- Laboratory of Health Psychology and Clinical Neuropsychology, Department of Applied Psychology and Psychotherapy, Faculty of Psychology and Educational Sciences, University of Bucharest, Bucharest, Romania
We aimed to investigate the working memory (WM) and language separate contributions to verbal learning and memory in patients with unilateral drug-resistant temporal lobe epilepsy (drTLE); additionally, we explored the mediating role of WM on the relationship between the number of antiepileptic drugs (AEDs) and short-term verbal memory. We retrospectively enrolled 70 patients with left (LTLE; n = 44) and right (RTLE; n = 26) drTLE. About 40 similar (age and education) healthy controls were used to determine impairments of groups at WM, language (naming and verbal fluency), and verbal learning and memory (five trials list-learning, story memory—immediate recall). To disentangle the effect of learning from the short-term memory, we separately analyzed performances at the first trial, last trial, and delayed-recall list-learning measures, in addition to the total learning capacity (the sum of the five trials). Correlation and regression analyses were used to assess the contribution of potential predictors while controlling for main clinical and demographic variables, and ascertain the mediating role of WM. All patients were impaired at WM and story memory, whereas only LTLE showed language and verbal learning deficits. In RTLE, language was the unique predictor for the most verbal learning performances, whereas WM predicted the results at story memory. In LTLE, WM was the sole predictor for short-term verbal learning (list-learning capacity; trial 1) and mediated the interaction between AED number and the performance at these measures, whereas language predicted the delayed-recall. Finally, WM confounded the performance at short-term memory in both groups, although at different measures. WM is impaired in drTLE and contributes to verbal memory and learning deficits in addition to language, mediating the relationship between AED number and short-term verbal memory in LTLE. Clinicians should consider this overlap when interpreting poor performance at verbal learning and memory in drTLE.
Introduction
Patients with temporal lobe epilepsy show heterogeneous cognitive phenotypes, including various deficits not only in verbal memory and language (1, 2) but also in working memory (WM) (3). WM, a dedicated system for short-term active manipulation of the information, serves as a foundation for various cognitive processes, including verbal learning, language comprehension, and production (4, 5).
Although WM and the short-term verbal memory span concepts significantly overlap (6), they represent distinct entities (7), functionally correlated within a multi-store memory model (8). Short-term memory span refers to the retention of a limited number of data (in the verbal domain, roughly the George Miller's “magical” number 7 ± 2 items) for a relatively short time (usually up to 30 s) (9). Instead, WM involves an active organization and manipulation of the stored information and relates to executive functioning (4).
Findings regarding the WM impairment in drug-resistant temporal lobe epilepsy (drTLE) are discrepant (10–13), most probably due to the different complexity of the tasks used to measure WM. The WM capacity is commonly measured with complex supra-span tasks involving both storage and processing components (14), and it highly depends on the item semantic grouping and complexity (15). Thus, verbal memory tests used in the drTLE assessment, like list-learning and story-learning (16), are differently influenced by WM, having different demands on the semantic processing (17). Although the verbal memory and WM were found to have a high degree of overlap for both tasks (18), other findings suggest that an executive dysfunction impacts the list-learning task performance more than the story memory task (19). In particular, the unrelated list-learning task puts a heavy accent on the rote learning and is considered to be critically dependent on WM (20).
At the same time, language is a powerful predictor for verbal learning and memory (21, 22) and interferes with the verbal memory performance in language-dominant drTLE (23, 24). Therefore, language abilities must be considered when interpreting the verbal memory performance (17, 25), particularly in patients with word-finding difficulties (26).
The poor cognitive outcome is generally associated with an early onset and a long duration of the disease, and with poor seizure control (27, 28). In particular, seizure-related variables, like age at onset, disease duration, and seizure frequency, seem to be more critical than sociodemographic variables, like age and intelligence quotient (IQ), when interpreting results of neuropsychological tests (29). Nevertheless, polypharmacy induces attentional or arousal deficits (30), affecting executive functioning and broader neuropsychological functions (31–33), contributing to cumulating deficits in learning and memory. Executive function and memory showed higher negative correlations with the number of AEDs than with the defined daily dose; therefore, the AED number is a sensitive measure for side effects (34). Executive functions are particularly sensitive to drug load, whereas learning and memory are less vulnerable (35, 36); therefore, the relationship between polytherapy and verbal memory is most likely mediated by executive functions, including the WM capacity (33).
This study aims to understand the unique contributions of the verbal/auditory WM and language in various stages of the verbal learning and memory (i.e., short-term, delayed) of patients with left (LTLE) or right (RTLE) foci, beyond the influence of the primary demographic and disease characteristics. Additionally, we explored the mediating effect of WM on the relationship between the AED number and the short-term verbal memory. We expect the performance at the last list-learning trial and delayed-recall measures to be predicted mainly by language. An opposite pattern of predictors (WM as the main predictor) is expected for the first list-learning trial and story memory (immediate recall), both measures of the short-term verbal memory. We emphasize the importance of the integrated interpretation of the neuropsychological tests used in the pre-surgical assessment of patients with drTLE to unravel the reasons behind the poor verbal memory performance of a patient, thus improving the postoperative cognitive decline prediction and informing the rehabilitation programs.
