- 1SHARE Military Initiative, Shepherd Center, Atlanta, GA, United States
- 2School of Medicine, Department of Emergency Medicine, Emory University, Atlanta, GA, United States
- 3School of Medicine, Department of Rehabilitation Medicine, Emory University, Atlanta, GA, United States
- 4Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, United States
Objective: To explore the use of person-centered goals (PCGs) to direct interdisciplinary care to support PCG attainment in military service members and Veterans (SM/Vs) with chronic mild traumatic brain injury (mTBI) and co-occurring psychological conditions.
Methods: A retrospective chart review was completed for 146 United States military SM/Vs reporting chronic symptoms following mTBI and co-occurring psychological conditions who received care in the SHARE Military Initiative intensive outpatient program, a donor-funded program administered by a not-for-profit hospital, between April 1, 2015 and March 31, 2019. PCGs were used to direct care consisting of individual and group-based interventions and therapies delivered by an interdisciplinary, co-located team including behavioral health, case management, neurology or physiatry, nursing, occupational therapy, physical therapy, recreation therapy, speech-language pathology, and transition support. The primary outcome measure was PCG attainment measured via goal attainment scaling.
Results: Increased PCG attainment was demonstrated at program discharge and throughout the first year following program discharge. Predictors of goal attainment at discharge included longer participation in treatment, greater reduction in depressive symptoms and greater improvement in adjustment at discharge, male gender, and higher cognitive and physical abilities on admission.
Conclusions: This sample of military SM/Vs with mTBI and co-occurring psychological conditions who received intensive, interdisciplinary, PCG directed care demonstrated increased PCG attainment at program discharge which further increased with transition support over the year post-discharge. Results suggest PGC goal directed care is a feasible, promising methodology of individualizing treatment in this population. This exploratory study lays a foundation for future prospective, controlled, comparative effectiveness research that will further understanding of the effectiveness of intensive, interdisciplinary, PCG directed care.
Introduction
Traumatic Brain Injury (TBI), the “signature injury” of post-9/11 military service members and Veterans (SM/Vs), can cause significant deficits in physical, behavioral, emotional, social, and cognitive functioning. The Traumatic Brain Injury Center of Excellence reports 453,919 documented TBI's among U.S. service members between 2000 and 2021, the majority of which are classified as mild TBI (mTBI) (1). While most recover from a single mTBI within weeks, as many as 20% of adults have a more prolonged course of recovery, negatively affecting health, function, and participation in important roles (2, 3).
Management of chronic mTBI symptoms in military populations has been a challenge for healthcare systems, in part because of the multifactorial and mutually reinforcing nature of the physical, cognitive, and emotional deficits that require treatment (4–6). Termed the “polytrauma clinical triad,” rates of co-occurrence of TBI, Posttraumatic Stress Disorder (PTSD), and chronic pain are high among SM/Vs (7). Veterans Healthcare Administration records indicate that most Operation Iraqi Freedom/Operation Enduring Freedom/Operation New Dawn Veterans diagnosed with TBI also have a mental health disorder and about half have both PTSD and pain (8). Psychological variables are known predictors of outcomes in the SM/V mTBI population (9, 10).
Specialized, comprehensive care provided through a collaborative multi- or interdisciplinary team approach has been shown to improve outcomes among military populations experiencing chronic effects of mTBI and co-occurring conditions (11–13). Given the heterogeneity of symptom presentations and the potential for individual pre-injury and contextual factors to influence outcomes, rehabilitation outcomes may also be improved by employing person-centered goal (PCG) setting in the context of interdisciplinary care (6, 14). The use of PCGs scaled with goal attainment scaling (GAS) to drive interdisciplinary care has been shown to be efficacious in the management of non-military moderate-severe TBI (15–17); however, this has not previously been examined in military or non-military multidisciplinary management of mTBI (18) with or without psychological comorbidities. PCGs are identified by the person served and address meaningful, motivating aspects of participation and quality of life. GAS is a standardized method of developing a scale to measure progress toward a goal by comparing goal attainment to the person's baseline (19, 20). PCGs scaled by GAS can be used to guide interdisciplinary team collaboration and can support providers in explicitly linking interventions to PCGs when discussing treatments with patients. This model can drive rehabilitation outcomes by empowering patients, motivating helpful behaviors, and connecting treatment engagement to personal goals (21, 22).
Given the intensive resources required to provide PCG directed interdisciplinary care, it is important to evaluate program outcomes and understand factors that contribute to success. This retrospective analysis of military SM/Vs with chronic mTBI and co-occurring psychological conditions who participated in intensive, interdisciplinary, PCG directed care had the following aims: (1) assess changes in PCG attainment following intensive, interdisciplinary, PCG directed care; (2) assess goal attainment in the year following program participation; and (3) identify demographic, injury characteristic, and clinical variables predicting goal attainment. We hypothesized that SM/Vs would report increased goal attainment at program discharge as compared to intake, and that goal attainment would be maintained over time in the year post-discharge. Further, we hypothesized that participant injury characteristics including number of and mechanism of injury, community participation, and psychological functioning including degree of depression, sleep, and PTSD symptoms may predict degree of goal attainment.
Methods
Institutional review board approval was obtained from Shepherd Center to complete this retrospective chart review.
Participants
Electronic medical records of a convenience sample of participants who received care in the SHARE Military Initiative (SHARE) intensive outpatient program (IOP), a donor-funded program administered by a not-for-profit hospital, between April 1, 2015 and March 31, 2019 (n = 182) were reviewed. Inclusion criteria: (1) unrestricted medical record for review; (2) diagnosis of mTBI assigned during clinical evaluation based on presence of at least one qualifier (23) with patient-report of symptoms persisting greater than 6 months post injury; (3) United States military SM/V; and (4) received treatment in the SHARE IOP. Exclusion criteria were: (1) history of brain injury other than mTBI (n = 12); (2) active psychosis (n = 0); and (3) discharge PCG GAS ratings unavailable (n = 24).
