
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 13 January 2022
Sec. Neurological Biomarkers
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.795376
This article is part of the Research Topic Diagnostic Biomarker Development Phases: From Discovery to Clinical Application View all 6 articles
Introduction: Recent reports revealed that higher serum glucose-potassium ratio (GPR) levels at admission were significantly associated with poor outcomes at 3 months following aneurysmal subarachnoid hemorrhage (aSAH). This study aimed to investigate the association between GPR and the risk of rebleeding following aSAH.
Methods: This single-center retrospective study of patients with aSAH was conducted in our hospital between January 2008 and December 2020. Patients meeting the inclusion criteria were divided into the rebleed group and the non-rebleed group. Univariate and multivariate analyses were implemented to assess the association between risk factors of rebleeding and outcomes.
Results: A total of 1,367 patients experiencing aSAH, 744 patients who met the entry criteria in the study [mean age (54.89 ± 11.30) years; 60.50% female patients], of whom 45 (6.05%) developed rebleeding. The patients in the rebleed group had significantly higher GPR levels than those of patients without rebleeding [2.13 (1.56–3.20) vs. 1.49 (1.23–1.87); p < 0.001]. Multivariable analysis revealed that higher mFisher grade and GPR were associated with rebleeding [mFisher grade, odds ratios (OR) 0.361, 95% CI 0.166–0.783, p = 0.01; GPR, OR 0.254, 95% CI 0.13–0.495, p < 0.001]. The receiver operating characteristics (ROCs) analysis described that the suitable cut-off value for GPR as a predictor for rebleeding in patients with aSAH was determined as 2.09 (the area under the curve [AUC] was 0.729, 95% CI 0.696–0.761, p < 0.0001; the sensitivity was 53.33%, and the specificity was 83.98%). Pearson correlation analysis showed a significant positive correlation between GPR and mFisher grade, between GPR and Hunt–Hess grade (mFisher grade r = 0.4271, OR 0.1824, 95% CI 0.3665–0.4842, p < 0.001; Hunt–Hess grade r = 0.4248, OR 0.1836, 95% CI 0.3697–0.4854, p < 0.001). The patients in the poor outcome had significantly higher GPR levels than those of patients in the good outcome [1.87 (1.53–2.42) vs. 1.45 (1.20–1.80); p < 0.001]. Multivariable analysis demonstrated that GPR was an independent predictor for poor prognosis. The AUC of GPR was 0.709 (95% CI 0.675–0.741; p < 0.0001) (sensitivity = 77.70%; specificity = 55.54%) for poor prognosis.
Conclusion: Higher preoperative serum GPR level was associated with Hunt–Hess grade, mFisher grade, rebleeding, and unfunctional outcome, and that they predicted preoperative rebleeding and the 90-days outcome of non-diabetic patients with aSAH, who had potentially relevant clinical implications in patients with aSAH.
Subarachnoid hemorrhage (SAH) from a ruptured intracranial aneurysm contributes to only 5% of stroke cases but occurs at a mean age of 55 years and is associated with a high mortality and disability rate (1, 2). The major early complication is aneurysmal rebleeding with an incidence of 4–13.6% during the first 24 h (3, 4). Early rebleeding is associated with poor-neurosurgical outcomes and mortality rates as high as 50–80% (3, 5). Some studies identified risk factors for preoperative rebleeding have included sex, advanced age, systolic blood pressure >160 mmHg, aneurysm size larger than 10 mm, neurological severity (high Fisher grade and high Hunt–Hess grade), and biomarkers (3–15). Although the mechanism of rebleeding is poorly understood and controversial, studies have pointed the inflammatory response after aSAH may be related to rebleeding (15, 16). When the aneurysm ruptures, inflammation occurs simultaneously, and the inflammation subsequently spreads throughout the brain (16). As the disease progresses, the entire brain continues to experience more severe inflammation, which may exacerbate the occurrence of rebleed. Biomarkers related to inflammation that is readily available on admission may help predict the early risk of a complex rebleeding process (15).
Previous studies reported that a higher serum glucose-potassium ratio (GPR) level at admission was significantly associated with poor outcome or mortality at 3 months following aSAH (17, 18). The existing literature left several crucial unanswered questions about whether the GPR was related to the risk of early brain injury. That is, whether elevated GPR is associated with the risk of vasospasm, delayed cerebral ischemia, and rebleeding. Similarly, the exact underlying mechanism is still unrevealed. Coincidentally, Zhang et al. demonstrate (19, 20) that elevated blood glucose level played a pathological role in active bleeding through inflammation pathway, and was associated with hematoma expansion and prevalence of island sign, blend sign in patients with intracerebral hemorrhage (ICH). A meta-analysis (21) reported that hyperglycemia on admission was related to an increased rate of spontaneous intracranial hemorrhage after mechanical thrombectomy in acute ischemic stroke patients. In addition, hypokalemia caused by aSAH may contribute to the occurrence of rebleeding by the increase of vasoconstrictor receptors and the functional impairment of KATP channels in cerebrovascular myocytes (22, 23).
Based on the aforementioned theory, we hypothesized that higher preoperative serum GPR levels might correlate with Hunt–Hess grade, modified Fisher grade (mFisher grade), and rebleeding. Moreover, GPR levels predicted rebleeding and 90-days outcomes in non-diabetic patients with aSAH.
This single-center retrospective observational study included all adult patients diagnosed with aSAH who were admitted to the department of neurosurgery in the Second Affiliated Hospital of Fujian Medical University between January 2008 and December 2020. The inclusion criteria included: (1) SAH and rebleeding were diagnosed by CT, and the presence of intracranial aneurysms was confirmed by CT angiography (CTA) or digital subtraction angiography (DSA); (2) baseline CT within 24 h after hemorrhage was performed in all patients, and a follow-up CT scan was performed within 72 h or in cases of the neurological function deterioration; and (3) primary outcome was whether patients developed rebleeding within 72 h following aSAH. The exclusion criteria were as follows: (1) age <19 years; (2) history of diabetes; (3) patients with intracranial tumors, cerebral arteriovenous malformations, moyamoya disease, trauma; (4) multiple intracranial aneurysms; (5) patient had no rebleeding within 72 h after aSAH and received surgical intervention; (6) acute kidney damage or chronic kidney disease; (7) concurrent systemic comorbidities including liver cirrhosis and malignancy; and (8) historical modified Rankin Scale (mRS) > 1. If the patient has rebleeding before surgery, surgical intervention can be performed within 72 h (after rebleeding). This study was approved by the institutional review board of the hospital and carried out in accordance with the principles of the Declaration of Helsinki revised in 2008. The detailed flow of the selection process is given in Figure 1.
