
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 30 September 2022
Sec. Neurogenetics
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1005670
 Min-Yu Lan1,2,3†
Min-Yu Lan1,2,3† Chin-Song Lu4,5†
Chin-Song Lu4,5† Shey-Lin Wu6,7
Shey-Lin Wu6,7 Ying-Fa Chen1,2†
Ying-Fa Chen1,2† Yueh-Feng Sung8†
Yueh-Feng Sung8† Min-Chien Tu9,10†
Min-Chien Tu9,10† Yung-Yee Chang1,2*†
Yung-Yee Chang1,2*†Hereditary spastic paraplegias (HSPs) are a heterogeneous group of neurodegenerative disorders clinically characterized by progressive lower-limb spasticity. Cerebellar ataxia commonly co-occurs with complicated HSPs. HSP with concurrent cerebellar ataxia has significant clinical and genetic overlaps with hereditary cerebellar ataxia (HCA) and other inherited neurological diseases, adding to the challenge of planning genetic testing for the disease. In this study, we characterized clinical features of a cohort of 24 patients (male/female: 15/9) from 22 families who presented spastic paraparesis combined with cerebellar involvement, with a median disease onset age 20.5 (range 5–53) years. Aside from the core phenotype, 18 (75%) patients had additional neuropsychiatric and systemic manifestations. A stepwise genetic testing strategy stratified by mode of inheritance, distinct neuroimaging features (e.g., thin corpus callosum), population-specific prevalence and whole-exome sequencing was utilized to investigate the genetic etiology. Causative mutations in up to 10 genes traditionally related to HSP, HCA and other neurogenetic diseases (autosomal recessive spastic ataxia of Charlevoix-Saguenay, neurodegeneration with brain iron accumulation, and progressive encephalopathy with brain atrophy and thin corpus callosum) were detected in 16 (73%) of the 22 pedigrees. Our study revealed the genetic complexity of HSP combined with cerebellar involvement. In contrast to the marked genetic diversity, the functions of the causative genes are restricted to a limited number of physiological themes. The functional overlap might reflect common underlying pathogenic mechanisms, to which the corticospinal tract and cerebellar neuron circuits may be especially vulnerable.
Hereditary spastic paraplegias (HSPs) comprise a heterogeneous group of inherited neurodegenerative diseases with the common clinical characteristics of progressive spasticity and weakness of the lower extremities. Pathophysiologically, HSP is a primary distal axonopathy that affects both the corticospinal and posterior column sensory tracts, with maximal degeneration at the end of the nerve fibers in these tracts. The genetic basis of HSP is also complex. To date, there have been 87 HSP genes or loci registered in Online Mendelian Inheritance of Men (1), suggesting diverse molecular pathogenetic mechanisms underlying this group of diseases. All modes of inheritance have been observed in HSPs. HSP is classified as a pure or complicated form according to the absence or presence of additional neurological or systemic features, such as cognitive impairment, epilepsy, cerebellar ataxia, thin corpus callosum, peripheral neuropathy, optic neuropathy, retinopathy, and deafness (2, 3). Pure forms of HSP tend to be inherited in an autosomal dominant (AD) pattern, whereas complicated HSPs are more frequently autosomal recessive (AR).
Cerebellar ataxia is a common concurrent neurological manifestation of complicated HSPs and is present in more than 30 disease entities and ~30% of complicated HSP patients (4–6). Conversely, hereditary cerebellar ataxia (HCA) may occasionally present with severe lower limb spasticity in the early stage of the disease, resembling HSP until the ataxic component becomes more prominent (7). Moreover, HSP has significant clinical and genetic overlaps with other categories of neurodegenerative disease, such as HCA, spastic ataxia (SPAX), genetic leukoencephalopathy, motor neuron disease and inherited neuropathy (7, 8). Because of the individual rarity, phenotype overlaps and genetic heterogeneity of the conditions, the molecular diagnosis of HSP and “HSP mimics” remains challenging.
In this study, we conducted a clinical and genetic characterization study in a cohort of Taiwanese patients whose main clinical presentation was spastic paraparesis combined with cerebellar dysfunction. Through a strategy incorporating inheritance and brain imaging-guided target gene testing, followed by screening for prevalent diseases and whole-exome sequencing (WES), genetic diagnosis was achieved in more than two-thirds of cases. In addition to demonstrating the effectiveness of customized genetic testing in the diagnosis of HSP combined with cerebellar involvement, this study provides insights into the genetic heterogeneity of neurodegenerative diseases with a phenotype of coexisting spasticity and ataxia.
A total of 30 patients of complicated HSP from 27 kindreds were recruited at or referred to Kaohsiung Chang Gung Memorial Hospital, Taiwan, between January 2015 and June 2021. Among them, 24 patients from 22 families had combined cerebellar involvement in addition to an HSP-like core phenotype. The clinical diagnosis of HSP was based on the following: (i) clinically spastic paraplegia as an early and prominent sign of a degenerative disease affecting the nervous system, or spastic tetraparesis with earlier and more severe affliction of the lower limbs; and (ii) no acquired causes of the presenting symptoms (9). Cerebellar involvement was defined as cerebellar ataxia demonstrated by neurological examination, or as atrophy of the cerebellum more than other brain regions on magnetic resonance imaging (MRI) when ataxia could not be demonstrated by neurological examination due to severe weakness or spasticity. Clinical information and brain MRI findings were collected. Ambulatory disability was scored on a six-level scale: 0 = asymptomatic, 1 = minimally impaired (abnormal gait but able to run), 2 = mildly impaired (able to walk independently but not run), 3 = moderately impaired (able to walk with an aid), 4 = severely impaired (wheelchair bound), and 5 = confined to bed. The study was approved by the Chang Gung Memorial Hospital Institutional Review Board (No. 100-4460C). Written informed consent was obtained from participants or, in the case of minors, from a parent.
To focus the analysis on the most phenotype-relevant genes, reducing cost and time consumed and ensuring diagnostic yield at the same time, a stepwise genetic testing strategy was applied (Figure 1). In the first step, the mode of inheritance was considered. AD inheritance was defined as the presence of affected individuals in at least two successive generations in a family. The inheritance pattern was classified as AR if there were at least two affected siblings in a generation without the presence of affected members in two successive generations. First, the patients with AD inheritance were screened with a panel of spinocerebellar ataxia (SCA) genes, including SCA1 (ATXN1), SCA2 (ATXN2), SCA3 (ATXN3), SCA6 (CACNA1A), SCA7 (ATXN7), SCA17 (TBP) and dentatorubropallidoluysian atrophy (ATN1). In the second stage, we observed characteristic brain MRI features, such as thin corpus callosum (TCC), which might hint at probable HSP subtypes and thus guide sequencing analysis of the most relevant genes. Third, because SPG5 is the most common complicated form of HSP in Taiwan, attributed to a founder CYP7B1 mutation, c.334C>T (p.Arg112Ter), and usually combined with cerebellar ataxia (10, 11), genetic testing of the gene CYP7B1 was performed for the cases that tested negative in MRI-directed genetic testing and for those without specific brain MRI findings. Finally, WES was conducted for the remaining undiagnosed cases.