Intervention
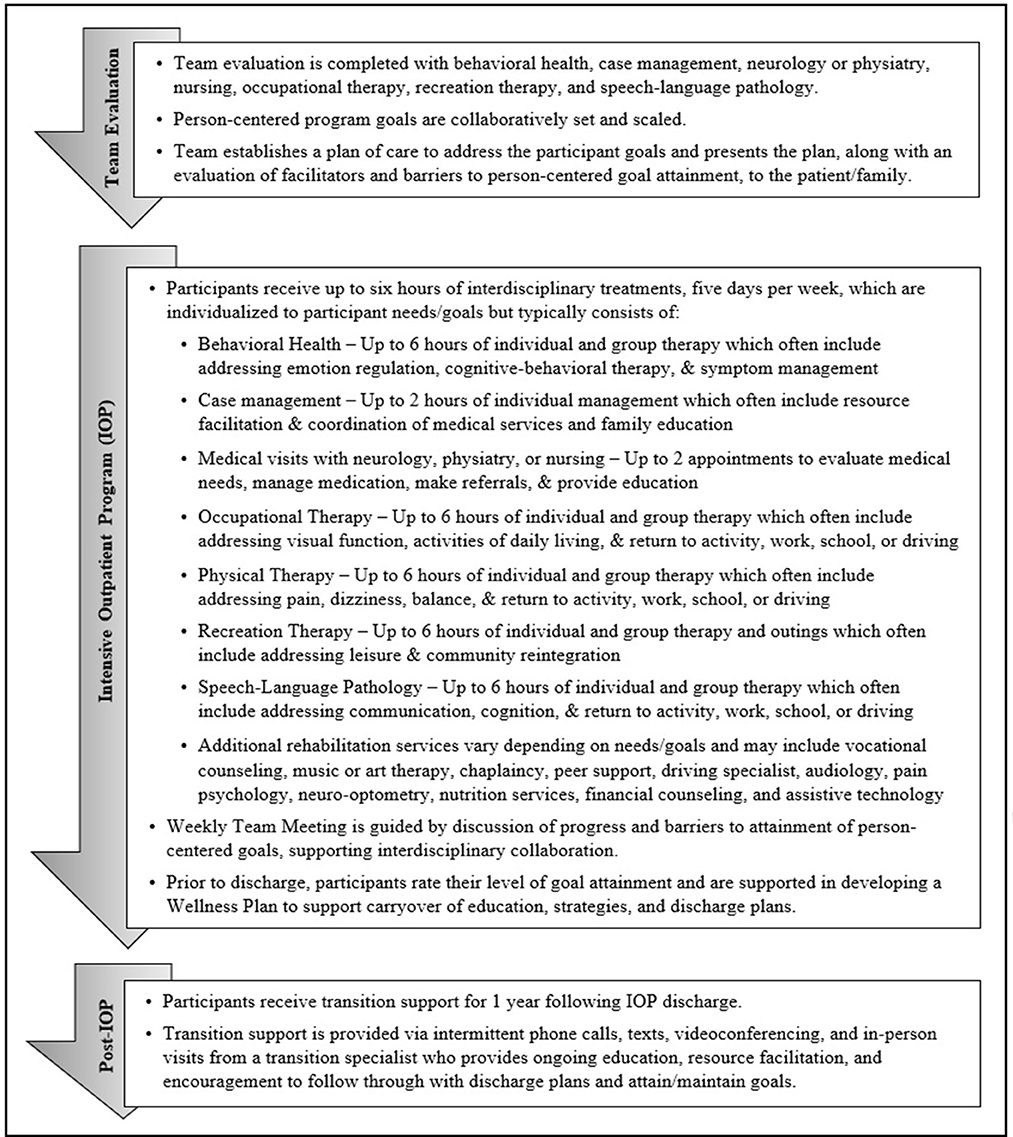
The SHARE IOP provides interdisciplinary outpatient rehabilitation for U.S. SM/Vs experiencing symptoms of brain injury. SHARE IOP participants identify between 1–3 person-centered program goals for which co-located, interdisciplinary team members including behavioral health, case management, neurology or physiatry, nursing, occupational therapy, physical therapy, recreation therapy, and speech-language pathology evaluate facilitators and barriers to develop a comprehensive, PCG directed plan of care (see Figure 1). Program length of stay and the type and amount of therapy provided are individualized given strengths, barriers, symptoms, resources, comorbidities, and other contextual factors unique to each patient (6, 14). Treatment interventions address barriers to goal attainment, and additional services are added (e.g., vocational counseling, art therapy, or chaplaincy) matched to participant PCGs. Program participants receive up to 6 h of individual and group therapy, 5 days per week, delivered by the interdisciplinary team. Following program completion, participants receive support for 1 year from a transition support specialist who provides coaching to adhere to discharge recommendations and maintain goal attainment via remote and in-person visits. Program participants collaborate with the care team to apply GAS to their goals on admission (20, 24). Participants then rate their goal attainment at program discharge, and six, nine, and 12 months after discharge.
Procedures
Two trained researchers conducted chart reviews of medical records to collect demographic, injury, and medical data not electronically abstracted. Number of TBI events were coded as 0, 1, 2, and 3 or more due to ambiguity in records describing greater than three TBIs. Extracted medical record data were recorded into an Excel spreadsheet, and 20% of cases were reviewed by the lead author who measured interrater reliability at 0.96 (Cohen's kappa). Standardized assessment data were electronically extracted for clinical measures administered at admission and discharge. Goal attainment data were electronically extracted for admission, discharge, and timepoints collected post-discharge. Goals were reviewed and categorized by two authors, who first together determined the most common categories based on review of the goals, and then separately assigned each goal to a category. This resulted in discrepancies for 2% of the goals of which 100% were resolved following discussion.
Measures
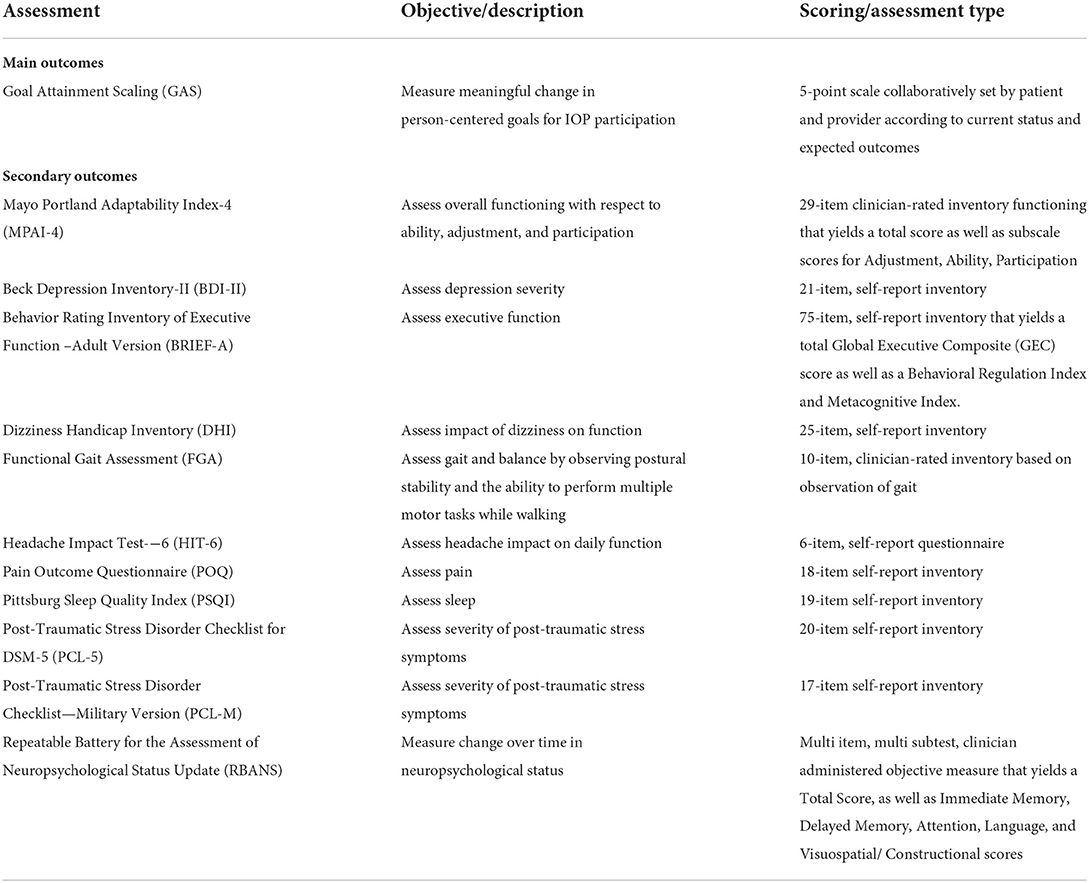
Measures are described in Table 1. GAS was used as the primary outcome measure to assess meaningful change in PCGs (19). Participants set program goals and rated goal attainment as outlined above.
