
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 06 December 2021
Sec. Dementia and Neurodegenerative Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.789840
This article is part of the Research Topic Horizon in Frontotemporal Lobar Degeneration Related Disorder View all 14 articles
Objective: Uric acid as an antioxidant plays an important role in neurodegenerative disease. Our objective is to investigate the relationship between plasma uric acid and cognitive impairment in patients with amyotrophic lateral sclerosis (ALS).
Methods: In this cross-sectional study, 124 ALS patients were screened by the Edinburgh Cognitive and Behavioral Screen (ECAS) and classified according to the revised Strong's criteria. Additionally, based on total ECAS cut-off score patients were categorized into those with cognitive impairment (ALS-cie) and those without cognitive impairment (ALS-ncie), and clinical data and uric acid level were compared between the two groups. Parameters with significant differences were further included in a multivariate linear regression analysis with ECAS score as a dependent variable. Hold-out validation was performed to evaluate the fitness of regression model.
Results: Up to 60% of ALS patients showed cognitive or/and behavioral impairment. The ALS-cie group had lower education level (p < 0.001), older age at symptom onset (p = 0.001), older age at testing (p = 0.001), and lower plasma uric acid (p = 0.01). Multivariate analysis showed increased uric acid (β = 0.214, p = 0.01), lower age at testing (β = −0.378, p < 0.001), and higher education level (β = 0.424, p < 0.001) could predict higher ECAS score (F = 19.104, R2 = 0.381, p < 0.0001). Validation analysis showed that predicted ECAS score was significantly correlated with raw ECAS score in both the training set (rs = 0.621, p < 0.001) and the testing set (rs = 0.666, p < 0.001).
Conclusions: Cognitive impairment was a common feature in our Chinese ALS patients. Plasma uric acid might help evaluate the risk of cognitive impairment in ALS patients when combined with education level and age at testing.
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disorder characterized by progressive loss of upper and lower motor neurons. Although ALS was initially considered only to involve the motor system, cognitive impairment has been increasingly recognized as one feature of ALS (1). While up to half of ALS patients had mild cognitive impairment, 15% of patients fulfilled the diagnostic criteria of frontotemporal dementia (FTD) (2, 3). The overlapping clinical (4), neuroimaging (5), neuropathological (6), and genetic features (7) suggested that ALS and FTD might constitute a disease spectrum. Since cognitive impairment could increase the caregiver burden and shorten survival (8), early detection of cognitive abnormalities in ALS patients is essential.
Diagnosis of cognitive impairment has been largely based on comprehensive neuropsychological tests (9), which could be limited by resource and time. Additionally, completion of those tests might be restricted by the physical disabilities of ALS patients. In contrast, blood biomarkers have the potential of being readily available. One study on hormonal peptides showed that plasma neuropeptide Y and leptin levels might be markers of cognitive changes measured by Addenbrooke's Cognitive Examination-Revised in ALS patients (10). Another study found significant differences in the plasma levels of 20 proteins by mass spectrometry analysis in 36 ALS patients with or without cognitive impairment using Addenbrooke's Cognitive Examination-III (11).
Oxidative stress was involved in pathological processes leading to neuronal damage in ALS (12). The neuroprotective role of plasma uric acid as an antioxidant has been studied widely in neurodegenerative diseases including ALS (13). Two case-control studies showed that ALS patients had significantly lower plasma uric acid than healthy controls, and the lower uric acid level was associated with faster disease progression (14, 15). Another two randomized clinical trials reported that ALS patients with a higher uric acid level at baseline had prolonged survival advantages (16, 17). Additionally, the uric acid level was reversely correlated with disease stages and significantly decreased during disease progression (18). Furthermore, a prospective study involving 319,617 participants found that uric acid level was inversely related to ALS risk in healthy individuals (19). However, the relationship between plasma uric acid level and cognitive impairment of ALS patients is still unknown.
In this study, we aimed to identify whether plasma uric acid could help evaluate cognitive impairment in Chinese ALS patients.