Materials and Methods
Participants and Procedure
We retrospectively enrolled 70 consecutive patients with unilateral drTLE, further divided according to the seizure-onset lateralization into LTLE (n = 44) and RTLE (n = 26). Patients (right-handed adults, 55.71% male, age: M = 31.77, SD = 9.65 years; age at seizure onset: M = 20.66, SD = 11.69 years; disease duration: M = 10.80, SD = 9.43 years; IQ > 80) were candidates for epilepsy surgery and have been referred between 2019 and 2021 for neuropsychological evaluation by the epileptologists from the Epilepsy Monitoring Unit of the University Emergency Hospital Bucharest. The medical records of patients concluded the diagnosis of unilateral focal drTLE based on the following factors: case history, seizure semiology, interictal and/or ictal video-EEG, and brain imaging (1.5- or 3-T MRI, interictal [18F] fluorodeoxyglucose–PET). Exclusion criteria: moderate or severe mood disorder (e.g., anxiety, depression, and stress); bilateral TLE features; incomplete data. About 40 healthy right-handed individuals (50% female; age: M = 33.90, SD = 9.98) were recruited from the broader community to provide an age and education equivalent control sample to the patient sample. Exclusion criteria for the controls comprised any history of neurologic disease or major psychiatric illness, current psychoactive medication, and IQ < 80. Controls signed a consent form detailing the study procedure under the guidelines from the local ethical committee.
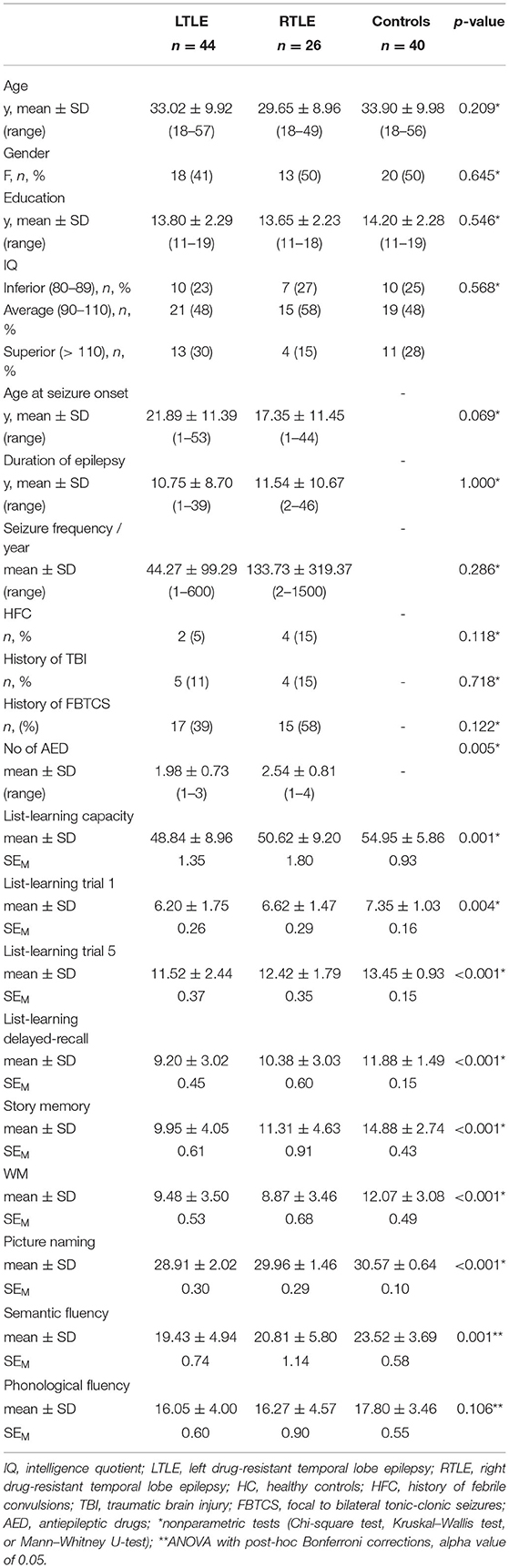
The sociodemographic, clinical, and neuropsychological characteristics of participants are summarized in Table 1. There are no significant differences between groups in sex, χ2(2) = 0.88, p = 0.645, ϕ = 0.09, age at assessment, χ2(2) = 3.09, p = 0.213, ϕ = 0.21, education years, χ2(2) = 1.63, p = 0.443, ϕ = 0.12, and IQ level, χ2(2) = 1.13, p = 0.568, ϕ = 0.10.
Patient groups show similar disease characteristics for epilepsy duration, U = 572, z = 0.00, p = 1.000, frequency of seizures per year, U = 484.5, z = −1.07, p = 0.286, history of febrile convulsions, χ2(1) = 2.45, p = 0.118, ϕ = 0.19, history of secondary generalized seizures, χ2(1) = 2.39, p = 0.122, ϕ = 0.18, history of encephalopathy, χ2(1) = 0.24, p = 0.627, ϕ = 0.06, and age at onset, U = 721.5, z = −1.82, p = 0.069, except higher number of AED in RTLE, U = 357.5, z = −2.78, p = 0.005.
Measures
Verbal memory refers to storing verbally presented information, and tasks include learning word lists (i.e., list-learning) and short stories. The information can be recalled immediately after stimuli presentation or, usually, after 30 min (i.e., delayed recall) (37). Verbal memory was assessed with two tasks: five-trial unrelated list-learning and story memory (immediate recall). The unrelated list-learning test used in our study is an unpublished Romanian adaptation from Ray Auditory Verbal Learning test (38). The subject hears a list of 15 concrete, semantically unrelated words five times and, after each repetition, the subject is asked to immediately recall as many words as possible within 1 min, and freely recall the list after 30 min delay since the last repetition. To disentangle the effect of the short-term memory from learning, we analyzed the performance of patients in three stages of the list-learning test: trial 1—a measure of short-term verbal memory span; trial 5—a measure of learning ability; and delayed-recall, a measure of the retrieval capacity (39), and for the total number of correctly recalled items over the fifth trials (maximum 75), an indicator of the overall verbal learning capacity. The story memory test was an item of the Romanian Mini-Mental State Examination (MMSE)-−2 Expanded Version (40), where subjects must immediately repeat as accurately as possible a short story that is read to them (one trial). The number of correctly rendered keywords (maximum 25) is considered a measure of short-term semantic memory.