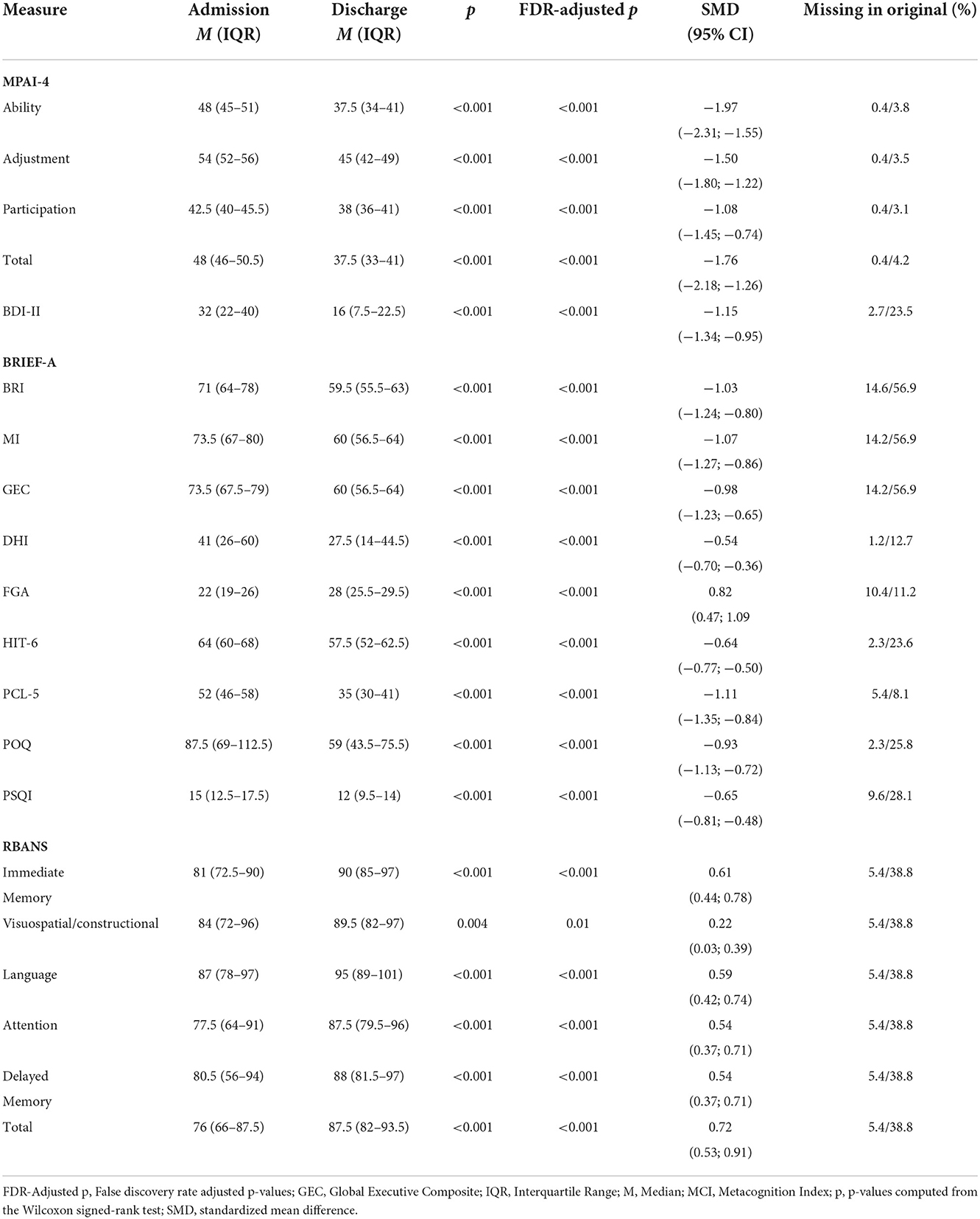
Clinical measures collected as part of standard care were used in a set of secondary analyses. Clinicians completed Mayo Portland Adaptability Index-4 (MPAI-4) ratings on participants within their first 3 days of program participation, and then again in the last week to evaluate Ability, Adjustment, and Participation as related to body movements, thinking skills, emotions, behavior, and social skills (25). Participants completed clinical measures within their first 2 weeks of program participation and then again in their last week of program participation. Clinical measures included the Beck Depression Inventory-II (BDI-II) (26), Behavior Rating Inventory of Executive Function – Adult Version (BRIEF-A) (27), Dizziness Handicap Inventory (DHI) (28), Functional Gait Assessment (FGA) (29), Headache Impact Test-6 (HIT-6) (30), the Post-Traumatic Stress Disorder Checklist for DSM-5 (PCL-5) (31), Post-Traumatic Stress Disorder Checklist—Military Version (PCL-M) (32), Pittsburg Sleep Quality Index (PSQI) (33), Pain Outcome Questionnaire (POQ) (34), and Repeatable Battery for the Assessment of Neuropsychological Status Update (RBANS) (35) (see Table 1). Some participant records contained PCL-M scores vs. PCL5, which were converted to PCL5 scores for analyses (36).
Statistical analysis
Categorical and ordinal variables were described using frequencies and percentages. Continuous and scale variables were described using medians and interquartile ranges.
The primary research questions aimed to determine how patient goal attainment, measured using GAS T-scores, changed at program completion and in the year following discharge. A fixed effects linear regression assessment was used to evaluate changes in GAS T-score following discharge by including time since discharge as a predictor using an autoregressive variance/covariance structure to account for multiple measurements per participant. In order to adjust for potential confounding, this analysis was repeated with the following covariates: age, gender, race, ethnicity, education, MPAI-4 subscales measured at admission, change in MPAI-4 subscales from admission to discharge, time since the index TBI, time since the first TBI, time since the most recent TBI, number of known instances of loss of consciousness, BDI-II measured at admission, the change in BDI-II from admission to discharge, the PSQI measured at admission, the change in PSQI from admission to discharge, the PCL measured at admission, the change in PCL from admission to discharge, and the length of stay in the IOP. Both regression coefficients (i.e., the expected change in the outcome per unit change in the predictor in raw units) and standardized regression coefficients (i.e., the expected change in the outcome per unit change in the predictor in standard deviations), as well as 95% confidence intervals, are presented. P-values and 95% CIs were computed using bias-corrected and accelerated bootstrapping (10,000 resamples).
In addition to the primary analysis, a number of secondary research questions were considered. First, variation in discharge GAS scores across PCG categories (e.g., fitness-related goals, education-related goals, etc.) were evaluated. Raw GAS values (a 5-point scale) were submitted to a fixed-effects ordinal logistic regression. A fixed-effects ordinal logistic regression was used to account for inclusion of multiple GAS values per participant, as individuals set up to three goals. Goal category was weighted-effects coded so that each odds ratio represents the difference between a given category and the average category. “Other” was chosen as the leave-out category. P-values and 95% CIs were computed using bias-corrected and accelerated bootstrapping (10,000 resamples).
