- 1Department of Neurology and Intensive Care Medicine, Schoen Clinic Bad Aibling, Bad Aibling, Germany
- 2German Center for Vertigo and Balance Disorders, Ludwig-Maximilians University (LMU), Munich, Germany
- 3Pettenkofer School of Public Health, Institute for Medical Information Processing, Biometry, and Epidemiology, Ludwig-Maximilians University (LMU), Munich, Germany
Background: The COVID-19 disease frequently causes neurological symptoms. Critically ill patients often require neurorehabilitation for manifestations like intensive care unit (ICU) acquired weakness or encephalopathy. The outcome of these patients, however, is largely unknown. Here we report the clinical course of critical affected COVID-19 patients from hospital admission to discharge from inpatient neurorehabilitation.
Methods: Prospective cohort study. COVID-19 patients admitted to neurorehabilitation were included based on a laboratory-confirmed SARS-CoV-2 infection. Assessments [modified Rankin Scale (mRS), Barthel-Index, Fatigue-Severity-Scale-7 and health-related quality of life (EQ-5D-5L)] were conducted at admission and before discharge from inpatient care. Data were compared to the preclinical health status.
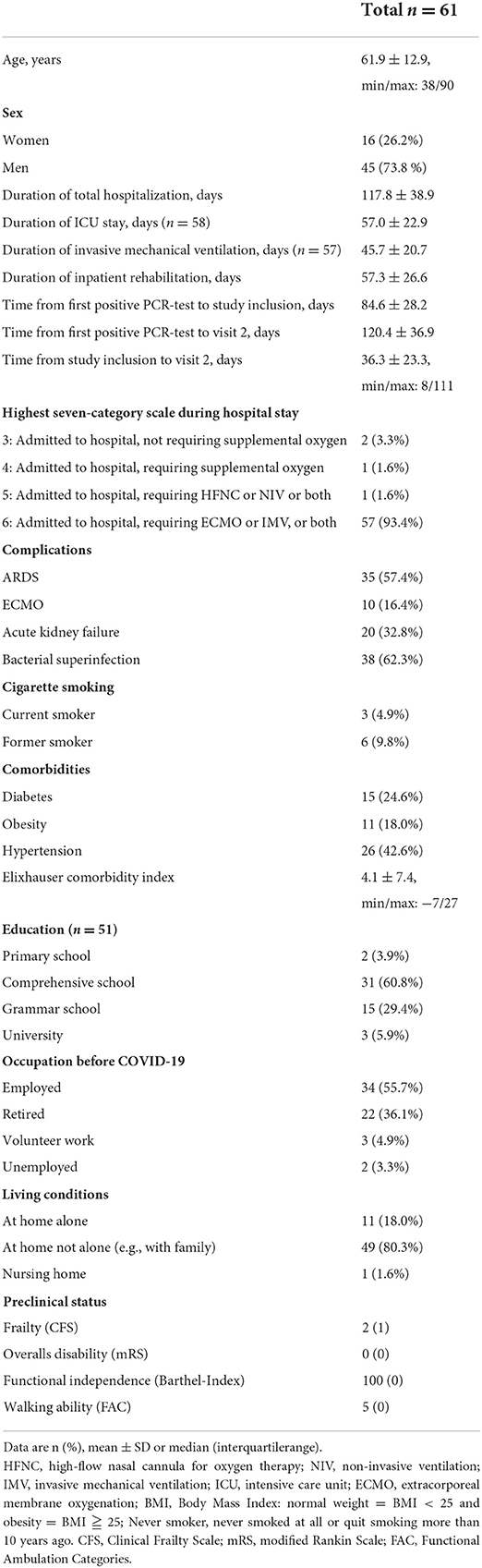
Results: Sixty-one patients (62 ± 13 years, 16 female) were included in the analysis. Most patients had been treated on ICU (n = 58; 57 ± 23 days) and had received invasive ventilation (n = 57; 46 ± 21 days). After discharge from ICU, patients spent on average 57 ± 26 days in neurorehabilitation. The most frequent neurological diagnoses were ICU-acquired weakness (n = 56) and encephalopathy (n = 23). During rehabilitation overall disability improved [mRS median (IQR) 4.0 (1.0) at inclusion and 2.0 (1.0) at discharge]. However, the preclinical health state [mRS 0.0 (0.0)] was not regained (p < 0.001). This was also reflected by the Barthel-Index [preclinical 100.0 (0.0), at inclusion 42.5 (35.0), at discharge 65.0 (7.5); p < 0.001]. Patients had only minor fatigue during inpatient care. Quality of life generally improved but was still low at discharge from hospital.
Conclusion: Patients with neurological sequelae after critical COVID-19 disease showed substantial deficits at discharge from inpatient care up to 4 months after the initial infection. They were restricted in activities of daily living and had reduced health-related quality of life. All patients needed continued medical support and physical treatment.
Introduction
The Corona pandemic has resulted in millions of infections with SARS-CoV-2 worldwide and continues to cause numerous new infections. By August 2022, more than 6.4 million people had died in association with SARS-CoV-2 infection (https://covid19.who.int/, as of August 04, 2022). Although many patients recover within days to weeks, a substantial proportion develops long-standing symptoms (long-COVID) ranging from mild fatigue and reduced physical fitness to immobility and long-term disability. The presumed number of patients affected by long-COVID ranges from 2.3 to 53.0% in the current literature (1). Different parameters, such as multiple organ involvement during the acute phase of the disease, persistent reservoirs of the virus in different tissues, as well as manifestation in the central nervous system and immune system dysregulation, are hypothesized to contribute to symptom persistence (2, 3).
