- 1Department of Orthopedics, Huashan Hospital, Fudan University, Shanghai, China
- 2Spine Center Fudan University, Shanghai, China
- 3Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China
- 4Department of Orthopedics, Shanghai Fifth People's Hospital, Fudan University, Shanghai, China
Hirayama disease (HD) is characterized by the juvenile onset of unilateral or asymmetric weakness and amyotrophy of the hand and ulnar forearm and is most common in males in Asia. A perception of compliance with previous standards of diagnosis and treatment appears to be challenged, so the review is to update on HD. First, based on existing theory, the factors related to HD includes, (1) cervical cord compression during cervical flexion, (2) immunological factors, and (3) other musculoskeletal dynamic factors. Then, we review the clinical manifestations: typically, (1) distal weakness and wasting in one or both upper extremities, (2) insidious onset and initial progression for 3–5 years, (3) coarse tremors in the fingers, (4) cold paralysis, and (5) absence of objective sensory loss; and atypically, (1) positive pyramidal signs, (2) atrophy of the muscles of the proximal upper extremity, (3) long progression, and (4) sensory deficits. Next, updated manifestations of imaging are reviewed, (1) asymmetric spinal cord flattening, and localized lower cervical spinal cord atrophy, (2) loss of attachment between the posterior dural sac and the subjacent lamina, (3) forward displacement of the posterior wall of the cervical dural sac, (4) intramedullary high signal intensity in the anterior horn cells on T2-weighted imaging, and (5) straight alignment or kyphosis of cervical spine. Thus, the main manifestations of eletrophysiological examinations in HD include segmental neurogenic damages of anterior horn cells or anterior roots of the spinal nerve located in the lower cervical spinal cord, without disorder of the sensory nerves. In addition, definite HD needs three-dimensional diagnostic framework above, while probable HD needs to exclude other diseases via “clinical manifestations” and “electrophysiological examinations”. Finally, the main purpose of treatment is to avoid neck flexion. Cervical collar is the first-line treatment for HD, while several surgical methods are available and have achieved satisfactory results. This review aimed to improve the awareness of HD in clinicians to enable early diagnosis and treatment, which will enable patients to achieve a better prognosis.
Introduction
Hirayama disease (HD), which is also referred to as juvenile muscular atrophy of the distal upper extremities or monomelic amyotrophy, is a special neurological disorder that was first reported by a Japanese neurologist, Keizo Hirayama (1). HD is a regional and male-prone disorder that is characterized by the juvenile onset of unilateral or asymmetric weakness and amyotrophy of the hand and forearm muscles supplied by myotomes C7-T1, with sparing of the brachioradialis muscles and no objective sensory disturbance or lower limb involvement.
Most HD patients have characteristic abnormal forward-shifting of the posterior dura (crescent-shaped loss of attachment behind the posterior dura) during neck flexion on magnetic resonance imaging (MRI), and both autopsy and neuropathologic studies have demonstrated that major lesions of HD occur primarily in locations such as the cervical anterior horn and ventral roots. Therefore, restricting neck flexion (e.g., neck collar support and surgical treatment) has become the main method of treating HD. More importantly, an increasing number of studies have demonstrated that this benign disease, if not treated early and reasonably, may cause serious dysfunction with loss of productivity (2–7). However, both neck collar support and surgical treatment have been applied in only a small number of cases due to the lack of awareness of this disease, although clinician-led guidelines on the diagnosis and treatment of HD were published in 2020 (8).
The names given to the entity herein described as Hirayama disease vary, and it is urgent to standardize the name to make it possible for physicians to identify the disease category. To our knowledge, there exist 14 different names describing the entity. We classified these into six distinct types to better understand them. First, some names containing “distal,” for instance, juvenile muscular atrophy of (a) distal upper extremity, benign juvenile muscular atrophy of the distal upper extremity, distal bimelic amyotrophy, juvenile amyotrophy of the distal upper extremity, and segmental muscular atrophy of distal upper extremity with juvenile onset, are incomplete descriptions due to the presence of proximal alone or combined distal atrophy in a great number of cases; the use of such names will mislead clinicians, likely leading to misdiagnosis or delays in diagnosis (1, 9–12). Second, monomelic amyotrophy (MMA) is a broader concept that includes crural MMA and brachial MMA; the latter includes other diseases that are characterized by atrophy of the upper limbs (13). It could be more appropriate to use the name “MMA” for similar cases that do not meet the characteristics and pathogenesis of HD and do not display other precisely defined disorders (10). Third, another term, benign focal amyotrophy, similar to MMA, involves either proximal or distal portions of the upper or lower extremities, with no compressive or traumatic etiology and arising later in life. Therefore, benign focal amyotrophy encompasses a more heterogeneous group of amyotrophies (14). Fourth, some names that include the term “spinal muscular atrophy” (SMA), for example, benign juvenile brachial SMA, distal SMA, and juvenile asymmetric segmental SMA, are not appropriate, for the reason discussed above (9–11). Fifth, cervical flexion (induced) myelopathy might be a suitable name for the entity, but it has been used relatively rarely in studies (15). We recommend that the condition continue to be designated “Hirayama disease,” or juvenile benign muscular atrophy of upper extremity, according to current knowledge of the entity. Because, as adapted from the statements of Haluk Yavuz et al., HD is a type of juvenile muscular atrophy of the upper extremity with proximal, distal, and proximodistal involvement due to radiologically proven and arrested flexion myelopathy without sensory disturbance or with minimal sensory disturbance (10). From the perspective of pathogenesis that cervical flexion plays an important part in, it may be also called as juvenile cervical flexion myelopathy properly.
In the present study, we mainly review the studies published in the last 10 years, including previous clinician-led guidelines, and report the current strategies for diagnosis and management of HD.
Epidemiology
HD was first reported in 12 cases by Hirayama in 1959, who termed it “juvenile muscular atrophy of the unilateral upper extremity.” In 1982, the hypothesis was proposed that HD is caused by the compression of the lower cervical spinal cord as a result of an imbalance of the distance between the spinal cord and the vertebral canal (16). Importantly, in 1987, the first autopsy of a patient with HD was performed, which revealed anterior horn cell (AHC) shrinkage that was most severe at C7 and C8, indicating that HD is a disease of AHCs rather than a subtype of motor neuron disease (MND) (17).
In 2006, Tashiro et al. conducted a nationwide survey of patients with HD in Japan that included information on epidemiology, period of disease progression, neurological, neuroradiological, electrophysiological and cerebrospinal fluid findings, and treatment. This is the only complete epidemiological survey that has been completed to date (18). Most HD patients are from Asia. During the last two decades, more than 900 patients with Hirayama disease have been included in clinical studies conducted in mainland China (19–23). There have been reports of increasing numbers of cases in other countries and regions including India, North America, Europe, and Australia (3, 24–27). Inadequate understanding of the disease by pediatric neurologists in North America may decrease the rate of diagnosis of HD, and there is no doubt that the number of HD patients has increased (26). In studies of HD, the reported male-to-female ratio varies: 8.3:1 (281 males to 34 females) and 20:1 in Japan; 2.8:1 in India; and 11:1 (67 males to 6 females) and 31.6:1 (348 males to 11 females) in China (18, 20, 28–30). The concealment of onset in females may also be the cause of the inconsistency of onset between males and females. Most patients suffer from HD in their late second or early third decades, predominantly beginning at the age of 17–19 (18).
Pathogenesis
In early studies, HD was recognized as a subtype of SMA and described as “juvenile asymmetric segmental spinal muscular atrophy” because the clinical manifestations were similar to those of SMA. However, genetic studies demonstrated that the form of survival motor neuron gene deletion found in patients with SMA does not occur in HD patients (31), and significant cervical structural abnormalities and the relatively good clinical prognosis of HD patients further highlighted the difference between the two diseases. Similarly, unlike patients with amyotrophic lateral sclerosis (ALS), patients with HD also present with no superoxide dismutase 1 gene deletion (32). Furthermore, through whole-exome sequencing, Lim et al. found that only two variant genes, KIAA1377 and C5orf42, are associated withHD. Thus, clinicians established HD as a new entity that differs from SMA, ALS and other MNDs (33).
Taking into account the characteristic abnormal forward shifting of the posterior dura during neck flexion in MRI, cervical cord compression during cervical flexion was considered one of the possible pathogenic conditions leading to HD. (1) Dural sac dysplasia: dural sac dysplasia could arise from the disproportionate lengths of the spinal cord and the spinal canal due to the growth spurt that occurs during puberty, leading to tightness of the spinal dura mater; this is why HD is self-limiting after puberty, and the difference in the growth rates of males and females also explains the predominance of HD in males (34–36). The anterior shifting of the tight spinal dura mater during neck flexion causes the cervical spinal cord to oppress the posterior portions of the cervical vertebrae (37). The resulting compression leads to deficits in circulation through the spinal anterior artery, and infarctions involving the AHCs in C7-T1 occur (38–40). (2) Nerve root dysplasia: the nerve root dysplasia observed in patients might be related to the short length of the cervical roots. During neck extension, slackness of the dorsal roots disappears on the affected side, on which the arm grows faster than on the other side. On neck flexion, the right dorsal roots cannot extend, and this causes the cord to be drawn anteriorly to the affected side (41). (3) Structural abnormalities of the spinal ligament: the tightness of the posterior dura mater may also be caused by loss of fibrous connective tissue, including loss of both elastic fibers and the normal wavy structure of the dura and loss of the epidural ligament (42, 43). Asymmetry of the epidural ligaments is the reason why atrophy is usually unilateral or asymmetric. (4) Venous dysplasia: engorgement of the venous plexus is considered another possible cause of compression of the spinal cord. There are three mechanisms through which the venous plexus engorges: negative pressure in the posterior epidural space due to anterior displacement of the dura; impaired venous drainage in the jugular veins during cervical flexion and consequent diversion of flow toward the posterior epidural plexus; and shifting of blood to the posterior epidural compartment from the compressed anterior venous plexus (44). Importantly, both autopsies and neuropathologic studies have demonstrated that major lesions of HD occur primarily in locations such as the cervical anterior horn and the ventral roots. Therefore, ischemic injury of the cervical anterior horn and/or nerve root caused by excessive forward displacement of the posterior dura during neck flexion has become the main current hypothesis regarding the pathogenic mechanism leading to HD.