For the assessment of verbal/auditory WM, we selected an adapted version (41) of the Letter-Number Sequencing (LNS), a subtest of the fourth edition of the Wechsler Adult Intelligence Scale (42). Subjects listen to an auditory presentation of several series of mixed letters and numbers, increasing in length, and then reorder the numbers in ascending order and letters in alphabetical order, in increasing difficulty. LNS is a reliable complex span measure for WM used in both clinical and research settings (14, 43, 44).
Naming (or confrontation naming) is defined as the ability to label visually presented stimuli; the picture naming task requires the retrieval of the phonological and semantic information from the memory system (45). Naming abilities were assessed with the Neuropsychological Assessment Battery (NAB) Naming Test (46). The participants need to name the objects in a series of 31 colored pictures.
Verbal fluency is a cognitive function that facilitates information retrieval from memory, measured by asking subjects to retrieve specific information from a specific category or starting with a specific letter, usually within the 1-min time limit (47). Verbal fluency (phonemic and semantic) was assessed by asking participants to say as many words as possible within 1 min, starting with the letter “c,” respectively from the “animals” category. The letter “c” was chosen based on the analysis of the frequency of words in the Romanian dictionary as proven to reflect a high-frequency category of words.
Intelligence quotient was assessed with the RAVEN Progressive Matrices Standard test (48), a reliable measure of fluid intelligence. Raw scores were categorized as inferior (corresponding to an IQ range between 80 and 89), average (IQ range between 90 and 110), and superior (IQ > 110). The mood was assessed with Depression, Anxiety, Stress Scale (49), the 21 self-report items version, with the following cutoff scores: 13 for depression, 9 for anxiety, and 17 for stress.
Data Analysis
Demographic characteristics were assessed using one-way univariate ANOVA with the groups (LTLE, RTLE, and controls) as between-subject factors. Patient groups were compared with t-tests or Mann–Whitney U-test on continuous clinical variables and chi-square on categorical variables.
We computed analyses in three steps. In the first step, we compared participant groups using ANOVA with post-hoc comparisons (Bonferroni corrections), based on an alpha of 0.05, supplemented with the Kruskal–Wallis test. The Spearman correlation analysis (alpha value 0.05) was used to explore associations between each criterion variables (verbal memory measures) and predictor variables (sociodemographic and disease characteristics: WM and language), and between predictor variables in each patient group. Kendall or point biserial correlation analyses were conducted between scale and ordinal, and between scale and categorical variables.
In the second step, hierarchical linear regressions were conducted in each patient group, with the verbal memory and learning variables as the dependent variable. The stepwise method was used, and standardized beta (β) values were reported. The hierarchical regression analysis results consist of model comparisons and a model interpretation based on an alpha of 0.05. Each step in the hierarchical regression was compared to the previous step using F-tests. The coefficients of the model in the final step were interpreted. For step 1, the relevant sociodemographic and disease characteristics were entered as predictor variables into the null model. Only sociodemographic and disease characteristics variables showing significant correlations with study variable (r > 0.30, p < 0.05) were first introduced in the regression models. Furthermore, depending on the specific research question for each criterion variable, either WM or language variables were separately added into the model in step 2 and step 3, respectively. At each step, variance inflation factors (VIFs) were calculated to detect the presence of multicollinearity between predictors. Then, variables generating the highest VIF values were subsequently excluded until all VIF values were <2. The following set of sociodemographic and disease-characteristic variables emerged as the most robust predictors in patient groups: education (years), age (at assessment), age at onset, epilepsy duration, frequency of seizures per year, and number of AEDs. However, age at onset was eliminated from almost all regression models as generating very high multicollinearity (VIF > 10). Where only one significant predictor was identified, linear regression was performed.
A Baron and Kenny mediation analysis (50) was conducted in each patient group to assess if WM mediates the relationship between the number of AED and short-term verbal memory measures: list-learning trial 1, learning capacity, and story memory. To determine whether a mediating relationship was supported by the data, three regressions were conducted. For mediation to be supported, four items must be met: (1) the independent variable (AED number) must be related the dependent variable (short-term verbal memory measure), (2) the independent variable (AED number) must be related to the mediator variable (WM), (3) the mediator must be related to the dependent variable but in the presence of the independent variable, and (4) the independent variable should no longer be a significant predictor of the dependent variable in the presence of the mediator variable (41). Only mediations supported by the data were further described in the results section.
Based on findings from the previous two steps, in the third step, ANCOVA was used to compare again the performance of groups at verbal memory while controlling for the relevant predictors.
Effect sizes were reported based on Cohen's standards (51), where coefficients between 0.10 and 0.29 represent a small effect size, coefficients between 0.30 and 0.49 represent a moderate effect size, and coefficients above 0.50 indicate a large effect size. There were no missing data. Correlation and regression analyses were performed with Intellectus Statistics™ (52). All other analyses were performed with SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA) (53). Post-hoc effect size and achieved power for regression analyses were computed using G*Power 3.1.9.7 tool (54).