A SureSelect Human All Exon 50 Mb Kit (Agilent Technologies) was used to capture the exome library, and sequencing was performed on an Illumina HiSeq2000 (Illumina). Approximately 52 million paired reads were mapped to the human reference genome. Burrows-Wheeler Aligner and Genome Analysis Toolkit were used for read mapping and variant calling. A coverage analysis revealed that 90% of the target regions were covered by ≥ 35 reads.
To determine causative variants, we prioritized WES variants with the following process. First, variants in genes that were included in the lists of HSP/HSP-like genes, HCA genes and genetic leukoencephalopathy genes (Supplementary material 1) or those that were novel or coded as “pathogenic” or “likely pathogenic” according to ClinVar and related to a relevant neurological disease were selected. Second, only variants with a predicted functional impact on coding regions (e.g., missense, nonsense, insertion, deletion or splicing site variation) were considered. Third, based on the Genome Aggregation Database (https://gnomad.broadinstitute.org/), Exome Variant Server (https://evs.gs.washington.edu/EVS/), and Taiwan Biobank (https://taiwanview.twbiobank.org.tw/index), variants with frequencies >0.1% in any population were discarded. Finally, mode of inheritance and clinical data were taken into account to evaluate the relevance of the variants to the phenotypes.
The impact of a missense variant on encoded protein structure or function was predicted by SIFT (https://sift.bii.a-star.edu.sg/), PROVEAN (http://provean.jcvi.org/index.php), and CADD (https://cadd.gs.washington.edu/). The impact of splicing site variants on RNA transcription was interpreted with the Spice Site Prediction by Neural Network (https://www.fruitfly.org/seq_tools/splice.html). Variants that were interpreted as “pathogenic” or “likely pathogenic” based on the American College of Medical Genetics and Genomics and Association of Molecular Pathology (ACMG-AMP) guidelines (12) were considered to be significantly pathogenic unless otherwise specified. The novelty of the pathogenic variant was checked with the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php) and ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/). All reported variants were confirmed by Sanger sequencing. The parental origin of the detected variants was investigated by sequencing the genes in each proband's parents and siblings where available.
There were 24 patients (male/female: 15/9) from 22 families, including four cases from four AD families (D1–D4), six cases from four AR families (R1–R4), and 14 sporadic cases (S1–S14) (Table 1). The median age was 36 (range 13–65) years at the time of examination, and the median disease onset age was 20.5 (range 5–53) years. Twenty patients presented both cerebellar ataxia in neurological examination and cerebellar atrophy on MRI, one patient presented cerebellar ataxia in neurological examination with normal brain MRI (S12), and three patients (R2, R3-2 and S10) showed cerebellar atrophy on MRI (Supplementary material 2) while ataxia could not be checked by neurological examination due to severe weakness or spasticity. Aside from the core feature of spastic paraparesis combined with cerebellar ataxia or atrophy, 18 (75%) patients had additional neuropsychiatric manifestations, including cognitive impairment (n = 8), parkinsonism or other extrapyramidal signs (dystonia, myoclonus, or tremor) (n = 8), polyneuropathy (n = 6), ophthalmoplegia (n = 4), intellectual disability (n = 1), optic atrophy (n = 1) and bipolar disorder (n = 1). For systemic comorbidity, one patient (R1-2) had rheumatoid arthritis, and another had insulin-dependent diabetes mellitus (S9).
The first tier of genetic testing was the SCA gene panel for the four patients with a family history of autosomal dominant inheritance, and it revealed pathogenic CAG repeat expansion in ATXN3 in three of them (15/83 in D1, 15/67 in D2, and 20/72 in D3). Then, relevant genes were tested in three patients with characteristic brain MRI features that suggested specific disease entities. For patient R2, brain MRI showed horizontal hypointensity strips, a hyperintensity zone in the lateral pons, a hyperintense peri-thalamic rim on T2W and FLAIR imaging, and TCC (Figures 2A–C). These findings were typical of autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) (13). Sequencing analysis of SACS revealed a homozygous frameshift variant, c.8621_8624 del (p.Ser2874TrpfsTer), which confirmed the diagnosis. Brain MRI of patients S2 and S13 showed TCC. Patient S13 also presented T2-hyperintense streaks in the forceps minor of the corpus callosum (“ears of the lynx” sign) (14) and a box-shaped calloso-caudate angle (Figures 2D–F). For the two cases, sequencing analysis for genes of the three HSPs most frequently associated with TCC, SPG11, ZFYVE26 (SPG15 gene) and SPG21, was performed. SPG11 was confirmed for patient S13 with the detection of two novel heterozygous variants, c.1602+1 G>C and c.3175_3176 delinsTG (p.Ala1059Ter), in SPG11, while no pathogenic variant was detected in the three genes in patient S2. Afterward, sequencing analysis of CYP7B1 was performed in the remaining probands, and SPG5 was confirmed in three cases (S3, S4 and S12). All three SPG5 cases carried the CYP7B1 variant c.334 C>T (p.Arg112Ter), which was homozygous in patients S3 and S12 and was heterozygous and combined with a missense variant c.1316 T>G (p.Leu439Arg) in patient S4. Although the variant c.1316 T>G was categorized as a “variant of uncertain significance” based on the ACMG-AMP criteria, it has been reported previously in another Taiwanese SPG5 patient (11).
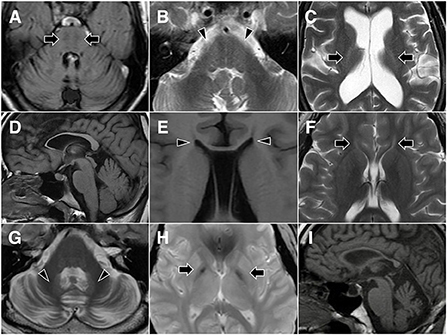
Figure 2. Representative brain MR images. Patient R2 shows horizontal hypointensity strips on FLAIR imaging (A, arrows), along with a hyperintense zone in the lateral pons on T2-weighted imaging (B, arrowheads) and a hyperintense peri-thalamic rim (C, arrows). Patient S13 shows thin corpus callosum (D) and a box-shaped calloso-caudate angle (E, arrowheads) on T1-weighted imaging, as well as hyperintense streaks in the forceps minor of the corpus callosum (“ears of the lynx” sign) on T2-weighted imaging (F, arrows). Patient D4 demonstrates cerebellar atrophy and T2 hyperintensity of the dentate nucleus (G, arrowheads), as well as T2 gradient-echo hypointensity of the medial globus pallidus (H, arrows). Midsagittal T1-weighted imaging of patient S2 shows thin corpus callosum and cerebellar atrophy (I).