One hundred and ninety ALS patients (possible, probable, or definite ALS according to the El Escorial criteria) (20) who were admitted to Department of Neurology, Tongji Hospital in Wuhan between August 2017 and October 2020 were screened for this cross-sectional study. Exclusion criteria included other neurological disorders affecting cognitive function, such as stroke, traumatic brain injury, epilepsy; psychiatric disorders; major organ dysfunction; loss of both language and writing ability, and alcohol and drug abuse. Out of the 190 patients, 124 patients (65.3%) were finally included in this study (Figure 1). The study was approved by the Ethics Committee of Tongji Hospital, and all patients provided written informed consent to participate.
Clinical information including sex, age, disease duration, education time, body mass index (BMI), and site of onset was collected. Patients were evaluated at their first visit by the Chinese version of Edinburgh Cognitive and Behavioral Screen (ECAS) (21), a screening tool for the comprehensive assessment of cognitive status of ALS patients. It consists of five cognitive domains, including language (28 points), executive functions (48 points), verbal fluency (24 points), memory (24 points), and visuospatial functions (12 points), which make up a total score of 136 points (21, 22). The cut-off total score (81.92 points) and subdomains scores were calculated as two standard deviations below the corresponding mean score of the healthy Chinese population (22). Additionally, ECAS includes a behavioral assessment of patients by caregivers (10 points), based on the key behavioral criteria of diagnosing behavioral variant FTD (21). It evaluates five behavioral domains including disinhibition (three points), apathy (one point), loss of sympathy (two points), perseveration (two points), and changes in eating behaviors (two points), as well as psychotic symptoms (three points). The severity of physical disability was measured by the amyotrophic lateral sclerosis functional rating scale-revised (ALSFRS-R), which evaluated bulbar, upper limb, lower limb, and respiratory function, with a higher score (total score 48 points) representing better physical function (23). Plasma uric acid level was measured via enzymatic colorimetric method using the Cobas c701 automatic analyzer (Roche) in the Department of Clinical Laboratory of Tongji Hospital.
All patients were screened into two groups based on total ECAS score, i.e., the group with cognitive impairment (ALS-cie group, total score <81.92) and the group without cognitive impairment (ALS-ncie group, total score ≥81.92) (22). Additionally, patients with abnormality in at least one behavioral domain were considered to have behavioral abnormality.
Patients were further categorized into five classical groups according to the revised Strong's criteria: (1) ALS with cognitive impairment (ALSci), if patients had at least one abnormal symptom in the domain of language, executive function, or verbal fluency; (2) ALS with behavioral impairment (ALSbi), if patients had apathy or at least two other behavioral/psychotic symptoms; (3) ALS with combined cognitive and behavioral impairment (ALScbi), if patients met the criteria of both ALSci and ALSbi; (4) ALS-FTD, if patients had at least three abnormal symptoms of cognitive or behavior domain which were progressive; (5) ALS with normal cognition (ALSns), if patients did not meet any of the above mentioned criteria (24).
All statistical analyses were performed by SPSS statistical software (version 22.0). Normally distributed data were presented as the mean ± SD, while non-normally distributed data were showed as median (range). The distribution of categorical data was reported as frequencies and percentages. To compare clinical parameters and uric acid level between the two groups, the Chi-square test was used for categorical data, the independent t-test was used for normally distributed continuous data, and the Mann-Whitney-U-test for non-normally distributed continuous data. Significantly different parameters were further included in a multivariate linear regression analysis as independent variables, with the total ECAS score as a dependent variable. Education level was dichotomized into lower education (≤9 years) and higher education (>9 years) according to the median value. Moreover, the hold-out validation analysis was performed to assess the fitness of regression model by dividing patients randomly into two subsets, i.e., 80% as training set and 20% as testing set. After obtaining predicted ECAS scores through the regression model, correlations between predicted and raw ECAS scores were calculated in both training set and testing set. Additionally, correlations between plasma uric acid level and ECAS total/subdomain scores were analyzed by Pearson correlation or Spearman correlation in case of normally distributed data or non-normally distributed data, respectively. All statistical analyses were two-sided, and p < 0.05 was considered statistically significant.
Of 124 ALS patients, 52 (41.9%) were women, and 72 (58.1%) were men. The mean age at onset was 53.6 ± 10.8 years, and the mean age at testing was 54.6 ± 10.9 years. The site of onset was limb onset in 93 patients (75%), followed by bulbar onset in 22 patients (17.7%), mixed onset in 8 patients (6.5%), and respiratory onset in 1 patient (0.8%). The median education time was 9 years, the median duration of illness was 11 months, the median BMI was 21.6, and the median ALSFRS-R score was 41 (Table 1).