Results
Verbal Learning and Memory Performance in Patient Groups
Except for phonemic fluency, F(2, 107) = 2.297, p = 0.106, ηp2 = 0.04, participant groups performance analysis (Table 1) revealed significant differences between groups at all neuropsychological tests: list-learning capacity, χ2(2) = 13.73, p = 0.001; list-learning trial 1, χ2(2) = 11.02, p = 0.004; list-learning trial 5, χ2(2) = 14.94, p < 0.001; list-learning delayed-recall, χ2(2) = 19.03, p < 0.001; story memory, χ2(2) = 28.34, p < 0.001; picture naming, χ2(2) = 23.64, p < 0.001; semantic fluency, F(2, 107) = 7.90, p < 0.001, ηp2 = 0.13; and WM, χ2(2) = 19.12, p < 0.001. Pairwise post-hoc comparisons revealed that, compared to controls, both patient groups had impaired WM (LTLE: p = 0.002; RTLE: p < 0.001), but only the LTLE group was impaired at verbal memory and language measures: learning capacity (p = 0.001), trial 1 (p = 0.003), trial 5 (p < 0.001), delayed-recall (p < 0.001), story memory (p < 0.001), picture naming (p < 0.001), and semantic fluency (p < 0.001), whereas the RTLE group showed only a story memory impairment (p = 0.002). Compared to the RTLE group, LTLE showed a lower performance only at picture naming test (p = 0.017).
Correlations Between Study Variables
Detailed correlations analysis results are included in Supplementary Material 1 for all participant groups, including 95% CI. In LTLE, WM showed a strong correlation with list-learning trial 1 (rs = 0.53, p = 0.002), and moderate correlations with list-learning capacity (rs = 0.46, p < 0.001) and trial 5 (rs = 0.39, p = 0.009). Language variables showed moderate correlations with all verbal memory measures (rs between 0.31 and 0.39, p < 0.05). In RTLE, WM showed a strong correlation only with story memory (rs = 0.66, p < 0.001), whereas language (mainly picture naming) showed moderate and strong correlations with all verbal memory measures (rs between 0.39 and 0.51, p < 0.05). We found no correlation between WM and language measures in either patient groups. Potential predictors showed no correlation with demographic and disease-characteristics in either patient groups, except a strong correlation between verbal fluency measures and education years in the RTLE group (rs between 0.54 and 0.56, p < 0.01), and a moderate correlation between WM and AED number in the LTLE group (rs = −0.37, p = 0.014). The AED number showed moderate correlations with all verbal learning and memory measures only in the LTLE group (rs between −0.33 and −0.41, p < 0.05). IQ level (three levels: inferior, average, and superior) showed small-to-moderate correlations with the list-learning measures not only in LTLE (rk between 0.33 and 0.45, p < 0.05) but also having a strong correlation with education years (rk = 0.64, p < 0.001). Education had moderate correlations with list-learning capacity and trial 5 only in LTLE, and with story memory in RTLE, whereas age moderately correlated with list-learning delayed-recall in both groups, and with list-learning capacity in LTLE (rs between 0.34 and 0.48, p < 0.05). We found no significant correlations between sex, history of traumatic brain injury (TBI) or focal to bilateral tonic-clonic seizures (FBTCS), and verbal memory variables in any patient group, except a moderate correlation between history of febrile convulsion (HFC) and list-learning capacity, trial 1 and delayed-recall in LTLE (rpb between 0.31 and 0.41, p < 0.05).
In healthy control group, both WM and language showed moderate and strong correlations with verbal memory variables (rs between 0.32 and 0.80, p < 0.001). In addition, WM had strong correlations with all language measures (rs between 0.61 and 0.67, p < 0.001). Education had strong positive correlations with all verbal memory measures (rs between 0.68 and 0.79, p < 0.001), whereas age showed moderate correlations only with list-learning measures (rs between 0.31 and 0.49, p < 0.05). The IQ level strongly correlated with all verbal memory measures (rk between 0.61 and 0.72, p < 0.001), and with education (rk = 0.77, p < 0.001).
Predictors of the Verbal Learning and Memory in Participant Groups
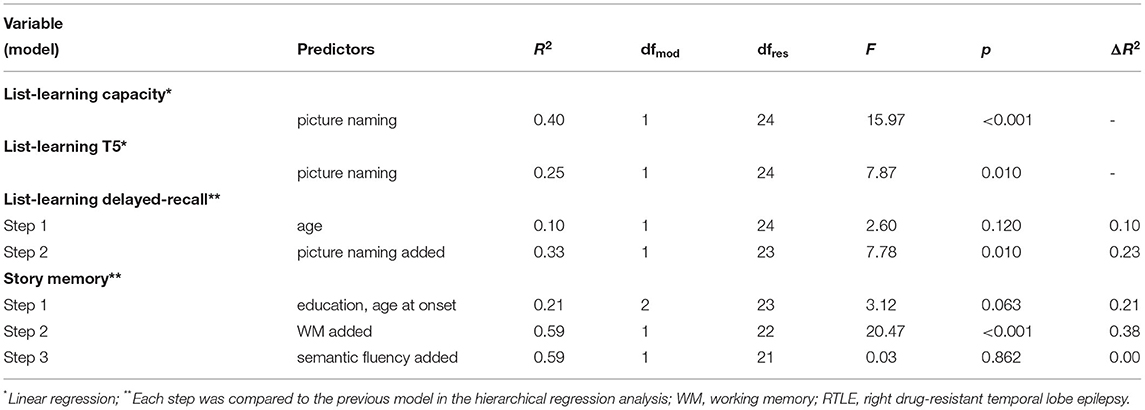
The results of regression analyses for variables predicting verbal learning and memory in each participant group are described in Supplementary Material 2. Tables 2–4 summarize the model comparisons for variables predicting verbal learning and memory in each participant groups. Each step was compared to the previous model in the hierarchical regression analysis. After controlling for relevant demographic and disease-characteristic variables, we found an opposite pattern of predictors in patient groups. In LTLE, we found WM as the sole predictor for learning capacity, explaining an additional 10% of the variation (f2 = 1.23, achieved power 0.99), and for trial 1 performance (additional 17%; f2 = 0.51, achieved power 0.98). Semantic fluency was the unique predictor for delayed-recall, explaining an additional 9% of the variation (f2 = 0.24, achieved power 0.80). No significant predictors were identified for trial 5 and story memory. In contrast, in RTLE, we found picture naming as the unique predictor for all list-learning measures (except for trial 1), explaining an additional 40% of the learning capacity variation (f2 = 0.33, achieved power 0.81), an additional 25% of the list-learning trial 5 variation (f2 = 0.35, achieved power 0.83), and an additional 23% of delayed-recall variation (f2 = 0.60, achieved power 0.92). WM was the unique predictor for story memory, explaining an additional 38% of the variation (f2 = 1.33, achieved power 0.99). No significant predictor was identified for list-learning trial 1.