WES was then performed in the remaining patients who tested negative in the previous genetic analysis. Significant variants in the spasticity or ataxia-relevant genes were detected in all of the one AD and 3 AR cases and 4 of the remaining 10 sporadic cases. A novel heterozygous variant c.101A>G (p.Asn34Ser) in STUB1, a gene linked to SCA48, was detected in Patient D4. The patient's father had a phenotype of slowly progressive pure cerebellar dysfunction and carried the same STUB1 variant (see Case reports). The R1 proband (R1-1) had two heterozygous variants in GBA2, including c.2618 G>A (p.Arg873His), a previously reported disease-causing variant (15), and a novel mutation c.2635 C>T (p.Arg879Trp), and SPG46 was diagnosed. The R3 proband (R3-1) was heterozygous for two reported variants, c.991 G>T (p.Asp331Tyr) and c.1427+1 G>A in PLA2G6 (16, 17), and PLA2G6-associated neurodegeneration (PLAN) was diagnosed. The same GBA2 and PLA2G6 variants were also detected in the affected siblings of the two probands. Pathogenic variants in CAPN1 (SPG76 gene) were detected in two patients, including a homozygous novel nonsense variant c. 894 G>A (p.Trp298Ter) in patient R4 and two previously reported heterozygous variants, c.188 dup (p.Val64GlyfsTer) and c.1493 C>T (p.Pro498Leu) (18, 19), in patient S11. Patient S2 had a variant c.1340 C>T (p.Ala447Val) (“likely pathogenic” registered in ClinVar) and a previously reported variant c.3365 C>T (p.Pro1122Leu) (20) in TBCD, a gene linked to progressive encephalopathy with brain atrophy and thin corpus callosum (PEBAT, OMIM:617193). Two heterozygous missense variants in KIF1A (SPG30 gene) were detected in two patients, including a novel variant c.1031 C>T (p.Thr344Met) in patients S6 and a previously reported variant c.761 G>A (p.Arg254Gln) (21) in patient S10. De novo mutations were considered for both patients, as the corresponding KIF1A variants were not detected in their parents. In all, genetic mutation was detected in 16 (73%) of the 22 probands of this cohort (Figure 1; Table 2).
The 32-year-old security guard had an unsteady gait that had developed approximately one-half to 1 year prior. Meanwhile, slurred speech, hand awkwardness and slower general movements developed insidiously during the period. Neurological examination revealed normal cognitive function (a Mini-Mental State Examination score of 29), combined nuclear and supranuclear ophthalmoplegia, dysmetric saccades, increased muscle tone in the lower limbs, general hyperreflexia, four limb and trunk ataxia, a positive Romberg test, and a mixed ataxic and spastic gait. Brain MRI showed diffuse cerebellar atrophy, T2 hyperintensity of the dentate nucleus, and T2 gradient-echo hypointensity of the medial globus pallidus (Figures 2G,H). The peripheral nerve conduction study was normal. WES detected a heterozygous missense variant, c.101A>G (p.Asn34Ser), in STUB1.
The patient's father, 63 years old, had unsteady gait with slow progression in severity since the early 40 years of age. Unlike the proband, he presented with lower-limb- and trunk-predominant cerebellar ataxia without ophthalmoplegia or spasticity. Brain MRI showed pancerebellar atrophy, and genetic testing revealed heterozygosity for the same STUB1 variant. The proband's mother and siblings were normal in neurological examination and did not carry the STUB1 variant (Supplementary material 3). The intrafamilial segregation of the phenotype and the genetic variant was consistent with AD inheritance, and thus SCA48 was diagnosed.
The proband, a 37-year-old male, had unsteady gait since 20 years ago. He had intellectual disability and delayed developmental milestones and received integrated rehabilitation from 2 to 5 years of age. He had developed psychiatric symptoms including delusions, visual hallucinations and emotional lability in his early 20s and had been diagnosed with bipolar disorder. Slow movements, dysuria and urine incontinence appeared along with progression of gait disturbance in the following few years. Neurological examination showed weakness of the distal four limbs, increased lower limb muscle tone, general hyperreflexia, bilateral extensor plantar reflex and a right spastic gait, apart from cognitive impairment, ophthalmoplegia, hypophonic dysarthria, four limb dysmetria and general hypokinesia. Brain MRI showed marked pancerebellar atrophy. WES detected compound heterozygous variants, c.991 G>T (p.Asp331Tyr) and c.1427+1 G>A, in PLA2G6, and PLAN was diagnosed accordingly.
His 39-year-old sister (R3-2) had a much later disease-onset age. She presented with rapid progression of motor and cognitive symptoms since the onset 2 years ago. Neurological examination showed general hyperreflexia, bradykinesia, rigidity and dystonia as well as a spastic gait. Brain MRI showed diffuse cerebellar atrophy. Genetic testing revealed identical compound heterozygous variants in PLA2G6.
The 42-year-old man had intellectual disability since early childhood and delayed early development (able to stand at 24 months and walk at 30 months). Intrauterine or perinatal events were denied by his parents. Unsteady gait was noted at 33 years of age at first, and it became more severe with time and led to repeated falls. Family history was not contributory. Neurological examination revealed impaired cognitive function, optic atrophy, strabismus, increase in lower limb muscle tone, muscle atrophy at distal limbs, general hyperreflexia and right side extensor plantar reflex, four limb and trunk ataxia and bilateral spastic gait. A nerve conduction study showed axonal polyneuropathy in the lower limbs. Brain MRI was remarkable for a thin corpus callosum (Figure 2I) and pancerebellar atrophy. WES detected compound heterozygous variants c.1340 C>T (p.Ala447Val) and c.3365 C>T (p.Pro1122Leu) in TBCD.
The 49-year-old male born to consanguineous (3rd degree) parents had insidious development of gait abnormality that progressed gradually in the past 5 years. He felt stiffness of the leg muscles and had difficulty walking fast and easy tripping. Speech, swallowing and upper limb functions were not affected. Symptoms of bladder control were denied. Neurological examination showed normal cognitive function, cranial nerves and muscle bulk and strength of the four limbs. Marked muscle tone increase in the lower limbs, general hyperreflexia, bilateral ankle clonus with extensor plantar reflex, prominent impairment of posterior column sensations at distal lower limbs, lower limb and trunk ataxia, Romberg's sign, and a mixed spastic and wide-based gait pattern were noted. Sequence analysis for CYP7B1 revealed homozygosity for c.334 C>T (p.Arg112Ter) and SPG5 was confirmed.