Neurological and neuropsychiatric complications of SARS-CoV-2 infections frequently occur, with the utmost prevalence reported for anosmia (43.1%), muscular weakness (40.0%), and fatigue (37.8%). Other common neurologic or neuropsychiatric symptoms include headache, dysgeusia, myalgia, depression as well as sleep disorder (4). Within a study including nearly 5,000 hospitalized patients with COVID-19, a 38% increased hazard of in-hospital death and a decreased likelihood of discharge home among patients diagnosed with a neurological disorder was reported (5). The pooled prevalences of severe complications such as stroke (2%) and encephalopathy (7%) might be lower (6), however, they can cause substantial disability and often trigger neurorehabilitation. Furthermore, patients with critical COVID-19 disease and prolonged invasive ventilation are at high risk of developing neuromuscular weakness. This was shown in an observational study in 110 critically ill patients with COVID-19 treated in the intensive care unit (ICU). All patients showed ICU-acquired weakness (ICUAW) on awakening (7). In another study with patients requiring intubation due to COVID-19, neurologic outcomes were investigated 3 and 6 months after ICU discharge. Cognitive impairment, muscle weakness, and psychologic symptoms were frequent and 74% still required follow-up interventions like physiotherapy or neuropsychological therapy (8). These cases emphasize the necessity of neurorehabilitation in most cases (9, 10). However, studies about rehabilitation after COVID-19 often focused on pulmonary rehabilitation, reported on rehabilitation in less severely affected patients, investigated therapies in patients during the acute phase or with community-dwelling participants or outpatients (11–15).
The rehabilitation of critically ill patients after COVID-19 with neurologic symptoms has not yet been described. Therefore, the objective of this study was to describe the clinical course of critically ill patients with COVID-19 and neurological sequelae during inpatient neurorehabilitation. We hypothesized that patients would show incomplete recovery and that disability and fatigue at discharge can be predicted by the severity of the disease, i.e., the length of stay on ICU.
Methods
Study population
Patients for this prospective cohort study were recruited at one of the largest neurorehabilitation centers in Germany (Schoen Clinic Bad Aibling). Adult patients (≥18 years) with laboratory-confirmed COVID-19 (nasal or pharyngeal swab for SARS-CoV-2, evaluated by real-time reverse transcriptase-polymerase chain reaction (PCR) assay) were included after being tested non-infectious (two negative PCR-tests) and discharged from the ICU. Exclusion criteria were insufficient communication skills (that would interfere with the accomplishment of the questionnaires and assessments) and patients treated with a main diagnosis different than COVID-19 during hospitalization. Data represent the interim analysis of an ongoing larger cohort study with follow-up assessments up to 1 year after hospital discharge. Patients who completed the first two study visits (at study inclusion and at hospital discharge) are entailed in this analysis. All patients received at least 100 min per day of neurorehabilitation therapies including physiotherapy (gait rehabilitation, aerobic, endurance and resistance training, balance training, physical therapy etc.), occupational therapy (training of gross and fine motor skills of the upper extremities, training of activities of daily living like grooming, dressing, using the toilet, resistance training, treatment of sensory deficits etc.) dysphagia therapy, respiratory therapy and neuropsychology (therapy for deficits of attention, concentration, processing speed, memory and executive functions, supportive conversations for coping with the illness and relaxation training). Treatment distribution was tailored to individual patient necessities and therapies were conducted in single or group settings.
Standard protocol approvals, registrations, and patient consents
The study was approved by the medical ethics committee of the Ludwig-Maximilians University of Munich (project no. 20-0478) and the study conforms with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all participants (or their legal guardians). The study was registered at the German Clinical Trials Register (No. DRKS00025606).
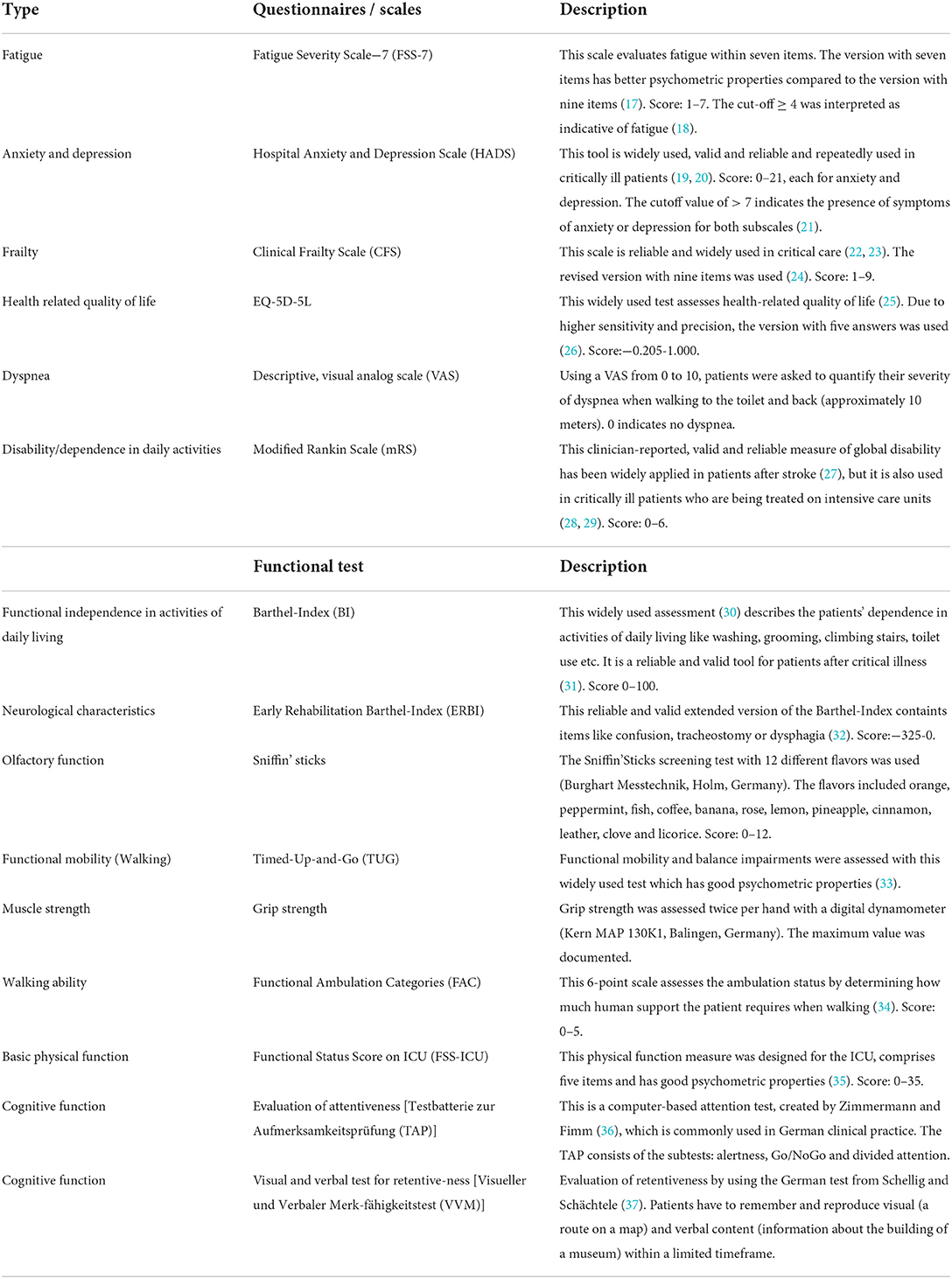
Procedures, scales and scores
Disease severity was categorized by the Seven-Category Ordinal Scale (16). The first two categories describe patients not being hospitalized, categories three to six comprise patients being hospitalized with increasing disease severity (e.g., need for non-invasive mechanical ventilation) and the seventh category includes death.