We also conducted an exploratory analysis to identify potential predictors of the primary outcome, T-Scores computed from GAS of PCGs measured at discharge (19). In addition to participant demographic and injury characteristics, select clinical variables were included as potential predictors. MPAI-4 scores were included to assess the relationship of functioning commonly impacted by TBI. BDI, PCL-5, and PSQI scores were included to assess the relationship of the severity of symptoms for psychological comorbidities most commonly experienced by SHARE IOP participants, namely depression, PTSD, and sleep difficulties. For each clinical measure, both severity at admission and the degree of change were included in the model. All participants received the same model of care but varying LOS, therefore, LOS was included in the model to determine if the amount of care received influenced goal attainment. Predictors were evaluated using a linear regression and Shapley scores resulting from that analysis. Shapley scores describe the average marginal contribution of each variable to the model output (37). As a result, Shapley scores can be used to evaluate the importance of a given variable with respect to predicting an outcome. Shapley values were scaled so that they sum to 1. Additionally, we present coefficients, standardized coefficients, and 95% confidence intervals.
Finally, an additional analysis evaluated pre-post changes in clinical measures that occurred between admission and discharge. These comparisons were conducted using the Wilcoxon signed-rank test and the standardized mean difference for paired comparisons (i.e., the change from pre- to post expressed in terms of standard deviations). 95% confidence intervals for the standardized mean difference were computed using bias-corrected and accelerated bootstrapping (10,000 resamples).
For the secondary analyses, the false discovery rate was formally controlled (38). Across all variables of interest, a median of 2.7% of data points were missing (mean 11.2%, range 0–58%). The primary outcome, GAS T-Scores, was available for all patients. Ten complete data sets were imputed using fully conditional specification, a multiple imputation method which draws values from each variable's conditional distribution using Markov Chain Monte Carlos (39). All available variables were included in the imputation model. Statistical analyses were conducted using R (v3.6.3) (40).
Because this was a retrospective analysis of existing data, no a priori power calculation was conducted. However, the final sample size results in sufficient power (80%) to detect odds ratios >2.6 (assuming maximal variance for a binomial variable), a between-groups standardized mean difference of 0.5 (assuming the minimal asymptotic efficiency of a non-parametric test), a within-groups standardized mean differences of 0.25 (assuming the minimal asymptotic efficiency of a non-parametric test), or a Cohen's f of 0.23.
Results
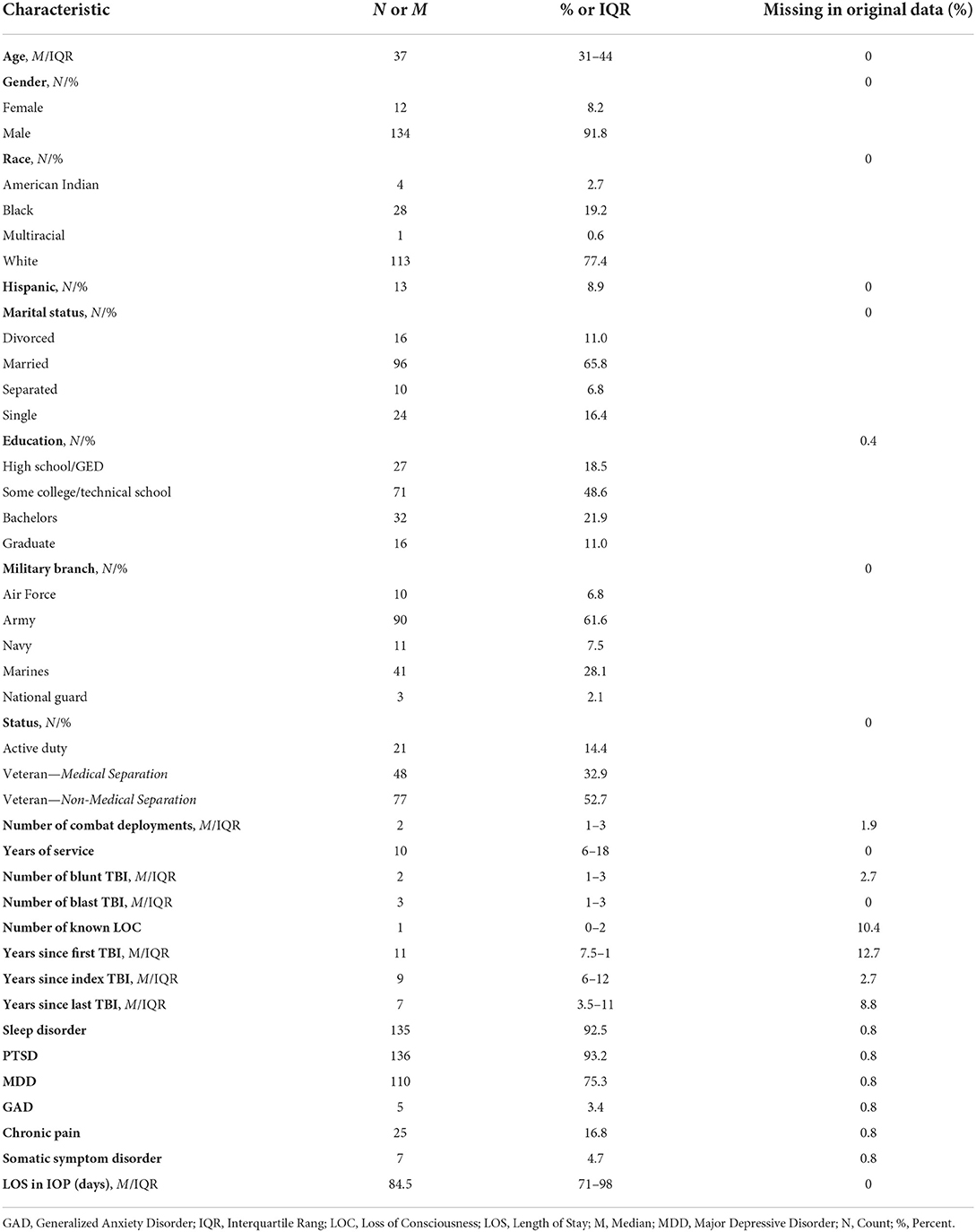
The sample included 146 participants who were mostly male (91.8%), with a median age of 37 (IQR 31–44; range 23–60). All participants served in the military post 9–11. Most were either members of the Army (61.6%) or Marines (28.1%) and were separated from service (85.6%). Participants served a median of 10 years (IQR 6–18; range 3–30) and reported a median of two combat deployments (IQR 1–3; range 0–14). The majority had history of multiple TBIs overall (84.4%), multiple blast-TBIs (66.9%), multiple blunt TBIs (51.9%), and at least one injury resulting in loss of consciousness (75.3%). Common co-occurring conditions included sleep disorders (92.5%), PTSD (93.2%), and major depressive disorder (75.3%). Length of program participation ranged from 31 to 148 days (median = 84.5; IQR: 71–98). See Table 2 for details on participant characteristics.
Goal attainment
The 146 participants set 281 goals of which 252 were met or exceeded (89.68%) by program discharge. GAS outcomes included goal attainment at much less than expected (0.4%), less than expected (10.4%), expected (32.3%), more than expected (37.3%), and much more than expected (19.65%). The mean T-Score was 57.7 (SD = 9.3) at discharge, indicating better than average goal attainment across participants.