Patients in the ALS-cie group had shorter education time (7.5 vs. 11 years, p < 0.001), older age at symptom onset (57.5 ± 10.4 vs. 51.1 ± 10.4 years, p = 0.001), older age at testing (58.6 ± 10.3 vs. 52.1 ± 10.5 years, p = 0.001), and lower plasma uric acid level (289.3 ± 70.0 vs. 323.8 ± 77.1, p = 0.01) than those in the ALS-ncie group. Sex ratio, disease duration, site of onset, BMI values, and ALSFRS-R score did not differ between the two groups (Table 1).
Forty-eight (38.7%) patients had abnormal ECAS total scores based on a cut-off value of 81.92. Of five cognitive domains, executive impairment was the most frequent (46.8%), followed by visuospatial (39.5%) and memory (29.0%) impairment. Around one-fifth of patients had language (25.8%) and verbal fluency (21.0%) impairment (Figure 2A).
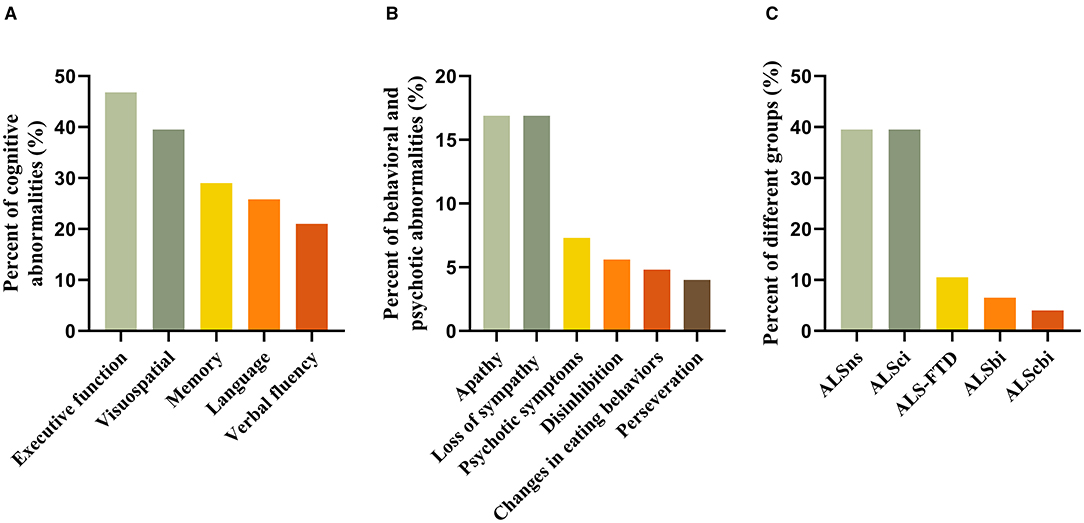
Figure 2. (A–C) Frequency of cognitive and behavioral subdomain impairment as well as ALS grouping according to the revised Strong's criteria.
Behavioral and psychotic abnormalities were observed in 39 (31.5%) of patients, with 21 (16.9%) showing abnormalities in one, 10 (8.1%) in two, and 7 (5.6%) in three or more domains. Apathy (16.9%) and loss of sympathy (16.9%) were the most common, followed by psychotic symptoms (7.3%), disinhibition (5.6%), changes in eating behaviors (4.8%), and perseveration (4.0%) (Figure 2B).
According to the revised Strong's criteria, 49 patients (39.5%) had ALSns, 49 (39.5%) patients had ALSci, 13 (10.5%) had ALS-FTD, 8 (6.5%) had ALSbi, and 5 (4.0%) had ALScbi (Figure 2C).
Plasma uric acid level was weakly associated with scores of language (rs = 0.268, p = 0.003) and executive domain (rs = 0.179, p = 0.047). No significant correlations between uric acid and verbal fluency, memory, and visuospatial functions were identified (Table 2).