During rehabilitation, two study visits were conducted. The first visit (visit 1) was scheduled at study inclusion after patients had been transferred from ICU to the early neurorehabilitation unit of our hospital. The second visit was conducted at discharge from inpatient care (visit 2). Study visits included comprehensive questionnaires and tests (Table 1). The study visits were predominantly conducted by a physiotherapist (M.Sc.) with 6 year experience in the conduction of clinical trials (ME) or by a medical student after 5 years of medical school (CW).
In order to comprehensively determine the patient's clinical course, we retrospectively assessed the preclinical status with regard to preclinical health status, walking ability and frailty. Furthermore, level of education, living and working conditions were recorded. This information was collected during the personal interview of visit 1. Data regarding symptom onset, ICU length of stay, duration of invasive mechanical ventilation, neurological diagnoses as well as pre-existing diseases, Barthel-Index (BI), and Early Rehabilitation Barthel-Index (ERBI) were extracted from patient's health records (32).
Cognitive evaluation was conducted by experienced neuropsychologists (see Table 1). Spirometry was performed to evaluate pulmonary function and to identify restrictive lung diseases.
Medical records were screened for complications and neurological diagnoses, symptoms, and syndromes (e.g., ICUAW, peripheral neuropathy, critical illness polyneuropathy (CIP), critical illness myopathy (CIM), delirium, tetraparesis, dysphagia). The diagnosis ICUAW was defined “as the acute development of generalized weakness in a critically ill patient that cannot be explained by other causes” (38, 39). It encompasses pathologies including critical illness polyneuropathy (CIP), critical illness myopathy (CIM), the combination critical illness neuromyopathy (CINM) and / or muscle atrophy. The diagnose ICUAW based on the medical reports of our or the referring hospital, where the diagnose was set either by the clinical manifestation (weakness, atrophy) or by an electrophysiological investigation. If the patient was transferred from another hospital to ours, we validated the ICUAW diagnose by a clinical investigation of muscle strength (mean strength ≤ 4/5 according to the MRC scale). In inconclusive cases, electrophysiological investigations were conducted (including nerve conduction studies and electromyography were appropriate).
Statistical analysis
Clinical characteristics are presented as absolute values and percentages, as mean values and standard deviations or as median and interquartile range, as appropriate.
Cognitive impairment was evaluated in percentages according to normative age-dependent values. If the subtest results in the test for attentiveness (TAP) were below 16%, the patient was classified as having limitations concerning attentiveness. If the result of the test for retentiveness (VVM) was below 16%, the patient was classified as having memory and retentiveness limitations. If one result of both test parts was noticeably low, a patient was classified as having general cognitive limitations.
The pulmonary function tests were evaluated to classify the grade of restrictive lung disease. Forced vital capacity (FVC) was set in relation to normative age-dependent values. Percentages ≤ 40% were classified as severe, 41–60% as moderate, 61–80% as light and >80% as no restrictive lung disease. Additionally, FEV1 (forced expiratory volume in 1 s) divided by FVC was used to exclude obstructive lung diseases (with formula values >70%).
For the comparison of symptoms between visit 1 and visit 2 paired t-tests were used for interval scaled values, Wilcoxon tests were applied for ordinal scaled values. Friedman-tests with post-hoc analysis via Dunn-Bonferroni test (including corrected p-values) were used for categorical values and comparisons of more than two time points.
A binary logistic regression was calculated to analyze predictors for a high degree of disability and dependence in daily activities (mRS ≥3) at discharge. The independent variables were entered hierarchically: Model 1: age, gender; Model 2: Elixhauser Comorbidity Index, diabetes; Model 3: length of invasive mechanical ventilation. Another hierarchical binary logistic regression model was applied to investigate coefficient predicting whether a subject developed severe fatigue (FSS-7 ≥4 (18)): Model 1: age, gender; Model 2: Elixhauser Comorbidity Index, diabetes; Model 3: length of total hospitalization. A linear multiple regression analysis was used to investigate predictors for hospitalization length. The independent variables were entered hierarchically: Model 1: age, gender; Model 2: Elixhauser Comorbidity Index, diabetes, smoking (within the last 10 years); Model 3: preclinical frailty (CFS).
Statistical analyses were performed using IBM SPSS Statistics 19. A p < 0.05 was considered significant. Missing data were not replaced.
Results
Study population and disease severity
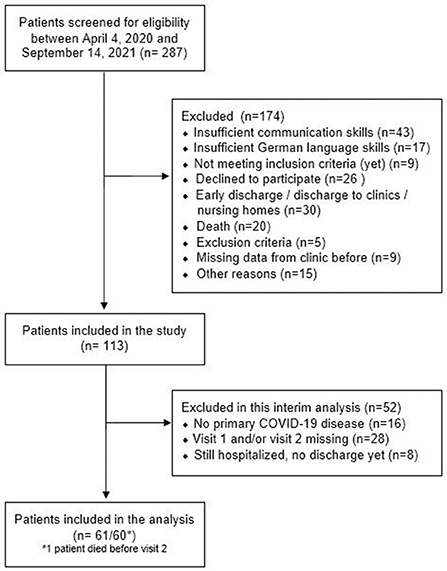
Of the 287 patients with COVID-19 admitted to our hospital between April 2020 and September 2021, 113 were enrolled in the study and 61 were included in the current interim analysis. Reasons for exclusion are shown in Figure 1. Patients were included in the study on average 84.6 ± 28.2 days and performed visit 2 on average 120.4 ± 36.9 days after their first positive PCR test.