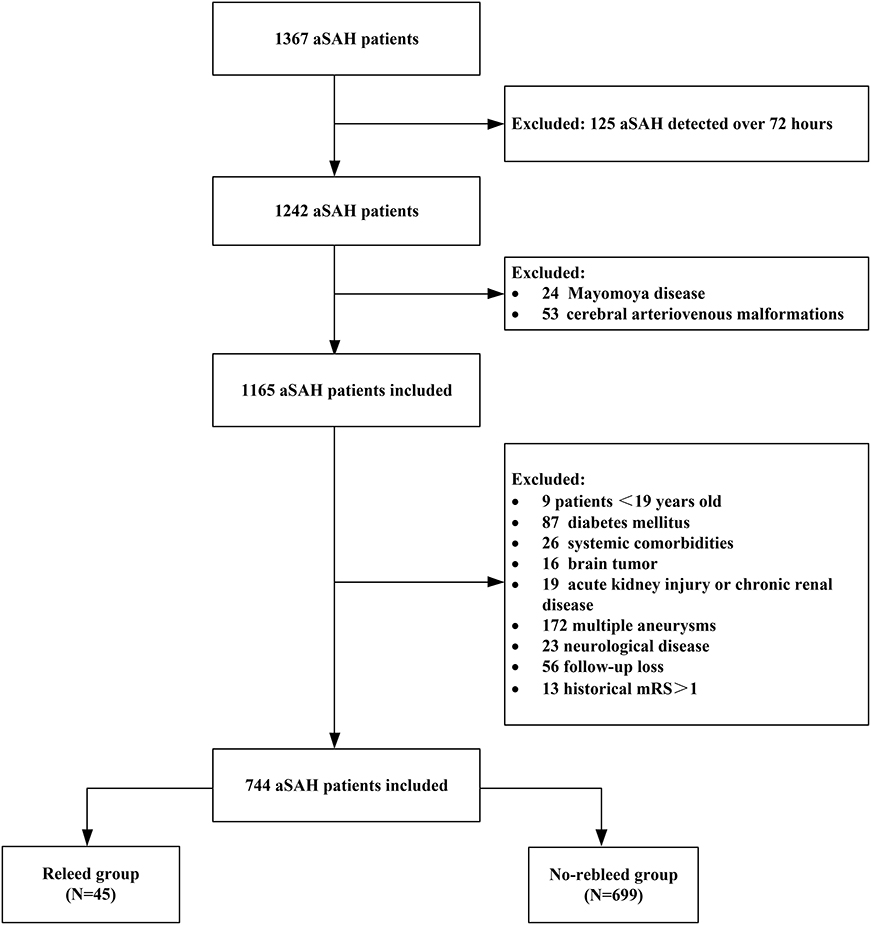
Figure 1. Flow diagram of the patient selection process. aSAH, aneurysmal subarachnoid hemorrhage; mRS, modified Rankin Scale.
Baseline clinical and demographic characteristics, including age, sex, admission blood pressure, hypertension, time from onset to admission, mFisher scale, Hunt–Hess grade, aneurysm location, admission laboratory, and GPR, were recorded. For the laboratory tests, peripheral venous blood samples were drawn at our neurosurgery department within 1 h of hospitalization. Neurological status was assessed with the Hunt–Hess grade at the initial presentation after bleeding without sedation, and patients were grouped into severe clinical conditions (Hunt–Hess grade 4-5) or mild clinical conditions (Hunt–Hess grade 1-3). Admission CT scans were classified using the modified Fisher grade, and patients were classified as severe aSAH (mFisher grade 3-4) or mild aSAH (mFisher grade 1-2). The clinical prognostic assessment was measured using the mRS score at 90 days (24). The favorable outcome was defined as mRS 0–2, while a poor outcome was mRS 3–6.
Based on the previous literature (7), we defined rebleeding as a sudden deterioration of neurological function, such as sudden headache and coma, accompanied by radiological signs of increased bleeding compared with previous CT imaging. All the suspected cases of rebleeding underwent the follow-up CT to confirm the clinical diagnosis.
Continuous variables with normal distribution were presented as mean ± SD and non-normal distribution as median (interquartile range), whereas categorical variables were expressed as count (percentage). The data were presented as a scatter plot with the median. Student's t-test or ANOVA carried out comparisons between groups for normally distributed variables, the Mann–Whitney U test and Kruskall-Wallis test for non-normally distributed variables, or chi-squared test for categorical parameters. Analysis of correlation was performed using the Pearson correlation test. Risk factors with p < 0.1 in univariable models were entered into the multivariable logistic regression models. GPR was dichotomized as “ ≤ optimal cutoff value” and “> optimal cutoff value” in the multivariate model. The receiver operating characteristics (ROCs) curve and the area under the curve (AUC) were used to assess the sensitivity and specificity of potential parameters for predicting rebleeding. SPSS software (version 25.0, IBM SPSS, IBM Corp, USA), GraphPad Prism 8 (GraphPad Software, San Diego, California, USA), and MedCalc version 20.0.4 (MedCalc Software, Ostend, Belgium, UK) were used for statistical analysis. p < 0.05 was considered as statistically significant (ns: p > 0.05, *: p < 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001).
A total of 1,367 patients experiencing aSAH, 744 patients who met the entry criteria in the study [mean age (54.89 ± 11.30) years; 60.50% female patients], of whom 45 (6.05%) developed rebleeding (Table 1 and Figure 1). The time from admission to rebleed was 25 (15.25–35.0) h. A total of 341 patients (45.8%) were in severe clinical condition. A total of 317 patients (42.6%) had severe aSAH on admission. Poor prognosis at 90 days was observed in (18.68%) 139 patients. A total of 744 patients meeting the inclusion criteria were divided into the rebleed group (n = 45) and the non-rebleed group (n = 699).