Education level, age at testing (age at onset was not selected due to their collinearity relationship), and plasma uric acid level were included in a lineal regression analysis. Increased uric acid (β = 0.214, p = 0.01), lower age at testing (β = −0.378, p < 0.001), and higher education level (β = 0.424, p < 0.001) were significant predictors of higher ECAS score (F = 19.104, R2 = 0.381, p < 0.0001) (Table 3). Validation analysis showed predicted ECAS scores were significantly correlated with raw ECAS scores in both the training set (rs = 0.621, p < 0.001) and the testing set (rs = 0.666, p < 0.001), indicating reasonable model fitness.
Our findings for the first time showed that cognitive or behavioral dysfunction occurred in around 60% of Chinese ALS patients according to the revised Strong's criteria (24). Among all cognitive and behavioral subdomains, executive dysfunction was the most common subtype of cognitive impairment, while apathy and loss of sympathy were the main subtypes of behavioral abnormality. Importantly, we found that low plasma uric acid could help predict cognitive impairment in ALS patients along with aging and low educational level.
While the percentages of ALSbi and ALScbi in our ALS cohort were similar to previously reported data, ALSci (39.5% vs. around 16%) was more than doubled and ALS-FTD (10.5% vs. around 20%) was around half compared to the Italian populations (mean age 66) (25, 26). Another study showed the percentage of overall cognitive impairment (36 vs. 22%, ECAS total cut-off score) as well as executive dysfunction (47 vs. 20%) is higher among the ALS patients from China (mean age 55) than those from Germany (mean age 60), while the authors stated that the language used in ECAS questionnaire could not fully account for the differences (27). Interestingly, the percentage of cognitive dysfunction from our ALS cohort (mean age 55) based on the ECAS criteria was highly consistent with the data of Chinese patients recruited in another major academic center (22). Although other factors including educational level might influence ECAS performance, these findings support that race related differences in neurobiological networks might play a role in the different performances of Chinese and Caucasian populations (27). While executive dysfunction was the most common cognitive change in ALS patients consistent with previous studies (22), our study found that cognitive domains including memory and visual space were also frequently affected (28, 29). While some studies proposed that memory impairment was due to the failure of encoding secondary to executive impairment (30), a recent finding suggested that memory impairment was a primary dysfunction due to temporal lobe involvement in ALS patients (31). Similarly, poor visuospatial performance might be associated with temporal lobe involvement (32).
Our ALS patients were significantly younger and had lower bulbar onset percentage (18 vs. 33%) than the Italian cohort (53.6 vs. 65.5 years), which could contribute to the lower prevalence of ALS-FTD (26). In addition, C9orf72 gene mutation was strongly associated with ALS-FTD (26). The lower percentage of ALS-FTD in our ALS cohort could be partly attributed to the significantly lower prevalence of C9orf72 gene mutation in the Chinese than the Caucasian populations (33). In line with previous studies (34–36), apathy and loss of sympathy were common behavioral changes in ALS patients.
Our study showed lower plasma uric acid level was an independent predictor of cognitive impairment. Additionally, plasma uric acid level was positively, although weakly, correlated with executive and language domains of ECAS. Uric acid is the end-product of purine metabolism (37). Hyperuricemia is associate with gout with the deposition of monosodium urate in joints (38). Additionally, uric acid has been shown to act as a major antioxidant in the human body by scavenging reactive oxygen species (ROS) and peroxynitrite (39) and inhibiting iron-mediated oxidation through chelation (40). A prospective study involving 4,618 participants aged 55 years or older found that higher plasma uric acid level was associated with better cognitive function and a decreased risk of dementia after adjusting for cardiovascular risk factors (41). Another study conducted on 111 patients with tauopathies, including FTD, Alzheimer's disease (AD), and progressive supranuclear palsy, found lower plasma uric acid in patients compared to healthy controls and demonstrated that plasma uric acid level was inversely associated with risk of tauopathies independent from age and gender (42). One previous study using astroglial cultures showed that uric acid could boost glutathione production through astrocytic molecular pathways (43) and might play a protective role in reducing oxidative stress in tauopathies (44).