Patients in our study were profoundly affected by the disease: They overwhelmingly needed long-term critical care therapy (mean ICU length of stay 57.0 ± 22.9 days) with prolonged invasive mechanical ventilation (duration of mechanical ventilation: 45.7 ± 20.7 days) or even extracorporeal membrane oxygenation (ECMO) therapy (16.4%). The length of neurologic rehabilitation after ICU discharge added up to an average of 57.3 ± 26.6 days. Table 2 displays the detailed clinical characteristics of the study population.
Pulmonary dysfunction
Forty-seven patients underwent a lung function test at study inclusion (87.5 ± 31.4 days after the first positive PCR test), of which four had to be excluded due to a lack of cooperation. Of the remaining 43, only two showed signs of obstructive lung disease, whereas most patients (n = 31; 72.1%) were diagnosed with restrictive lung disease of varying severity [light: n = 16 (37.0%); moderate: n = 12 (27.9%); severe: n = 3 (7%)]. Only 10 patients (23.2%) displayed a normal lung function test.
Neurological disorders and cognitive impairment after severe COVID infection
All patients had severe neurological deficits requiring intense neurological rehabilitation. ICUAW (CIP/CIM) was the hallmark diagnosis (n = 56; 91.8%), followed by delirium in 19 (31.1%), and encephalopathy in 11 patients (18.0%). Other neurological diagnoses were cerebral ischemia (n = 5; 8.2%), epileptic seizures (n = 4; 6.6%), and Guillain-Barré-Syndrome (n = 2; 3.3%).
In accordance with the high prevalence of CIP/CIM, most patients suffered from incomplete tetraparesis. Other common symptoms were dysphagia (n = 28; 45.9%), hypoesthesia/paresthesia/neuropathic pain (n = 9; 14.8%), paresis (n = 7; 11.5%; hemiparesis, facial palsy or monoparesis due to peripheral nerve lesions), and tremor (n = 2; 3.3%).
At study inclusion, 47 participants underwent cognitive testing, with n = 36 (76.6%) showing cognitive impairments. Deficits in the memory and retentiveness component were apparent in n = 26 of 44 participants (59.0%). Regarding the attentiveness component (conducted in 46), n = 26 (56.6%) showed deficits. Problems in both components were apparent in n = 16 (37.2%, n = 43 completed both tests). The 14 remaining patients were either not able to perform the tasks of cognitive testing due to language barriers or due to an insufficient functional ability to use the computer or to hold a pencil.
Clinical course and health status at discharge
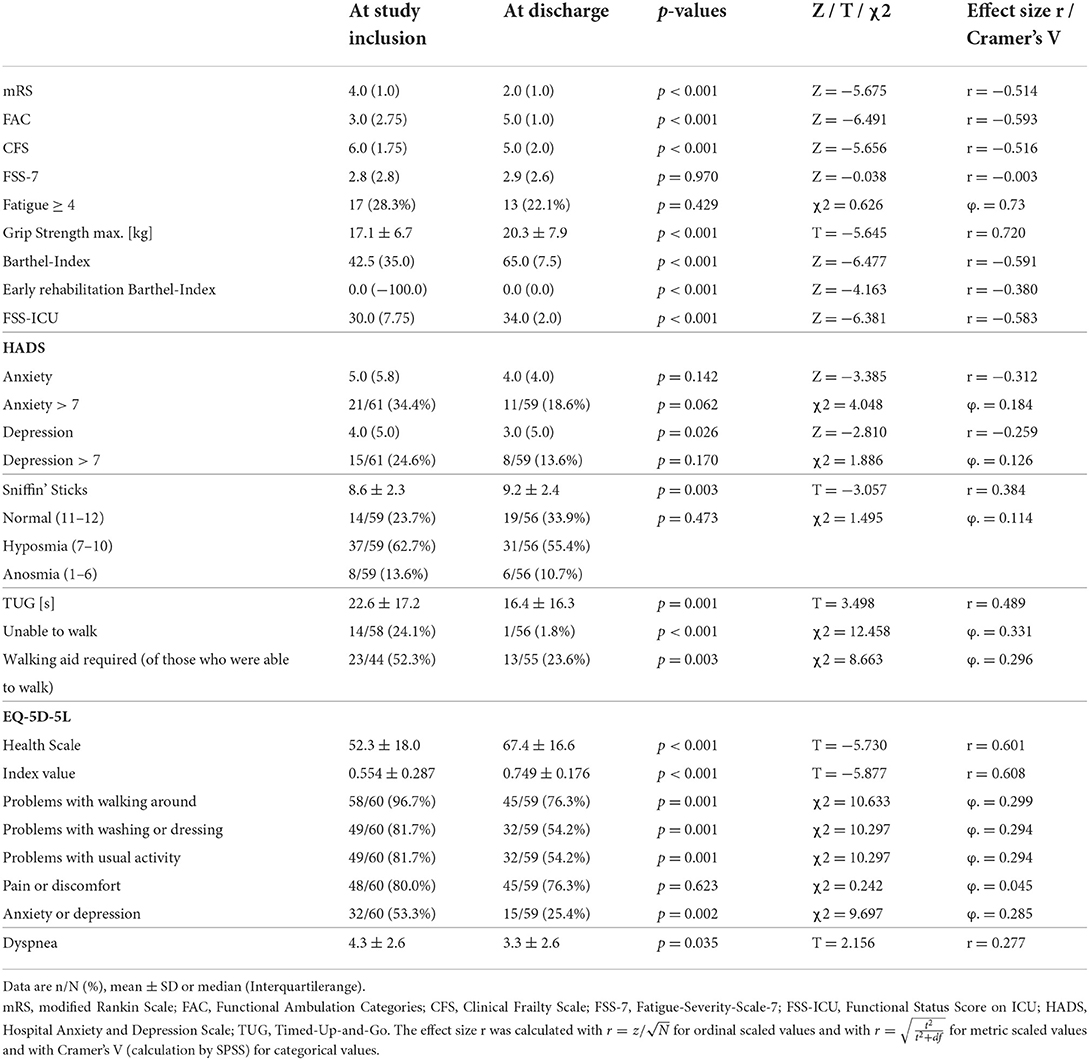
Results of assessments and questionnaires are shown in Table 3. From study inclusion to discharge, patient's health status significantly improved in all measured categories except for fatigue and anxiety. Figure 2 illustrates this for the mRS, where most of the patients improved their status [at admission most patients were classified into category 3 (38%) or 4 (36%), whereas at discharge most patients improved to category 2 (51%) and 3 (36%)]. However, health status and body function remained substantially reduced at discharge, represented by reduced mobility, restricted independence in activities of daily living, muscle strength, and breathing. Furthermore, impairments were observed for the olfactory sense with 37/56 (66.1%) being impaired, and pain/ discomfort with 45/60 (75%) being affected. Altogether, patients' health-related quality of life improved significantly during neurorehabilitation, but was still impaired at discharge.
