in the lower right corner illustrated an aschematic diagram of the MCP process of the IA (A–C).
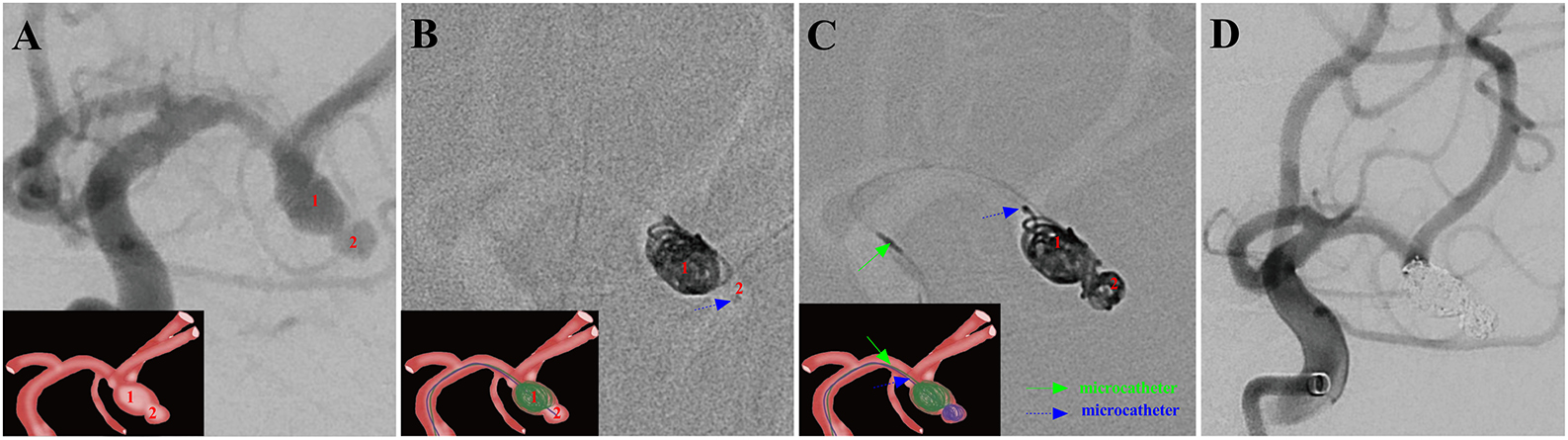
Figure 3. A ruptured right MCA aneurysm (Hunt-Hess grade II) in a 46-year-old female was treated using the MCP technique. Arabic numerals indicate the ascus of an IA. (A) Right ICA DSA (working angle) displayed a gourd-like irregular aneurysm with a daughter sac at the MCA. (B) Dual microcatheters were introduced in the IA sac. Embolization was performed with a microcatheter (blue arrow) navigating into the upper daughter sac and coils packing (image of digital non-subtraction angiography). (C) After packing the upper sac, the lower daughter sac was coiled via the other microcatheter (green arrow). (D) The working angle view of postoperative embolization showed complete occlusion of MCA aneurysm (Raymond grade I).
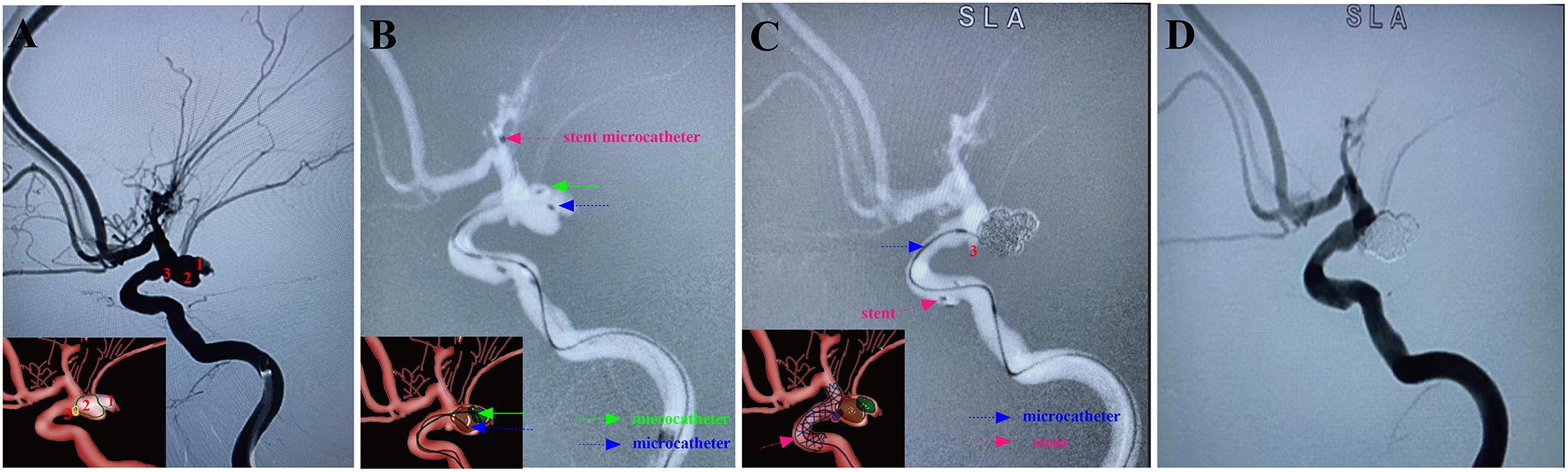
Figure 4. A ruptured right PcomA aneurysm (Hunt-Hess grade IV) in a 66-year-old female was treated using the stent-assisted MCP technique. In addition, the patient had coexisting Moyamoya disease. Arabic numerals indicate the ascus of an IA. (A) DSA (working angle) showed a cauliflower-shaped irregular aneurysm of PcomA with three daughter sacs. (B) The stent-microcatheter (pink arrow) was placed at the beginning of the M1 segment of the MCA. Double-coil microcatheters (blue and green arrows) were introduced in different sacs of the IA at appropriate surgical angles. (C) We used the Jailing technique to coil the aneurysm with the assistance of an Enterprise 2 stent (Codman Neurovascular, Raynham, Massachusetts, USA). Embolization was first performed with a microcatheter (green arrow) navigating into the upper daughter sac and coils packing (image of digital non-subtraction angiography). The coils were passed through the other microcatheter (blue arrow) to embolize the middle sacs, and then the microcatheter was withdrawn into the lower sac for coil embolization. (D) Post-embolization digital subtraction angiograms at the working angle exhibited complete occlusion at Raymond grade I. The inserts in the lower right corner illustrated an aschematic diagram of the MCP process of the IA (A–C).
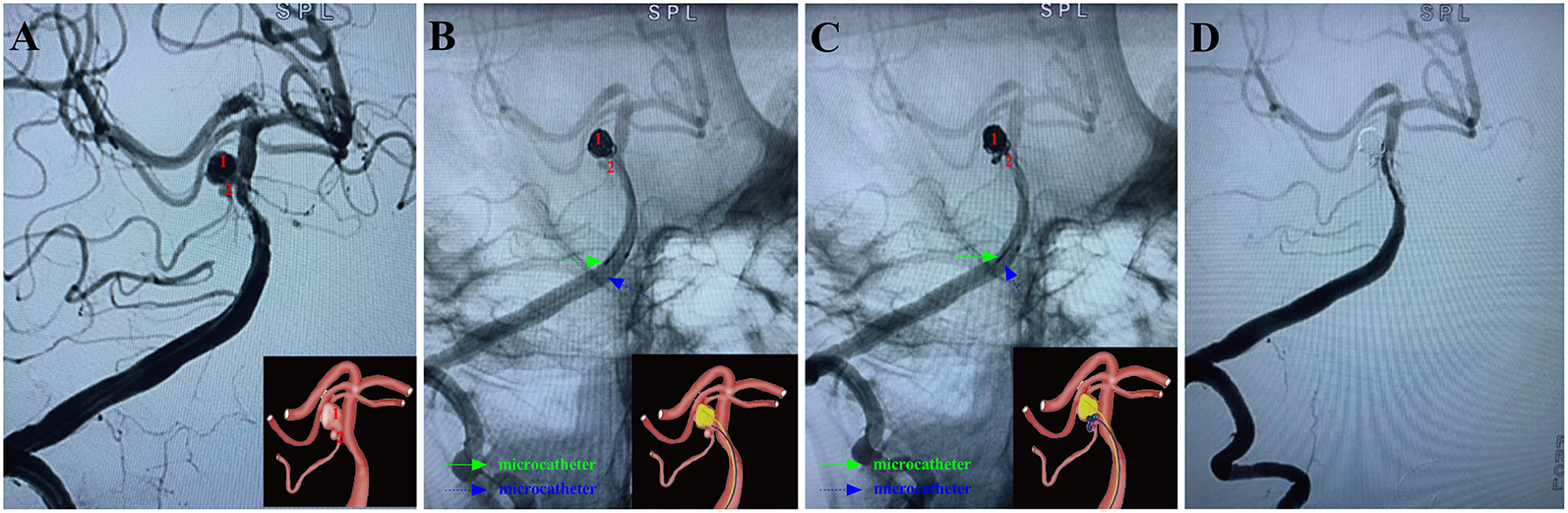
Figure 5. A ruptured right anterior inferior cerebellar artery aneurysm (Hunt-Hess grade III) in a 59-year-old female was treated using the MCP technique. Arabic numerals indicate the ascus of an IA. (A) Right vertebral artery digital subtraction angiography (working angle) showed an irregular aneurysm with a daughter sac at the bifurcation of the basilar artery and right superior cerebellar artery. (B) Double-coil microcatheters (blue and green arrows) were introduced in different sacs of the IA. Embolization was first performed with a microcatheter (green arrow) navigating into the upper daughter sac and coils packing (image of digital non-subtraction angiography). The other microcatheter completed the remaining part of the bottom of the aneurysm sac (blue arrow). (C) The other microcatheter completed the remaining part of the bottom of the aneurysm sac (blue arrow). After packing the upper sac, the microcatheter (blue arrow) enters the lower sac. Subsequently, the lower daughter sac was coiled (blue arrow). (D) Post-embolization digital subtraction angiograms at the working angle exhibited complete occlusion at Raymond grade I. The inserts in the lower right corner illustrated an aschematic diagram of the MCP process of the IA (A–C).