Mean GAS T-Score increased from 57.7 (SD = 9.3) at discharge to 59.5 (SD = 8.2) at 6 months post-discharge, 59.7 (SD = 8.3) at 9 months post-discharge, and 62.8 (SD = 8.5) at 12 months post-discharge. The observed increase in attainment from discharge to 12 months post treatment was significant (β = 0.36, 95% CI: 0.18–0.54, Standardized β = 0.19, p < 0.001). This remained significant following adjustment for the covariates listed in Table 3 (β = 0.30, 95% CI: 0.13–0.46, Standardized β = 0.16, p < 0.001).
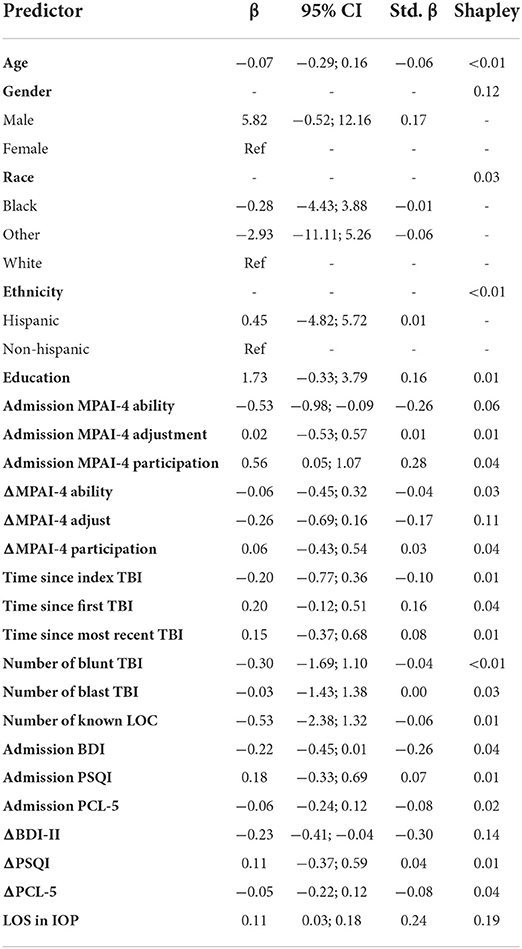
Table 3. Predicting discharge goal attainment measured by GAS as a function of demographic and clinical characteristics.
Goal attainment by category
A fixed-effects regression was used to evaluate the relationship between PCGGAS categories and attainment as measured by GAS (see Table 4). Goal categories were weighted-effects coded such that coefficients can be interpreted as the difference between a given goal category and the average goal category. PCGs related to being active in the community were associated with greater GAS improvement (OR = 5.16, 95% CI: 1.94–13.69, p =0.001). Improving fitness (OR = 0.37, 95% CI: 0.17–0.83, p = 0.02) and success at school (OR = 0.28, 95% CI: 0.14–0.59, p = 0.001) were associated with below average GAS achievement.
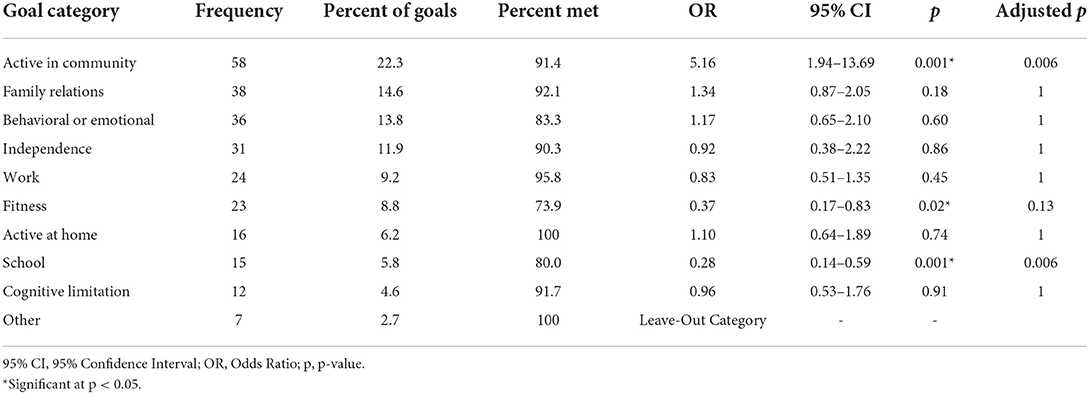
Table 4. Person-centered goal attainment by category type + fixed-effects regression predicting GAS scores as a function of goal categories.
Predictors of goal attainment
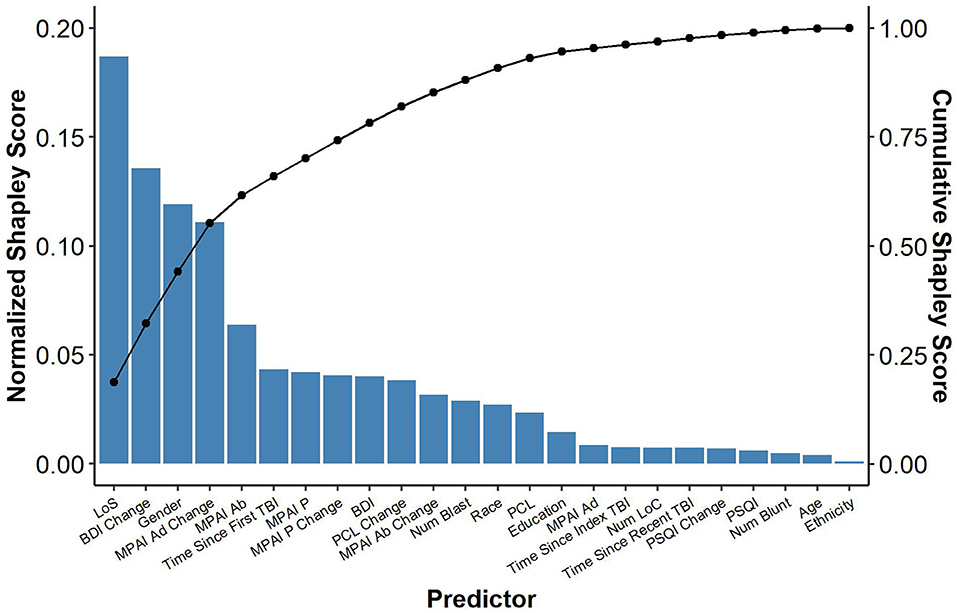
Participant length of stay (longer) in the program was the most important predictor in the model with longer lengths of stay resulting in greater goal attainment (Shapley = 0.19; β = 0.11, 95% CI = 0.03; 0.18, Std. β = 0.24). This was followed by change in BDI-II scores from admission to discharge (Shapley = 0.14; β = −0.23, 95% CI = −0.41; −0.04; Std. β = −0.30), gender (Shapley = 0.12; β = 5.82, 95% CI = −0.52; 12.16, Std. β = 0.17), change in MPAI-4 Adjustment from admission to discharge (Shapley = 0.11; β = −0.26, 95% CI = −0.69; 0.16, Std. β = −0.17), and MPAI-4 Ability measured at admission (Shapley = 0.06; β = −0.53, 95% CI = −0.98; −0.09, Std. β = −0.26) with greater improvement in depression and adjustment, male gender, and higher ability at admission resulting in greater goal attainment. Time since index TBI, MPAI-Participation measured at admission, change in MPAI-Participation from admission to discharge, BDI-II measured at admission, change in PCL from admission to discharge, change in MPAI-Ability form admission to discharge, number of blast injuries, patient race, and PCL measured at admission also made contributions but to a lesser degree. The remaining variables made relatively negligible contributions (see Table 3 and Figure 2).