With a genetic testing strategy stratified by mode of inheritance, characteristic neuroimaging features, population-specific prevalence and WES, we detected causative mutations in disease-relevant genes in 73% (16/22) of the index cases of combined spastic paraparesis and cerebellar ataxia. In addition, half of the cases with molecular diagnosis could be confirmed before implementation of WES. It has been demonstrated that by integrating mode of inheritance and pertinent clinical data, disease-related genetic testing followed by multigene panels may improve diagnostic yield for HSP and HCA (22). Furthermore, this and our study suggest that the integrated testing flowchart not only helps to guide a time and cost-efficient molecular testing in the new generation sequencing era, but also facilitates genotype-phenotype correlation in diagnosing the heterogeneous neurogenetic disorders.
HSP and HCA are traditionally designed as two disparate groups of inherited neurodegenerative disorders. With the main clinical manifestations related to degeneration of the corticospinal tract and the cerebellar circuit, each of them encompasses an ever-expanding list of causative genes. However, with the progress of next generation sequencing and genomic studies in the past decade, genetic overlap between HSP and HCA has been increasingly recognized. In the HCA cohorts with exclusion of repeat-expansion mutations, exome-based genetic studies revealed that mutations in HSP (SPG) genes represented ~20% (ranging from 18.5 to 30%) of cases with molecular diagnosis (23–27). In fact, some genes have been linked to both HSP and HCA loci. For instance, PNPLA6 has been identified as a cause of complicated HSP combined with motor neuropathy (SPG39) (28), and it has also been linked to AR cerebellar ataxia (29). These discoveries led the concept of a phenotype spectrum, with spasticity at one end and ataxia at the other, rather than considering HSP and HCA separately as two groups of diseases. Moreover, it is also the case that the phenotype of combined spasticity and ataxia is related to genes that classically belong to other disease categories, such as leukodystrophy, familial Parkinson's disease, frontotemporal dementia, and neurodegeneration with brain iron accumulation (NBIA) (30). These diseases have been more frequently considered jointly as “ataxia-spasticity spectrum diseases (ASSD)” (31) in replacement of the conventional but overlapping disease classifying systems.
Despite a small study cohort, causative mutation in up to 10 genes related to various neurogenetic diseases were detected in this study, indicating that etiologies are remarkably heterogeneous for spastic paraparesis combined with cerebellar ataxia. First, SCA3 was diagnosed in three cases, and SCA48 was diagnosed in one patient, supporting that HCA and HSP lie in two ends of the broad continuum of ASSD. SCAs may present predominantly with spastic paraparesis at the early stage of the disease. This distinct phenotype is more frequently observed in SCA3 cases of eastern Asian origin who carry greatly expanded ATXN3 trinucleotide repeats (32). STUB1 encodes CHIP, a protein that acts as a ubiquitin ligase and cochaperone and is highly expressed in the brain, especially in Purkinje cells (33). STUB1 mutations could be presented with HCA of AR (SCAR16) and AD (SCA48) inheritance. The variant detected in this study, c.101 A>G (p.Asn34Ser), is predicted to be deleterious to the functions or structure of the encoded protein by in silico analysis and cosegregated with affected individuals compatible with an AD inherited mode in the reported kindred. The amino acid residue altered by this variant is located in the tetratricopeptide repeat domain, which interacts with heat shock proteins and guides them to refold misfolded proteins (34), suggesting its pathogenic role. In addition, two variants affecting the neighboring residues, p.Gly33Ser and p.Arg35Ser, have been linked to SCA48 and SCAR16, respectively (35, 36).
Five diseases belonging to HSP loci were detected, including SPG5, SPG11, SPG30, SPG46, and SPG76. SPG5 is the most common AR HSP in Taiwan and is attributed to a founder CYP7B1 mutation, c.334C>T (p.Arg112Ter) (10, 11). All three SPG5 patients discovered in this study carried the ethnicity-specific CYP7B1 variant. The phenotype of SPG5 related to this variant is characterized by remarkable dorsal column sensory deficits and may present with spastic paraparesis and combined cerebellar and sensory ataxia (10), as in our patients. The CYP7B1 gene encodes the enzyme cytochrome P450 oxysterol 7α-hydroxylase, which is widely expressed in human tissues and acts on hydroxylating endogenous steroids, including the two neurosteroids dehydroepiandrosterone and pregnelonone (37). The two SPG30 patients discovered in this study were associated with de novo mutations of KIF1A. Apart from cerebellar ataxia, the two cases had additional neurological or systemic features, including polyneuropathy, cataract and cognitive decline. KIF1A mutations underlie a wide spectrum of neurodegenerative disorders, broadly termed KIF1A-associated neurological diseases (38). Among them, SPG30 could have a pure or complicated HSP phenotype and could be caused by de novo KIF1A mutations or inherited with an AD or AR mode (39). The KIF1A gene encodes a neuron-specific kinesin protein called kinesin 1a, which governs anterograde axonal transport of synaptic vesicle precursors (40). Regardless of phenotype, most of the reported cases with monoallelic mutation were related to missense variants that affect the kinesin motor domain (amino acid residues 1–365) and disrupt its binding with microtubules to process transport (41). The cohort also included two SPG46 patients from an AR family with compound GBA2 mutations. GBA2 encodes β-glucosidase 2, an enzyme catalyzing the cleavage of sphingolipid glucosylceramide. One of the detected mutations, c.2618 G>A (p.Arg873His), has been shown to be associated with a decrease in β-glucosidase 2 activity (42). GBA2 mutations have been reported in patients with HSP and HCA with spasticity (15, 43). Additional clinical features may include cognitive impairment, axonal neuropathy and hearing loss. SPG76 was confirmed in AR and sporadic patients caused by CAPN1 mutations. CAPN1 encodes calpain-1, a calcium-dependent proteinase highly expressed in cerebellar neurons that is involved in several neurobiological functions, such as synaptic plasticity, axonal maintenance and neuronal migration and maintenance (18, 44). CAPN1 mutations were first linked to a spastic ataxia phenotype in 2016. Since that time, there have been a significant number of SPG76 cases of east Asian origin reported in the literature (19, 45–49).