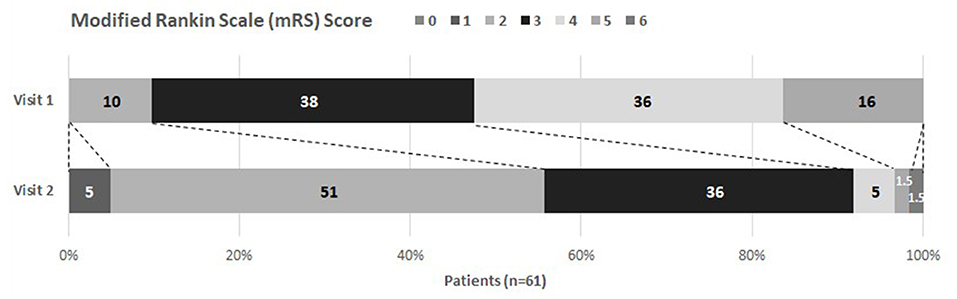
Figure 2. Modified Rankin Scale at visit 1 and 2. Visit 1 took place at study inclusion, visit 2 at discharge from inpatient care. Percentages of each category are given. A shift of mRS values can be noticed (depicted with dotted lines) showing improvement between visits.
Figure 3 shows a significant improvement of the BI from initial admission (median 17.5; IQR 30.0) via visit 1 (median 42.5; IQR 35.0) to visit 2 (median 65.0; IQR 7.5) [χ2(3) = 167.7; p < 0.001]. Notably, there was still a significant gap regarding the BI between the patients' condition at hospital discharge and their preclinical condition (median 100.0; IQR 0.0, p < 0.001). Post-hoc analysis revealed significant differences between all measurements of the BI.
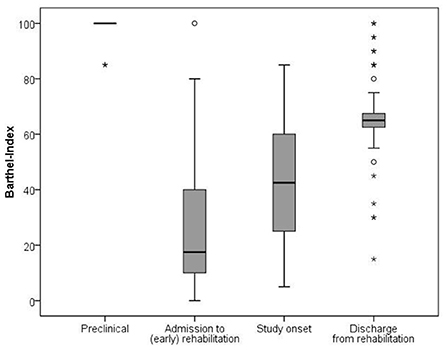
Figure 3. Development of the Barthel Index from preclinical state throughout rehabilitation. The comparison between the four time points showed significant differences and large effect sizes (Friedman-test and effect size with r = z/√N): preclinical-hospital admission (p < 0.001, r = −0.612); preclinical-study onset (p < 0.001, r = −0.616); preclinical-hospital discharge (p < 0.001, r = −0.615); hospital admission-study onset (p = 0.035; r = −0.487); hospital admission-hospital discharge (p < 0.001, r = −0.600); study onset-hospital discharge (p < 0.001, r = −0.591).
As shown in Figure 4, the condition of the patients was still limited at hospital discharge in comparison to the preclinical state. The FAC showed complete independence in preclinical walking but a significantly restricted ability at discharge (Z = −3.862, p < 0.001). As presented with the CFS, patients did not achieve their preclinical state of frailty (Z = −6.570, p < 0.001). The mRS underlines the overall significantly impaired health state at discharge (Z = −6.885, p < 0.001).
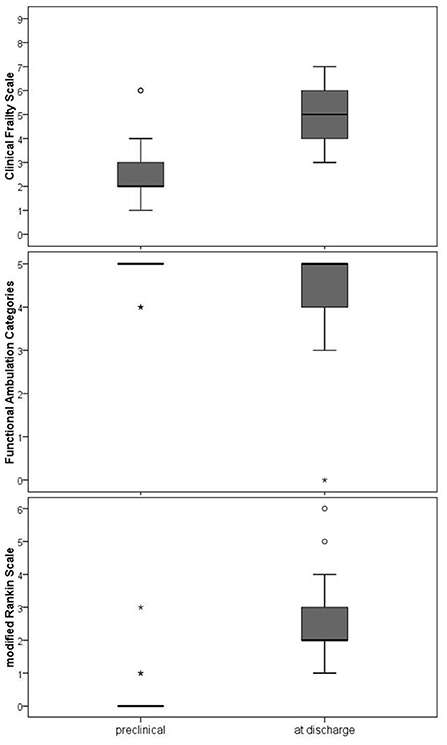
Figure 4. Walking ability, frailty and overall disability at clinical discharge compared to the preclinical condition. FAC (Functional Ambulation Categories), Frailty (Clinical Frailty Scale), mRS (modified Rankin Scale). The Wilcoxon test was used to compare the preclinical state and the state at discharge. All three comparisons differed statistically significant (p < 0.001).
Forty-four patients (73.3%) were discharged to their homes, 14 (23.3%) were discharged to another rehabilitation facility (not to hospitals), and 2 (3.3%) returned to their nursing home and assisted living facility. Among those participants employed before disease onset, all were discharged as incapable of working.
Predictors for disability, fatigue and hospitalization length
Regression analyses did not result in significant models (p > 0.18); none of the coefficients was found to be predictive for the disability at discharge, fatigue at discharge, or the length of hospitalization (p > 0.10).
Medical and assistive devices
At study inclusion, 15 patients (25.0%) needed oxygen, 15 (25%) had a permanent bladder catheter, 13 (21.7%) required a tracheal tube, 4 (6.7%) had a percutaneous endoscopic gastrostomy, 3 (5.0%) needed an ileostomy, 2 (3.3%) had a nasogastric tube, and 1 (1.7%) required negative pressure wound therapy.
At discharge, 7 participants (11.7%) still needed oxygen, 6 (10%) had a permanent bladder catheter, and 2 (3.3%) needed an ileostomy.