Twenty-eight patients presenting with subarachnoid hemorrhage (SAH) secondary to ruptured complex intracerebral aneurysms (RCIAs) were enrolled in treatment with the MCP technique based on the inclusion criteria between January 2021 and January 2022. Patient demographics and aneurysm characteristics are summarized in Table 1. This study included 28 patients (20 women and 8 men) aged from 29 to 79 years, with a mean age of 55.96. Five (17.9%) patients were in severe clinical condition (Hunt-Hess grade 4–5), and five (17.9%) patients had severe aSAH (mFisher grade 3–4) on admission. The aneurysms had sizes ranging from 2.80 to 19.87 mm (mean 7.53 mm), necks of 4.10 to 10.50 mm (mean 5.96 mm), and dome-to-neck ratios of 0.51 to 4.01 (mean 1.29). Twenty-one (75.0%) aneurysms presented irregular shapes. Sixteen (64.3%) aneurysms were located in the anterior circulation, and the other 10 (35.7%) were in the posterior circulation. Pre-treatment mRS score was 0.
Endovascular embolization of ARCIAs using the MCP technique showed technical success in all patients. Seventeen (60.7%) aneurysms were treated with the double-microcatheter MCP technique, and 11 (39.1%) were treated with the single-microcatheter MCP technique (Table 2). Thirteen (46.4%) aneurysms were treated with coil only, and 15 (53.6%) aneurysms received stent-assisted coiling. Of 28 patients treated with the MCP technique, 24 (85.7%) aneurysms were considered complete occlusion (Raymond I) based on the immediate postembolization angiogram results (Table 2). In contrast, four (14.3%) aneurysms were considered Raymond II. Figures 2–5 illustrate anterior communicating artery (AComA), middle cerebral artery (MCA), posterior communicating artery (PcomA), and anterior inferior cerebellar artery (AICA) aneurysms embolization process.
Complications occurred in 2/28 treatments, including guidewire perforation with SAH (directly related to the procedure) and cerebral vasospasm-related cerebral infarction. Aneurysm perforation occurred due to trauma caused by the pushing guide wire of a small daughter sac located above the treated aneurysm. As a result, the patient developed a small SAH. Postoperatively, the patient presented with headache symptoms, which almost wholly subsided after treatment, and the mRS score at discharge was 0. The DSA or MRA follow-up at 6 months post-embolization was available for all 28 survivors, demonstrating complete occlusion in 25/28 (89.3%) aneurysms, and the recanalization rate was 3.57% (Table 3). Twenty-six (92.9%) patients had a favorable 90-day outcome (mRS 0–2) after the endovascular coil embolization.
ARCIAs have always been a tremendous challenge for surgical clipping and endovascular coiling due to their complex vascular anatomy. In clinical practice, we introduced an MCP technique for ARCIAs and applied it to aneurysm embolization. Here, we listed four clinical applications of the MCP technique for ARCIAs embolization (Figures 2–5). The principal findings obtained in the present study are as follows. Of 28 patients successfully treated with the MCP technique, 24 (85.7%) aneurysms were considered as complete occlusions, and an angiography follow-up at 6 months post-embolization demonstrated complete occlusion in 25/28 aneurysms. The rate of recanalization at 6 months post-embolization was 3.57%. Moreover, we proposed a hypothetical model diagram in Figure 6 to embolize an aneurysm using the MCP technique, where the microcatheters were placed in a different compartment within the aneurysm to deploy the coils simultaneously or sequentially. Our report presents initial clinical experiences with the MCP technique, which provides a safe, reliable, and practical approach to the endovascular embolization of ARCIAs.