Some studies have shown that immunological factors may contribute to the pathological process. Kira et al. reported a high frequency of coexistent atopic disorders, hyperIgEaemia, and mite antigen-specific IgE, together with a family history of allergic disorders, in their patients, strongly suggesting the presence of an atopic tendency in HD (45). Ito et al. found that hyperIgEaemia was related to severe disabilities in HD patients and that there might be a negative correlation between IgE levels and the duration of disease progression (46). In addition to IgE, Petrova et al. found that IgA deficiency may contribute to vulnerability of the spinal cord to ischemia in HD patients (47). Anti-GQ1b antibody, an antiganglioside antibody that has also been found in patients, appeared not to be reliable for HD diagnosis and unsuitable as either a diagnostic or a prognostic factor (48). To our knowledge, all immunological studies related to HD have been retrospective studies of small sample groups; therefore, we are unable to assess the causality between immunological factors and outbreaks of HD.
In recently published studies, Khadilkar et al. reported that their patients had longer necks than did normal patients (49). Li et al. found that the imbalance between the strengths of flexor and extensor muscles that was found in HD patients during cervical flexion resulted in dynamic instability and showed that it was a risk factor for spinal cord atrophy. However, all of the above findings need to be confirmed in future studies (50). Wang et al. found that the neutral and flexion Cobb angles in HD patients decreased from C2/3 to C5/6 but increased at C6/7; this may be another cause of the observed dynamic instability (51).
Clinical Manifestations
Typical Clinical Manifestations
Since HD was first reported, clinicians have continuously updated their understanding of the clinical manifestations of the disease. In the past 10 years in particular, the continuous improvement in clinicians' awareness of the disease and the numerous relevant studies have enabled the compiling of the common clinical manifestations of HD (1, 8, 18, 23, 29), which comprise five primary symptoms and manifestations: (1) distal weakness and wasting, predominantly on the ulnar side, in one upper extremity or asymmetrically in both upper extremities. Most patients show more severe muscle atrophy on the ulnar side, a condition termed “reverse split hand syndrome,” which may be a feature that distinguishes HD from ALS. The AHCs that innervate the hypothenar muscles are most susceptible to this type of damage, whereas death due to apoptosis appears to more prominently affect the AHCs that innervate the radial muscles in ALS (52); (2) insidious onset and initial progression for 3–5 years, followed by arrest of the disease or a relatively benign period (1). (3) irregular, coarse tremors in the fingers of the affected hand(s); (4) mild transient worsening of symptoms when the individual is exposed to a cold environment. A previous study reported that cold paresis was observed in 97% of HD patients (53), patients with cold paresis have a longer strength-duration time constant and higher refractoriness than patients without cold paresis. It is possible that cooling the nerve causes a decrease in sodium-potassium adenosine triphosphant activity, which results in transient depolarization due to the inactivation of the electrogenic sodium pump (54); (5) absence of objective sensory loss (Figure 1).
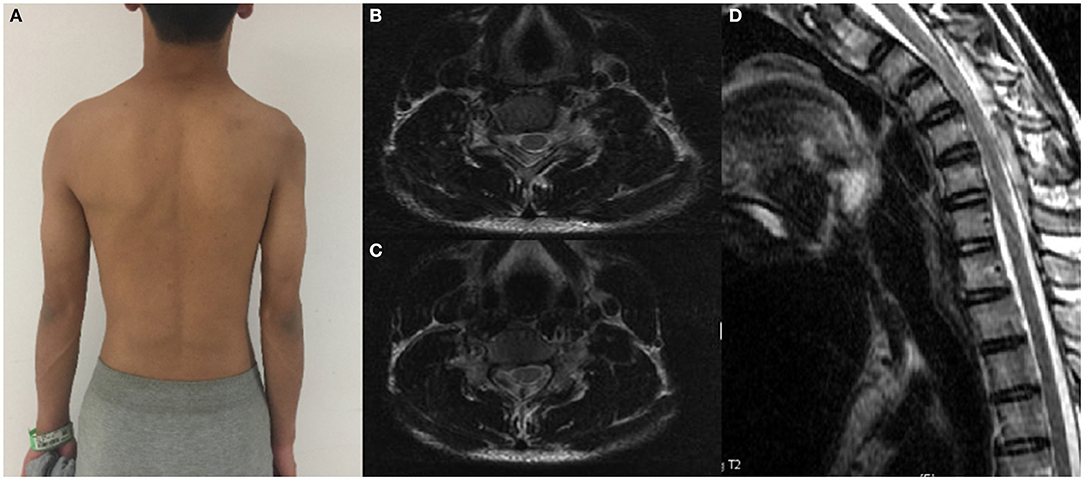
Figure 1. A patient with significant atrophy of the right proximal muscles of the upper extremity and his cervical flexion MRI. (A) Proximal muscle atrophy of right upper extremity. (B,C) Spinal cord flattening in horizontal view. (D) LOA in sagittal view.
The key problem with the above list is that an increasing number of cases with atypical symptoms are diagnosed by MRI or EMG. In such cases, we should recognize and intervene to avoid poor prognosis due to misdiagnosis and delay in treatment. With the progression of the disease, these symptoms will appear when HD reaches a severe stage rather than remaining absolutely atypic.
Atypical Clinical Manifestations
Pyramidal Signs
HD is considered a disease of AHCs, but pyramidal signs, especially the Hoffmann sign, appear in many patients. It was found in 2.4% of patients in Japan and 10.6% in China (18, 29). The pyramidal sign is assumed to be a consequence of a severe degree of spinal cord injury, and whether or not the pyramidal tract shows disorders depends on the severity of HD (6, 55).
Atrophy of the Muscles of the Proximal Upper Extremity
The compression associated with HD is usually restricted to the lower cervical spinal cord, mainly at the C7-T1 level; thus, the atrophy observed in most patients is located in the distal upper extremity. However, in not a few reported cases the symptoms emerge proximally, and some of these patients were subclinical cases (13, 18, 32) (Figure 2). Preethish-Kumar et al. showed that distal, proximal, and proximodistal bimelic amyotrophy are all myelopathies that can be induced by cervical flexion (40). Thus, the functional outcome of proximal forms is likely worse and more uncertain than that of distal forms (14, 56).
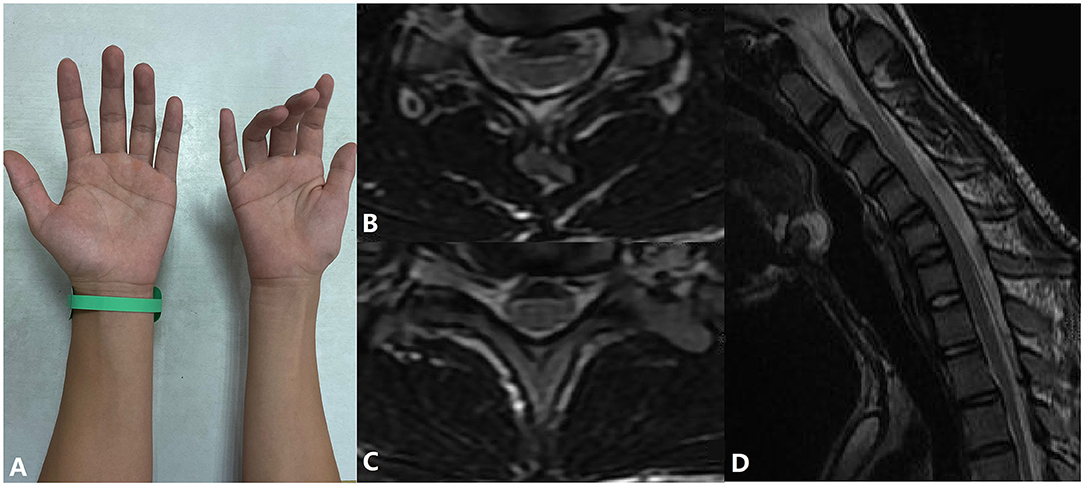
Figure 2. A patient with atrophy of the muscles of the right hand and forearm and his cervical flexion MRI. (A) Inability to extend fingers and muscle atrophy in the patient's right hand. (B,C) Spinal cord flattening and LOA in horizontal view. (D) LOA in sagittal view.
Long Progression
In the majority of HD patients, the progression of the disease is self-limiting, where progression occurs over <5 years. However, there have indeed been cases in which the disease progressed over more than 10 years, where the patient's condition continued to deteriorate after a stable period (44). The changes observed on imaging do not improve as the disease progresses, which indicates that HD may not be a self-limiting disease in some cases (57).