Demographic characteristics, clinical features, and laboratory data of patients with aSAH in the two groups were summarized in Table 2. There were significant statistical differences in Hunt–Hess grade, mFisher grade, blood glucose, serum potassium concentration, and GPR in univariate analysis between the rebleed group and the non-rebleed group. Higher blood glucose levels were detected in patients with rebleeding compared with those without [8.22 (5.80–10.52) vs. 5.74 (4.94–6.90) mmol/l, p < 0.001; Table 2], while lower serum potassium [3.71 (3.34–4.01) vs. 3.94 (3.65–4.20) mmol/l, p < 0.001; Table 2]. The patients in the rebleed group had significantly higher GPR than those of patients without rebleeding [2.13 (1.56–3.20) vs. 1.49 (1.23–1.87); p < 0.001] (Figure 2A). Adjustment of OR was made for all outcomes on the SBP, mFisher grade, Hunt–Hess grade, serum calcium, and higher mFisher grade and GPR were associated with rebleeding (mFisher grade OR 0.361, 95% CI 0.166–0.783, p = 0.01; GPR, OR 0.254, 95% CI 0.13–0.495, p < 0.001; Table 2), while Hunt–Hess grade was not associated with rebleeding.
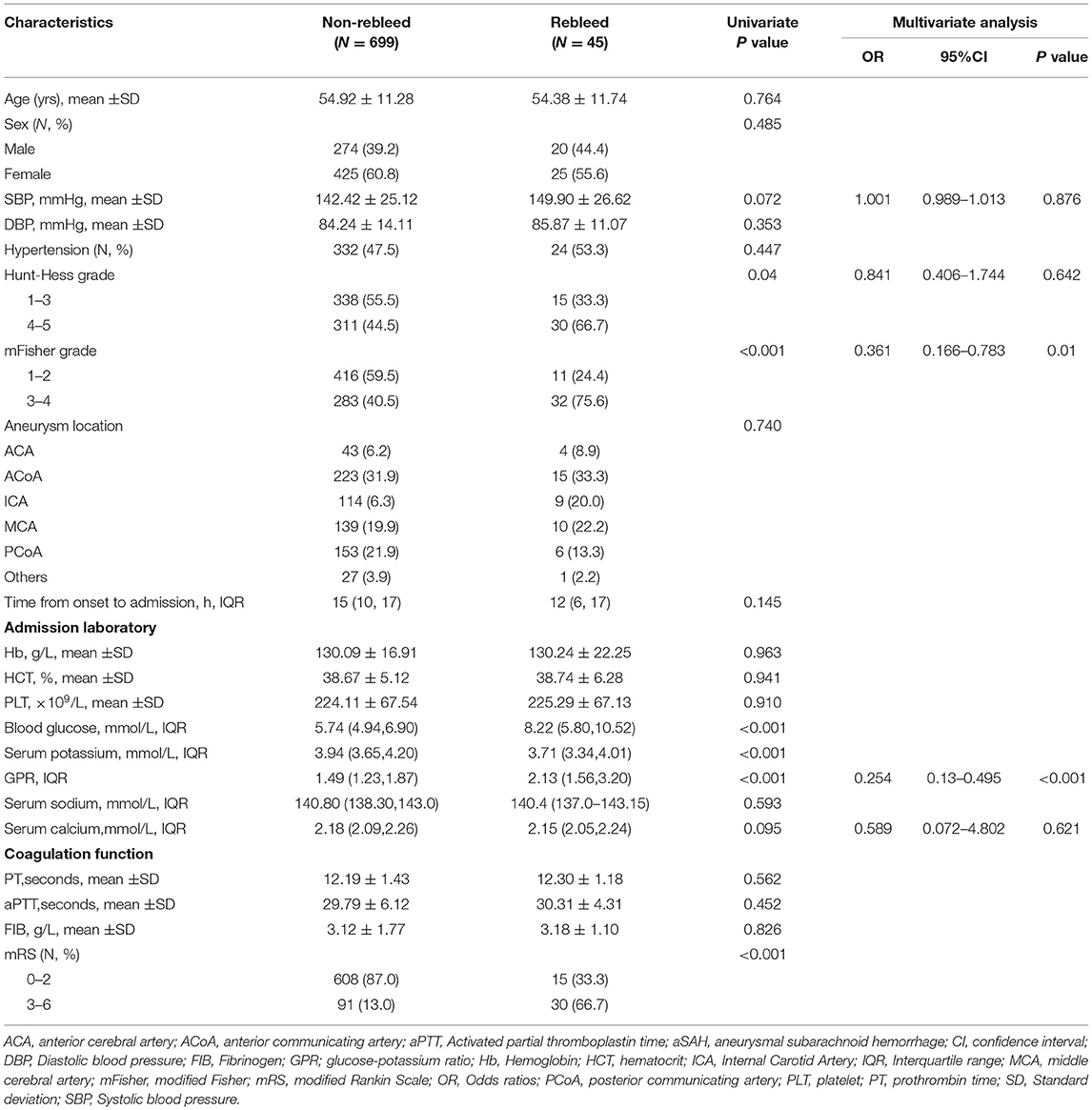
Table 2. Univariate and multivariate analysis of risk factors related to rebleeding in patients with aSAH.