The most frequently identified pathology in the ALS-frontotemporal spectrum disorder is the accumulation of transactive response DNA-binding protein 43 (TDP-43) in the cytoplasm of neurons (6). One study found a significant difference in the severity of TDP-43 pathology between ALS-FTD and non-demented ALS patients (45). Another study further confirmed that ALS-specific cognitive impairment of verbal fluency, language, and executive function based on ECAS was highly correlated with TDP-43 pathology in the corresponding functional lobal areas in ALS patients without clinically diagnosed dementia (46). A post-mortem ALS study found that TDP-43 aggregation showed temporal progression patterns across different disease stages with the TDP-43 pathology expanding from the motor cortex to the prefrontal and temporal lobe (47).
Of note, mitochondria dysfunction and oxidative stress have been reported to play an important role in TDP-43 pathology of cellular and animal models (48, 49). Basic in vivo and in vitro studies showed that TDP-43 could induce mitochondrial ROS production and negatively affect neuronal survival and function (50). The nuclear factor erythroid 2-related factor 2 (Nrf2) was a main regulatory factor in preventing the accumulation of ROS and reducing oxidative stress (51). Overexpression of Nrf2 in astrocytes could delay symptom onset and prolong survival in ALS mouse models (52). Protective effects of uric acid on motor neurons could be exerted by activating Nrf2 expression in ALS models, leading to increased glutathione, and decreased oxidative damage (53). Furthermore, deficiency of Nrf2 expression could aggravate the impairment of recognition memory in a neuroinflammatory mouse model (54), while induction of Nrf2 expression alleviated cognitive impairment in an AD mouse model (55). We thus postulate that higher plasma uric acid level could help attenuate ROS production and oxidative stress in brain areas critical to maintaining normal cognition in ALS patients. One study proposed that lower uric acid levels in ALS patients might be due to malnutrition attributed to the bulbar onset and longer disease duration (56). Our study showed no difference in BMI values, disease duration, ALSFRS-R score, and bulbar onset between ALS-ncie and ALS-cie, thus not supporting a correlation between nutrition and uric acid level in our ALS patients. Inosine, the urate precursor, was proven to increase serum uric acid level safely and tolerably when administered orally or via feeding tubes in a small pilot trial of ALS patients (57). It would be interesting to explore whether inosine helps prevent or mitigate cognitive decline in ALS patients in future longitudinal studies.
The current study had several limitations. The cognitive and behavioral status of our patients was only evaluated by ECAS, thus not fully fulfilling the technical requirements of the revised Strong's criteria for accurate classification, for example, primary progressive aphasia and loss of insight could not be evaluated in a standardized way. Our study has a small sample size due to the low incidence of ALS and all participants were recruited in a single tertiary center, making referral bias possible. In addition, our study had a cross-section design and plasma uric acid was not dynamically measured, weakening its predictive strength. Furthermore, genetic features including C9orf72 mutation status that could affect the cognitive and behavioral status of ALS patients (26, 58) were not obtained due to limited resources. Of note, age was one of the major factors affecting cognitive function. It had been showed that hypertension, diabetes, cardiovascular disease, smoking, systemic inflammation, and stress were associated with cognitive impairment during the aging process (59), and these factors were not included and comprehensively evaluated in our regression model. Lastly, we did not have the neuroimaging data to explore potential correlation between cognitive/behavioral status and specific brain areas' structural or functional changes (47).
Cognitive impairment was a common feature in the Chinese ALS patients. Decreased plasma uric acid was an independent risk factor of cognitive dysfunction in ALS patients apart from aging and low educational level. Longitudinal studies on the dynamic changes in plasma uric acid and cognitive status of ALS patients could help further clarify their relationship during disease progression which might provide potential therapeutic options.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Hospital. The patients/participants provided their written informed consent to participate in this study.
JT, MZ, and FD contributed to the study design. JT, YY, ZG, ZL, and LH contributed to data acquisition. JT and ML contributed to data interpretation and statistical analysis. JT drafted the manuscript. ML and MZ revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the clinical research program of Bethune Charitable Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all patients and caregivers for their participation in this study, and doctors and nurses from the Department of Neurology, Tongji Hospital for their support. We thank Dr. Guo Li for his advice on statistical analysis.