Sensory Deficits
A number of patients with sensory deficits have been reported. These deficits occur because compression injures the tracts that conduct information from the sensory organs, and the deficits likely become more severe due to higher and higher stress (25). According to Tashiro et al., 64 of 333 (19.2%) HD patients had sensory deficits (18).
Importantly, the clinician-led guidelines have added some of these symptoms to the list of symptoms that should be considered in the diagnosis of HD, and as physicians, we should reevaluate our stereotypes (8). First, some patients with HD may experience disease progression after an interval in which the disease remains at a stable stage. Second, in some patients with HD, the duration of the disease may be more than 5 years. Third, HD allows the presence of clinical symptoms (including atrophy, weakness, and denervation) of symmetrical functional involvement of both upper limbs. In addition, a small number of patients with HD may experience subjective paresthesia in the upper limbs during the early stage of the disease. Finally, pyramidal tract signs can be present, in a certain percentage of patients.
Imaging Manifestations
Almost all HD patients show straight alignment or kyphosis of the cervical spine; this is common but not specific to HD. Myelography shows forward movement of the posterior dural wall during neck flexion. According to Chen et al., some signs that appear on neutral-position MRI require our attention (58). These are (1) asymmetric cord flattening and localized lower cervical cord atrophy due to compression by the tight posterior dural wall, mostly at the C4 to C7 level; (2) abnormal cervical curvature (straight or kyphotic cervical spine alignment); (3) loss of attachment between the posterior dural sac and the subjacent lamina (however, this is considered a neck-flexion MRI sign in some studies because it also manifests in normal people when they are in the cervical neutral position) (59); (4) non-compressed intramedullary high signal intensity on T2-weighted imaging (T2WI), a sign that can be caused by ischemia or necrosis of AHC. A symmetrical bilateral high-signal-intensity lesion on axial T2WI is found in some HD patients; this sign has been termed “snake-eye appearance” because it resembles the face of a snake. Furthermore, the presence of snake-eye appearance indicates an irreversible lesion, a poor prognosis and the need for timely surgical intervention (19).
Neck-flexion MRI is considered the most important imaging examination for the diagnosis of HD; it reveals the pathogenesis of HD and compression of the lower cervical spinal cord due to the abnormal tightness of the dural sac during cervical flexion. Signs include forward displacement of the posterior wall of the cervical dural sac, cord flattening, atrophy, and the presence of a crescent-shaped high-intensity mass.
In HD, lower cervical cord flattening and atrophy are present in the neck-flexion MRI, similar to the neutral position (30, 60). Another hallmark sign is the forward displacement of the spinal cord due to the presence of a shorter dural sac. The disproportionate distance between the vertebrae and their contents due to the juvenile growth spurt and the suspended dura mater anchored only at C2 to C3 and coccyx result in tightness of the dural sac during neck flexion, causing forward displacement of the posterior wall of the cervical dural sac followed by forward displacement of the cervical spinal cord and cord flattening. Consequently, microcirculatory disturbances in the region served by the anterior spinal artery caused by long-term compression bring about ischemia and necrosis of AHCs; as a result, the cord appears thin due to atrophy. In addition, a crescent-shaped high-intensity mass with curvilinear flow-void signals inside it appears in the posterior epidural space during neck flexion. The mass disappears when the neck returns to its normal position, revealing congestion of the venous plexus rather than vascular malformation or tumors (61). The congestion of the venous plexus is due to the three factors discussed above. Postgadolinium T1 fat-suppressed images show an enhanced posterior epidural venous plexus with flow voids within it at the affected levels of the spinal cord and asymmetric flattening of the affected hemicord (62). Furthermore, forward displacement of the posterior wall of the cervical dural sac, LOA, and the presence of a crescent-shaped high-intensity mass are different imaging manifestations of the same cause.
All signs detected on neck-flexion MRI indicate the pathogenesis of HD and correspond to clinical manifestations. Regardless of the severity of the patient's symptoms and the neutrality of the position MRI shown in the MRI, neck-flexion MRI is required to obtain a definite diagnosis of HD. Although there are no optimal flexion requirements for cervical-flexion MRI, a cervical flexion angle of 35° is recommended to achieve the best appearance and an accurate diagnosis (37).
Diffusion tensor imaging (DTI), a functional imaging technique, is based on the Brownian motion exhibited by water molecules in tissues and can potentially be utilized in the diagnosis of HD (63) A higher apparent diffusion coefficient (a measure of the magnitude of water molecular diffusion) and lower fractional anisotropy (the fraction of the total diffusion that is measured in one specific voxel) are potential diagnostic parameters for HD in DTI (64). DTI is used not only to evaluate spinal cord injury and the prognosis of spinal cord diseases involving HD but also to choose the level at which to operate. Functional imaging is an important issue for future research, and further work is required to establish the usefulness of DTI in diagnosing HD accurately and as early as possible. Blood oxygenation level dependent-functional MRI of HD patients showed significant ipsilateral activation more frequently in symptomatic hand tasks than in asymptomatic hand tasks, and activation of both the contralateral and ipsilateral primary motor areas was stronger during symptomatic hand tasks. In addition, the activation was reduced after surgery (65).
Electrophysiological Examinations
Electrophysiological examinations are important for the diagnosis and differentiation of HD. The main manifestations of EMG include segmental neurogenic damage of the AHCs or anterior root of the spinal nerves located in lower cervical spinal cord, without disorders of the sensory nerves. EMG is commonly used in the neurological examination of HD patients. It shows neurogenic lesions in patients in whom the disease has been present for a short period of time (these are indicated by fibrillations and positive sharp waves) and decreased motor unit number estimation (MUNE) and large potentials (due to denervation and re-innervation) in patients in whom the disease has been present for a longer period (66). The presence of spontaneous potentials indicates that the disease is in the progressive phase (20, 67). More importantly, cases with subclinical unilateral symptoms on the affected contralateral side were found, perhaps reminding us that the illness will eventually affect the upper extremities bilaterally if the intervention is not effective or does not occur in time (20).
Motor nerve conduction studies have shown decreased amplitudes and delayed latencies in the affected upper limbs muscles without abnormal conduction velocities (68). The ulnar/median compound muscle action potential ratio is decreased in HD patients, which is consistent with symptoms that indicate that the ulnar side of the affected limb is more severely affected. In MND patients, an increase in the ulnar/median-ratio has been reported. Sensory nerve conduction studies typically yield normal results in HD patients, which correspond to the clinical manifestation and pathogenesis of the disease (20, 69). Thus, generally, conduction velocities do not change, although if abnormal conduction velocities are found, peripheral nerve lesions should first be considered.
F waves in HD patients show decreased frequency and conduction velocity but normal latency (20). F-wave studies in HD patients have yielded varied result: Hassan et al. found that F-wave conduction velocities normal in HD, whereas Zheng et al. reported a delay in F-wave latency in HD (68, 70). The latter study reported a significant increase in the percentage of median repeater F waves, which may be attributed to reinnervation by a distal (and/or proximal) axonal sprouting mechanism or supramaximal stimulation, which preferentially activates large motor neurons that produce the F wave during neck flexion (70). Moreover, repeater F waves were observed, the percentage of ulnar and median repeater F waves significantly increased, and higher average peak-to-peak amplitude of F-waves expressed as a percentage of the maximal M-wave amplitude were observed on the symptomatic side during neck flexion, compared with during a neutral posture., which supports the idea that remnant AHCs are inhibited during cervical flexion. Delayed F-wave latency, reduced conduction velocity, and lower persistence are expected in some cases of cervical spondylotic myelopathy, whereas normal or lower persistence is expected in MND, which helps practitioners distinguish between these conditions. Results of studies that examined somatosensory evoked potentials (SEP) and motor evoked potentials (MEP) in HD patients vary considerably. Abraham et al. found that the latency of SEPs was normal in HD, whereas Park et al. reported that the N13–N20 interpeak latency during neck flexion was significantly correlated with the presence of HD (71, 72). For MEP, decreased amplitude, longer central motor conduction time, and delayed latency have been observed in HD patients, as well as a significant reduction in the upper limb amplitude of MEPs on cervical stimulation compared with that in the neutral position, although conclusions drawn in several studies are inconsistent (71, 73). In contrast, SEPs are not show any significantly different between the neutral and cervical positions in HD patients (71, 73). Recently, it was found that the amplitude of intraoperative MEP increases during surgery, and this persists until the end of the surgical procedure. Therefore, the amplitude of intraoperative MEP may help define an adequate fixation position (74).
MUNE is a quantitative method that can be used to estimate the number of motor units innervating a muscle or a muscle group. This provides a direct index through which to measure the severity and progress of HD and the effects of treatment. MUNE values are negatively correlated with illness duration, and a decreased MUNE value in a patient with normal muscle strength indicates that the MUNE value changed before the symptoms emerged (75). The motor unit number index also shows subclinical changes that appear as a decrease before muscle strength changes (76).