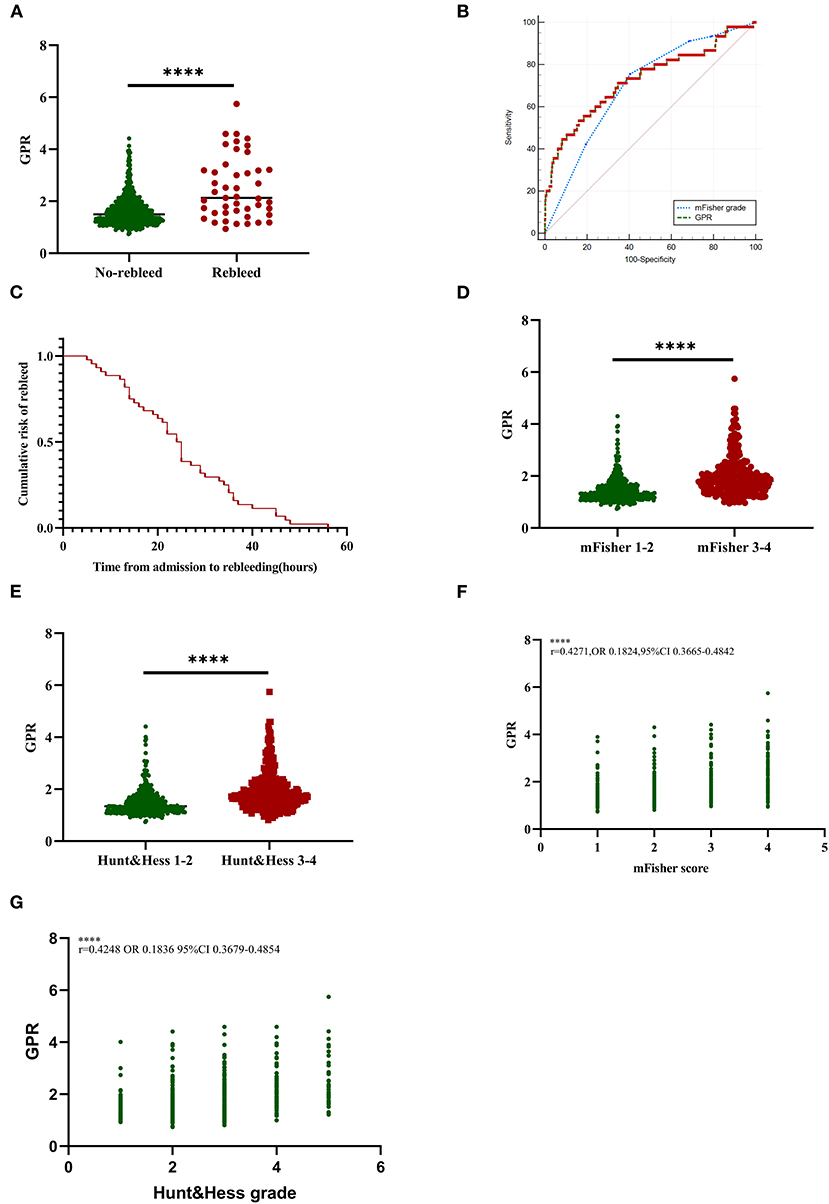
Figure 2. Association of GPR levels with rebleed and initial clinical status in aSAH patients. (A) GPR levels in patients with rebleed and non-rebleed. (B) The ROC curve analyses comparing admission mFisher grade and GPR for predicting rebleeding in patients with aSAH. The AUC of mFisher grade, GPR were 0.698 (95% CI 0.664–0.731, p < 0.0001; the sensitivity was 75.56% and the specificity was 59.51%), 0.729 (95% CI 0.696–0.761, p < 0.0001; the sensitivity was 53.33% and the specificity was 83.98%). Z-test illustrated that AUC of mFisher grade was slightly lower than that of GPR, but no statistically significant difference existed (Z = 0.843, p = 0.3993). (C) Cumulative rebleeding rate using the Kaplan–Meier analysis within 72 h after admission in the rebleed group. (D) GPR levels in patients with mild (mFisher1-2) and severe (mFisher3-4) aSAH. (E) GPR levels in patients with mild (Hunt-Hess grade 1-3) and severe (Hunt-Hess grade 4-5) clinical conditions. (F) The correlation of GPR levels with mFisher grade. (G) The correlation of GPR levels with Hunt–Hess grade. Median with IQR was shown for scatter-dot plot in (A,D,E). Groups were compared using the Mann–Whitney U tests. Correlations were determined using Pearson's correlation analysis. aSAH, aneurysmal subarachnoid hemorrhage; AUC, area under the curve; GPR, glucose-potassium ratio; mFisher, modified Fisher; ROC, receiver operating characteristic. ****p ≤ 0.0001.
The ROC analysis described that the suitable cutoff value for GPR as a predictor for rebleeding in patients with aSAH was determined as 2.09 (the AUC was 0.729, 95% CI 0.696–0.761, p < 0.0001; the sensitivity was 53.33%, and the specificity was 83.98%; Figure 2B). The predictive performances of the mFisher grade (the AUC was 0.698, 95% CI 0.664–0.731, p < 0.0001; the sensitivity was 75.56%, and the specificity was 59.51% based on the best threshold of two) was also illustrated by the ROC analysis. The AUC of GPR levels and the AUC of mFisher grade were comparable by Z-test and the AUC of mFisher grade was slightly lower than that of GPR, but no statistically significant difference existed (Z = 0.843, p = 0.3993; Figure 2B). Cumulative rebleeding rate using Kaplan-Meier analysis within 72 h after admission in the rebleed group is present in Figure 2C.
In order to investigate the relationship between different levels of GPR and rebleeding, we grouped the patients based on the quartile range of GPR levels. Of all the clinical and radiological variables, age, Hunt–Hess grade, mFisher grade, rebleed, and poor outcomes were significantly different among different levels of GPR (p < 0.05; Table 3). GPR ≥ 1.941 was found in 183 patients (24.60%) and observed in 24 patients with rebleeding(14.2%) (Table 3). GPR was significantly higher in patients with aSAH with mFisher 3–4 compared with mFisher 1–2 [1.81 (1.49–2.28) vs. 1.33 (1.16–1.62), p < 0.0001; Figure 2D]. In addition, patients with the severe clinical condition on admission (Hunt–Hess 4-5) had a higher GPR [1.76 (1.44–2.17) vs. 1.34 (1.16–1.65), Figure 2E]. Pearson correlation analysis showed a significant positive correlation between GPR and mFisher grade, between GPR and Hunt–Hess grade (mFisher grade r = 0.4271, OR 0.1824, 95% CI 0.3665–0.4842, p < 0.001; Hunt–Hess grade r = 0.4248, OR 0.1836, 95% CI 0.3697–0.4854, p < 0.001) (Figures 2F,G).