At discharge, the majority of participants still needed assistive devices for their activities of daily living. Only 14 participants (23.3%) did not require any aids and appliances. Most frequently used were walker-rollators [33 (55.0%)], wheelchairs [16 (26.7%)], toilet and shower chairs [14 (23.3%)], ankle-foot orthosis [7 (11.7%)], nursing beds [6 (10.0%)], bathroom handles [6 (10.0%)] and oxygen concentrators [5 (8.3%)].
Discussion
We here report on the clinical course in a cohort of the most severely affected patients with COVID-19 disease who required long-term inpatient care and rehabilitation because of neurological deficits. After the ICU-treatment, patients showed mainly muscular weakness (ICUAW, CIP/CIM) and cognitive deficits (delirium, encephalopathy). Our main findings are: (1) Patients with critical COVID-19 spent on average 4 months in hospital; most of them showed relevant muscular weakness due to ICUAW. (2) Patients improved over time, but still suffered from substantial deficits at discharge. Patients in general did not reach the preclinical health status, as indicated by the overall disability (mRS) and frailty (CFS). (3) We could not identify any predictors for the degree of disability and fatigue at discharge or for the length of hospitalization.
Previous studies reported on benefits and effectiveness of rehabilitation in patients after COVID-19. However, these studies focused on less severely affected patients, shorter rehabilitation periods, earlier rehabilitation phases, less intensive rehabilitation, or other organ systems (e.g., pulmonary rehabilitation) (11, 12, 40, 41).
Despite the long duration of rehabilitation and total hospitalization in our cohort, the functional and health status at discharge was worse compared to other studies (12, 40, 41). This most likely reflects the prolonged treatment on ICU with mechanical ventilation in our sample. The maximum number of days on ICU reported before were 18–22 days (40, 42, 43).
The impaired health status at discharge is clearly represented by the gap in mRS and the CFS between the preclinical state and the state at discharge. Both assessments, as well as the BI, indicate the patients' need for help in nearly all their activities of daily living (ADL). This clearly affects their level of independence. Limitations in ADLs after the acute phase of COVID-19 were previously reported (44). Our results show that ADL limitations after critical COVID-19 disease last for a prolonged period of time. Furthermore, health-related quality of life was still substantially impaired compared to a sample of healthy German seniors, although we found a significant improvement over time (45). Our values in this domain were also lower than those reported in a study on 47 patients after COVID-19 who required mechanical ventilation for a median of 12 days. In that study, the VAS in the EQ-5D-5L was 80 (70–90) 3 months after hospital admission and only 40% reported problems in the dimension of mobility (compared to 75% in our cohort) (46). This difference again can be explained by the critical course of disease with a prolonged length of hospitalization in our cohort. Our EQ-5D-5L results are in a similar range as reported for (1) patients with chronic conditions like cardiovascular disease or depression (VAS: 64±23) (47) and (2) general critical illness survivors with a median length of 10 days for mechanical ventilation [e.g., VAS: 64 ± 23; index: 0.73 (IQR = 0.3)] (48). However, those patients reported higher values of anxiety (as measured by the HADS; median = 7.0) compared to our patients (median = 4.0). This might be explained by feelings of relief and gratefulness for surviving COVID-19, which were frequently mentioned by our participants. In contrast, reported depression values were quite similar to the results of our study (48). Our values for anxiety and depression are in accordance with HADS values reported in a cohort ~4 months after hospital admission due to COVID-19 (median duration of mechanical ventilation: 19 days) (42).
Regarding fatigue, we expected higher values in our group of critically ill patients, similar to reported values in several studies on patients post-COVID (4). In a meta-analysis on hospitalized patients, 38.4% suffered from fatigue (95% CI 30.4–47.4) over 90 days after symptom onset (49). The mild fatigue score in our group (noticeable in only ~25%) might be explained by the fact that our patients were still in a hospital setup without the challenging responsibilities, duties or long-lasting physical activities required for ADL.
Our results show that even after an average of >100 days of hospitalization including >50 days of intensive neurorehabilitation, the health state and functional capacity after severe COVID-19 disease is limited. Therefore, a long-term disability can be anticipated, especially when considering the sequelae reported in less severely affected patients. Huang et al. (50) reported in a trial on 1,733 hospitalized patients post COVID-19 that 76% experienced at least one symptom like fatigue or muscle weakness, sleep difficulties, anxiety and depression 6 months after infection. This percentage increased to 86% in a subgroup of patients who needed (non-)invasive ventilation (50). Within a cohort of 246 ICU survivors after critical COVID-19, Heesakkers et al. (43) reported than 74.3% experienced physical symptoms, 26.2% experienced mental symptoms and 16.2% experienced cognitive symptoms even 1 year after ICU treatment (43). Therefore, further evaluations of symptoms and their impact on activity and participation in daily life are urgently needed.
Our study has some limitations. First, a non-COVID-19 control group with similar motor and cognitive deficits would have been of value to compare outcomes. It is not clear how specific our findings are for COVID-19. Furthermore, evaluation of lung function, cognitive impairment and nerve conduction studies were only conducted once. Thus, no assertions can be made about their potential improvement during rehabilitation. Additionally, a high number of screened patients were not included in the study (226 of 287) mainly due to insufficient communication skills. Furthermore, 19 patients were excluded, because we were only able to conduct one study visit due to a rapid discharge (~ 1–5 days) after the first study visit. We did an analysis to investigate the characteristics of those 19 excluded patients compared to the 61 included patients. Patients who were excluded were significantly younger (mean age 54.3 vs. 61.9 years), had significantly less comorbidities, were significantly shorter on ICU (44.1 vs. 57.0 days), had significantly less days of mechanical ventilation (26.9 vs. 45.7 days) and had a significantly shorter duration of complete hospitalization (78.6 cs. 119.4 days). However, both patient groups did not significantly differ in their health status at discharge regarding any assessment (e.g., Barthel Index, HADS, EQ-5D-5L, mRS, CFS, FSS-ICU, FAC, FSS-7). It can be concluded, that younger patients with a better preclinical health status required less intensive care and recovered faster. However, their health status at discharge was as limited as the health status of patients with a longer hospitalization period and a worse preclinical health status. Finally, single-center studies bear the risk of bias, which for example becomes apparent as our center included only critically affected patients.