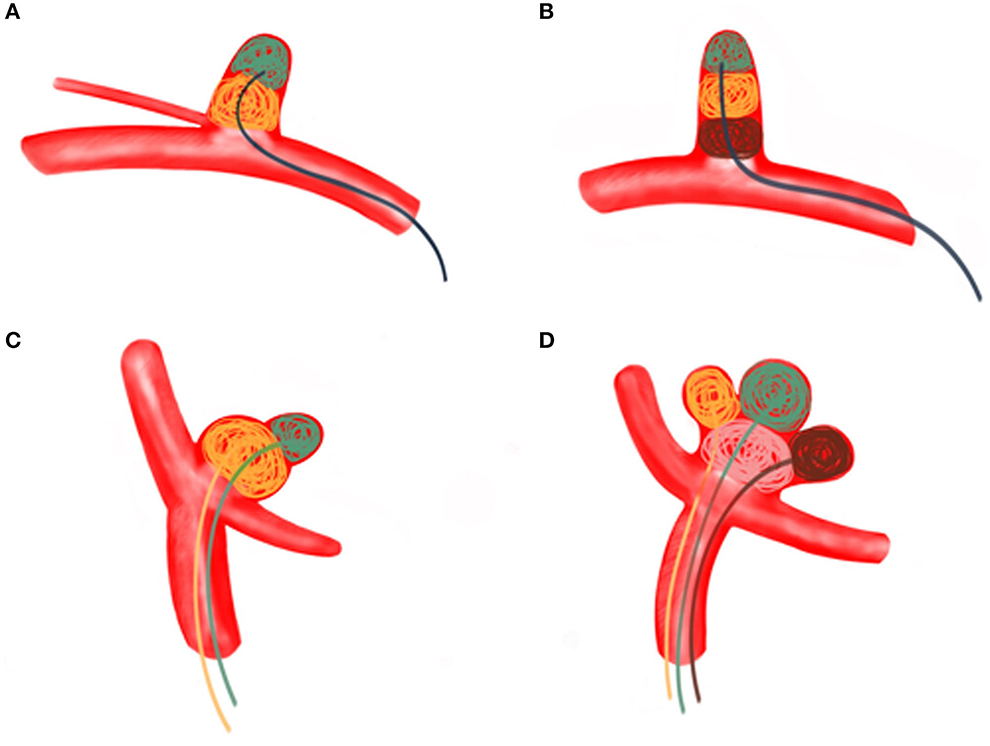
Figure 6. (A) A regular wide-necked aneurysm was treated using the MCP technique with a single microcatheter. Aneurysm embolization proceeds from the distal part of the aneurysm to the proximal part. (B) The MCP technique of a single microcatheter was used for long, regular arteries involving branch vessels. Aneurysm embolization proceeds from the distal part of the aneurysm toward the proximal part. (C) Dual microcatheter MCP technique was used to treat bifurcated irregular polysaccular aneurysms. The small ruptured sac is embolized first, followed by the embolization of the larger sac (the aneurysm). (D) Irregular polysaccular aneurysm was treated with multi-microcatheter MCP technique. Aneurysm embolization is performed from the individual sub-sacs of the aneurysm.
Several pieces of literature have demonstrated the use of microcatheters to temporarily alter the configuration of the aneurysm neck and bifurcation branches (also known as microcatheter protection techniques) to assist in aneurysm embolization (15, 16, 19). The present study differs from the previous literature in several ways. First, the MCP technique used in our case series was used to deliver coils. Second, our case series illustrated that the intraoperative microcatheter is adjusted according to the embolization strategy, and the microcatheter can be adjusted according to the embolization area of the expected aneurysm, even with the single microcatheter technique. Third, more 3-dimensional (3-D) coils were used intraoperatively to be woven into a basket based on a preset microcatheter varying with the size and shape of the aneurysm in our case series. Finally, coil-assisted embolization can be used for aneurysms with multiple daughter sacs and wide necks. The rims in the second partition must climb several times within the first partition to increase the stability of the rims in the second partition.
Compared with surgical clipping, endovascular treatment has become a preferred method in most cases for treating unruptured and ruptured IAs—over 50% of IAs are treated via endovascular coiling (7, 29, 30). Difficulties in traversing the microcatheter through tortuous vasculature, guiding the microcatheter tip to the 1/2 or 2/3 position within the aneurysm, and maintaining it in a stable position account for many coil embolization procedures fail. These causes include incomplete aneurysm occlusion (16, 31, 32), aneurysm recanalization (31), coil misalignment (33), and intraoperative rupture (34). These failures can be attributed to unfavorable vessel tortuosity and CIAs geometry. CIAs of varying shapes and sizes, and the stability of the intra-aneurysm embolization coil after intervention remain challenges for endovascular embolization techniques (7). Based on the MCP technique, the neurointerventionalist may advance the coil filling at the working projection without hesitation and may not worry about moving the microcatheter into the parent vessel or filling the coil into the parent vessel.