Three different diseases presenting with TCC, including SPG11, PEBAT and ARSACS, were detected in the study cohort. HSP combined with TCC is characterized to belong to a group of distinctive HSP subtypes (50). For this special HSP group, SPG11 is the common subtype, accounting for 33 and 41% of the cases in the genetic investigations of two large cohorts (51, 52). Aside from TCC, abnormality of the forceps minor of the corpus callosum may be present (14). These characteristic brain imaging features guided us to achieve diagnosis in a patient by phenotype-directed gene analysis. Clinically, SPG11 is typically associated with complicated phenotypes, including intellectual disability, progressive cognitive decline, axonal neuropathy and cerebellar signs (51, 52). Notably, we detected compound heterozygous TBCD mutations in the other HSP patient combined with TCC, adding a novel member in this distinctive HSP subtype. TBCD mutations were first linked to a rare early-onset neurodevelopmental disease (20, 53, 54), which was later termed PEBAT, in 2016. Patients with the disease typically present with copious neurodegenerative features, including microcephaly, developmental delay, intellectual disability, seizure, optic atrophy, spastic quadriparesis, ataxia and prominent brain imaging abnormalities such as brain atrophy, hypomyelination and TCC. TBCD encodes tubulin folding cofactor D, a tubulin-specific chaperone required for the formation of α- and β-tubulin heterodimers. Reduced TBCD levels or defective β-tubulin binding to mutant TBCD proteins results in decrease of soluble α/β tubulin levels and accelerates microtubule polymerization (20), suggesting that altered microtubule dynamics underlie the pathogenic mechanism for PEBAT.
Aside from PEBAT, two diseases not traditionally referred to as HSP, including ARSACS and PLAN, were also found in this cohort. ARSACS was first reported as a prevalent, early-onset neurodegenerative disease in Quebec, Canada, with rather homogenous clinical features, including spasticity, ataxia, optic nerve hypermyelination and intellectual disability (55). Cases of ARSACS discovered in other regions of the world may present with different phenotypes, such as later onset of disease or absence of spasticity (56). Despite the variations in clinical phenotypes, brain MR images showed distinctive features, including horizontal hypointensity in a bulky pons, longitudinal hyperintensity in the lateral pons, TCC, hyperintensity in the lateral thalamic rim on T2 images, and upper-vermis-predominant cerebellar atrophy, which serve as imaging hallmarks for ARSACS (13). PLAN is linked to PLA2G6 mutations and belongs to NBIA, a heterogeneous group of neurodegenerative diseases characterized by iron accumulation in the basal ganglia and substantia nigra due to various disrupted cellular mechanisms (57). PLA2G6 encodes calcium-dependent phospholipase A2, which catalyzes the hydrolysis of glycerophospholipids. PLAN comprises a continuum of distinctive diseases with overlapping clinical features (58). Patients with classical infantile neuroaxonal dystrophy and atypical neuroaxonal dystrophy present progressive neurocognitive regression during the infantile period to early childhood and axonal spheroids secondary to deranged cellular membranes in the central and peripheral nervous systems. PLA2G6-related dystonia-parkinsonism, on the other hand, typically has an onset of psychiatric symptoms, cognitive decline, pyramidal, extrapyramidal, and cerebellar features in young adulthood. Rarely, PLAN may present with early- or adult-onset spastic paraparesis combined with cerebellar ataxia, intellectual disability or Parkinsonism (59–61), adding complicated HSP to the PLAN phenotype spectrum. Intrafamilial variability of phenotype for PLA2G6-assocaited neurodegeneration has been reported previously (62). The exact mechanisms are not yet known although it is suggested that differences of genetic variants in other genes linked to neurodegenerative diseases between the affected siblings may contribute to the phenotypic heterogeneity.
A total of 10 different diseases caused by mutations in the corresponding genes were detected in this study. In contrast to the broad spectrum of genetically defined disorders for the investigated phenotype, the functions of these causative genes may fall within a relatively small number of common physiological themes at the cellular level, including microtubule maintenance (TBCD, SACS), axonal transport (KIF1A), phospholipid and fatty acid metabolism (CYP7B1, GBA2, PLA2G6), the endosome-lysosome-autophagosome system (ATXN3, STUB1, SPG11, SCAS), myelination (GBA2) and mitochondrial functions (ATXN3, SACS) (56, 63–66). Except that, endoplasmic reticulum and intracellular membrane trafficking are also associated with some genes that are involved in ASSD (e.g., KIF1C, VAMP1) (31), although genetic mutation of this category was not detected in this study. The functional overlap underlying the numerous ASSDs might reflect common underlying pathogenic mechanisms, to which the corticospinal tract and cerebellar neuron circuits are especially vulnerable. This pathogenicity-driven approach might not only inspire a novel mechanism-based disease classification system but also trigger pathway-targeting disease exploration and therapeutic strategies (30).
Several limitations of the study need to be addressed. First, this study was based on a relatively small cohort collected from single medical center in Taiwan. Diagnostic yield of the stratified genetic testing strategy as well as genetic spectrums for the phenotype of combined spastic paraparesis and cerebellar ataxia need to be checked by nationwide surveys with large study cohorts. Second, some patients were included in the study based on the observation of prominent cerebellar atrophy on MRI. However, brain volumetry compared with normal controls was not performed to ascertain significant decrease of cerebellar volume in these patients. Third, genetic testing methods used in this study cannot detect variants located in deep intron or exon deletion or duplication. To fill the diagnostic gap, the stepwise genetic testing protocol must be complemented with long-read whole genome sequencing for the last tier of test. Fourth, a cross-sectional data of a six-level ambulatory disability scale is insufficient to reflect severity and clinical course of the disease. Standardized systems such as mini-mental state examination, Spastic Paraplegia Rating Scale (67) and Scale for the Assessment and Rating of Ataxia (68) are more appropriate for the purpose.
In conclusion, spastic paraparesis combined with cerebellar involvement is the phenotype of a group of genetically heterogeneous diseases, which are traditionally referred to as HSP, HCA and other inherited neurodegenerative conditions. Recognition of inheritance mode and distinct clinical and neuroimaging features and incorporation of population-specific genetic information may help to guide efficient molecular testing and facilitate genotype-phenotype correlation. Improvement in knowledge regarding biological functions shared by these ASSD genes might open the way for therapeutic approaches targeting a handful of dysregulated cellular pathways instead of single genes or disease symptoms.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/articles/dataset/Clinico-genetic_characterization_of_spastic_ataxia/20392284.
The studies involving human participants were reviewed and approved by Chang Gung Memorial Hospital Institutional Review Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
M-YL, C-SL, and Y-YC: project conceptualization and design. M-YL, C-SL, S-LW, Y-FS, Y-FC, and M-CT: data collection. M-YL and Y-YC: methodology. M-YL and C-SL: genetic testing and data analysis. Y-FC: figure and image editing. M-YL: writing of the first draft. C-SL and Y-YC: manuscript review and critique. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1005670/full#supplementary-material
1. Online Mendelian Inheritance in Man, OMIM®. Baltimore, MD: McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (2022). Available online at: https://omim.org/ (accessed July 10, 2022).