Diagnosis and Classification
Diagnostic Criteria
Recently, numerous reports have shown that HD patients exhibit the pyramidal sign, a long period of disease progression, and sensory deficits, which suggests that the diagnostic criteria above are no longer suitable for the current clinical understanding of HD. Previously, in clinical practice, we found that when clinical manifestations are used as the primary basis for diagnosis, diagnosis is delayed. However, it is now widely recognized that medical imaging and EMG, especially neck-flexion MRI, are of significant value in the diagnosis of HD. Thus, it was necessary to update HD diagnostic criteria, which led to the development of the Huashan diagnostic criteria for HD. These criteria take into account clinical manifestations, medical imaging manifestations, and electrophysiological examinations, based on recent reports and our experiences (29). The details are as follows (Table 1).
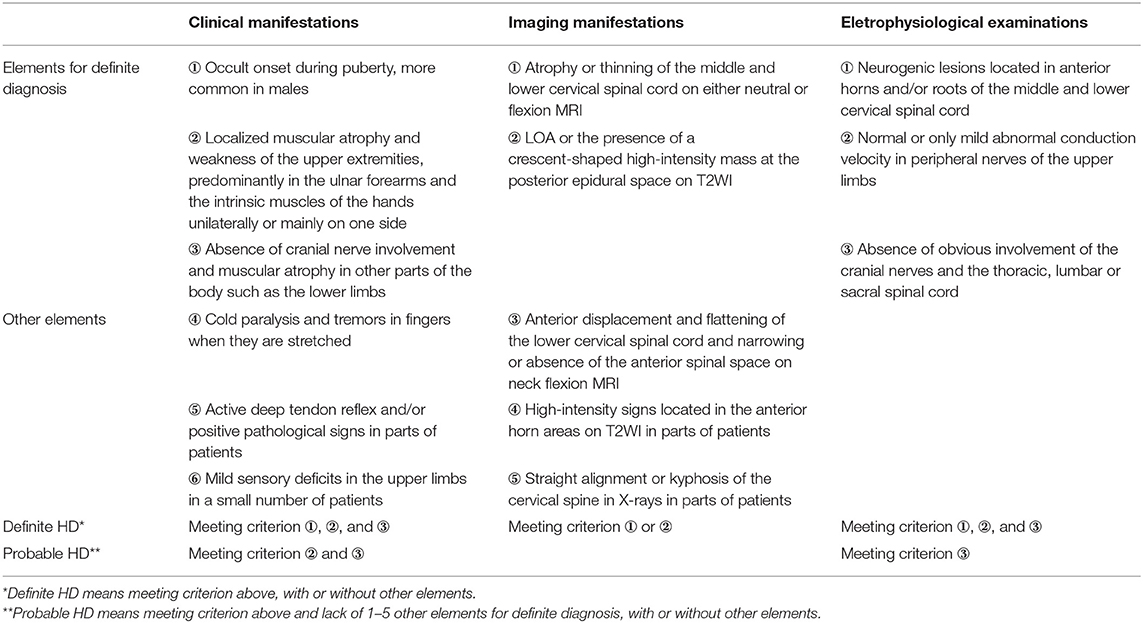
Table 1. The Huashan diagnostic criteria for Hirayama disease (29).
Definite HD refers to individuals with all ①–③ elements for definite diagnosis in both “clinical manifestations” and “electrophysiological examinations”, and one of the two elements for definite diagnosis in “imaging manifestations”, with or without some of the other elements. Probable HD is defined by: meeting criterion ②, ③ in “clinical manifestations” and criterion ③ in “electrophysiological examinations”, but missing 1–5 of the other elements for definite diagnosis, with or without some of the other elements.
In brief, definite HD needs 3-dimensional diagnostic framework, while probable HD needs to exclude other diseases via “clinical manifestations” and “electrophysiological examinations”.
Compared the new diagnostic criteria with diagnostic criteria proposed by Hirayama (1), “progressive aggravation within 3–5 years after onset” is not mentioned, and localized muscular atrophy of the distal upper limbs, lack of sensory disturbance, and lack of pyramidal tract signs are no longer emphasized. The new criteria specify a clear move forward of the cervical spinal cord, flatten of the spinal cord under MRI during neck flexion, a crescent-shaped hyperintensity shadow (LOA) on T2WI, and segmental nerve injuries limited to the lower cervical segment and appearing mostly in segments C7/8–T1.
Differential Diagnosis
HD is a local abnormality of the cervical spine, with atrophy and weakness of the upper limb muscles as the main clinical manifestations, which lead to neurological dysfunction. Therefore, it is necessary to perform a differential diagnosis from common diseases that cause muscular atrophy of the upper limbs, such as ALS-related diseases, cervical spondylosis, peripheral nerve compression syndrome, (e.g., carpal tunnel, cubital tunnel, supinator, and thoracic outlet syndromes), syringomyelia, intramedullary neoplasm, and ossification of the posterior longitudinal ligament of the cervical spine.
Using age of onset, alongside cervical MRI, computed tomography, and other imaging examinations, HD can be clinically distinguished from syringomyelia, intramedullary neoplasm, and ossification of the posterior longitudinal ligament of the cervical spine. Moreover, upper limb peripheral nerve compression diseases can be excluded with electrophysiological examinations.
Cervical spondylotic amyotrophy and ALS can be easily confused with HD in the early stage of the disease (8, 77–79). Clinical identification needs to be considered from numerous perspectives, such as age of onset, clinical manifestations, and imaging and electrophysiological examination results (Table 2).
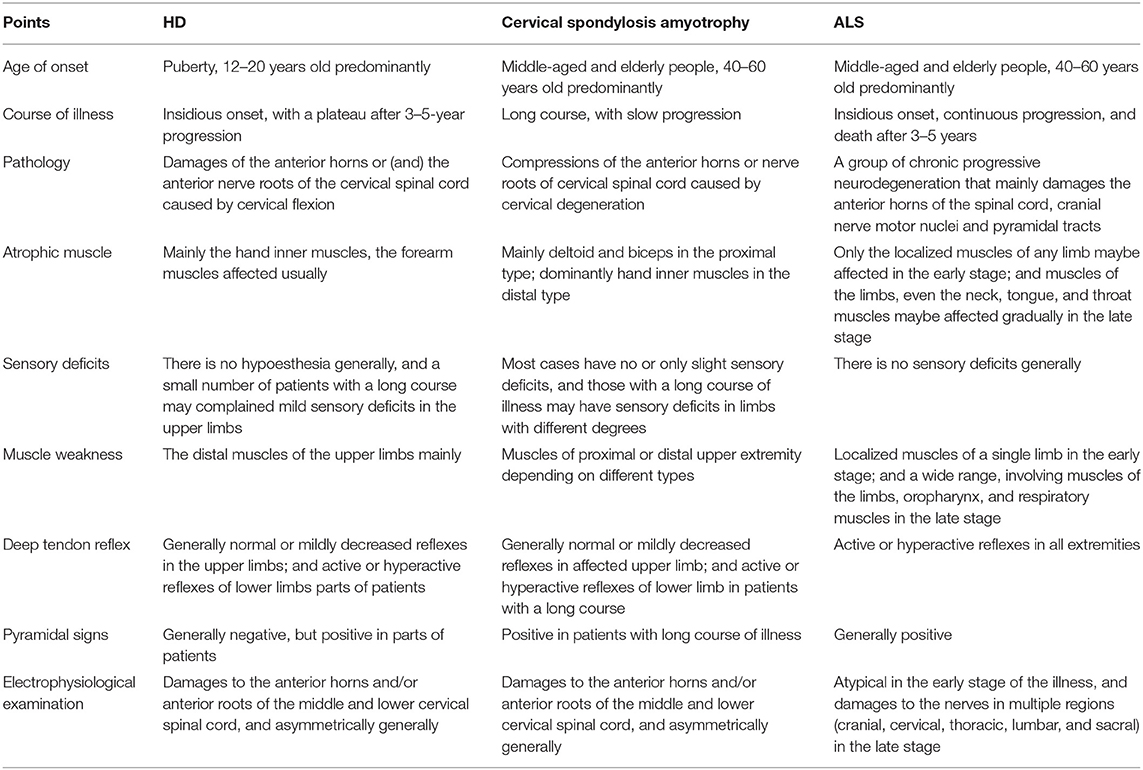
Table 2. Clinical differential diagnoses among HD, cervical spondylotic amyotrophy, and ALS (8, 77, 78).
Clinical Classification
With the advances in clinical understanding of HD, it has been found that there is considerable variation in the clinical manifestation and severity between HD patients. Moreover, whether HD patients are in the advanced or stationary stage significantly influences the choice of intervention options; thus, appropriate clinical classifications must be adopted. As such, several scholars have attempted to classify HD into three types, to classify and evaluate HD patients (29, 80). Type I patients display atrophy of the inner muscles of the hand and forearm in the upper limb unilaterally or mainly on one side. Depending on whether the symptoms have progressed and whether electrophysiological examinations have been conducted in the past 6 months, type I can be further divided into a stable period (subtype Ia) or a progression period subtype (subtype Ib). Type II patients exhibit atrophy of the inner muscles of the hand and forearm in the upper limb unilaterally or mainly on one side, as well as pyramidal tract injury (active or hyperactive knee reflex, and positive Hoffmann signs). Type III represents atypical HD, in which there may be atrophy of the proximal muscles of the upper limb, symmetrical and bilateral muscle atrophy of the upper limbs, and sensory disturbances associated with upper limb numbness (Table 3). Patients whose symptoms fit more than one classification should be preferentially assigned the higher-level classification.