1. Strong MJ. The syndromes of frontotemporal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. (2008) 9:323–38. doi: 10.1080/17482960802372371
2. Burrell JR, Halliday GM, Kril JJ, Ittner LM, Götz J, Kiernan MC, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. (2016) 388:919–31. doi: 10.1016/s0140-6736(16)00737-6
3. Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry. (2015) 86:168–73. doi: 10.1136/jnnp-2013-307223
4. Beeldman E, Raaphorst J, Klein Twennaar M, de Visser M, Schmand BA, de Haan RJ. The cognitive profile of ALS: a systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry. (2016) 87:611–9. doi: 10.1136/jnnp-2015-310734
5. Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL, et al. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology. (2005) 65:75–80. doi: 10.1212/01.wnl.0000167602.38643.29
6. Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. (2006) 351:602–11. doi: 10.1016/j.bbrc.2006.10.093
7. Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC Hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. (2011) 72:245–56. doi: 10.1016/j.neuron.2011.09.011
8. Chio A, Vignola A, Mastro E, Giudici AD, Iazzolino B, Calvo A, et al. Neurobehavioral symptoms in ALS are negatively related to caregivers' burden and quality of life. Eur J Neurol. (2010) 17:1298–303. doi: 10.1111/j.1468-1331.2010.03016.x
9. Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. (2013) 12:368–80. doi: 10.1016/S1474-4422(13)70026-7
10. Ahmed RM, Phan K, Highton-Williamson E, Strikwerda-Brown C, Caga J, Ramsey E, et al. Eating peptides: biomarkers of neurodegeneration in amyotrophic lateral sclerosis and frontotemporal dementia. Ann Clin Transl Neurol. (2019) 6:486–95. doi: 10.1002/acn3.721
11. Xu Z, Lee A, Nouwens A, Henderson RD, McCombe PA. Mass spectrometry analysis of plasma from amyotrophic lateral sclerosis and control subjects. Amyotroph Lateral Scler Frontotemporal Degener. (2018) 19:362–76. doi: 10.1080/21678421.2018.1433689
12. Bolanos JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv Drug Deliv Rev. (2009) 61:1299–315. doi: 10.1016/j.addr.2009.05.009
13. Paganoni S, Schwarzschild MA. Urate as a marker of risk and progression of neurodegenerative disease. Neurotherapeutics. (2017) 14:148–53. doi: 10.1007/s13311-016-0497-4
14. Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. (2009) 285:95–9. doi: 10.1016/j.jns.2009.06.002
15. Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. (2012) 51:1501–8. doi: 10.2169/internalmedicine.51.7465
16. Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, et al. The PRO-ACT database design, initial analyses, and predictive features. Neurology. (2014) 83:1719–25. doi: 10.1212/Wnl.0000000000000951
17. Paganoni S, Nicholson K, Chan J, Shui A, Schoenfeld D, Sherman A, et al. Urate levels predict survival in amyotrophic lateral sclerosis: analysis of the expanded pooled resource open-access ALS clinical trials database. Muscle Nerve. (2018) 57:430–4. doi: 10.1002/mus.25950
18. Chen X, Wei QQ, Chen Y, Cao B, Ou R, Hou Y, et al. Clinical disease stage related changes of serological factors in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2019) 20:53–60. doi: 10.1080/21678421.2018.1550516
19. O'Reilly EJ, Bjornevik K, Schwarzschild MA, McCullough ML, Kolonel LN, Le Marchand L, et al. Pre-diagnostic plasma urate and the risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2018) 19:194–200. doi: 10.1080/21678421.2017.1418005
20. Brooks BR, Miller RG, Swash M, Munsat TL. El escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) 1:293–9. doi: 10.1080/146608200300079536
21. Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. (2014) 15:9–14. doi: 10.3109/201678421.2013.805784
22. Ye S, Ji Y, Li CY, He J, Liu XL, Fan DS. The Edinburgh cognitive and behavioural ALS screen in a chinese amyotrophic lateral sclerosis population. PLoS ONE. (2016) 11:e0155496. doi: 10.1371/journal.pone.0155496