In summary, our findings stress the need for intensive neurorehabilitation in patients with severe neurological symptoms after critical COVID-19 disease. We cannot determine the specific effect of rehabilitation. However, deficits are pronounced and do not resolve on a short time scale. We report substantial and long-lasting limitations regarding the general health status, dependence in ADL and health related quality of life even at discharge. As persistent limitations after critical COVID-19 disease are a socioeconomic and medical challenge, further research characterizing the neurological aspects of the pandemic disease and developing tailored rehabilitation and home care programs is of paramount interest.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Medical Faculty of the Ludwig-Maximilians University of Munich. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CW: data curation (equal), formal analysis (equal), investigation (equal), resources (support), visualization (equal), writing—original draft preparation (equal), and writing—review and editing (equal). ME: conceptualization (equal), data curation (equal), formal analysis (equal), funding acquisition (support), investigation (lead), methodology (lead), project administration (support), resources (lead), validation (equal), visualization (equal), writing—original draft preparation (equal), and writing—review and editing (equal). JB: formal analysis (equal), funding acquisition (lead), methodology (support), project administration (lead), validation (equal), and writing—review and editing (equal). VH: validation (equal), resources (support), writing—original draft (support), and writing—review and editing (equal). FM: conceptualization (equal), methodology (support), supervision (support), validation (equal), and writing—review and editing (equal). KJ: conceptualization (equal), funding acquisition (support), methodology (support), project administration (lead), supervision (lead), validation (equal), visualization (support), writing—original draft preparation (equal), and writing—review and editing (equal). All authors contributed to the article and approved the submitted version.
Funding
This work was partly funded by the Else Kröner-Fresenius-Stiftung (2021_EKEA.78).
Acknowledgments
The authors thank Dr. Thomas Weber for the help with the appraisal of the lung function data. We thank Dipl.-Psych. Josef Metsch and team for the selection of suitable cognitive tests and the help with the interpretation of their results. Furthermore, we thank Katie Göttlinger for copy-editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nittas V, Gao M, West EA, Ballouz T, Menges D, Wulf Hanson S, et al. Long COVID through a public health lens: an umbrella review. Public Health Rev. (2022) 43:1604501. doi: 10.3389/phrs.2022.1604501
2. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12: 698169. doi: 10.3389/fmicb.2021.698169
3. Moghimi N, Di Napoli M, Biller J, Siegler JE, Shekhar R, McCullough LD, et al. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep. (2021) 21:44. doi: 10.1007/s11910-021-01130-1
4. Rogers JP, Watson CJ, Badenoch J, Cross B, Butler M, Song J, et al. Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry. (2021) 92:932–41. doi: 10.1136/jnnp-2021-326405
5. Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. (2021) 96:e575–86. doi: 10.1212/WNL.0000000000011609
6. Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology. (2021) 97:e2269–81. doi: 10.1212/WNL.0000000000012930
7. McWilliams D, Weblin J, Hodson J, Veenith T, Whitehouse T, Snelson C. Rehabilitation levels in patients with COVID-19 admitted to intensive care requiring invasive ventilation. An observational study. Ann Am Thorac Soc. (2021) 18:122–9. doi: 10.1513/AnnalsATS.202005-560OC
8. Jaquet P, Legouy C, Le Fevre L, Grinea A, Sinnah F, Franchineau G, et al. Neurologic outcomes of survivors of COVID-19-associated acute respiratory distress syndrome requiring intubation. Crit Care Med. (2022) 50:e674–e82. doi: 10.1097/CCM.0000000000005500
9. Ceravolo MG, Arienti C, de Sire A, Andrenelli E, Negrini F, Lazzarini SG, et al. Rehabilitation and COVID-19: the cochrane rehabilitation 2020 rapid living systematic review. Eur J Phys Rehabil Med. (2020) 56:642–51. doi: 10.23736/S1973-9087.20.06501-6
10. Wasilewski MB, Cimino SR, Kokorelias KM, Simpson R, Hitzig SL, Robinson L. Providing rehabilitation to patients recovering from COVID-19: a scoping review. PM R. (2022) 14:239–58. doi: 10.1002/pmrj.12669
11. Gloeckl R, Leitl D, Jarosch I, Schneeberger T, Nell C, Stenzel N, et al. Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ open Res. (2021) 7:00108–2021. doi: 10.1183/23120541.00108-2021
12. Udina C, Ars J, Morandi A, Vilaró J, Cáceres C, Inzitari M. Rehabilitation in adult post-COVID-19 patients in post-acute care with therapeutic exercise. J Frailty Aging. (2021) 10:297–300. doi: 10.14283/jfa.2021.1
13. Piquet V, Luczak C, Seiler F, Monaury J, Martini A, Ward AB, et al. Do patients with COVID-19 benefit from rehabilitation? Functional outcomes of the first 100 patients in a COVID-19 rehabilitation unit. Arch Phys Med Rehabil. (2021) 102:1067–74. doi: 10.1016/j.apmr.2021.01.069
14. de Sire A, Andrenelli E, Negrini F, Lazzarini SG, Cordani C, Ceravolo MG, et al. Rehabilitation and COVID-19: update of the rapid living systematic review by cochrane rehabilitation field as of february 28th, 2022. Eur J Phys Rehabil Med. (2022) 58:498–501. doi: 10.23736/S1973-9087.22.07593-1
15. Negrini F, de Sire A, Andrenelli E, Lazzarini SG, Patrini M, Ceravolo MG, et al. Rehabilitation and COVID-19: update of the rapid living systematic review by cochrane rehabilitation field as of december 31st, 2021. Eur J Phys Rehabil Med. (2022) 58:328–31. doi: 10.23736/S1973-9087.22.07497-4
16. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
17. Johansson S, Kottorp A, Lee KA, Gay CL, Lerdal A. Can the fatigue severity scale 7-item version be used across different patient populations as a generic fatigue measure–a comparative study using a rasch model approach. Health Qual Life Outcomes. (2014) 12:24. doi: 10.1186/1477-7525-12-24
18. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. (2008) 31:1601–7. doi: 10.1093/sleep/31.11.1601
19. Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. (2016) 43:23–9. doi: 10.1016/j.genhosppsych.2016.08.005
20. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
21. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. (2003) 1:29. doi: 10.1186/1477-7525-1-29
22. Pugh RJ, Battle CE, Thorpe C, Lynch C, Williams JP, Campbell A, et al. Reliability of frailty assessment in the critically ill: a multicentre prospective observational study. Anaesthesia. (2019) 74:758–64. doi: 10.1111/anae.14596
23. Shears M, Takaoka A, Rochwerg B, Bagshaw SM, Johnstone J, Holding A, et al. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. (2018) 45:197–203. doi: 10.1016/j.jcrc.2018.02.004
24. Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. (2020) 23:210–5. doi: 10.5770/cgj.23.463
25. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. (1990) 16:199–208. doi: 10.1016/0168-8510(90)90421-9
26. Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics. (2018) 36:675–97. doi: 10.1007/s40273-018-0623-8
27. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. (2007) 38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6
28. Cantier M, Morisot A, Guérot E, Megarbane B, Razazi K, Contou D, et al. Functional outcomes in adults with tuberculous meningitis admitted to the ICU: a multicenter cohort study. Crit Care. (2018) 22:210. doi: 10.1186/s13054-018-2140-8
29. Schubert J, Brämer D, Huttner HB, Gerner ST, Fuhrer H, Melzer N, et al. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e514. doi: 10.1212/NXI.0000000000000514
30. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. (1965) 14:61–5. doi: 10.1037/t02366-000
31. Dos Reis NF, Figueiredo F, Biscaro RRM, Lunardelli EB, Maurici R. Psychometric properties of the barthel index used at intensive care unit discharge. Am J Crit Care. (2022) 31:65–72. doi: 10.4037/ajcc2022732
32. Rollnik JD. The early rehabilitation barthel index (ERBI). Rehabilitation. (2011) 50:408–11. doi: 10.1055/s-0031-1273728
33. Christopher A, Kraft E, Olenick H, Kiesling R, Doty A. The reliability and validity of the timed up and go as a clinical tool in individuals with and without disabilities across a lifespan: a systematic review. Disabil Rehabil. (2021) 43:1799–813. doi: 10.1080/09638288.2019.1682066
34. Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. (2007) 88:1314–9. doi: 10.1016/j.apmr.2007.06.764
35. Huang M, Chan KS, Zanni JM, Parry SM, Neto SG, Neto JA, et al. Functional status score for the ICU: an international clinimetric analysis of validity, responsiveness, and minimal important difference. Crit Care Med. (2016) 44:e1155–64. doi: 10.1097/CCM.0000000000001949
36. Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP). Herzogenrath: Psytest (1993).
37. Schellig D, Schächtele B. Visueller und Verbaler Merkfähigkeitstest (VVM, Visual and Verbal Memory Test) – Manual. Frankfurt am Main: Pearson Assessment and Information GmbH (2009).
38. Qin ES, Hough CL, Andrews J, Bunnell AE. Intensive care unit-acquired weakness and the COVID-19 pandemic: a clinical review. PM R. (2022) 14:227–38. doi: 10.1002/pmrj.12757
39. Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. (2009) 37:S299–308. doi: 10.1097/CCM.0b013e3181b6ef67
40. Puchner B, Sahanic S, Kirchmair R, Pizzini A, Sonnweber B, Wöll E, et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med. (2021) 57:189–98. doi: 10.23736/S1973-9087.21.06549-7
41. Curci C, Negrini F, Ferrillo M, Bergonzi R, Bonacci E, Camozzi DM, et al. Functional outcome after inpatient rehabilitation in post-intensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur J Phys Rehabil Med. (2021) 57:443–50. doi: 10.23736/S1973-9087.20.06660-5
42. van Gassel RJJ, Bels J, Remij L, van Bussel BCT, Posthuma R, Gietema HA, et al. Functional outcomes and their association with physical performance in mechanically ventilated Coronavirus Disease 2019 survivors at 3 months following hospital discharge: a cohort study. Crit Care Med. (2021) 49:1726–38. doi: 10.1097/CCM.0000000000005089
43. Heesakkers H, van der Hoeven JG, Corsten S, Janssen I, Ewalds E, Simons KS, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. (2022) 327:559–65. doi: 10.1001/jama.2022.0040
44. Pizarro-Pennarolli C, Sánchez-Rojas C, Torres-Castro R, Vera-Uribe R, Sanchez-Ramirez DC, Vasconcello-Castillo L, et al. Assessment of activities of daily living in patients post COVID-19: a systematic review. PeerJ. (2021) 9:e11026. doi: 10.7717/peerj.11026
45. Marten O, Greiner W. EQ-5D-5L reference values for the German general elderly population. Health Qual Life Outcomes. (2021) 19:76. doi: 10.1186/s12955-021-01719-7
46. Carenzo L, Protti A, Dalla Corte F, Aceto R, Iapichino G, Milani A, et al. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann Intensive Care. (2021) 11:91. doi: 10.1186/s13613-021-00881-x
47. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Quality Life Res. (2013) 22:1717–27. doi: 10.1007/s11136-012-0322-4
48. Batterham AM, Bonner S, Wright J, Howell SJ, Hugill K, Danjoux G. Effect of supervised aerobic exercise rehabilitation on physical fitness and quality-of-life in survivors of critical illness: an exploratory minimized controlled trial (PIX study). Br J Anaesth. (2014) 113:130–7. doi: 10.1093/bja/aeu051
49. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. (2021) 92:55–70. doi: 10.1016/j.ejim.2021.06.009
Keywords: COVID-19, SARS-CoV-2, neurological rehabilitation, critical care outcomes, neurological manifestations
Citation: Wimmer C, Egger M, Bergmann J, Huge V, Müller F and Jahn K (2022) Critical COVID-19 disease: Clinical course and rehabilitation of neurological deficits. Front. Neurol. 13:1012685. doi: 10.3389/fneur.2022.1012685
Received: 05 August 2022; Accepted: 06 October 2022;
Published: 28 October 2022.
Edited by:
Luigi Tesio, University of Milan, ItalyReviewed by:
Alessandro de Sire, University of Magna Graecia, ItalyStefano Scarano, University of Milan, Italy
Copyright © 2022 Wimmer, Egger, Bergmann, Huge, Müller and Jahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Klaus Jahn, a2xhdXMuamFobiYjeDAwMDQwO21lZC51bmktbXVlbmNoZW4uZGU=
†These authors have contributed equally to this work and share first authorship
 Corinna Wimmer1,2†
Corinna Wimmer1,2† Marion Egger
Marion Egger Jeannine Bergmann
Jeannine Bergmann Klaus Jahn
Klaus Jahn