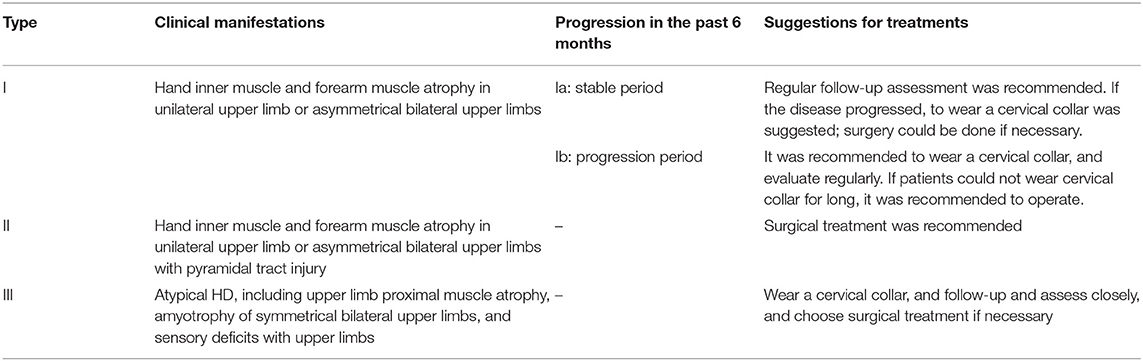
Table 3. The Huashan clinical classification system for HD and the suggestions of treatment (29, 80).
Treatment
Non-surgical Treatment
Although HD is self-limiting, it is necessary to reach an early diagnosis and intervene effectively. Non-surgical treatment using cervical collars is the first-line treatment for halting the progression of the illness in many patients; this method prevents the further progression of HD by restricting neck movement and preventing compression injury to the spinal cord during flexion (81). Cortese et al. found that the venous plexus decreased in size after this treatment (82). According to Rajeev et al. and Rajesh et al., subjective muscle strength increases after the use of cervical braces (83, 84). Narayana et al. reported that cervical collar treatment was effective for patients with Hirayama disease (85). Tokumaru et al. evaluated the therapeutic effects of the use of cervical collars by 38 patients with Hirayama disease by comparing them with 45 patients with untreated Hirayama disease (81). The results showed that the duration of the disease decrease with cervical collar treatment, and 15 patients who were within 2.5 years of onset even experienced relief of muscle weakness and cold paralysis, suggesting that cervical collar treatment is effective. At the same time, early diagnosis is very important, and it was pointed out that early diagnosis and clinical intervention might reduce the dysfunction that typically develops in adolescent patients (82). A shorter disease duration compared to the untreated group was also reported in numerous studies. Nonsurgical treatment is still the most commonly used treatment in HD patients.
There are several problems with the use of cervical collars. First, the collar must be worn for 24 h daily for 3–4 years, and this could be unbearable to a high number of patients (86, 87). Second, in some cases, the cervical collar will halt the progression of the disease but will not improve the objective indicators due to the decrease in MUNE (88). The reason why muscle strength increases after treatment is that the reinnervation process can maintain strength (89). Third, a few reports show that the disease can worsen during a period of conservative treatment (3). The results would probably be irreversible if significant deterioration occurred while patients were receiving treatment with a cervical collar.
In addition, it has been shown that drugs are ineffective (90). Exercise therapy aimed at strengthening the cervical spine muscles is widely applied to improve cervical spine function and has been proven to be beneficial (91).
Surgical Treatment
A study of 73 HD patients showed that nearly 80% of patients wore it for less than 6 months, indicating poor compliance with wearing a neck brace (92). Therefore, in recent decades, surgical procedures have been performed. The effects of surgery include not only subjective symptom relief but also improvement on objective examination. On the one hand, surgery shortens the natural duration of the illness, as shown by the clinical signs and EMG, lack of recurrence of venous congestion, and improved grip strength attributed to the recovery of muscles other than the atrophic intrinsic muscles (3, 21). On the other hand, intraoperative EMG showed immediate changes with surgical intervention, including variations in abnormal F waves and MEP (74, 93). At the same time, some improvements visible on imaging also appeared after the surgeries, including reduced range of cervical flexion motion and angle mobility of the cervical spine and increased cervical lordosis and spinal cord area (21). The idea that surgery plays an important role in the treatment of HD is agreed upon by experts in various fields (8, 21).
Surgical treatment is effective; the indications for surgery might include (1) progressive course and intolerance of the cervical collar; (2) ineffectiveness of conservative treatment; and (3) the presence of serious symptoms, a serious degree of spinal cord atrophy, the occurrence of spinal cord atrophy in rare segments, and positive pyramidal signs (8, 29).
Various surgical approaches have been utilized in clinical practice during the last two decades. These surgical approaches include laminectomy, duraplasty, corpectomy, discectomy, decompression, and fusion (3, 6, 21, 34). One or more of these approaches are selected. The procedures used can be divided into two categories: posterior approaches and anterior approaches. Posterior approaches include multilevel posterior cervical facetal fixation, short-segment posterior cervical fixation without vertebra fusion or removal of the internal fixation, cervical laminectomy and micro-resection of the posterior venous plexus, and posterior lateral mass screw fixation without decompression or fusion but with removal of the internal fixation after four years of follow-up. Anterior approaches include anterior cervical fusion or anterior cervical discectomy and fusion using intervertebral disc fusion polyetheretherketone cages or autogenous iliac bone placement (3, 21, 88, 94, 95). Various scholars have reported on the fusion of segments 1–4 based on different considerations (5, 21, 88, 96).
Early surgical treatment may not only prevent the progressive loss of motor units but also restore motor units that have undergone functional inhibition and incomplete necrosis in some HD patients (93). However, a meta-analysis showed that it is still unclear what type of surgery produces optimal results (97). Not all patients benefit from surgery, and caution should be employed when selecting patients for surgery (98).
Prognosis
Most HD patients are given a benign prognosis for 3–5 years, followed by arrest of the disease (8). The management of cervical collar immobilization has been shown to be effective in 57.2% of cases (18). Furthermore, 46.3–87.6% of cases who accepted surgery reached an ideal stage at follow-up (34, 97, 99–101). Nervertheless, there still exist a few cases have a poor prognosis whose spastic gait persists after treatment due to non-timely treatment (2). Moreover, some patients experience atrophy or weakness of the upper limb again following a stable period (102).
Conclusion and Future Direction
HD is a rare benign disease characterized by muscular atrophy of the unilateral or asymmetric bilateral upper extremities. It predominantly affects juvenile Asian males. As cases have been reported all over the world and research on this disease has been conducted at a rapid pace during the last two decades, some novelties need to be discussed. These problems have received a great deal of attention.
The pathogenesis of HD is still unknown, but most scholars believe that it is related to a mismatch between the length of the spinal cord and that of the spinal canal that occurs due to a growth spurt. Neck flexion MRI is the main auxiliary examination for HD; on such examination, affected individuals show spinal cord flatness, atrophy, LOA, and T2WI hyperintensity. Electrophysiologically, HD is mainly characterized by neurogenic damage without abnormal nerve conduction velocity. In terms of treatment, long-term cervical braces are the mainstay. For individuals who cannot tolerate a cervical brace or who experience short-term disease progression, surgical treatment is feasible, but the optimal surgical treatment has not been identified. The entity needs to be recognized by clinicians.
In the future, further research on the pathogenesis of HD is needed to gain a better understanding of the disease and develop more effective treatments. The surgical approach remains controversial, and thus, research prospects are broad. Additionally, a more far-reaching exploration of the optimization of the selection of surgical segments is crucial. We hope to improve clinicians' understanding of HD to allow early detection and treatment, which will offer better prognoses.
Author Contributions
HwW and HlW conceptualized the review. HwW, YT, and JW did the literature search and analysis, and wrote original draft. CZ, SL, CS, CN, and HlW did review and editing. XX, XM, FL, JJ, and HlW were responsible for supervision. All authors contributed to the article and approved the submitted version.
Funding
Clinical Research Plan of SHDC (No. SHDC2020CR4030), Clinical Technology Innovation Project of SHDC (No. SHDC12019X26), National Natural Science Foundation of China (No. 82072488), and AO Spine National Research Grant 2020 [No. AOSCN(R)2020-9].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHCs, anterior horn cells; ALS, amyotrophic lateral sclerosis; DTI, diffusion tensor imaging; EMG, electromyography; HD, Hirayama disease; LOA, loss of attachment; MEP, motor evoked potentials; MMA, monomelic amyotrophy; MND, motor neuron disease; MRI, magnetic resonance imaging; MUNE, motor unit number estimation; SEP, somatosensory evoked potentials; SMA, spinal muscular atrophy; T2WI, T2-weighted imaging.
References
1. Hirayama K, Toyokura Y, Tsubaki T. Juvenile muscular atrophy of unilateral upper extremity: a new clinical entity. Psychiatric Neurol Jpn. (1959) 61:2190–7.