The MCP technique has several unique strengths. First, it exploits the superior tracking capabilities of the microcatheter, allowing flexible and maneuverable embolization of aneurysms in tortuous vasculature or smaller distal arteries. This technique can avoid remodeling and repositioning the microcatheter later and reduce surgical time and postoperative complications. Second, the MCP technique facilitates intraoperative selection of coil size. In the compartments of the aneurysm, the coils are gradually and orderly piled up, stabilizing the coils, and making them less likely to come out of the vessel. On the other hand, the microcatheter is simultaneously placed in the aneurysm sac, keeping the coils in place, and intertwined to reduce the chance of protruding. Third, the MCP technique facilitates dense embolization. It allows placing many coils in the aneurysm sac, creating a stable build before separating them. During the procedure, the distal tips of the microcatheter are steam-shaped in different shapes according to the shape of IA, and the tips of the microcatheters are implanted in different parts of IAs. ARCIAs are then densely embolized by using the microcatheters simultaneously or alternately. It should be noted that when stent-assisted embolization is used, anti-platelet aggregation drugs such as tirofiban must be used. At this time, forming an intratumoral thrombus in the aneurysm is complex, and ARCIAs can achieve the purpose of dense embolization of the aneurysm with the MCP technique. On the other hand, if stent-assisted embolization is necessary, using the MCP technique can effectively reduce coils escaping from the aneurysm cavity, increase coil packing density, and reduce aneurysm recurrence. In a literature review of stent-assisted coil embolization for ARCIAs, 63% of aneurysms exhibited complete occlusion (35). A published series with a large population using stent-assisted coil embolization for ARCIAs showed immediate occlusion in 87.5% of patients (36–39). From a multitude of perspectives, when coils are loosely packed on ruptured aneurysms, they appear to increase the risk of recurrent SAH, re-ruptured, and postoperative recurrence (15, 16). In the present study, satisfactory aneurysm occlusion was achieved in 89.3% of cases at 6 months postembolization, and the recanalization rate of 3.57% was low. The MCP technique can effectively increase the coil packing density, reduce perioperative re-rupture or rebleeding, and minimize the risk of postoperative recurrence.
The MCP treatment for ARCIAs is a double-edged sword, raising three serious concerns. First, placing two or more microcatheters into the aneurysm may increase the risk of rupture. In this study, a guidewire-induced aneurysm perforation occurred, resulting in SAH. The previous literature reported that the incidence of rebleeding caused by two or more microcatheters was 3% (14, 15), which is similar to the 2.0% to 7.3% reported incidence for all aneurysms regardless of size or presence of subarachnoid hemorrhage (14, 31). Second, coiling ARCIAs without a stent or balloon may increase the risk of coil protrusion and parent artery occlusion (36–40). Although no similar complication occurred in our cases, it still requires concern. Third, coiling ARCIAs without stent or balloon assistance may reduce packing density and increase recurrence rates (36, 38–41). In our case, complete occlusion (Raymond I) was achieved in 89.3% of cases at 6 months postembolization, and we look forward to long-term follow-up results. Taken together, neurointerventionalists need to carefully balance the risk of aneurysm recurrence with the inherent risk of stent or balloon remodeling. Nevertheless, it is undeniable that stent-assisted coil embolization has been increasing for ARCIAs (25, 42).
Notably, not all ARCIAs can be treated endovascularly. Up to 25% of surgical neurointerventions fail in attempted endovascular treatment of intracranial aneurysms (7). Some ARCIAs require revascularization techniques, including extracranial-to-intracranial carotid artery bypass with aneurysm clipping or distal or proximal arterial occlusion, intracranial-to-intracranial bypass, and excision of the diseased segment with direct vessel revascularization (in situ bypass) (1, 43). Future studies are needed to explore whether the MCP technique can guide the choice of surgical modalities for the revascularization of such CIAs. Moreover, we did not obtain a long-term follow-up result in our patients even though we obtained a good packing density and stable short-term outcome. Further follow-up and application in more patients will be required. Third, since the sample size in the present study was small, larger-scale clinical trials are needed to verify the generalizability of the present findings. Fourth, for treating giant aneurysms, the use of MCP technology requires the use of bilateral femoral artery sheath and multi-catheter system technology, which may increase surgical complexity, heighten the risk of intraoperative complications, and increase operative times.