23. Balendra R, Jones A, Jivraj N, Knights C, Ellis CM, Burman R, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS functional rating scale. Amyotroph Lateral Scler Frontotemporal Degener. (2014) 15:279–84. doi: 10.3109/21678421.2014.897357
24. Strong MJ, Abrahams S, Goldstein LH, Woolley S, McLaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:153–74. doi: 10.1080/21678421.2016.1267768
25. Chio A, Moglia C, Canosa A, Manera U, Vasta R, Brunetti M, et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology. (2019) 93:e984–94. doi: 10.1212/WNL.0000000000008063
26. Chiò A, Moglia C, Canosa A, Manera U, D'Ovidio F, Vasta R, et al. ALS phenotype is influenced by age, sex, and genetics. Neurology. (2020) 94:e802–10. doi: 10.1212/wnl.0000000000008869
27. Ye S, Rosenbohm A, Bohm S, Uttner I, Ji Y, Ludolph AC, et al. Cognitive and behavioral impairments in German and Chinese ALS populations - a post-hoc comparison of national study data. Amyotroph Lateral Scler Frontotemporal Degener. (2019) 20:28–36. doi: 10.1080/21678421.2018.1542535
28. Wei QQ, Chen XP, Zheng ZZ, Huang R, Guo XY, Cao B, et al. Screening for cognitive impairment in a Chinese ALS population. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:40–5. doi: 10.3109/21678421.2014.966311
29. Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. (2012) 83:102–8. doi: 10.1136/jnnp-2011-300188
30. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. (2007) 6:994–1003. doi: 10.1016/S1474-4422(07)70265-X
31. Machts J, Bittner V, Kasper E, Schuster C, Prudlo J, Abdulla S, et al. Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: a comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neurosci. (2014) 15:83. doi: 10.1186/1471-2202-15-83
32. Sarro L, Agosta F, Canu E, Riva N, Prelle A, Copetti M, et al. Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: a diffusion tensor tractography study. AJNR Am J Neuroradiol. (2011) 32:1866–72. doi: 10.3174/ajnr.A2658
33. Jiao B, Tang BS, Liu XY, Yan XX, Zhou L, Yang Y, et al. Identification of C9orf72 repeat expansions in patients with amyotrophic lateral sclerosis and frontotemporal dementia in mainland China. Neurobiol Aging. (2014) 35:936.e19–22. doi: 10.1016/j.neurobiolaging.2013.10.001
34. Lule D, Burkhardt C, Abdulla S, Bohm S, Kollewe K, Uttner I, et al. The Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:16–23. doi: 10.3109/21678421.2014.959451
35. Poletti B, Solca F, Carelli L, Madotto F, Lafronza A, Faini A, et al. The validation of the Italian Edinburgh cognitive and behavioural ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener. (2016) 17:489–98. doi: 10.1080/21678421.2016.1183679
36. Benbrika S, Desgranges B, Eustache F, Viader F. Cognitive, emotional and psychological manifestations in amyotrophic lateral sclerosis at baseline and overtime: a review. Front Neurosci. (2019) 13:951. doi: 10.3389/fnins.2019.00951
37. Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Semin Nephrol. (2011) 31:394–9. doi: 10.1016/j.semnephrol.2011.08.002
38. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. (2016) 388:2039–52. doi: 10.1016/S0140-6736(16)00346-9
39. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. (1981) 78:6858–62. doi: 10.1073/pnas.78.11.6858
40. Waugh WH. Inhibition of iron-catalyzed oxidations by attainable uric acid and ascorbic acid levels: therapeutic implications for Alzheimer's disease and late cognitive impairment. Gerontology. (2008) 54:238–43. doi: 10.1159/000122618
41. Euser SM, Hofman A, Westendorp RGJ, Breteler MMB. Serum uric acid and cognitive function and dementia. Brain. (2009) 132:377–82. doi: 10.1093/brain/awn316
42. Schirinzi T, Di Lazzaro G, Colona VL, Imbriani P, Alwardat M, Sancesario GM, et al. Assessment of serum uric acid as risk factor for tauopathies. J Neural Transm. (2017) 124:1105–8. doi: 10.1007/s00702-017-1743-6