2. Fujimori T, Tamura A, Miwa T, Iwasaki M, Oda T. Severe cervical flexion myelopathy with long tract signs: a case report and a review of literature. Spinal Cord Ser Cases. (2017) 3:17016. doi: 10.1038/scsandc.2017.16
3. Brandicourt P, Sol JC, Aldea S, Bonneville F, Cintas P, Brauge D. Cervical laminectomy and micro resection of the posterior venous plexus in Hirayama disease. Neurochirurgie. (2018) 64:303–9. doi: 10.1016/j.neuchi.2018.04.004
4. Chiba S, Yonekura K, Nonaka M, Imai T, Matumoto H, Wada T. Advanced hirayama disease with successful improvement of activities of daily living by operative reconstruction. Intern Med. (2004) 43:79–81. doi: 10.2169/internalmedicine.43.79
5. Dohzono S, Toyoda H, Tamura A, Hayashi K, Terai H, Nakamura H. Surgical treatment of a patient with prolonged exacerbation of hirayama disease. Spine Surg Relat Res. (2019) 3:95–7. doi: 10.22603/ssrr.2018-0037
6. Wu W, Wang S, Lin JA. 34-year-old female patient with hirayama disease complicated by severe spinal cord injury. World Neurosurg. (2019) 130:84–8. doi: 10.1016/j.wneu.2019.06.208
7. Kumar M, Athwal PSS, Rhandhawa S, Kahlon S, Shiv Kumar J. Hirayama's disease in a young male: a rare case report. Cureus. (2019) 11:e6204. doi: 10.7759/cureus.6204
8. Lyu F, Zheng C, Wang H, Nie C, Ma X, Xia X, et al. Establishment of a clinician-led guideline on the diagnosis and treatment of Hirayama disease using a modified Delphi technique. Clin Neurophysiol. (2020) 131:1311–9. doi: 10.1016/j.clinph.2020.02.022
9. Pradhan S. Bilaterally symmetric form of Hirayama disease. Neurology. (2009) 72:2083–9. doi: 10.1212/WNL.0b013e3181aa5364
10. Yavuz H. A proposal for the definition of Hirayama disease and monomelic amyotrophy. J Child Neurol. (2012) 27:815–6; author reply 7–8. doi: 10.1177/0883073812442591
11. Vitale V, Caranci F, Pisciotta C, Manganelli F, Briganti F, Santoro L, et al. Hirayama's disease: an Italian single center experience and review of the literature. Quant Imaging Med Surg. (2016) 6:364–73. doi: 10.21037/qims.2016.07.08
12. Preethish-Kumar V, Nalini A, Singh RJ, Saini J, Prasad C, Polavarapu K, et al. Distal bimelic amyotrophy (DBMA): phenotypically distinct but identical on cervical spine MR imaging with brachial monomelic amyotrophy/Hirayama disease. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:338–44. doi: 10.3109/21678421.2015.1039546
13. Nalini A, Gourie-Devi M, Kandavel T, Ramalingaiah A. Monomelic amyotrophy: clinical profile and natural history of 279 cases seen over 35 years (1976-2010). Amyotroph Lateral Scler Frontotemporal Degener. (2014) 15:1–9. doi: 10.3109/21678421.2014.903976
14. Cintas P. Benign focal amyotrophy. Rev Neurol. (2017) 173:338–44. doi: 10.1016/j.neurol.2017.03.016
15. Polavarapu K, Preethish-Kumar V, Nashi S, Vengalil S, Prasad C, Bhattacharya K, et al. Intrafamilial phenotypic variations in familial cases of cervical flexion induced myelopathy/Hirayama disease. Amyotroph Lateral Scler Frontotemporal Degener. (2018) 19:38–49. doi: 10.1080/21678421.2017.1374977
16. Yada K. Spinal cord lesion due to relative imbalance of cervical spine and cervical cord (author's transl.). 1981 Annual Report of Prevention and Treatment for the Congenital Anomalies of the Spine and Spinal Cord. (1982), 48–55.
17. Hirayama K, Tomonaga M, Kitano K, Yamada T, Kojima S, Arai K. Focal cervical poliopathy causing juvenile muscular atrophy of distal upper extremity: a pathological study. J Neurol Neurosurg Psychiatry. (1987) 50:285–90. doi: 10.1136/jnnp.50.3.285
18. Tashiro K, Kikuchi S, Itoyama Y, Tokumaru Y, Sobue G, Mukai E, et al. Nationwide survey of juvenile muscular atrophy of distal upper extremity (Hirayama disease) in Japan. Amyotroph Lateral Scler. (2006) 7:38–45. doi: 10.1080/14660820500396877
19. Xu H, Shao M, Zhang F, Nie C, Wang H, Zhu W, et al. Snake-Eyes Appearance on MRI occurs during the late stage of hirayama disease and indicates poor prognosis. Biomed Res Int. (2019) 2019:9830243. doi: 10.1155/2019/9830243
20. Wang X-n, Cui L-y, Liu M-s, Guan Y, Li B-h, Du H. A clinical neurophysiology study of Hirayama disease. Chin Med J. (2012) 125:1115–20. doi: 10.3760/cma.j.issn.0366-6999.2012.06.027
21. Zhang H, Wang S, Li Z, Shen R, Lin R, Wu W, et al. Anterior cervical surgery for the treatment of Hirayama disease. World Neurosurg. (2019) 127:e910–e8. doi: 10.1016/j.wneu.2019.03.295
22. Fu Y, Sun Q, Han H, Hou C, Zhang J, Kang D, et al. Study of association between hyperlgEaemia and Hirayama disease. Natl Med J China. (2010) 90:2629–32. doi: 10.3760/cma.j.issn.0376-2491.2010.37.010
23. Zhou B, Chen L, Fan D, Zhou D. Clinical features of Hirayama disease in mainland China. Amyotroph Lateral Scler. (2010) 11:133–9. doi: 10.3109/17482960902912407
24. Sim K, Gaillard F, Day T. Anterior displacement of the spinal cord on flexion views in a patient with unilateral arm weakness. Hirayama's disease. J Clin Neurosci. (2014) 21:487–539. doi: 10.1016/j.jocn.2013.06.028
25. Dejobert M, Geffray A, Delpierre C, Chassande B, Larrieu E, Magni C. Hirayama disease: three cases. Diagn Interv Imaging. (2013) 94:319–23. doi: 10.1016/j.diii.2012.10.008
26. Ghosh PS, Moodley M, Friedman NR, Rothner AD, Ghosh D. Hirayama disease in children from North America. J Child Neurol. (2011) 26:1542–7. doi: 10.1177/0883073811409226
27. Boruah D, Sanyal S, Prakash A, Achar S, Dhingani D, Sarmah B. Bimelic symmetric Hirayama disease: spectrum of magnetic resonance imaging findings and comparative evaluation with classical monomelic amyotrophy and other motor neuron disease. Iran J Neurol. (2017) 16:136–45.
28. Hashimoto O, Asada M, Ohta M, Kuroiwa Y. Clinical observations of juvenile nonprogressive muscular atrophy localized in hand and forearm. J Neurol. (1976) 211:105–10. doi: 10.1007/BF00313354
29. Wang H, Zheng C, Jin X, Lyu F, Ma X, Xia X, et al. The Huashan diagnostic criteria and clinical classification of Hirayama disease. Chin J Orthop. (2019) 39:458–65. doi: 10.3760/cma.j.issn.0253-2352.2019.08.003
30. Hirayama K. Juvenile Muscular atrophy of distal upper extremity (Hirayama Disease). Intern Med. (2000) 39:283–90. doi: 10.2169/internalmedicine.39.283
31. Misra U, Kalita J, Mishra V, Kesari A, Mittal BA. Clinical, magnetic resonance imaging, and survival motor neuron gene deletion study of Hirayama disease. Arch Neurol. (2005) 62:120–3. doi: 10.1001/archneur.62.1.120
32. Robberecht W, Aguirre T, Van Den Bosch L, Theys P, Nees H, Cassiman J-J, et al. Familial juvenile focal amyotrophy of the upper extremity (Hirayama disease): superoxide dismutase 1 genotype and activity. Arch Neurol. (1997) 54:46–50.