The MCP technique is simple, safe, and effective, achieving good packing density and initial occlusion rate when used for the treatment of ARCIAs, and may serve as an option for treating ARCIAs. Further studies are necessary to evaluate its long-term effectiveness.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University. The Ethics Committee waived the requirement of written informed consent for participation.
Y-BZ, D-ZK, and S-FZ designed the study. Y-BZ, B-SX, H-JW, and S-XH collected, analyzed data, and drafted the manuscript. W-JF, MZ, G-RC, P-SY, L-SD, and L-HY helped with the figures and result in interpretation. D-LW, L-SD, L-HY, D-ZK, and S-FZ reviewed and revised the manuscript. L-SD, D-ZK, and S-FZ were identified as the guarantor of the article, taking responsibility for the integrity of the work as a whole. All authors contributed to the article and approved the submitted version.
The study was supported by the National Natural Science Foundation of China (Nos. 81870930 and 82171327) and Qihang Fund Project of Fujian Medical University (2019QH1262).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ravina K, Rennert RC, Brandel MG, Strickland BA, Chun A, Lee Y, et al. Comparative Assessment of Extracranial-to-Intracranial and Intracranial-to-Intracranial In Situ Bypass for Complex Intracranial Aneurysm Treatment Based on Rupture Status: a Case Series. World Neurosurg. (2021) 146:e122–38. doi: 10.1016/j.wneu.2020.10.056
2. Nussbaum ES, Kallmes KM, Lassig JP, Goddard JK, Madison MT, Nussbaum LA. Cerebral revascularization for the management of complex intracranial aneurysms: a single-center experience. J Neurosurg. (2018) 131:1297-1307. doi: 10.3171/2018.4.JNS172752
3. Lawton MT, Lang MJ. The future of open vascular neurosurgery: perspectives on cavernous malformations, AVMs, and bypasses for complex aneurysms. J Neurosurg. (2019) 130:1409–25. doi: 10.3171/2019.1.JNS182156
4. Pierot L, Barbe C, Nguyen HA, Herbreteau D, Gauvrit JY, Januel AC, et al. Intraoperative Complications of Endovascular Treatment of Intracranial Aneurysms with Coiling or Balloon-assisted Coiling in a Prospective Multicenter Cohort of 1088 Participants: Analysis of Recanalization after Endovascular Treatment of Intracranial Aneurysm (ARETA) Study. Radiology. (2020) 295:381–9. doi: 10.1148/radiol.2020191842
5. Al-Mufti F, Amuluru K, Gandhi CD, Prestigiacomo CJ. Flow Diversion for Intracranial Aneurysm Management: A New Standard of Care. Neurotherapeutics. (2016) 13:582–9. doi: 10.1007/s13311-016-0436-4
6. Nomura M, Mori K, Tamase A, Kamide T, Seki S, Iida Y, et al. Thromboembolic complications during endovascular treatment of ruptured cerebral aneurysms. Interv Neuroradiol. (2018) 24:29–39. doi: 10.1177/1591019917739448
7. Gopesh T, Wen JH, Santiago-Dieppa D, Yan B, Pannell JS, Khalessi A, et al. Soft robotic steerable microcatheter for the endovascular treatment of cerebral disorders. Sci Robot. (2021) 6:eabf0601. doi: 10.1126/scirobotics.abf0601
8. Nelson PK, Sahlein D, Shapiro M, Becske T, Fitzsimmons BF, Huang P, et al. Recent steps toward a reconstructive endovascular solution for the orphaned, complex-neck aneurysm. Neurosurgery. (2006) 59:S77-92. doi: 10.1227/01.NEU.0000240664.00611.BB
9. Agarwal A, Gokhale S, Gupta J, Raju R, Nimjee S, Smith T, et al. Use of pipeline flow diverting stents for wide neck intracranial aneurysms: a retrospective institutional review. Asian J Neurosurg. (2014) 9:3–6. doi: 10.4103/1793-5482.131057
10. Sluzewski M. Rooij WJv, Slob MJ, Bescós JO, Slump CH, Wijnalda D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. (2004) 231:653–8. doi: 10.1148/radiol.2313030460
11. Ota K, Matsubara N, Miyachi S, Izumi T, Ito M, Asai T, et al. Evaluation of the characteristics of various types of finishing coils for the embolization of intracranial aneurysms in an experimental model with radiolucent coils. Interv Neuroradiol. (2017) 23:143–50. doi: 10.1177/1591019916685713
12. Baxter BW, Rosso D, Lownie SP. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. Am Soc Neuroradiol. (1998) 19:1176–8.