A total of 605 patients with aSAH (81.32%) had a good prognosis, whereas 139 patients (18.68%) had a poor prognosis. Significant statistical differences were detected in the age, SBP, diastolic blood pressure (DBP), hypertension, Hunt–Hess grade, mFisher grade, blood glucose, serum potassium concentration, serum calcium, aPTT, GPR, and rebleed in univariate analysis. The patients in the poor outcome had significantly higher GPR levels than those of patients in the good outcome [1.87 (1.53–2.42) vs. 1.45 (1.20–1.80); p < 0.001; Table 4 and Figure 3A]. Adjustment of OR was made for all outcomes on the age, SBP, DBP, hypertension, Hunt–Hess grade, mFisher grade, serum calcium, aPTT, rebleed (Table 4). The multivariable analysis demonstrated that higher mFisher grade (3-4), higher Hunt–Hess (4-5), rebleed, and GPR were independent predictors for poor prognosis (Table 4). The AUC of GPR was 0.709 (95% CI 0.675–0.741; p < 0.0001) (Sensitivity = 77.70%; Specificity = 55.54%) for poor outcome based on a cutoff value of 1.51 (Figure 3B).
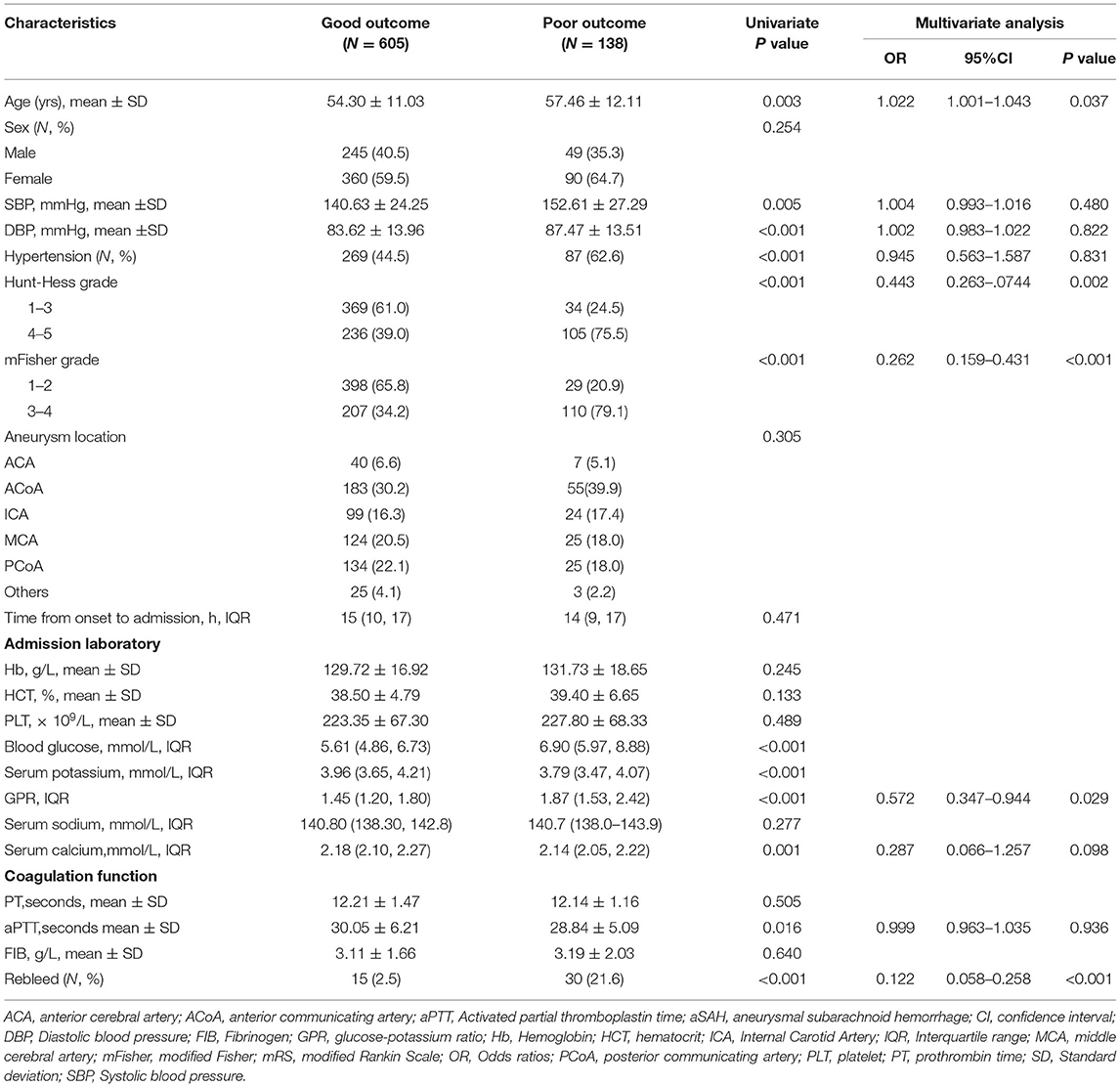
Table 4. Univariate and multivariate analysis of risk factors related to outcome in patients with aSAH.
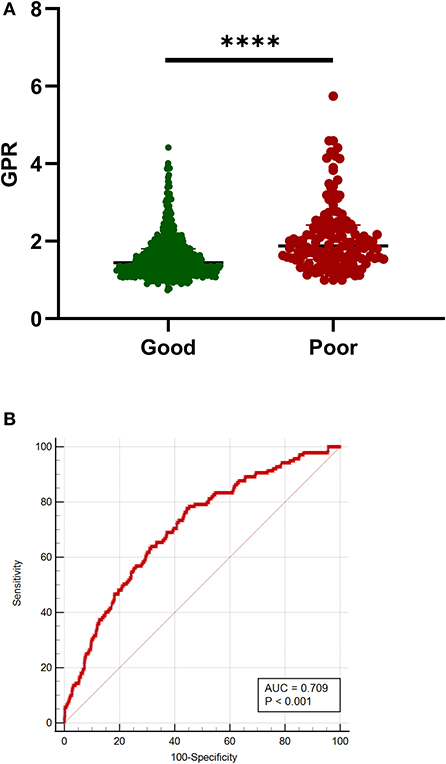
Figure 3. Association of GPR with outcome in patients with aSAH. (A) G. GPR levels in patients with good (mRS 0-2) and poor (mRS 3-6) outcomes. (B) The ROC curve analysis of GPR for predicting poor prognosis. The AUC of GPR was 0.709 (sensitivity = 77.70%; specificity = 55.54%) for poor prognosis based on a cutoff value of 1.51. Median with IQR was shown for scatter-dot plot in (A). Groups were compared using Mann–Whitney U tests. aSAH, aneurysmal subarachnoid hemorrhage; AUC, area under the curve; GPR, glucose-potassium ratio; mRS, modified Rankin Scale; ROC, receiver operating characteristic. ****p ≤ 0.0001.