2. Harding AE. Hereditary spastic paraplegias. Semin Neurol. (1993) 13:333–6. doi: 10.1055/s-2008-1041143
3. Fink JK. Advances in the hereditary spastic paraplegias. Exp Neurol. (2003) 184:S106–10. doi: 10.1016/j.expneurol.2003.08.005
4. Kara E, Tucci A, Manzoni C, Lynch DS, Elpidorou M, Bettencourt C, et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain. (2016) 139:1904–18. doi: 10.1093/brain/aww111
5. Lynch DS, Koutsis G, Tucci A, Panas M, Baklou M, Breza M, et al. Hereditary spastic paraplegia in Greece: characterisation of a previously unexplored population using next-generation sequencing. Eur J Hum Genet. (2016) 24:857–63. doi: 10.1038/ejhg.2015.200
6. Schüle R, Wiethoff S, Martus P, Karle KN, Otto S, Klebe S, et al. Hereditary spastic paraplegia: clinicogenetic lessons from 608 patients. Ann Neurol. (2016) 79:646–58. doi: 10.1002/ana.24611
7. Hedera P. Hereditary myelopathies. Continuum. (2018) 24:523–50. doi: 10.1212/CON.0000000000000580
8. Timmerman V, Clowes VE, Reid E. Overlapping molecular pathological themes link Charcot-Marie-Tooth neuropathies and hereditary spastic paraplegias. Exp Neurol. (2013) 246:14–25. doi: 10.1016/j.expneurol.2012.01.010
9. Fink JK, Heiman-Patterson T, Bird T, Cambi F, Dubé MP, Figlewicz DA, et al. Hereditary spastic paraplegia: advances in genetic research. Hereditary Spastic Paraplegia Working group. Neurology. (1996) 46:1507–14. doi: 10.1212/WNL.46.6.1507
10. Lan MY, Yeh TH, Chang YY, Kuo HC, Sun HS, Lai SC, et al. Clinical and genetic analysis of Taiwanese patients with hereditary spastic paraplegia type 5. Eur J Neurol. (2015) 22:211–4. doi: 10.1111/ene.12407
11. Chou CT, Soong BW, Lin KP, Tsai YS, Jih KY, Liao YC, et al. Clinical characteristics of Taiwanese patients with Hereditary spastic paraplegia type 5. Ann Clin Transl Neurol. (2020) 7:486–96. doi: 10.1002/acn3.51019
12. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
13. Prodi E, Grisoli M, Panzeri M, Minati L, Fattori F, Erbetta A, et al. Supratentorial and pontine MRI abnormalities characterize recessive spastic ataxia of Charlevoix-Saguenay. A comprehensive study of an Italian series. Eur J Neurol. (2013) 20:138–46. doi: 10.1111/j.1468-1331.2012.03815.x
14. Riverol M, Samaranch L, Pascual B, Pastor P, Irigoyen J, Pastor MA, et al. Forceps minor region signal abnormality “ears of the lynx”: an early MRI finding in spastic paraparesis with thin corpus callosum and mutations in the spatacsin gene (SPG11) on chromosome 15. J Neuroimaging. (2009) 19:52–60. doi: 10.1111/j.1552-6569.2008.00327.x
15. Hammer MB, Eleuch-Fayache G, Schottlaender LV, Nehdi H, Gibbs JR, Arepalli SK, et al. Mutations in GBA2 cause autosomal-recessive cerebellar ataxia with spasticity. Am J Hum Genet. (2013) 92:245–51. doi: 10.1016/j.ajhg.2012.12.012
16. Lu CS, Lai SC, Wu RM, Weng YH, Huang CL, Chen RS, et al. PLA2G6 mutations in PARK14-linked young-onset parkinsonism and sporadic Parkinson's disease. Am J Med Genet B Neuropsychiatr Genet. (2012) 159B:183–91. doi: 10.1002/ajmg.b.32012
17. Wu Y, Jiang Y, Gao Z, Wang J, Yuan Y, Xiong H, et al. Clinical study and PLA2G6 mutation screening analysis in Chinese patients with infantile neuroaxonal dystrophy. Eur J Neurol. (2009) 16:240–5. doi: 10.1111/j.1468-1331.2008.02397.x
18. Wang Y, Hersheson J, Lopez D, Hammer M, Liu Y, Lee KH, et al. Defects in the CAPN1 gene result in alterations in cerebellar development and cerebellar ataxia in mice and humans. Cell Rep. (2016) 16:79–91. doi: 10.1016/j.celrep.2016.05.044
19. Lu YQ, Dong EL, Yang WQ, Lai LL, Lin XH, Ma LX, et al. Generation of an integration-free induced pluripotent stem cell line, FJMUi001-A, from a hereditary spastic paraplegia patient carrying compound heterozygous pP498L and pR618W mutations in CAPN1 (SPG76). Stem Cell Res. (2019) 34:101354. doi: 10.1016/j.scr.2018.11.015
20. Flex E, Niceta M, Cecchetti S, Thiffault I, Au MG, Capuano A, et al. Biallelic mutations in TBCD, encoding the tubulin folding cofactor D, perturb microtubule dynamics and cause early-onset encephalopathy. Am J Hum Genet. (2016) 99:962–73. doi: 10.1016/j.ajhg.2016.08.003
21. Ohba C, Haginoya K, Osaka H, Kubota K, Ishiyama A, Hiraide T, et al. De novo KIF1A mutations cause intellectual deficit, cerebellar atrophy, lower limb spasticity and visual disturbance. J Hum Genet. (2015) 60:739–42. doi: 10.1038/jhg.2015.108
22. Riso V, Rossi S, Nicoletti TF, Tessa A, Travaglini L, Zanni G, et al. Application of a clinical workflow may lead to increased diagnostic precision in hereditary spastic paraplegias and cerebellar ataxias: a single center experience. Brain Sci. (2021) 11:246. doi: 10.3390/brainsci11020246
23. Fogel BL, Lee H, Deignan JL, Strom SP, Kantarci S, Wang X, et al. Exome sequencing in the clinical diagnosis of sporadic or familial cerebellar ataxia. JAMA Neurol. (2014) 71:1237–46. doi: 10.1001/jamaneurol.2014.1944
24. Pyle A, Smertenko T, Bargiela D, Griffin H, Duff J, Appleton M, et al. Exome sequencing in undiagnosed inherited and sporadic ataxias. Brain. (2015) 138(Pt 2):276–83. doi: 10.1093/brain/awu348
25. van de Warrenburg BP, Schouten MI, de Bot ST, Vermeer S, Meijer R, Pennings M, et al. Clinical exome sequencing for cerebellar ataxia and spastic paraplegia uncovers novel gene-disease associations and unanticipated rare disorders. Eur J Hum Genet. (2016) 24:1460–6. doi: 10.1038/ejhg.2016.42