13. Nakahara T, Kutsuna M, Yamanaka M, Sakoda K. Coil embolization of a large, wide-necked aneurysm using a double coil-delivered microcatheter technique in combination with a balloon-assisted technique. Neurol Res. (1999) 21:324–6. doi: 10.1080/01616412.1999.11740939
14. Durst CR, Starke RM, Gaughen JR, Geraghty S, Kreitel KD, Medel R, et al. Single-center experience with a dual microcatheter technique for the endovascular treatment of wide-necked aneurysms. J Neurosurg. (2014) 121:1093–101. doi: 10.3171/2014.7.JNS132237
15. Cho YD, Rhim JK, Kang HS, Park JJ, Jeon JP, Kim JE, et al. Use of triple microcatheters for endovascular treatment of wide-necked intracranial aneurysms: a single center experience. Korean J Radiol. (2015) 16:1109–18. doi: 10.3348/kjr.2015.16.5.1109
16. Yoon PH, Lee JW, Lee YH, Kwon YS, Yang KH. Dual microcatheter coil embolization of acutely ruptured wide-necked intracranial aneurysms. Interv Neuroradiol. (2017) 23:477–84. doi: 10.1177/1591019917708570
17. Trasimeni G, Laurino F, Lamusta D, Limbucci N, Mangiafico S. Double micro-guide-wire technique to facilitate microcatheter navigation through tortuous intracranial vasculature. J Neuroradiol. (2018) 45:333–5. doi: 10.1016/j.neurad.2018.06.004
18. Liu Y, Li Z, Duan P, Liu D. E Y. Dual microcatheter “cross-regional” embolization technique for the treatment of intracranial irregular aneurysms. J Interv Radiol. (2018) 27:199–202.
19. Starke RM, Durst CR, Evans A, Ding D, Raper DM, Jensen ME, et al. Endovascular treatment of unruptured wide-necked intracranial aneurysms: comparison of dual microcatheter technique and stent-assisted coil embolization. J Neurointerv Surg. (2015) 7:256–61. doi: 10.1136/neurintsurg-2014-011159
20. Olli I. Tähtinen, Ritva L Vanninen, Hannu I Manninen, Riitta Rautio, Arto Haapanen, Tero Niskakangas, Jaakko Rinne, Keski-Nisula L. Wide-necked intracranial aneurysms: treatment with stentassisted coil embolization during acute (72 hours) subarachnoid hemorrhage—experience in 61 consecutive patients. Radiology. (2009) 253:199–208. doi: 10.1148/radiol.2531081923
21. Zhang Y, Zheng S, Wang H, Chen G, Li C, Lin Y, et al. Admission Lower Serum Phosphate Ion Levels Predict Acute Hydrocephalus of Aneurysmal Subarachnoid Hemorrhage. Front Neurol. (2022) 12. doi: 10.3389/fneur.2021.759963
22. Nishido H, Piotin M, Bartolini B, Pistocchi S, Redjem H, Blanc R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol. (2014) 35:339–44. doi: 10.3174/ajnr.A3658
23. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. (2003) 34:1398–403. doi: 10.1161/01.STR.0000073841.88563.E9
24. Shirakawa M, Yoshimura S, Uchida K, Yamada K, Sakamoto D, Iida T, et al. Coil Embolization for Cerebral Aneurysms Using a Semi-Jailing Technique and Open-Cell Stent. World Neurosurg. (2019) 125:e16–21. doi: 10.1016/j.wneu.2018.12.065
25. Nii K, Inoue R, Morinaga Y, Mitsutake T, Hanada H. Evaluation of Acute In-stent Thrombosis during Stent-assisted Coil Embolization of Unruptured Intracranial Aneurysms. Neurol Med Chir (Tokyo). (2018) 58:435–41. doi: 10.2176/nmc.oa.2018-0131
26. Poncyljusz W, Kubiak K, Sagan L, Limanowka B, Kolaczyk K. Evaluation of the Accero Stent for Stent-Assisted Coiling of Unruptured Wide-Necked Intracranial Aneurysm Treatment with Short-Term Follow-Up. J Clin Med. (2020) 9. doi: 10.3390/jcm9092808
27. Zhang Y, Huang QH, Fang Y, Yang P, Xu Y, Hong B, et al. Novel Flow Diverter (Tubridge) for the Treatment of Recurrent Aneurysms: A Single-Center Experience. Korean J Radiol. (2017) 18:852–9. doi: 10.3348/kjr.2017.18.5.852
28. Connolly ES. Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart. Assoc. Am. Stroke Assoc. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