Changes in symptoms and community participation
MPAI-4, BDI-II, BRIEF-A, DHI, HIT-6, PCL-5, POQ, and PSQI scores decreased between admission and discharge (all p < 0.001), indicating improvement in cognitive and physical abilities, adjustment, community participation, depression, executive function, dizziness, headache, post-traumatic stress symptoms, pain, and sleep. Similarly, RBANS and FGA scores increased between admission and discharge (all p < 0.004) demonstrating objective gains in cognitive function and dynamic gait and balance. All comparisons remained significant following the false discovery rate correction (all p < 0.01; see Table 5).
Discussion
This sample of SM/Vs with mTBI and co-occurring psychological conditions demonstrated PCG attainment following participation in intensive, interdisciplinary, PCG directed care. Most participants met or exceeded scaled expectations for PCGs using standardized GAS methodology.
Results demonstrate that goal attainment increased throughout the first year following IOP discharge. The present study design does not allow for evaluation of the impact of post-discharge transition support on outcomes, nor does it consider other healthcare services received in the year following treatment. Nevertheless, maintaining and even improving upon goal attainment after discharge suggests maintenance and translation of gains post treatment into home and community environments. Further, while maintenance of gains following intensive, interdisciplinary TBI rehabilitation has been previously demonstrated (13, 41), an increase in treatment gains has only previously been reported after intensive rehabilitation within 6 months of TBI (42).
The most important predictors of goal attainment at program discharge were longer length of stay in the treatment program greater reduction in depressive symptoms and greater improvement in adjustment at discharge, gender, and higher cognitive and physical abilities on admission.
Longer length of stay was the most important predictor of greater goal attainment. This is consistent with other studies examining moderate and severe TBI outcomes which found that longer treatment course and intensity of services positively correlate with improved function and increased community re-integration (43, 44). Repetitive task-specific training drives plasticity leading to improved outcomes in neurological populations (45), and a longer length of stay may facilitate the repetitions and practice needed to create change and make functional improvements. A longer stay may also permit more opportunities to practice use of compensatory strategies in different functional contexts, increasing potential for mastery.
Greater reduction in depressive symptoms and greater improvement in adjustment were also important predictors of greater goal attainment. Behavioral theories of depression posit a bidirectional relationship between mood and activity levels, such that those who are depressed engage in less rewarding activity, which in turn maintains or worsens depression (46, 47). The interdisciplinary rehabilitation included interventions and experiences known to improve depression and adjustment, such as Cognitive Behavioral Therapy skills (48), behavioral activation (49), community outings and engagement in leisure activities targeting self-efficacy and environmental reward (46), contact with supportive peers (50), and increased physical activity (51). Given the participation-oriented nature of many PCGs, it is possible that improvements in depression supported attainment, but also that simultaneously addressing cognitive, emotional, and physical mTBI symptoms that pose barriers to community participation and behavioral activation led to both goal attainment and improvement in mood and adjustment. Future prospective research could help ascertain the nature of the relations between change in overall psychological well-being and goal attainment.
Gender was identified as an important variable predicting goal attainment with male gender resulting in greater goal attainment. This finding may have limited generalizability given the small number of females in the sample. However, other studies have found poorer outcomes to be associated with female gender in chronic mTBI (52, 53) and more research is needed to understand the specific healthcare needs of females post mTBI. Higher cognitive and physical abilities as measured by the MPAI-4 Ability score at admission was also an important predictor. Given that higher cognitive functioning supports goal directed behavior (54), participants with higher cognitive abilities on admission may have been more engaged by the PCG directed care model, thereby leading to greater goal attainment. Higher cognitive functioning may also be supportive of greater success implementing other rehabilitation strategies and interventions (55). Both higher cognitive and physical abilities may be indicative of lower global impairment, thereby contributing to more favorable outcomes for those participants (56). Further, 52.3% of goals identified by participants targeted activity and participation (e.g., active in the community, fitness, and active at home) with active in the community being the most common category of goals set and the category associated with greatest goal attainment, and it is likely participants with greater abilities experienced fewer barriers to increasing activity and participation. Further study is necessary to determine specific subgroups and injury characteristics that benefit most from PCG and GAS focused management.
An exploratory analysis of clinical measures revealed participants also demonstrated improvements in measures assessing participation, as well as cognitive (e.g., memory, attention, executive function), psychological (e.g., depression, PTSD, sleep), and physical (e.g., pain, headaches, dizziness) impairment. These results cannot be interpreted as resultant of the treatment intervention given the lack of a control group. However, gains were significant in these pre-post comparisons. MPAI-4 results were in the mild to moderate impairment range on admission (40–50) and improved to the mild impairment range at discharge (30–40) (57). In other TBI studies, this degree of change correlated with improved independence, return to work, and goal attainment (16, 58). The observed increased PCG and improvements across symptom and participation measures are notable because all participants were experiencing chronic mTBI symptoms, many of them for years post injury, and the sample includes participants with potentially more severe injuries and/or comorbidities given that one-third were medically separated from service.
Study limitations
This study is marked by some limitations that should be noted in interpretation of these results. A limitation of the study design is a lack of control condition, and accordingly these findings do not definitively conclude that the intervention used resulted in the improvements noted. Improvements demonstrated in pre-post comparisons may be attributed to factors unrelated to the intervention, such as response to attention given by clinicians or spontaneous recovery. Further, generalizability of findings is limited by use of convenience sampling. Generalizability to other programs is also limited by the setting. While the SHARE IOP services SM/Vs, the program takes place in a civilian setting and may not be generalizable to military settings where SM/Vs more frequently seek care. Likewise, while the results may not be generalizable to non-military individuals with mTBI and co-occurring psychological conditions. Data for these analyses were obtained through retrospective chart review, and much was extracted from narratives in progress notes; therefore, it is probable that this data is incomplete. Injury count data was coded as, “0, 1, 2, 3 or more,” because often at the point of three or more of a type of TBI mechanism (blast, blunt force), it was unclear exactly how many were sustained. Many outcome measures were self-reported, which can introduce bias. Patient reported measures were collected by the participants' clinical providers, which may have introduced a reporting bias in which some participants may have rated their improvement as greater in order to please their providers. In addition, RBANS gains may be resultant of practice effects given the short time period between test administration. While the hypothesis tests included in the regression were conducted a priori, it did involve a large number of comparisons which may result in an elevated rate of false positives. Further, several predictors in the regression model were non-significant. While it is possible that these predictors do not contribute to goal attainment, it is also possible that the sample size resulted in insufficient power to detect these effects. The study sample was comprised predominantly of younger White males, and findings may not generalize to more diverse samples of SM/Vs. Finally, the impact of medications on treatment outcomes was not considered and could account for some of the variance in outcomes.