26. Coutelier M, Hammer MB, Stevanin G, Monin ML, Davoine CS, Mochel F, et al. Spastic paraplegia and ataxia network. Efficacy of exome-targeted capture sequencing to detect mutations in known cerebellar ataxia genes. JAMA Neurol. (2018) 75:591–9. doi: 10.1001/jamaneurol.2017.5121
27. Kim M, Kim AR, Kim JS, Park J, Youn J, Ahn JH, et al. Clarification of undiagnosed ataxia using whole-exome sequencing with clinical implications. Parkinsonism Relat Disord. (2020) 80:58–64. doi: 10.1016/j.parkreldis.2020.08.040
28. Rainier S, Bui M, Mark E, Thomas D, Tokarz D, Ming L, et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet. (2008) 82:780–5. doi: 10.1016/j.ajhg.2007.12.018
29. Teive HAG, Camargo CHF, Sato MT, Shiokawa N, Boguszewski CL, Raskin S, et al. Different cerebellar ataxia phenotypes associated with mutations of the PNPLA6 gene in Brazilian patients with recessive ataxias. Cerebellum. (2018) 17:380–5. doi: 10.1007/s12311-017-0909-y
30. Parodi L, Coarelli G, Stevanin G, Brice A, Durr A. Hereditary ataxias and paraparesias: clinical and genetic update. Curr Opin Neurol. (2018) 31:462–71. doi: 10.1097/WCO.0000000000000585
31. Synofzik M, Schüle R. Overcoming the divide between ataxias and spastic paraplegias: shared phenotypes, genes, and pathways. Mov Disord. (2017) 32:332–45. doi: 10.1002/mds.26944
32. Lin HC, Chang YY, Chang KH, Chen YF, Lan MY. Spastic paraparesis as the first manifestation of Machado-Joseph disease: a case report and review of the literature. Clin Neurol Neurosurg. (2018) 172:137–40. doi: 10.1016/j.clineuro.2018.06.037
33. Shi Y, Wang J, Li JD., Ren H, Guan W, He M, Yan W, et al. Identification of CHIP as a novel causative gene for autosomal recessive cerebellar ataxia. PLoS ONE. (2013) 8:e81884. doi: 10.1371/journal.pone.0081884
34. Shin Y, Klucken J, Patterson C. Hyman BT, McLean PJ. The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J Biol Chem. (2005) 280:23727–34. doi: 10.1074/jbc.M503326200
35. De Michele G, Lieto M, Galatolo D, Salvatore E, Cocozza S, Barghigiani M, et al. Spinocerebellar ataxia 48 presenting with ataxia associated with cognitive, psychiatric, and extrapyramidal features: a report of two Italian families. Parkinsonism Relat Disord. (2019) 65:91–6. doi: 10.1016/j.parkreldis.2019.05.001
36. Gazulla J, Izquierdo-Alvarez S, Sierra-Martínez E, Marta-Moreno ME, Alvarez S. Inaugural cognitive decline, late disease onset and novel STUB1 variants in SCAR16. Neurol Sci. (2018) 39:2231–3. doi: 10.1007/s10072-018-3545-5
37. Stiles AR, McDonald JG, Bauman DR, Russell DW. CYP7B1: one cytochrome P450, two human genetic diseases, and multiple physiological functions. J Biol Chem. (2009) 284:28485–9. doi: 10.1074/jbc.R109.042168
38. Kaur S, Van Bergen NJ, Verhey KJ, Nowell CJ, Budaitis B, Yue Y, et al. Expansion of the phenotypic spectrum of de novo missense variants in kinesin family member 1A (KIF1A). Hum Mutat. (2020) 41:1761–74. doi: 10.1002/humu.24079
39. Citterio A, Arnoldi A, Panzeri E, Merlini L, D'Angelo MG, Musumeci O, et al. Variants in KIF1A gene in dominant and sporadic forms of hereditary spastic paraparesis. J Neurol. (2015) 262:2684–90. doi: 10.1007/s00415-015-7899-9
40. Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. (1995) 81:769–80. doi: 10.1016/0092-8674(95)90538-3
41. Lee JR, Srour M, Kim D, Hamdan FF, Lim SH, Brunel-Guitton C, et al. De novo mutations in the motor domain of KIF1A cause cognitive impairment, spastic paraparesis, axonal neuropathy, and cerebellar atrophy. Hum Mutat. (2015) 36:69–78. doi: 10.1002/humu.22709
42. Sultana S, Reichbauer J, Schüle R, Mochel F, Synofzik M, van der Spoel AC. Lack of enzyme activity in GBA2 mutants associated with hereditary spastic paraplegia/cerebellar ataxia (SPG46). Biochem Biophys Res Commun. (2015) 465:35–40. doi: 10.1016/j.bbrc.2015.07.112
43. Martin E, Schüle R, Smets K, Rastetter A, Boukhris A, Loureiro JL, et al. Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am J Hum Genet. (2013) 92:238–44. doi: 10.1016/j.ajhg.2012.11.021
44. Gan-Or Z, Bouslam N, Birouk N, Lissouba A, Chambers DB, Vérièpe J, et al. Mutations in CAPN1 cause autosomal-recessive hereditary spastic paraplegia. Am J Hum Genet. (2016) 98:1038–46. doi: 10.1016/j.ajhg.2016.04.002
45. Peng F, Sun YM, Quan C, Wang J, Wu JJ. Two novel homozygous mutations of CAPN1 in Chinese patients with hereditary spastic paraplegia and literatures review. Orphanet J Rare Dis. (2019) 14:83. doi: 10.1186/s13023-019-1053-1
46. Chen Y, Cen Z, Zheng X, Xie F, Chen S, Luo W, et al. Novel homozygous CAPN1 pathogenic variant in a Chinese patient with pure hereditary spastic paraplegia. J Clin Neurol. (2019) 15:271–2. doi: 10.3988/jcn.2019.15.2.271
47. Shetty A, Gan-Or Z, Ashtiani S, Ruskey JA, van de Warrenburg B, Wassenberg T, et al. CAPN1 mutations: expanding the CAPN1-related phenotype: From hereditary spastic paraparesis to spastic ataxia. Eur J Med Genet. (2019) 62:103605. doi: 10.1016/j.ejmg.2018.12.010
48. Wei Q, Dong HL, Pan LY, Chen CX, Yan YT, Wang RM, et al. Clinical features and genetic spectrum in Chinese patients with recessive hereditary spastic paraplegia. Transl Neurodegener. (2019) 8:19. doi: 10.1186/s40035-019-0157-9
49. Xia ZC, Liu ZH, Zhou XX, Liu Z, Wang JL, Hu ZM, et al. Mutation analysis of CAPN1 in Chinese populations with spastic paraplegia and related neurodegenerative diseases. J Neurol Sci. (2020) 411:116691. doi: 10.1016/j.jns.2020.116691