29. Poncyljusz W, Kubiak K. Initial Experience with LVIS EVO Stents for the Treatment of Intracranial Aneurysms. J Clin Med. (2020) 9:3966. doi: 10.3390/jcm9123966
30. Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001-2008. AJNR Am J Neuroradiol. (2011) 32:1071–5. doi: 10.3174/ajnr.A2453
31. Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke. (2013) 44:2046–54. doi: 10.1161/STROKEAHA.113.000733
32. Jang LK, Alvarado JA, Pepona M, Wasson EM, Nash LD, Ortega JM, Randles A, Maitland DJ, Moya ML, Hynes WF. Three-dimensional bioprinting of aneurysm-bearing tissue structure for endovascular deployment of embolization coils. Biofabrication. (2020) 13:015006. doi: 10.1088/1758-5090/abbb9b
33. Khatri R, Chaudhry SA, Rodriguez GJ, Suri MF, Cordina SM, Qureshi AI. Frequency and factors associated with unsuccessful lead (first) coil placement in patients undergoing coil embolization of intracranial aneurysms. Neurosurgery. (2013) 72:452–458. doi: 10.1227/NEU.0b013e3182804ad1
34. Kocur D, Przybylko N, Bazowski P, Baron J. Rupture during coiling of intracranial aneurysms: Predictors and clinical outcome. Clin Neurol Neurosurg. (2018) 165:81–7. doi: 10.1016/j.clineuro.2018.01.006
35. Bodily KD, Cloft HJ, Lanzino G, Fiorella DJ, White PM, Kallmes DF. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol. (2011) 32:1232–6. doi: 10.3174/ajnr.A2478
36. Chalouhi N, Drueding R, Starke RM, Jabbour P, Dumont AS, Gonzalez LF, et al. In-stent stenosis after stent-assisted coiling: incidence, predictors and clinical outcomes of 435 cases. Neurosurgery. (2013) 72:390–6. doi: 10.1227/NEU.0b013e31828046a6
37. Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. (2013) 44:1348–53. doi: 10.1161/STROKEAHA.111.000641
38. Chalouhi N, Jabbour P, Tjoumakaris S, Dumont AS, Chitale R, Rosenwasser RH, et al. Single-center experience with balloon-assisted coil embolization of intracranial aneurysms: safety, efficacy and indications. Clin Neurol Neurosurg. (2013) 115:607–13. doi: 10.1016/j.clineuro.2012.07.028
39. Chalouhi N, Starke RM, Koltz MT, Jabbour PM, Tjoumakaris SI, Dumont AS, et al. Stent-assisted coiling versus balloon remodeling of wide-neck aneurysms: comparison of angiographic outcomes. AJNR Am J Neuroradiol. (2013) 34:1987–92. doi: 10.3174/ajnr.A3538
40. Pierot L, Cognard C, Spelle L, Moret J. Safety and efficacy of balloon remodeling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. Am J Neuroradiol. (2012) 33:12–5. doi: 10.3174/ajnr.A2403
41. Chen PR. Balloon-assisted coil embolization of small intracranial aneurysms. Neurosurg Focus. (2014) 37:1. doi: 10.3171/2014.V2.FOUS14192
42. Oishi H, Fujii T, Yatomi K, Teranishi K, Suzuki K, Mishima Y, et al. Stent-assisted coil embolization of unruptured middle cerebral artery aneurysms using LVIS Jr. Stents J Clin Neurosci. (2020) 80:87–91. doi: 10.1016/j.jocn.2020.07.070
Keywords: endovascular embolization, intracerebral aneurysm, subarachnoid hemorrhage, microcatheter, treatment, prognosis
Citation: Zhang Y-B, Xie B-S, Wang H-J, Huang S-X, Fan W-J, Zhu M, Chen G-R, Wang D-L, Yao P-S, Yu L-H, Dai L-S, Kang D-Z and Zheng S-F (2022) Microcatheter-guided compartment packing of acutely ruptured complex intracerebral aneurysms (ARCIAs): Preliminary experience and technical note. Front. Neurol. 13:1020013. doi: 10.3389/fneur.2022.1020013
Received: 15 August 2022; Accepted: 12 October 2022;
Published: 23 November 2022.
Edited by:
Tianxiao Li, Henan Provincial People's Hospital, ChinaReviewed by:
Kostadin L. Karagiozov, Jikei University School of Medicine, JapanCopyright © 2022 Zhang, Xie, Wang, Huang, Fan, Zhu, Chen, Wang, Yao, Yu, Dai, Kang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin-Sun Dai, RGFpbHNAc2luYS5jb20=; De-Zhi Kang, a2R6OTk5ODhAc2luYS5jb20=; Shu-Fa Zheng, enNmMjAwMjExMEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.