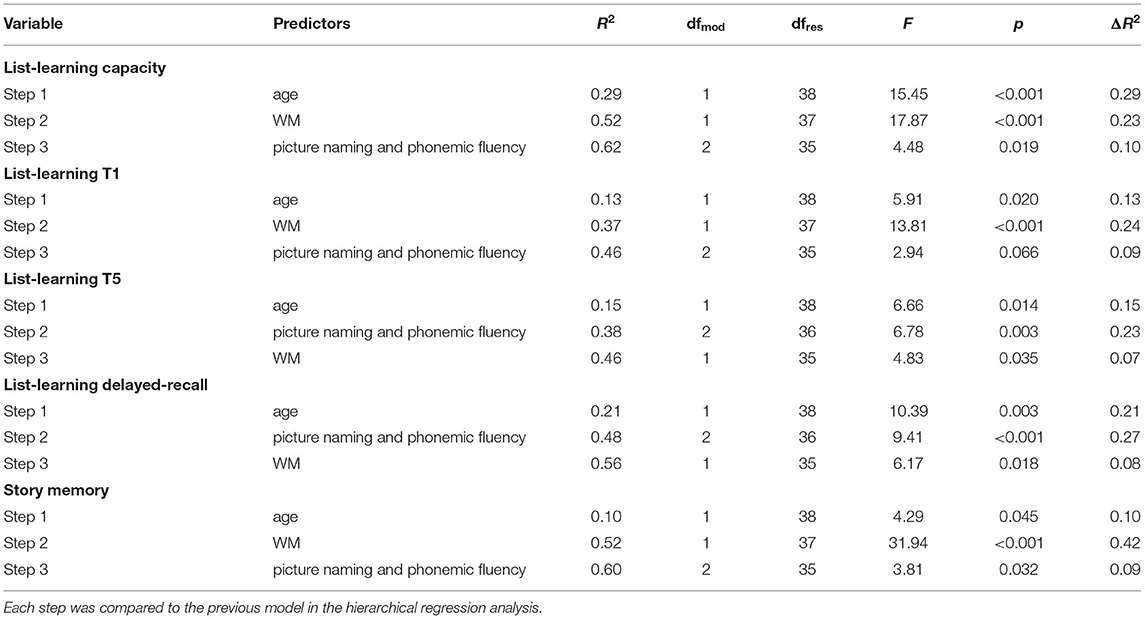
We additionally explored the relationships between WM, language, and verbal memory in the healthy control group. Education and age were introduced in the regression analyses in the first step. However, education was removed from all regression models as generating very high multicollinearity (VIF > 10). WM brought significant additional variation in all verbal memory measures: learning capacity (23%), trial 1 (24%), trial 5 (7%), delayed-recall (8%), and story memory (42%). From language measures, phonemic fluency added a significant amount of variation for all verbal memory measures except for trial 1: list-learning capacity (additional 10%), trial 5 (additional 23%), delayed-recall (additional 27%), and story memory (additional 7%). The regression model for list-learning trial 1 indicates that language did not account for a significant amount of additional variation (p = 0.066).
Table 5 summarizes the contribution of predictor variables to the verbal learning and memory performance (final model interpretation) in each of the participant groups (patients and healthy controls) after controlling for relevant disease characteristics and/or sociodemographic variables.

Table 5. Predictor variables for verbal learning and memory measures in participant groups, after controlling for disease characteristics and/or sociodemographic relevant variables (final model interpretation).
For the last step of our analysis, participant groups were again compared for verbal memory measures, taking relevant predictors as covariates. When controlling for WM, the results of the ANCOVA were not significant for list-learning trial 1, F(2, 106) = 2.709, p = 0.071, η2 = 0.05, and significant for story memory, F(2, 106) = 10.527, p < 0.001, η2 = 0.17; the mean of story memory for controls (M = 13.93, SD = 3.54) was significantly larger than for LTLE (M = 10.37, SD = 3.41), p < 0.001, but similar with RTLE (p > 0.05). WM confounds the performance at the short-term verbal memory measures: story memory in RTLE, and list-learning trial 1 in LTLE.
When controlling for age at assessment, education, picture naming, and WM, the results were not significant for learning capacity, F(2, 103) = 2.128, p = 0.124, η2 = 0.04. However, when each of the predictors were controlled separately, we found that only picture naming confounds the performance at the list-learning capacity measure, F(2, 106) = 2.157, p = 0.121, η2 = 0.04. When controlling for education years and picture naming, the results were significant for list-learning trial 5, F(2, 105) = 4.834, p = 0.01, η2 = 0.08; the mean of trial 5 for control (M = 13.08, SD = 1.71) was significantly larger than for LTLE (M = 11.86, SD = 1.73), p = 0.007. When controlling for age at assessment, picture naming, and semantic fluency, the results of the ANCOVA were significant for list-learning delayed-recall, F(2, 104) = 4.419, p = 0.014, η2 = 0.08; the mean of list-learning delayed-recall for control (M = 11.35, SD = 2.32) was significantly larger than for LTLE (M = 9.81, SD = 2.31), p = 0.014.