50. da Graça FF, de Rezende TJR, Vasconcellos LFR, Pedroso JL, Barsottini OGP, França MC Jr. Neuroimaging in hereditary spastic paraplegias: current use and future perspectives. Front Neurol. (2019) 9:1117. doi: 10.3389/fneur.2018.01117
51. Stevanin G, Azzedine H, Denora P, Boukhris A, Tazir M, Lossos A, et al. Mutations in SPG11 are frequent in autosomal recessive spastic paraplegia with thin corpus callosum, cognitive decline and lower motor neuron degeneration. Brain. (2008) 131(Pt 3):772–84. doi: 10.1093/brain/awm293
52. Pensato V, Castellotti B, Gellera C, Pareyson D, Ciano C, Nanetti L, et al. Overlapping phenotypes in complex spastic paraplegias SPG11, SPG15, SPG35 and SPG48. Brain. (2014) 137(Pt 7):1907–20. doi: 10.1093/brain/awu121
53. Miyake N, Fukai R, Ohba C, Chihara T, Miura M, Shimizu H, et al. Biallelic TBCD mutations cause early-onset neurodegenerative encephalopathy. Am J Hum Genet. (2016) 99:950–61. doi: 10.1016/j.ajhg.2016.08.005
54. Edvardson S, Tian G, Cullen H, Vanyai H, Ngo L, Bhat S, et al. Infantile neurodegenerative disorder associated with mutations in TBCD, an essential gene in the tubulin heterodimer assembly pathway. Hum Mol Genet. (2016) 25:4635–8. doi: 10.1093/hmg/ddw292
55. Richter A, Rioux JD, Bouchard JP, Mercier J, Mathieu J, Ge B, et al. Location score and haplotype analyses of the locus for autosomal recessive spastic ataxia of Charlevoix-Saguenay, in chromosome region 13q11. Am J Hum Genet. (1999) 64:768–75. doi: 10.1086/302274
56. Bagaria J, Bagyinszky E, An SSA. Genetics of autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) and role of sacsin in neurodegeneration. Int J Mol Sci. (2022) 23:552. doi: 10.3390/ijms23010552
57. Hayflick SJ, Kurian MA, Hogarth P. Neurodegeneration with brain iron accumulation. Handb Clin Neurol. (2018) 147:293–305. doi: 10.1016/B978-0-444-63233-3.00019-1
58. Guo YP, Tang BS, Guo JF. PLA2G6-associated neurodegeneration (PLAN): review of clinical phenotypes and genotypes. Front Neurol. (2018) 9:1100. doi: 10.3389/fneur.2018.01100
59. Ozes B, Karagoz N, Schüle R, Rebelo A, Sobrido MJ, Harmuth F, et al. PLA2G6 mutations associated with a continuous clinical spectrum from neuroaxonal dystrophy to hereditary spastic paraplegia. Clin Genet. (2017) 92:534–9. doi: 10.1111/cge.13008
60. Chen YJ, Chen YC, Dong HL Li LX, Ni W, Li HF, et al. Novel PLA2G6 mutations and clinical heterogeneity in Chinese cases with phospholipase A2-associated neurodegeneration. Parkinsonism Relat Disord. (2018) 49:88–94. doi: 10.1016/j.parkreldis.2018.02.010
61. Koh K, Ichinose Y, Ishiura H, Nan H, Mitsui J, Takahashi J, et al. PLA2G6-associated neurodegeneration presenting as a complicated form of hereditary spastic paraplegia. J Hum Genet. (2019) 64:55–9. doi: 10.1038/s10038-018-0519-7
62. Kim YJ, Lyoo CH, Hong S, Kim NY, Lee MS. Neuroimaging studies and whole exome sequencing of PLA2G6-associated neurodegeneration in a family with intrafamilial phenotypic heterogeneity. Parkinsonism Relat Disord. (2015) 21:402–6. doi: 10.1016/j.parkreldis.2015.01.010
63. Sleigh JN, Rossor AM, Fellows AD, Tosolini AP, Schiavo G. Axonal transport and neurological disease. Nat Rev Neurol. (2019) 15:691–703. doi: 10.1038/s41582-019-0257-2
64. Matos CA, de Almeida LP, Nóbrega C. Machado-Joseph disease/spinocerebellar ataxia type 3: lessons from disease pathogenesis and clues into therapy. J Neurochem. (2019) 148:8–28. doi: 10.1111/jnc.14541
65. Renvoisé B, Chang J, Singh R, Yonekawa S, FitzGibbon EJ, Mankodi A, et al. Lysosomal abnormalities in hereditary spastic paraplegia types SPG15 and SPG11. Ann Clin Transl Neurol. (2014) 1:379–89. doi: 10.1002/acn3.64
66. Akiyama H, Ide M, Nagatsuka Y, Sayano T, Nakanishi E, Uemura N, et al. Glucocerebrosidases catalyze a transgalactosylation reaction that yields a newly-identified brain sterol metabolite, galactosylated cholesterol. J Biol Chem. (2020) 295:5257–77. doi: 10.1074/jbc.RA119.012502
67. Schüle R, Holland-Letz T, Klimpe S, Kassubek J, Klopstock T, Mall V, et al. The Spastic Paraplegia Rating Scale (SPRS): a reliable and valid measure of disease severity. Neurology. (2006) 67:430–4. doi: 10.1212/01.wnl.0000228242.53336.90
Keywords: hereditary spastic paraplegia, spinocerebellar ataxia, whole-exome sequencing, thin corpus callosum, ataxia-spasticity spectrum diseases
Citation: Lan M-Y, Lu C-S, Wu S-L, Chen Y-F, Sung Y-F, Tu M-C and Chang Y-Y (2022) Clinical and genetic characterization of a Taiwanese cohort with spastic paraparesis combined with cerebellar involvement. Front. Neurol. 13:1005670. doi: 10.3389/fneur.2022.1005670
Received: 28 July 2022; Accepted: 12 September 2022;
Published: 30 September 2022.
Edited by:
Tim W. Rattay, University of Tübingen, GermanyReviewed by:
Jeannette Hübener-Schmid, University of Tübingen, GermanyCopyright © 2022 Lan, Lu, Wu, Chen, Sung, Tu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yung-Yee Chang, Y2hhbmd5eTdAZ21haWwuY29t
†ORCID: Min-Yu Lan orcid.org/0000-0001-7361-8475
Chin-Song Lu orcid.org/0000-0002-5297-5438
Ying-Fa Chen orcid.org/0000-0001-5604-6962
Yueh-Feng Sung orcid.org/0000-0001-5984-9277
Min-Chien Tu orcid.org/0000-0002-8883-1313
Yung-Yee Chang orcid.org/0000-0001-6840-8537
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.