We presented a single-center retrospective observational study of non-diabetic patients with aSAH that identified elevated admission GPR level as an independent risk factor for rebleeding. Furthermore, GPR also exhibited a comparable performance to mFisher grade in predicting rebleeding. In addition, we have also demonstrated significant correlations between GPR and the amount of intracranial blood produced by initial bleeding, measured by the mFisher grade, and the severity of clinical symptoms, measured by the Hunt–Hess grade. The correlation between elevated GPR levels and increased severity and outcome in patients with aSAH was observed. This is the first study to investigate the potential predictive power of elevated admission GPR levels for rebleeding in patients with aSAH to the best of our knowledge.
Previous literature reported that higher serum GPR level at admission was significantly associated with poor outcome or mortality at 3 months following aSAH (17, 18). However, the studies fail to answer whether the GPR levels are related to early brain injury risk. Similarly, the exact underlying mechanism is still unrevealed. Interestingly, a 3.94-fold difference in the risk of rebleeding and a 4.95-fold difference in unfavorable outcomes between the lowest GPR (≤1.235) and the highest GPR (≥1.941) quartiles (Table 3) was detected in this study. Therefore, this study identifies that elevated admission GPR level is an independent risk factor for rebleeding following aSAH and rebleeding is associated with poor functional outcomes.
Preoperative aneurysm rebleeding has traditionally been a significant reason contributing to morbidity and mortality after aSAH. Previously identified risk factors for preoperative rebleeding have included sex, systolic blood pressure > 160 mm Hg, high Fisher grade, aneurysm characteristics (location, size, and numbers), hyperglycemia, neurological severity (5–9, 11–13, 25–29). Rebleeding following aSAH is a complex and multifactorial event. The pathophysiological mechanisms of rebleeding are still not fully understood and may involve complex interactions between blood products, vasoactive substances, and inflammatory cascades. Previous literature suggests that a higher neutrophile lymphocyte ratio predicts the occurrence of rebleeding, indicating that direct and reactive inflammation may contribute to rebleeding following aSAH. Zhang et al. demonstrate that elevated blood glucose level plays a pathological role in active bleeding through inflammation pathway, and is associated with hematoma expansion and prevalence of island sign, blend sign in patients with ICH (19, 20). Coincidentally, this study also detects that the rebleed group has a higher blood glucose level than that of the non-rebleed group in patients with aSAH. In addition, hypokalemia caused by aSAH may contribute to the occurrence of rebleeding by the increase of vasoconstrictor receptors and the functional impairment of KATP channels in cerebrovascular myocytes (22, 23). Taken together, we propose that elevated GPR level at admission can predict early rebleeding following aSAH, and it is verified in our study.
Associations of elevated GPR levels with poor outcome and mortality have been described in patients with aSAH (17, 18) but never concerning rebleeding occurrence. How can elevated GPR levels (increased blood glucose and decreased potassium) cause rebleeding? Hyperglycemia often accompanies aneurysm rupture within 72 h after onset. Under stress and injuries, this hyperglycemia directly results from raised blood catecholamine levels or increasing glucagon secretion and inhibiting insulin secretion indirectly (27). Disturbance of glucose metabolism after aSAH reflects the degree of early brain damage, and the brain inflammatory response begins when the aneurysm ruptures (16). Venditti et al. reported that increased glucose levels could lead to a downstream microvascular thrombo-inflammation, blood-brain barrier disruption, and hemorrhagic transformation after thrombectomy in stroke (30). The detrimental effects of hyperglycemia accelerate the destruction of the blood–brain barrier, impair the integrity of the surrounding blood vessels, and promote continuous or emerging bleeding. Inflammation caused by hyperglycemia and the production of oxygen free radicals are also involved in the progress of aSAH and it is reported to be involved in the pathophysiological process of microvascular integrity damage and early rebleeding or hematoma expansion.
Furthermore, it is well-known that the inflammatory process of the aneurysm wall promotes the growth and rupture of intracerebral aneurysms (31–33). The presence and subsequent lysis of red blood cells after aSAH produce an immediate cerebral inflammatory response similar to the inflammatory cascade that characterizes the systemic inflammatory response syndrome (16), leading to local vasoconstriction and hypoxia, which may induce rebleeding. The presence of hyperglycemia and hypokalemia exacerbate the damage as mentioned earlier process.
Potassium is the major intracellular cation, and 98% of the potassium in the human body exists in the intracellular fluid. Potassium is actively transported from plasma to cells by the sodium-potassium adenosine triphosphatase pump (Na/K-ATPase). After aSAH, catecholamines, and insulin reduce serum potassium levels and activate the KATP channel, inflammation exacerbates this damaging process after aSAH. Reduced potassium may contribute to rebleed by the increase of vasoconstrictor receptors and the functional impairment of KATP channels in cerebrovascular myocytes (22). Studies from animal models of SAH demonstrated that the K channel is related to the dilation and contraction of the intracranial artery (22, 34). Another recent animal model also indicates that the inflammatory response after SAH leads to oxidative damage to cerebral arteries, weakening ATP-sensitive K channel currents in vascular smooth muscle, and changes in the morphology of basilar arteries (35). Based on this theory, we speculate that lower serum potassium caused by aSAH exacerbates the damage of intracranial arteries. Subsequently, rebleed events occurred on the damaged vessel wall.
The reported literature shows that strict early management of blood glucose, electrolytes, and fluid intake after ICH, can reduce the volume of hematoma growth or the incidence of rebleeding, thereby improving the prognosis (23, 36). Combined with our results, we believe that patients with aSAH with elevated GPR levels can partially reveal a higher risk of active bleeding or rebleeding. In addition, in line with existing literature, a higher mFisher grade is an independent factor in predicting rebleeding after aSAH. It is possible that the amount of bleeding is one of the crucial signs that affect the stability of the aneurysm wall in patients with aSAH after the aneurysm wall ruptures (7, 37). This study indicated that GPR levels also exhibited a comparable performance to mFisher grade in predicting rebleeding following aSAH. Significant correlations between GPR levels, Hunt–Hess grade, and mFisher grade were observed in our study, suggesting that GPR levels can reflect the severity of aSAH to the same extent.