WM as a Mediator Between AED Number and Short-Term Memory Measures
Our data analyses revealed that the mediating relationship was supported only for list-learning capacity and list-learning trial 1 in LTLE. No mediation relationships were identified in RTLE between WM, AED number, and any of the short-term verbal memory measures.
List-Learning Trial 1 (LTLE)
In step 1 of the mediation model, the regression of AED number on list-learning, ignoring the mediator, was significant, β = −0.95, t = −2.80, p < 0.01. Step 2 showed that the regression of the AED number on WM was also significant, β = −1.76, t = −2.57, p < 0.05. Step 3 showed that the effect of WM on the list-learning, controlling for AED number, was significant, β = 0.22, t = 3.16, p < 0.01, whereas in the presence of the mediator, the AED number was not a significant predictor of list-learning, β = −0.56, t = −1.71, p = 0.09. Complete mediation is supported.
List-Learning Capacity (LTLE)
In step 1 of the mediation model, the regression of AED number on list-learning capacity, ignoring the mediator, was significant, β = −5.32, t = −3.12, p < 0.01. In step 2, the regression of the AED number on WM was also significant, β = −1.76, t = −2.57, p < 0.05. Step 3 showed that effect of WM on the list-learning capacity, controlling for AED number, was significant, β = 0.89, t = 2.45, p < 0.05, and AED number was still a significant predictor of list-learning capacity, whereas in the presence of the mediator WM (β = −3.75, t = −2.16, p < 0.05). Partial mediation is supported.
Discussion
We conducted this study intending to understand the individual contributions of WM and language in the verbal learning and memory performance of patients with unilateral drTLE, beyond the influence of demographic and disease characteristics. Additionally, we explored the mediating role of the WM on the relationship between the number of AED and short-term verbal memory.
Similar to previous findings (10–12), we found patients with drTLE impaired at WM irrespective of their seizure onset lateralization. In line with the current neuropsychological literature, patients with LTLE were impaired at semantic-related language tasks (picture naming and semantic fluency) and at all verbal-learning measures when compared to healthy controls while showing lower naming abilities compared to the patients with RTLE. Compared to controls, both patient groups were impaired at the story memory test, which is similar to previous findings (17, 55–59).
As we hypothesized, in the two groups, the short-term verbal memory measures were predicted by WM, but differently: the total learning capacity and the first trial of the list-learning task in the LTLE group, and the story memory in the RTLE group. The final comparison with healthy controls revealed that WM confounds the performance at these measures in both groups. The last list-learning trial, a measure of learning ability, was predicted exclusively by language in the RTLE group, while showing no relevant predictor in the LTLE group. In RTLE group, picture naming was the unique predictor for most list-learning measures, whereas in the LTLE group, semantic fluency showed a significant but small contribution on the list-learning delayed-recall measure. Finally, after controlling for relevant predictors, patients with LTLE performed worse than controls only at list-learning last trial, delayed-recall, and story memory tests. In addition, in line with previous research (26), we found that naming abilities confounds the performance at the list-learning capacity measure in the LTLE group. Thus, in line with the literature, only patients with LTLE have verbal memory consolidation and retrieval deficits and a semantic-related impairment.
Many neuropsychological studies addressing the verbal memory performance in drTLE did not consider the contribution of other cognitive processes, like WM and language, on the verbal memory performance. We found both WM and language as significant predictors of various verbal memory components, with WM confounding the performance at the short-term memory measures. Our analyses show that the magnitude of the effect of WM on verbal learning seems to decrease over the list-learning trials, showing no effect on the last trial and the delayed-recall measures, but only in the LTLE group. Conversely, in the RTLE group, WM was associated only with story memory but not with any of the list-learning measures, most probably due to the protective and compensatory role played by the better language abilities in this group. In healthy controls, we found that WM contributes, in addition to language, to all verbal memory measures, whereas being the strongest predictor for the short-term memory measures (list-learning trial 1 and story memory—immediate recall). Thus, our findings support the multi-store memory model (60), where various memory components dynamically interact in the learning process, and control processes such as “rehearsal” play an essential role in the transfer of information from the short-term to the long-term memory (61).
In addition, the current study adds to the findings that semantic function, characterized by deficits in semantic information retrieval (naming, semantic verbal fluency) and impaired performance at semantic verbal memory task (i.e., story memory), is altered in language-dominant patients with drTLE (62). The interdependence between verbal memory and language networks within the left temporal lobe is supported by many neuroimaging studies (63).
Some essential observations can be drawn so far. Story memory is impaired in patients with LTLE independent of their WM abilities. In addition, the list-learning last trial can be considered a better verbal learning ability indicator than the overall learning capacity measure (i.e., the sum of the five trials), proving to be impaired in patients with LTLE independent of language and WM.
As previously suggested (33), our findings showed that WM mediates the interaction between the AED number and the short-term verbal learning measures (i.e., the list-learning first trial and the total learning capacity measure), but this effect was seen only in patients with LTLE. AED was shown to have a differential impact on verbal memory (64), particularly in patients with LTLE (65), mainly when administrated in high doses and polytherapy (66–69).
We found that none of the verbal memory measures differentiated LTLE from RTLE even after controlling for relevant predictors, consistent with other studies (17, 22, 55, 58, 70). However, the story memory task used in our study required immediate recall after only one presentation of the material; therefore, it is representative of the short-term verbal memory. The performance at memory for verbal passages in a learning paradigm showed a significant difference between patients with LTLE and RTLE (71). As such, it would be more informative to understand the contribution of WM and language on various stages of a story memory learning paradigm, including delayed-recall.
We observed that an opposite pattern of contributors emerged in patient groups. Several reasons might explain this discrepancy. First, the lexical-semantic retrieval adequacy (mainly naming abilities) may be the main reason for this discrepancy, playing a protective and compensatory role for patients with RTLE and overcoming their deficient WM.