33. Lim YM, Koh I, Park YM, Kim JJ, Kim DS, Kim HJ, et al. Exome sequencing identifies KIAA1377 and C5orf42 as susceptibility genes for monomelic amyotrophy. Neuromuscul Disord. (2012) 22:394–400. doi: 10.1016/j.nmd.2011.11.006
34. Ito H, Takai K, Taniguchi M. Cervical duraplasty with tenting sutures via laminoplasty for cervical flexion myelopathy in patients with Hirayama disease: successful decompression of a “tight dural canal in flexion” without spinal fusion. J Neurosurg Spine. (2014) 21:743–52. doi: 10.3171/2014.7.SPINE13955
35. Kohno M, Takahashi H, Yagishita A, Tanabe H. “Disproportion theory” of the cervical spine and spinal cord in patients with juvenile cervical flexion myelopathy—extension movements of the head and spine upon the spinal cord and nerve roots. Surg Neurol. (1998) 50:421–30. doi: 10.1016/S0090-3019(97)00451-5
36. Ding Y, Rong D, Wang X, Li C. The evaluate the cervical spine curvature and growth rate for studying the pathogenesis of Hirayama disease in adolescents. Chin J Intern Med. (2015) 54:721–4. doi: 10.3760/cma.j.issn.0578-1426.2015.08.015
37. Hou C, Han H, Yang X, Xu X, Gao H, Fan D, et al. How does the neck flexion affect the cervical MRI features of Hirayama disease? Neurol Sci. (2012) 33:1101–5. doi: 10.1007/s10072-011-0912-x
38. Fujimoto Y, Oka S, Tanaka N, Nishikawa K, Kawagoe H, Baba I. Pathophysiology and treatment for cervical flexion myelopathy. Eur Spine J. (2002) 11:276–85. doi: 10.1007/s005860100344
39. Elsheikh B, Kissel JT, Christoforidis G, Wicklund M, Kehagias DT, Chiocca EA, et al. Spinal angiography and epidural venography in juvenile muscular atrophy of the distal arm “Hirayama disease”. Muscle Nerve. (2009) 40:206–12. doi: 10.1002/mus.21307
40. Xu X, Han H, Gao H, Hou C, Fan D, Fu Y, et al. The increased range of cervical flexed motion detected by radiographs in Hirayama disease. Eur J Radiol. (2011) 78:82–6. doi: 10.1016/j.ejrad.2010.08.012
41. Toma S, Shiozawa Z. Amyotrophic cervical myelopathy in Adolocent. J Neurol Neurosurg Psychiatry. (1995) 58:56–64. doi: 10.1136/jnnp.58.1.56
42. Yoshiyama Y, Tokumaru Y, Arai K. Flexion-induced cervical myelopathy associated with fewer elastic fibers and thickening in the posterior dura mater. J Neurol. (2009) 257:149–51. doi: 10.1007/s00415-009-5329-6
43. Shinomiya K, Sato T, Spengler D, Dawson J. Isolated muscle atrophy of the distal upper extremity in cervical spinal cord compressive disorders. J Spinal Disord. (1995) 8:311–6. doi: 10.1097/00002517-199508040-00009
44. Ciceri EF, Chiapparini L, Erbetta A, Longhi L, Cicardi B, Milani N, et al. Angiographically proven cervical venous engorgement: a possible concurrent cause in the pathophysiology of Hirayama's myelopathy. Neurol Sci. (2010) 31:845–8. doi: 10.1007/s10072-010-0405-3
45. Kira J, Ochi H. Juvenile muscular atrophy of the distal upper limb (Hirayama disease) associated with atopy. J Neurol Neurosurg Psychiatry. (2001) 70:798–801. doi: 10.1136/jnnp.70.6.798
46. Ito S, Kuwabara S, Fukutake T, Tokumaru Y, Hattori T. HyperIgEaemia in patients with juvenile muscular atrophy of the distal upper extremity (Hirayama disease). J Neurol Neurosurg Psychiatry. (2005) 76:132–4. doi: 10.1136/jnnp.2003.031609
47. Petrova M, Grigorova O, Penev L, Traykov L. Hirayama disease and immunoglobulin A deficiency: a coincidence or a syndrome. J Neurol Sci. (2014) 344:243–4. doi: 10.1016/j.jns.2014.06.055
48. Alpaydin Baslo S, Erdogan M, Balcik ZE, Ozturk O, Atakli D. Is Hirayama a Gq1b disease? Neurol Sci. (2019) 40:1743–7. doi: 10.1007/s10072-019-03758-x
49. Khadilkar S, Patel B, Bhutada A, Chaudhari C. Do longer necks predispose to Hirayama disease? A comparison with mimics and controls. J Neurol Sci. (2015) 359:213–6. doi: 10.1016/j.jns.2015.11.005
50. Li Z, Zhang W, Wu W, Wei C, Chen X, Lin J. Is there cervical spine muscle weakness in patients with Hirayama disease? A morphological study about cross-sectional areas of muscles on MRI. Eur Spine J. (2020) 29:1022–8. doi: 10.1007/s00586-020-06290-1
51. Wang H, Sun C, Yang S, Jiang J, Lu F, Ma X, et al. Dynamic cervical radiographs in patients with hirayama disease: an unneglectable factor on the choice of surgery options. World Neurosurg. (2018) 114: e433–40. doi: 10.1016/j.wneu.2018.03.004
52. Singh RJ, Preethish-Kumar V, Polavarapu K, Vengalil S, Prasad C, Nalini A. Reverse split hand syndrome: dissociated intrinsic hand muscle atrophy pattern in Hirayama disease/brachial monomelic amyotrophy. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:10–6. doi: 10.1080/21678421.2016.1223140
53. Kijima M, Hirayama K, Nakajima Y. Symptomatological and electrophysiological study on cold paresis in juvenile muscular atrophy of distal upper extremity (Hirayama's disease) [in Japanese]. Clin Neurol. (2002) 42:841–8.
54. Sawai S, Misawa S, Kanai K, Isose S, Shibuya K, Noto Y, et al. Altered axonal excitability properties in juvenile muscular atrophy of distal upper extremity (Hirayama disease). Clin Neurophysiol. (2011) 122:205–9. doi: 10.1016/j.clinph.2010.06.015
55. Yoo SD, Kim HS, Yun DH, Kim DH, Chon J, Lee SA, et al. Monomelic amyotrophy (hirayama disease) with upper motor neuron signs: a case report. Ann Rehabil Med. (2015) 39:122–7. doi: 10.5535/arm.2015.39.1.122
56. Preethish-Kumar V, Polavarapu K, Singh RJ, Vengalil S, Prasad C, Verma A, et al. Proximal and proximo-distal bimelic amyotrophy: evidence of cervical flexion induced myelopathy. Amyotroph Lateral Scler Frontotemporal Degener. (2016) 17:499–507. doi: 10.3109/21678421.2016.1167912
57. Shao M, Yin J, Lu F, Zheng C, Wang H, Jiang J. The quantitative assessment of imaging features for the study of hirayama disease progression. Biomed Res Int. (2015) 2015:803148. doi: 10.1155/2015/803148
58. Chen C-J, Hsu H-L, Tseng Y-C, Lyu R-K, Chen C-m, Huang Y-C, et al. Hirayama flexion myelopathy: neutral-position MR imaging findings—importance of loss of attachment. Radiology. (2004) 231:39–44. doi: 10.1148/radiol.2311030004
59. Lai V, Wong YC, Poon WL, Yuen MK, Fu YP, Wong OW. Forward shifting of posterior dural sac during flexion cervical magnetic resonance imaging in Hirayama disease: an initial study on normal subjects compared to patients with Hirayama disease. Eur J Radiol. (2011) 80:724–8. doi: 10.1016/j.ejrad.2010.07.021
60. Bede P, Walsh R, Fagan AJ, Hardiman O. “Sand-watch” spinal cord: a case of inferior cervical spinal cord atrophy. J Neurol. (2014) 261:235–7. doi: 10.1007/s00415-013-7193-7
61. Huang YL, Chen CJ. Hirayama disease. Neuroimaging Clin N Am. (2011) 21:939–50, ix–x. doi: 10.1016/j.nic.2011.07.009
62. Boruah DK, Prakash A, Gogoi BB, Yadav RR, Dhingani DD, Sarma B. The importance of flexion MRI in Hirayama disease with special reference to laminodural space measurements. AJNR Am J Neuroradiol. (2018) 39:974–80. doi: 10.3174/ajnr.A5577
63. Ries M, Jones RA, Dousset V, Moonen CTW. Diffusion tensor MRI of the spinal cord. Magn Reson Med. (2000) 44:884–92. doi: 10.1002/1522-2594(200012)44:6<884::AID-MRM9>3.0.CO;2-Q
64. Sun C, Zhou S, Cui Z, Zhang Y, Wang H, Jiang J, et al. The evaluation on neural status of cervical spinal cord in normal and Hirayama disease using diffusion tensor imaging. Eur Spine J. (2019) 28:1872–8. doi: 10.1007/s00586-019-06013-1
65. Wang H, Wu Y, Song J, Jiang J, Lu F, Ma X, et al. Cortical activation changes in Hirayama disease following anterior cervical decompression and fusion. World Neurosurg. (2018) 116: e588–94. doi: 10.1016/j.wneu.2018.05.045
66. Zheng C, Jiang J. Application and research progress of neuroelectrophysiological techniques in Hirayama disease. Chin J Orthop. (2015) 035:91–5. doi: 10.3760/cma.j.issn.0253-2352.2015.01.014
67. Cerami C, Valentino F, Piccoli F, La Bella V. A cervical myelopathy with a Hirayama disease-like phenotype. Neurol Sci. (2008) 29:451–4. doi: 10.1007/s10072-008-1058-3
68. Hassan KM, Sahni H, Jha A. Clinical and radiological profile of Hirayama disease: a flexion myelopathy due to tight cervical dural canal amenable to collar therapy. Ann Indian Acad Neurol. (2012) 15:106–12. doi: 10.4103/0972-2327.94993
69. Jin X, Jiang J-Y, Lu F-Z, Xia X-L, Wang L-X, Zheng C-J. Electrophysiological differences between Hirayama disease, amyotrophic lateral sclerosis and cervical spondylotic amyotrophy. BMC Musculoskelet Disord. (2014) 15:349. doi: 10.1186/1471-2474-15-349
70. Zheng C, Zhu Y, Yang S, Lu F, Jin X, Weber R, et al. A study of dynamic F-waves in juvenile spinal muscular atrophy of the distal upper extremity (Hirayama disease). J Neurol Sci. (2016) 367:298–304. doi: 10.1016/j.jns.2016.06.032
71. Abraham A, Gotkine M, Drory VE, Blumen SC. Effect of neck flexion on somatosensory and motor evoked potentials in Hirayama disease. J Neurol Sci. (2013) 334:102–5. doi: 10.1016/j.jns.2013.07.2519
72. Park JS, Ko JY, Park D. The reversible effect of neck flexion on the somatosensory evoked potentials in patients with Hirayama disease: a preliminary study. Neurol Sci. (2019) 40:181–6. doi: 10.1007/s10072-018-3614-9
73. Misra UK, Kalita J, Mishra VN, Phadke RV, Hadique A. Effect of neck flexion on F wave, somatosensory evoked potentials, and magnetic resonance imaging in Hirayama disease. J Neurol Neurosurg Psychiatry. (2006) 77:695–8. doi: 10.1136/jnnp.2005.082362
74. Kuo YH, Kuo CH, Huang WC, Wu JC. Anterior cervical discectomy and fusion for Hirayama disease: a case report and literature review. Neurospine. (2019) 16:626–30. doi: 10.14245/ns.1836178.089
75. Zheng C, Zhu Y, Zhu D, Lu F, Xia X, Jiang J, et al. Motor unit number estimation in the quantitative assessment of severity and progression of motor unit loss in Hirayama disease. Clin Neurophysiol. (2017) 128:1008–14. doi: 10.1016/j.clinph.2017.03.007
76. Zheng C, Zhu Y, Yu Q, Zhu D, Li J, Lyu F, et al. Quantitative assessment of motor impairment and surgical outcome in Hirayama disease with proximal involvement using motor unit number index. Neurophysiol Clin. (2021) 51:375–86. doi: 10.1016/j.neucli.2021.02.002
77. Jiang SD, Jiang LS, Dai LY. Cervical spondylotic amyotrophy. Eur Spine J. (2011) 20:351–7. doi: 10.1007/s00586-010-1544-1
78. Es M, Hardiman O, Chió A, Al-Chalabi A, Pasterkamp J, Veldink J, et al. Amyotrophic lateral sclerosis. Lancet. (2017) 2017:390. doi: 10.1016/S0140-6736(17)31287-4
79. Fang J, Liu MS, Guan YZ, Du H, Li BH, Cui B, et al. Pattern differences of small hand muscle atrophy in amyotrophic lateral sclerosis and mimic disorders. Chin Med J. (2016) 129:792–8. doi: 10.4103/0366-6999.178953
80. Sun C, Xu G, Zhang Y, Cui Z, Liu D, Yang Y, et al. Interobserver and intraobserver reproducibility and reliability of the huashan clinical classification system for Hirayama disease. Front Neurol. (2021) 12:2181. doi: 10.3389/fneur.2021.779438
81. Tokumaru Y, Hirayama K. Cervical collar therapy for juvenile muscular atrophy of distal upper extremity (Hirayama disease): results from 38 cases. Clin Neurol. (2000) 41:173–8.