Fujiki et al. (18). reported that a high serum GPR in patients with aSAH would help predict a poor prognosis. Jung et al. (17)also suggested that higher plasma GPR levels on admission were considered a potential predictor of 3-month mortality in patients with aSAH. Likewise, our study demonstrated high-serum GPR level at admission in patients who developed rebleeding and was associated with unfavorable outcomes in patients with aSAH.
However, this study has several limitations, including those inherent to most retrospective observational studies, such as less power to estimate the cause-effect and not control all the confounding factors. Furthermore, laboratory data such as blood glucose and serum potassium are the initial values of admission. Dynamic monitoring of laboratory results may improve the accuracy of our research. Further study is needed to determine whether changes in GPR levels over time are related to the onset of rebleeding. Third, serum glucose and potassium levels may be affected by various factors, such as last food intake time, potassium-consuming diuretics, and cortisol, but we did not consider these factors. The management of blood glucose was not described in detail in this study. Fourth, to observe early rebleeding following aSAH, we excluded patients with aSAH undergoing emergency surgery within 72 h, resulting in a reduction in sample size, which might affect the accuracy of our study. Last but not least, other relevant inflammation markers, such as C-reactive protein, neutrophil-to-lymphocyte ratio, and white blood cell count, are related to rebleeding of aSAH. Unfortunately, this study failed to provide a comparison.
Higher preoperative serum GPR level was associated with Hunt–Hess grade, mFisher grade, rebleeding, and unfunctional outcome, ant that predicted preoperative rebleeding and the 90-days outcome of non-diabetic patients with aSAH, who had potentially relevant clinical implications in patients with aSAH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
JW and YZ: designed the study, drafted the manuscript, prepared the figures, and interpreted the results. QF and WQ: collected and analyzed the data. YZ: helped in the statistical analysis and result interpretation. HG: supervised the study and revised manuscript. YZ and HG: were identified as the guarantor of the article and taking responsibility for the integrity of the work as a whole. All the authors read and approved the final version of the manuscript.
This study was supported by the Science and Technology Planning Project of Health and Family Planning Commission of Fujian Province (No. 2018-CX-34 to HG) and the Young and Middle-aged Project of Fujian Provincial Department of Education (No. JAT190224 to JW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. The Lancet. (2017) 389:655–66. doi: 10.1016/S0140-6736(16)30668-7
2. Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. (2017) 377:257–66. doi: 10.1056/NEJMcp1605827
3. Foreman PM, Starke RM, Hendrix P, Harrigan MR, Fisher WSR, Vyas NA, et al. Endothelin polymorphisms as a risk factor for cerebral aneurysm rebleeding following aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. (2017) 157:65–9. doi: 10.1016/j.clineuro.2017.04.007
4. Molyneux A. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. The Lancet. (2002) 360:1267–74. doi: 10.1016/S0140-6736(02)11314-6
5. Alfotih GT, Li F, Xu X, Zhang S. Risk factors for re-bleeding of aneurysmal subarachnoid hemorrhage: meta-analysis of observational studies. Neurol Neurochir Pol. (2014) 48:346–55. doi: 10.1016/j.pjnns.2014.08.002
6. Starke RM, Connolly ES Jr. Participants in the international multi-disciplinary consensus conference on the critical care management of subarachnoid H: rebleeding after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2011) 15:241–6. doi: 10.1007/s12028-011-9581-0
7. Lu VM, Graffeo CS, Perry A, Carlstrom LP, Rangel-Castilla L, Lanzino G, et al. Rebleeding drives poor outcome in aneurysmal subarachnoid hemorrhage independent of delayed cerebral ischemia: a propensity-score matched cohort study. J Neurosurg. (2019) 1–9. doi: 10.3171/2019.4.JNS19779
8. Wu TC, Tsui YK, Chen TY, Lin CJ, Wu TC, Tzeng WS. Rebleeding of aneurysmal subarachnoid hemorrhage in computed tomography angiography: risk factor, rebleeding pattern, and outcome analysis. J Comput Assist Tomogr. (2012) 36:103–8. doi: 10.1097/RCT.0b013e31823f2e57
9. Brilstra EH, Rinkel GJ, Algra A, van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology. (2000) 55:1656–60. doi: 10.1212/WNL.55.11.1656
10. Inagawa T, Yahara K, Ohbayashi N. Risk factors associated with cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Neurol Med Chir. (2014) 54:465–73. doi: 10.2176/nmc.oa.2013-0169
11. Cong W, Zhongxin Z, Tiangui L, Zhang Y, Min H, Chao Y. Risk factors for rebleeding of aneurysmal subarachnoid hemorrhage based on the analysis of on-admission information. Turk Neurosurg. (2012) 22:675–81. doi: 10.5137/1019-5149.JTN.5054-11.1
12. Hellingman CA, van den Bergh WM, Beijer IS, van Dijk GW, Algra A, van Gijn J, et al. Risk of rebleeding after treatment of acute hydrocephalus in patients with aneurysmal subarachnoid hemorrhage. Stroke. (2007) 38:96–9. doi: 10.1161/01.STR.0000251841.51332.1d
13. Molyneux AJ, Kerr RSC, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. (2009) 8:427–33. doi: 10.1016/S1474-4422(09)70080-8
14. Zheng J, Xu R, Guo Z, Sun X. Small ruptured intracranial aneurysms: the risk of massive bleeding and rebleeding. Neurol Res. (2019) 41:312–8. doi: 10.1080/01616412.2018.1563737