Conclusion
This sample of SM/Vs with mTBI and co-occurring psychological conditions demonstrated increased goal attainment following intensive, interdisciplinary, PCG directed care which further increased with transition support over the year post-discharge. Results suggest PGC goal directed care is a feasible, promising methodology of individualizing treatment in this population. The retrospective chart review design used in this study does not allow us to determine which components of the care model influenced outcomes, but this exploratory study lays a foundation for future prospective, controlled, comparative effectiveness research that can further our understanding of the effectiveness of intensive, interdisciplinary, PCG directed care. Participant goal attainment mirrored improvements demonstrated on traditional clinical measures of TBI outcomes, suggesting PCG directed care is a promising methodology of individualizing treatment in this complex patient population and should be further explored in both military and non-military mTBI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Shepherd Center Research Review Committee (RRC). A waiver of written informed consent was granted by the RRC, and abstracted medical records did not contain those who opted out of outcomes research at the time of registration.
Author contributions
TW and RG conceptualized the study. TW, AH, SP, and RG deigned the study. TW, AH, SP, and MW collected the data. TW, KM, AH, TM, SP, MW, and RG analyzed and interpreted the data and drafted the manuscript. TW, TM, and MW designed the tables and figure. All authors contributed to the article and approved the submitted version and agree to be accountable for the content of the work.
Funding
Open access publication fee funding provided by Shepherd Center.
Acknowledgments
We thank the generous donors, clinical staff, and operations staff at Shepherd Center's SHARE Military Initiative who supported the clinical programming described.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Department of Defense. DoD TBI Worldwide Numbers. (2022). Available online at: https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers (accessed June 15, 2022).
2. Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health. (2012) 4:147–54. doi: 10.1177/1941738111433673
3. Quinn DK, Mayer AR, Master CL, Fann JR. Prolonged postconcussive symptoms. Am J Psychiatry. (2018) 175:103–11. doi: 10.1176/appi.ajp.2017.17020235
4. Cooper DB, Bunner AE, Kennedy JE, Balldin V, Tate DF, Eapen BC, et al. Treatment of persistent post-concussive symptoms after mild traumatic brain injury: a systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging Behav. (2015) 9:403–20. doi: 10.1007/s11682-015-9440-2
5. Klyce DW, West SJ, Perrin PB, Agtarap SD, Finn JA, Juengst SB, et al. Network analysis of neurobehavioral and post-traumatic stress disorder symptoms one year after traumatic brain injury: a veterans affairs traumatic brain injury model systems study. J Neurotrauma. (2021) 38:3332–40. doi: 10.1089/neu.2021.0200
6. Polinder S, Cnossen MC, Real RGL, Covic A, Gorbunova A, Voormolen DC, et al. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol. (2018) 9:1113. doi: 10.3389/fneur.2018.01113
7. Peixoto C, Hyland L, Buchanan DM, Langille E, Nahas R. The polytrauma clinical triad in patients with chronic pain after motor vehicle collision. J Pain Res. (2018) 11:1927–36. doi: 10.2147/JPR.S165077
8. Cifu DX, Taylor BC, Carne WF, Bidelspach D, Sayer NA, Scholten J, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND Veterans. J Rehabil Res Dev. (2013) 50:1169–76. doi: 10.1682/JRRD.2013.01.0006
9. Ramanathan-Elion DM, Baydoun HA, Johnstone B. Psychological predictors of functional outcomes in service members with traumatic brain injury. Brain Inj. (2020) 34:1183–92. doi: 10.1080/02699052.2020.1793387
10. Mattson EK, Nelson NW, Sponheim SR, Disner SG. The impact of PTSD and mTBI on the relationship between subjective and objective cognitive deficits in combat-exposed veterans. Neuropsychology. (2019) 33:913–21. doi: 10.1037/neu0000560
11. Janak JC, Cooper DB, Bowles AO, Alamgir AH, Cooper SP, Gabriel KP, et al. Completion of multidisciplinary treatment for persistent postconcussive symptoms is associated with reduced symptom burden. J Head Trauma Rehabil. (2017) 32:1–15. doi: 10.1097/HTR.0000000000000202
12. Kontos AP, Collins MW, Holland CL, Reeves VL, Edelman K, Benso S, et al. Preliminary evidence for improvement in symptoms, cognitive, vestibular, and oculomotor outcomes following targeted intervention with chronic mTBI patients. Mil Med. (2018) 183:333–8. doi: 10.1093/milmed/usx172
13. DeGraba TJ, Williams K, Koffman R, Bell JL, Pettit W, Kelly JP, et al. Efficacy of an interdisciplinary intensive outpatient program in treating combat-related traumatic brain injury and psychological health conditions. Front Neurol. (2021) 11:580182. doi: 10.3389/fneur.2020.580182
14. World Health Organization. The International Classification of Functioning, Disability and Health (ICF). Geneva: WHO (2001).
15. Turner-Stokes L, Williams H, Johnson J. Goal attainment scaling: does it provide added value as a person-centred measure for evaluation of outcome in neurorehabilitation following acquired brain injury? J Rehabil Med. (2009) 41:528–35. doi: 10.2340/16501977-0383
16. Malec JF. Impact of comprehensive day treatment on societal participation for persons with acquired brain injury. Arch Phys Med Rehabil. (2001) 82:885–95. doi: 10.1053/apmr.2001.23895
17. Gerber GJ, Gargaro J. Participation in a social and recreational day programme increases community integration and reduces family burden of persons with acquired brain injury. Brain Inj. (2015) 29:722–9. doi: 10.3109/02699052.2015.1004745
18. Malec JF. Polytrauma transitional rehabilitation in the veterans administration: implementing the principles of person-centered, participation-oriented rehabilitation. J Head Trauma Rehabil. (2019) 34:135–40. doi: 10.1097/HTR.0000000000000456
19. Malec JF. Goal attainment scaling in rehabilitation. Neuropsychol Rehabilit. (1999) 9:253–75. doi: 10.1080/096020199389365
20. Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide [published correction appears in Clin Rehabil. 2010 Feb;24(2):191]. Clin Rehabil. (2009) 23:362–70. doi: 10.1177/0269215508101742
21. Jesus TS, Papadimitriou C, Bright FA, Kayes NM, Pinho CS, Cott CA. Person-centered rehabilitation model: framing the concept and practice of person-centered adult physical rehabilitation based on a scoping review and thematic analysis of the literature. Arch Phys Med Rehabil. (2022) 103:106–20. doi: 10.1016/j.apmr.2021.05.005
22. Turner-Stokes L, Rose H, Ashford S, Singer B. Patient engagement and satisfaction with goal planning: Impact on outcome from rehabilitation. Int J Therapy Rehabilit. (2015) 22:210–6. doi.org/10.12968/ijtr.2015.22.5.210 doi: 10.12968/ijtr.2015.22.5.210
23. Mild Traumatic Brain Injury Committee, American Congress of Rehabilitation Medicine, Head Injury Interdisciplinary Special Interest Group. Definition of mild traumatic brain injury. J Head Trauma Rehabilit. (1993) 8:86–7. doi: 10.1097/00001199-199309000-00010
24. Working Group to Develop a Clinician's Guide to Cognitive Rehabilitation in mTBI. Application for Military Service Members and Veterans. Clinician's Guide to Cognitive Rehabilitation in Mild Traumatic Brain Injury: Application for Military Service Members and Veterans. Rockville, MD: American Speech-Language-Hearing Association (2016).
25. Malec J. The Mayo-Portland Adaptability Inventory. The Center for Outcome Measurement in Brain Injury. (2005). Available online at: www.tbims.org/combi/mpai (accessed June 15, 2022).