82. Cortese R, Gerevini S, Dicuonzo F, Zoccolella S, Simone I. Hirayama disease: the importance of an early diagnosis. Neurol Sci. (2015) 36:1049. doi: 10.1007/s10072-015-2080-x
83. Verma R, Lalla R, Patil TB, Gupta A. Hirayama disease: a frequently undiagnosed condition with simple inexpensive treatment. BMJ Case Rep. (2012) 2012:bcr2012007076. doi: 10.1136/bcr-2012-007076
84. Ojha R, Shahi S, Nepal G, Shakya A, Gajurel BP, Karn R, et al. The diagnostic quandary of magnetic resonance imaging-negative Hirayama disease: a case report. J Med Case Rep. (2020) 14:133. doi: 10.1186/s13256-020-02453-2
85. Narayana Gowda BS, Mohan Kumar J, Basim PK. Hirayama's disease—a rare case report with review of literature. J Orthop Case Rep. (2013) 3:11–4. doi: 10.13107/jocr.2250-0685.107
86. Lin M-S, Kung W-M, Chiu W-T, Lyu R-K, Chen C-J, Chen T-Y. Hirayama disease. J Neurosurg Spine. (2010) 12:629–34. doi: 10.3171/2009.12.SPINE09431
87. Fu Y, Qin W, Sun Q, Fan D. Investigation of the compliance of cervical collar therapy in 73 patients with Hirayama disease. Natl Med J China. (2016) 96:3485–8. doi: 10.3760/cma.j.issn.0376-2491.2016.43.009
88. Paredes I, Esteban J, Ramos A, Gonzalez P, Rivas JJA. severe case of Hirayama disease successfully treated by anterior cervical fusion. J Neurosurg Spine. (2014) 20:191–5. doi: 10.3171/2013.10.SPINE13508
89. Bromberg M, Brownell A. Motor unit number estimation in the assessment of performance and function in motor neuron disease. Phys Med Rehabil Clin North Am. (2008) 19:509–32, ix. doi: 10.1016/j.pmr.2008.02.006
90. Lu F, Wang H, Jiang J, Chen W, Ma X, Ma X, et al. Efficacy of anterior cervical decompression and fusion procedures for monomelic amyotrophy treatment: a prospective randomized controlled trial: clinical article. J Neurosurg Spine. (2013) 19:412–9. doi: 10.3171/2013.4.SPINE12575
91. Gross A, Paquin J-P, Dupont G, Blanchette S, Lalonde P, Cristie T, et al. Exercises for mechanical neck disorders: a cochrane review update. Manual Ther. (2016) 24:25–45. doi: 10.1016/j.math.2016.04.005
92. Fu Y, Qin W, Sun Q, Fan D. Study of association between hyperlgeaemia and Hirayama disease. Natl Med J China. (2016) 96:3485–8.
93. Zheng C, Nie C, Lei W, Zhu Y, Zhu D, Wang H, et al. CAN anterior cervical fusion procedures prevent the progression of the natural course of Hirayama disease? An ambispective cohort analysis. Clin Neurophysiol. (2018) 129:2341–9. doi: 10.1016/j.clinph.2018.08.024
94. Goel A, Dhar A, Shah A. Multilevel spinal stabilization as a treatment for Hirayama disease: report of an experience with five cases. World Neurosurg. (2017) 99:186–91. doi: 10.1016/j.wneu.2016.11.143
95. Marathe N, Raj A, Bhosale S, Srivastava S, Dhole K. Surgical management of hirayama disease: a rare entity with unusual clinical features. Asian J Neurosurg. (2020) 15:405. doi: 10.4103/ajns.AJNS_291_19
96. Guo X, Lu M, Xie N, Guo Q, Ni B. Multilevel anterior cervical discectomy and fusion with plate fixation for juvenile unilateral muscular atrophy of the distal upper extremity accompanied by cervical kyphosis. J Spinal Disord Tech. (2014) 27:E241–6. doi: 10.1097/BSD.0000000000000098
97. Bohara S, Garg K, Mishra S, Tandon V, Chandra PS, Kale SS. Impact of various cervical surgical interventions in patients with Hirayama's disease—a narrative review and meta-analysis. Neurosurg Rev. (2021) 44:3229–47. doi: 10.1007/s10143-021-01540-2
98. Zou F, Yang S, Lu F, Ma X, Xia X, Jiang J. Factors affecting the surgical outcomes of Hirayama disease: a retrospective analysis of preoperative magnetic resonance imaging features of the cervical spine. World Neurosurg. (2019) 122:e296–301. doi: 10.1016/j.wneu.2018.10.025
99. Lu X, Xu GY, Nie C, Zhang YX, Song J, Jiang JY. The relationship between preoperative cervical sagittal balance and clinical outcome of patients with hirayama disease treated with anterior cervical discectomy and fusion. Neurospine. (2021) 18:618–27. doi: 10.14245/ns.2142564.282
100. Song J, Wang HL, Zheng CJ, Jiang JY. Risk factors for surgical results of Hirayama disease: a retrospective analysis of a large cohort. World Neurosurg. (2017) 105:69–77. doi: 10.1016/j.wneu.2017.05.097
101. Sun Y, Liu X, Fan D, Fu Y, Pan S, Zhang F, et al. Midtern clinical outcomes and radiological results of surgical treatment for Hirayama disease. J Peking Univ. (2017) 49:1019–26. doi: 10.3969/j.issn.1671-167X.2017.06.015
Keywords: Hirayama disease, pathogenesis, clinical manifestations, medical imaging, electromyography, diagnosis criteria, treatment
Citation: Wang H, Tian Y, Wu J, Luo S, Zheng C, Sun C, Nie C, Xia X, Ma X, Lyu F, Jiang J and Wang H (2022) Update on the Pathogenesis, Clinical Diagnosis, and Treatment of Hirayama Disease. Front. Neurol. 12:811943. doi: 10.3389/fneur.2021.811943
Received: 09 November 2021; Accepted: 24 December 2021;
Published: 01 February 2022.
Edited by:
Mamede De Carvalho, University of Lisbon, PortugalReviewed by:
Satish Vasant Khadilkar, Bombay Hospital, IndiaMaria Claudia Torrieri, University of Turin, Italy
Copyright © 2022 Wang, Tian, Wu, Luo, Zheng, Sun, Nie, Xia, Ma, Lyu, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongli Wang, d2FuZ2hvbmdsaTAyMTJAMTYzLmNvbQ==; d2FuZ2hvbmdsaUBodWFzaGFuLm9yZy5jbg==; Jianyuan Jiang, amp5QGZ1ZGFuc3BpbmUuY29t; Feizhou Lyu, bHVmZWl6aG91QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Hongwei Wang
Hongwei Wang Ye Tian1,2†
Ye Tian1,2† Jianwei Wu
Jianwei Wu Chi Sun
Chi Sun Hongli Wang
Hongli Wang