43. Bakshi R, Zhang H, Logan R, Joshi I, Xu YH, Chen XQ, et al. Neuroprotective effects of urate are mediated by augmenting astrocytic glutathione synthesis and release. Neurobiol Dis. (2015) 82:574–9. doi: 10.1016/j.nbd.2015.08.022
44. Alavi Naini SM, Soussi-Yanicostas N. Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxid Med Cell Longev. (2015) 2015:151979. doi: 10.1155/2015/151979
45. Prudlo J, Konig J, Schuster C, Kasper E, Buttner A, Teipel S, et al. TDP-43 pathology and cognition in ALS: a prospective clinicopathologic correlation study. Neurology. (2016) 87:1019–23. doi: 10.1212/WNL.0000000000003062
46. Gregory JM, McDade K, Bak TH, Pal S, Chandran S, Smith C, et al. Executive, language and fluency dysfunction are markers of localised TDP-43 cerebral pathology in non-demented ALS. J Neurol Neurosurg Psychiatry. (2020) 91:149–57. doi: 10.1136/jnnp-2019-320807
47. Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. (2013) 74:20–38. doi: 10.1002/ana.23937
48. Braun RJ, Sommer C, Carmona-Gutierrez D, Khoury CM, Ring J, Buttner S, et al. Neurotoxic 43-kDa TAR DNA-binding protein (TDP-43) triggers mitochondrion-dependent programmed cell death in yeast. J Biol Chem. (2011) 286:19958–72. doi: 10.1074/jbc.M110.194852
49. Stribl C, Samara A, Trumbach D, Peis R, Neumann M, Fuchs H, et al. Mitochondrial dysfunction and decrease in body weight of a transgenic knock-in mouse model for TDP-43. J Biol Chem. (2014) 289:10769–84. doi: 10.1074/jbc.M113.515940
50. Wang P, Deng J, Dong J, Liu J, Bigio EH, Mesulam M, et al. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. (2019) 15:e1007947. doi: 10.1371/journal.pgen.1007947
51. Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. (2014) 20:574–88. doi: 10.1089/ars.2012.5116
52. Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. (2008) 28:13574–81. doi: 10.1523/JNEUROSCI.4099-08.2008
53. Zhang C, Yang Y, Liang W, Wang T, Wang S, Wang X, et al. Neuroprotection by urate on the mutant hSOD1-related cellular and Drosophila models of amyotrophic lateral sclerosis: implication for GSH synthesis via activating Akt/GSK3β/Nrf2/GCLC pathways. Brain Res Bull. (2019) 146:287–301. doi: 10.1016/j.brainresbull.2019.01.019
54. Liu L, Kelly MG, Yang XR, Fernandez TG, Wierzbicki EL, Skrobach A, et al. Nrf2 deficiency exacerbates cognitive impairment and reactive microgliosis in a lipopolysaccharide-induced neuroinflammatory mouse model. Cell Mol Neurobiol. (2020) 40:1185–97. doi: 10.1007/s10571-020-00807-4
55. Uruno A, Matsumaru D, Ryoke R, Saito R, Kadoguchi S, Saigusa D, et al. Nrf2 suppresses oxidative stress and inflammation in app knock-in Alzheimer's disease model mice. Mol Cell Biol. (2020) 40:e00467-19. doi: 10.1128/MCB.00467-19
56. Zoccolella S, Simone IL, Capozzo R, Tortelli R, Leo A, D'Errico E, et al. An exploratory study of serum urate levels in patients with amyotrophic lateral sclerosis. J Neurol. (2011) 258:238–43. doi: 10.1007/s00415-010-5735-9
57. Nicholson K, Chan J, Macklin EA, Levine-Weinberg M, Breen C, Bakshi R, et al. Pilot trial of inosine to elevate urate levels in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. (2018) 5:1522–33. doi: 10.1002/acn3.671
58. Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. (2012) 11:232–40. doi: 10.1016/S1474-4422(12)70014-5
Keywords: amyotrophic lateral sclerosis, cognitive impairment, biomarker, uric acid, ECAS
Citation: Tang J, Yang Y, Gong Z, Li Z, Huang L, Ding F, Liu M and Zhang M (2021) Plasma Uric Acid Helps Predict Cognitive Impairment in Patients With Amyotrophic Lateral Sclerosis. Front. Neurol. 12:789840. doi: 10.3389/fneur.2021.789840
Received: 05 October 2021; Accepted: 08 November 2021;
Published: 06 December 2021.
Edited by:
Liyong Wu, Capital Medical University, ChinaReviewed by:
Dongsheng Fan, Peking University Third Hospital, ChinaCopyright © 2021 Tang, Yang, Gong, Li, Huang, Ding, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mao Liu, bGl1bWFvMTk4N0BnbWFpbC5jb20=; Min Zhang, emhhbmdfbWluXzM0NjRAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.