15. Wang JY, Zhang XT, Wang JQ, Wang CY, Zheng WL, Pan ZM, et al. Admission neutrophil-lymphocyte ratio predicts rebleeding following aneurismal subarachnoid hemorrhage. World Neurosurg. (2020) 138:e317–22. doi: 10.1016/j.wneu.2020.02.112
16. Hanafy KA, Morgan Stuart R, Fernandez L, Schmidt JM, Claassen J, Lee K, et al. Cerebral inflammatory response and predictors of admission clinical grade after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. (2010) 17:22–5. doi: 10.1016/j.jocn.2009.09.003
17. Jung HM, Paik JH, Kim SY, Hong DY. Association of plasma glucose to potassium ratio and mortality after aneurysmal subarachnoid hemorrhage. Front Neurol. (2021) 12:661689. doi: 10.3389/fneur.2021.661689
18. Fujiki Y, Matano F, Mizunari T, Murai Y, Tateyama K, Koketsu K, et al. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J Neurosurg. (2018) 129:870–5. doi: 10.3171/2017.5.JNS162799
19. Zhang F, Li H, Qian J, Zhang S, Tao C, You C, et al. Hyperglycemia is associated with island sign in patients with intracerebral hemorrhage. World Neurosurg. (2018) 119:e703–9. doi: 10.1016/j.wneu.2018.07.251
20. Zhang F, Li H, Qian J, Tao C, Zheng J, You C, et al. Hyperglycemia predicts blend sign in patients with intracerebral hemorrhage. Med Sci Monit. (2018) 24:6237–44. doi: 10.12659/MSM.910024
21. Zang L, Zhang D, Yao Y, Wang Y. Symptomatic intracranial hemorrhage in patients with admission hyperglycemia and diabetes after mechanical thrombectomy: a systematic review and meta-analysis. Am J Emerg Med. (2021) 45:23–8. doi: 10.1016/j.ajem.2021.02.032
22. Kamp MA, Dibue M, Schneider T, Steiger HJ, Hanggi D. Calcium and potassium channels in experimental subarachnoid hemorrhage and transient global ischemia. Stroke Res Treat. (2012) 2012:382146. doi: 10.1155/2012/382146
23. Alimohamadi M, Saghafinia M, Alikhani F, Danial Z, Shirani M, Amirjamshidi A. Impact of electrolyte imbalances on the outcome of aneurysmal subarachnoid hemorrhage: a prospective study. Asian J Neurosurg. (2016) 11:29–33. doi: 10.4103/1793-5482.154978
24. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. (2020) 382:1981–93. doi: 10.1056/NEJMoa2001123
25. Yu Z, Zheng J, Guo R, Li M, Li H, Ma L, et al. The accuracy of aneurysm size in predicting rebleeding after subarachnoid hemorrhage: a meta-analysis. Neurol Sci. (2020) 41:1843–50. doi: 10.1007/s10072-020-04297-6
26. Boogaarts HD, van Lieshout JH, van Amerongen MJ, de Vries J, Verbeek AL, Grotenhuis JA, et al. Aneurysm diameter as a risk factor for pretreatment rebleeding: a meta-analysis. J Neurosurg. (2015) 122:921–8. doi: 10.3171/2014.12.JNS14931
27. Bian L, Liu L, Wang C, Hussain M, Yuan Y, Liu G, et al. Hyperglycemia within day 14 of aneurysmal subarachnoid hemorrhage predicts 1-year mortality. Clin Neurol Neurosurg. (2013) 115:959–64. doi: 10.1016/j.clineuro.2012.09.026
28. Yazawa Y, Ohira T, Itabashi R, Uchida K, Sakai N, Yamagami H, et al. Association of admission hyperglycemia with clinical outcomes in japanese patients with acute large vessel occlusion stroke: a post hoc analysis of the recovery by endovascular salvage for cerebral ultra-acute embolism japan registry 2. Cerebrovasc Dis. (2021) 50:12–9. doi: 10.1159/000511679
29. Yin C, Huang GF, Ruan J, He ZZ, Sun XC. The APOE promoter polymorphism is associated with rebleeding after spontaneous SAH in a Chinese population. Gene. (2015) 563:52–5. doi: 10.1016/j.gene.2015.03.009
30. Venditti L, Chassin O, Ancelet C, Legris N, Sarov M, Lapergue B, et al. Pre-procedural predictive factors of symptomatic intracranial hemorrhage after thrombectomy in stroke. J Neurol. (2021) 268:1867–75. doi: 10.1007/s00415-020-10364-x
31. Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. (2012) 32:1659–76. doi: 10.1038/jcbfm.2012.84
32. Fennell VS, Kalani MY, Atwal G, Martirosyan NL, Spetzler RF. Biology of saccular cerebral aneurysms: a review of current understanding and future directions. Front Surg. (2016) 3:43. doi: 10.3389/fsurg.2016.00043
33. Sawyer DM, Amenta PS, Medel R, Dumont AS. Inflammatory mediators in vascular disease: identifying promising targets for intracranial aneurysm research. Mediators Inflamm. (2015) 2015:896283. doi: 10.1155/2015/896283
34. Zuccarello M, Boccaletti R, Tosun M, Rapoport RM. Role of extracellular Ca2+ in subarachnoid hemorrhage-induced spasm of the rabbit basilar artery. Stroke. (1996) 27:1896–902. doi: 10.1161/01.STR.27.10.1896
35. Shi X, Zhen L, Ding H, Chen J, Zhang S, Fu Y. Role of ATP-sensitive potassium channels and inflammatory response of basilar artery smooth muscle cells in subarachnoid hemorrhage of rabbit and immune-modulation by shikonin. Food Chem Toxicol. (2019) 134:110804. doi: 10.1016/j.fct.2019.110804
36. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Strok. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
Keywords: glucose, potassium, aneurysm subarachnoid hemorrhage, rebleed, risk factor
Citation: Wang J, Feng Q, Zhang Y, Qiu W and Gao H (2022) Elevated Glucose-Potassium Ratio Predicts Preoperative Rebleeding in Patients With Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 12:795376. doi: 10.3389/fneur.2021.795376
Received: 15 October 2021; Accepted: 15 December 2021;
Published: 13 January 2022.
Edited by:
Jean-Charles Sanchez, Université de Genève, SwitzerlandReviewed by:
Damien Bouvier, Centre Hospitalier Universitaire de Clermont-Ferrand, FranceCopyright © 2022 Wang, Feng, Zhang, Qiu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinbin Zhang, enliMjAwOHF6QDE2My5jb20=; Hongzhi Gao, aG9uZ3poaV9nYW9AaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.