26. Beck AT, Steer RA, Brown GK. Manual for The Beck Depression Inventory Second Edition (BDI-II). San Antonio: Psychological Corporation (1996).
27. Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function - Adult Version (BRIEF-A). Lutz, FL: Psychological Assessment Resources (2005).
28. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. (1990) 116:424–7. doi: 10.1001/archotol.1990.01870040046011
29. Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. (2004) 84:906–18. doi: 10.1093/ptj/84.10.906
30. Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia. (2011) 31:357–67. doi: 10.1177/0333102410379890
31. Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD. (2013). Available online at: www.ptsd.va.gov (accessed June 15, 2022).
32. Weathers FW, Litz BT, Huska JA, Keane TM. PCL-M for DSM-IV. Boston: National Center for PTSD—Behavioral Science Division (1994).
33. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
34. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. (2003) 40:381–95. doi: 10.1682/JRRD.2003.09.0381
35. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status Update (RBANS Update). Bloomington, MN: NSC Pearson (2012).
36. Moshier SJ, Lee DJ, Bovin MJ, Gauthier G, Zax A, Rosen RC, et al. An empirical crosswalk for the PTSD checklist: translating DSM-IV to DSM-5 using a veteran sample. J Trauma Stress. (2019) 32:799–805. doi: 10.1002/jts.22438
37. Lundberg SM, Lee SI. A unified approach to interpreting model predictions. In: Guyon I, von Luxburg U, Bengio S, Wallach HM, Fergus R, Vishwanathan SVN, et al., editors. Advances in neural information processing systems, 30. Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA (2017) p. 1–10.
38. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. (2001) 29:1165–88. doi: 10.1214/aos/1013699998
39. Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. (2015) 4:287–95. doi: 10.6000/1929-6029.2015.04.03.7
40. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020).
41. Sander AM, Roebuck TM, Struchen MA, Sherer M, High WM Jr. Long-term maintenance of gains obtained in postacute rehabilitation by persons with traumatic brain injury. J Head Trauma Rehabil. (2001) 16:356–73. doi: 10.1097/00001199-200108000-00006
42. High WM Jr, Roebuck-Spencer T, Sander AM, Struchen MA, Sherer M. Early versus later admission to postacute rehabilitation: impact on functional outcome after traumatic brain injury. Arch Phys Med Rehabil. (2006) 87:334–42. doi: 10.1016/j.apmr.2005.11.028
43. Rosenbaum AM, Gordon WA, Joannou A, Berman BA. Functional outcomes following post-acute rehabilitation for moderate-to-severe traumatic brain injury. Brain Inj. (2018) 32:907–14. doi: 10.1080/02699052.2018.1469040
44. Arango-Lasprilla JC, Ketchum JM, Cifu D, et al. Predictors of extended rehabilitation length of stay after traumatic brain injury. Arch Phys Med Rehabil. (2010) 91:1495–504. doi: 10.1016/j.apmr.2010.07.010
45. Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. (2009) 16:175–89. doi: 10.1002/oti.275
46. Carvalho J, Trent LR, Hopko DR. The impact of decreased environmental reward in predicting depression severity: support for behavioral theories of depression. Psychopathology. (2011) 44:242–52. doi: 10.1159/000322799
47. Trew JL. Exploring the roles of approach and avoidance in depression: an integrative model. Clin Psychol Rev. (2011) 31:1156–68. doi: 10.1016/j.cpr.2011.07.007
48. Cantor J, Ashman T, Dams-O'Connor K, Dijkers MP, Gordon W, Spielman L, et al. Evaluation of the short-term executive plus intervention for executive dysfunction after traumatic brain injury: a randomized controlled trial with minimization. Arch Phys Med Rehabil. (2014) 95:1–9.e3. doi: 10.1016/j.apmr.2013.08.005
49. Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. (2007) 27:318–26. doi: 10.1016/j.cpr.2006.11.001
50. Hibbard MR, Cantor J, Charatz H, Rosenthal R, Ashman T, Gundersen N, et al. Peer support in the community: initial findings of a mentoring program for individuals with traumatic brain injury and their families. J Head Trauma Rehabil. (2002) 17:112–31. doi: 10.1097/00001199-200204000-00004
51. Perry SA, Coetzer R, Saville CWN. The effectiveness of physical exercise as an intervention to reduce depressive symptoms following traumatic brain injury: a meta-analysis and systematic review. Neuropsychol Rehabil. (2020) 30:564–78. doi: 10.1080/09602011.2018.1469417
52. King NS. A systematic review of age and gender factors in prolonged post-concussion symptoms after mild head injury. Brain Injury. (2014) 28:1639–45. doi: 10.3109/02699052.2014.954271
53. Levin HS, Temkin NR, Barber J, Nelson LD, Robertson C, Brennan J, et al. TRACK-TBI Investigators. Association of sex and age with mild traumatic brain injury-related symptoms: a TRACK-TBI study. JAMA Network Ppen. (2021) 4:e213046. doi: 10.1001/jamanetworkopen.2021.3046
54. Hart T, Evans J. Self-regulation and goal theories in brain injury rehabilitation. J Head Trauma Rehabil. (2006) 21:142–55. doi: 10.1097/00001199-200603000-00007
55. Ownsworth T, Fleming J. The relative importance of metacognitive skills, emotional status, and executive function in psychosocial adjustment following acquired brain injury. J Head Trauma Rehabil. (2005) 20:315–32. doi: 10.1097/00001199-200507000-00004
56. Kean J, Malec JF, Altman IM, Swick S. Rasch measurement analysis of the Mayo-Portland Adaptability Inventory (MPAI-4) in a community-based rehabilitation sample. J Neurotrauma. (2011) 28:745–53. doi: 10.1089/neu.2010.1573
57. Malec JF, Kragness M, Evans RW, Finlay KL, Kent A, Lezak MD. Further psychometric evaluation and revision of the Mayo-Portland Adaptability Inventory in a national sample. J Head Trauma Rehabil. (2003) 18:479–92. doi: 10.1097/00001199-200311000-00002
Keywords: concussion, Traumatic Brain Injury, mental health, military, rehabilitation, goals, interdisciplinary, person-centered
Citation: Wallace TD, McCauley KL, Hodge AT, Moran TP, Porter ST, Whaley MC and Gore RK (2022) Use of person-centered goals to direct interdisciplinary care for military service members and Veterans with chronic mTBI and co-occurring psychological conditions. Front. Neurol. 13:1015591. doi: 10.3389/fneur.2022.1015591
Received: 09 August 2022; Accepted: 26 October 2022;
Published: 16 November 2022.
Edited by:
Nada Andelic, University of Oslo, NorwayReviewed by:
Paul Perrin, University of Virginia, United StatesJuan Lu, Virginia Commonwealth University, United States
Laraine Winter, United States Department of Veterans Affairs, United States
Copyright © 2022 Wallace, McCauley, Hodge, Moran, Porter, Whaley and Gore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tracey D. Wallace, dHJhY2V5LndhbGxhY2VAc2hlcGhlcmQub3Jn
 Tracey D. Wallace
Tracey D. Wallace Katherine L. McCauley
Katherine L. McCauley April T. Hodge1
April T. Hodge1 Tim P. Moran
Tim P. Moran Russell K. Gore
Russell K. Gore