Second, the Letter-Number Sequencing task used to measure WM in this study might measure the verbal buffer, a domain-specific “slave system” (43), rather than measuring more complex executive functions (72). Strong neuroimaging evidence connects verbal WM with the left hemisphere (73). In particular, the neuroanatomical basis of the phonological loop is mainly in the left hemisphere, reflecting a strong connection with language networks (74). The verbal learning and memory tests used in this study were delivered to participants in the auditory presentation modality, which presumably relies on the phonological loop (75).
Third, most probable, the language tasks used in our study did not capture the complexity of the language–memory relationship involved by the story memory test. Traditionally, language impairment is well recognized in drTLE for verbal fluency (76) and naming abilities (77), whereas other important language expressive functions, like spontaneous speech and discourse abilities, are much less addressed by the literature (78). Although basic language functions are generally considered unaffected in patients with TLE, the narrative discourse seems to be affected (79), particularly concerning discourse and high-level language abilities (80), probably associated with low WM capacity (81). Patients with early-onset TLE show mild discourse production impairments not associated with other language measures, but correlating with WM (82). In healthy subjects, WM emerged as a robust predictor for high-level language abilities (83). Maintaining verbal information in the verbal WM system directly depends on the long-term representations and processes used in language comprehension and production (84, 85). Retelling a story must involve language production processes as it naturally involves the maintenance and ordering of linguistic information during spoken recall (86). LTLE is particularly associated with macro linguistic disturbances affecting discourse production (87) and spontaneous speech (88). Postoperatively, patients with LTLE were found to be impaired at the immediate recall of stories when compared to those with RTLE, although preoperatively they had similar impaired performance, and this result was not related to the WM capacity, being most probably due to the language network disturbance following left anterior temporal lobe resection (89). Similarly, Joo et al. (2005) (90) found correlations between the extent of left temporal lobe surgery and performance at the story memory test. Thus, the adequacy of the language system of patient, both receptive and expressive, critically influences the interictal verbal learning and memory and must be included in interpreting the memory tests results (78).
Clinical Implications
Working memory is impaired in drTLE and predicts verbal learning and memory deficit, in addition to the language. Clinicians should consider this overlap when interpreting poor performance in verbal learning and memory assessment by including a relevant WM task and examining the learning performance over the five trials of a list-learning test. This is particularly important when naming abilities or other language functions are within the normal range. Our findings suggest that the last list-learning trial is more informative for the verbal learning integrity of the language-dominant hemisphere than the traditional learning capacity measure (the sum of the five trials). Instead, the performance at the first trial showed the highest sensitivity to a WM impairment. Additionally, an impairment at the story memory test in the absence of a WM deficit may suggest that the functional integrity of the language-dominant hemisphere is affected. Concurrently, a deficit at the story memory test should be interpreted with caution when WM is impaired.
Limitations
Our patient group included various pathologies; therefore, we could not disentangle the effect of mesial or lateral temporal lobe epilepsy on study variables. In addition, besides hand-dominance, we did not use another modality to determine the language lateralization; therefore, we can only assume that the majority of our patients have the left hemisphere as language-dominant (91, 92). Although the sample sizes of patients are small, particularly for RTLE group, the post-hoc effect sizes and the achieved power for regression analyses showed significant results in each group. In addition to the AED number, further research should explore the mediating role of WM on the relationship between specific drugs (i.e., type, dosage, and combination) and verbal memory performance in drTLE.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: Mendeley Data, https://data.mendeley.com, doi: 10.17632/hhwh69wy6n.1.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Board of the University of Bucharest, Romania (notice no. 329/11.12.2019). The participants provided their written informed consent to participate in this study.
Author Contributions
MB and CI contributed to the conception, the design of the study, and its implementation. MB wrote the first draft of the manuscript. CI wrote sections of the manuscript. CI and EA reviewed the manuscript. All authors read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the epileptologists from the Epilepsy Monitoring Unit of the University Emergency Hospital Bucharest for their invaluable work in diagnosing patients with epilepsy, and for the trust they have placed in us.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.780086/full#supplementary-material
References
1. Baxendale S, Thompson P. The association of cognitive phenotypes with postoperative outcomes after epilepsy surgery in patients with temporal lobe epilepsy. Epilepsy Behav. (2020) 112:107386. doi: 10.1016/j.yebeh.2020.107386
2. Reyes A, Kaestner E, Ferguson L, Jones JE, Seidenberg M, Barr WB, et al. Cognitive phenotypes in temporal lobe epilepsy utilizing data- and clinically driven approaches: Moving toward a new taxonomy. Epilepsia. (2020) 61:1211–20. doi: 10.1111/epi.16528
3. Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. (2012) 98:1–13. doi: 10.1016/j.eplepsyres.2011.10.009
4. Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. (2003) 4:829–39. doi: 10.1038/nrn1201
5. Cowan N. Working memory underpins cognitive development, learning, and education. Educ Psychol Rev. (2014) 26:197–223. doi: 10.1007/s10648-013-9246-y
6. Aben B, Stapert S, Blokland A. About the distinction between working memory and short-term memory. Front Psychol. (2012) 3:301. doi: 10.3389/fpsyg.2012.00301
7. Miller EK, Lundqvist M. Bastos AM. Working memory 2.0. Neuron. (2018) 100:463–75. doi: 10.1016/j.neuron.2018.09.023
8. Atkinson RC, Shiffrin RM. The control of short-term memory. Sci Am. (1971) 225:82–90. doi: 10.1038/scientificamerican0871-82
9. Cowan N. George Miller's magical number of immediate memory in retrospect: Observations on the faltering progression of science. Psychol Rev. (2015) 122:536–41. doi: 10.1037/a0039035
10. Stretton J, Winston G, Sidhu M, Centeno M, Vollmar C, Bonelli S, et al. Neural correlates of working memory in Temporal Lobe Epilepsy — An fMRI study. Neuroimage. (2012) 60:1696–703. doi: 10.1016/j.neuroimage.2012.01.126
11. Wagner DD, Sziklas V, Garver KE, Jones-Gotman M. Material-specific lateralization of working memory in the medial temporal lobe. Neuropsychologia. (2009) 47:112–22. doi: 10.1016/j.neuropsychologia.2008.08.010
12. Zamarian L, Trinka E, Bonatti E, Kuchukhidze G, Bodner T, Benke T, et al. Executive functions in chronic mesial temporal lobe epilepsy. Epilepsy Res Treat. (2011) 2011: 596174. doi: 10.1155/2011/596174
13. Tudesco I de SS, Vaz LJ, Mantoan MAS, Belzunces E, Noffs MH, Caboclo LOSF, et al. Assessment of working memory in patients with mesial temporal lobe epilepsy associated with unilateral hippocampal sclerosis. Epilepsy Behav. (2010) 18:223–8. doi: 10.1016/j.yebeh.2010.04.021
14. Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW, et al. Working memory span tasks: A methodological review and user's guide. Psychon Bull Rev. (2005) 12:769–86. doi: 10.3758/bf03196772
15. Eriksson J, Vogel EK, Lansner A, Bergström F, Nyberg L. Neurocognitive architecture of working memory. Neuron. (2015) 88:33–46. doi: 10.1016/j.neuron.2015.09.020
16. McAndrews MP, Cohn M. Neuropsychology in temporal lobe epilepsy: influences from cognitive neuroscience and functional neuroimaging. Epilepsy Res Treat. (2012) 2012:925238. doi: 10.1155/2012/925238
17. Helmstaedter C, Wietzke J, Lutz MT. Unique and shared validity of the “Wechsler logical memory test”, the “California verbal learning test”, and the “verbal learning and memory test” in patients with epilepsy. Epilepsy Res. (2009) 87:203–12. doi: 10.1016/j.eplepsyres.2009.09.002
18. Duff K, Schoenberg MR, Scott JG, Adams RL. The relationship between executive functioning and verbal and visual learning and memory. Arch Clin Neuropsychol. (2005) 20:111–22. doi: 10.1016/j.acn.2004.03.003
19. Tremont G, Halpert S, Javorsky DJ, Stern RA. Differential impact of executive dysfunction on verbal list learning and story recall. Clin Neuropsychol. (2000) 14:295–302. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295
20. Constantinidou F, Zaganas I, Papastefanakis E, Kasselimis D, Nidos A, Simos PG. Age-related decline in verbal learning is moderated by demographic factors, working memory capacity, and presence of amnestic mild cognitive impairment. J Int Neuropsychol Soc. (2014) 20:822–35. doi: 10.1017/S1355617714000678
21. Hermann BP, Wyler AR, Steenman H, Richey ET. The interrelationship between language function and verbal learning/memory performance in patients with complex partial seizures. Cortex. (1988) 24:245–53. doi: 10.1016/s0010-9452(88)80033-9
22. Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Adequacy of language function and verbal memory performance in unilateral temporal lobe epilepsy. Cortex. (1992) 28:423–33. doi: 10.1016/S0010-9452(13)80152-9
23. Kim H, Yi S, Son EI, Kim J. Material-specific memory in temporal lobe epilepsy: Effects of seizure laterality and language dominance. Neuropsychology. (2003) 17:59–68. doi: 10.1037/0894-4105.17.1.59
24. Weber B, Fliessbach K, Lange N, Kügler F, Elger CE. Material-specific memory processing is related to language dominance. Neuroimage. (2007) 37:611–7. doi: 10.1016/j.neuroimage.2007.05.022
25. Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. (2009) 132:570–82. doi: 10.1093/brain/awp012
26. Busch RM, Chapin JS, Haut JS, Dulay MF, Naugle RI, Najm I. Word-finding difficulties confound performance on verbal cognitive measures in adults with intractable left temporal lobe epilepsy. Epilepsia. (2013) 54:e37–40. doi: 10.1111/epi.12088
27. Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. (2004) 3:663–72. doi: 10.1016/S1474-4422(04)00906-8
28. Höller Y, Trinka E. What do temporal lobe epilepsy and progressive mild cognitive impairment have in common? Front Syst Neurosci. (2014) 8:58. doi: 10.3389/fnsys.2014.00058
29. Goldstein LH. Neuropsychological Investigation of Temporal Lobe Epilepsy. J R Soc Med. (1991) 84:460–5. doi: 10.1177/014107689108400806
30. Drane DL, Meador KJ. Epilepsy, anticonvulsant drugs and cognition. Baillieres Clin Neurol. (1996) 5:877–85.
31. Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. (2011) 4:385–407. doi: 10.1177/1756285611417920
32. Witt J-A, Elger CE, Helmstaedter C. Adverse cognitive effects of antiepileptic pharmacotherapy: Each additional drug matters. Eur Neuropsychopharmacol. (2015) 25:1954–9. doi: 10.1016/j.euroneuro.2015.07.027
33. Witt J-A, Helmstaedter C. How can we overcome neuropsychological adverse effects of antiepileptic drugs? Expert Opin Pharmacother. (2017) 18:551–4. doi: 10.1080/14656566.2017.1309025
34. Helmstaedter C, Witt J-A. How neuropsychology can improve the care of individual patients with epilepsy. Looking back and into the future. Seizure. (2017) 44:113–20. doi: 10.1016/j.seizure.2016.09.010
35. Aldenkamp AP. Effects of antiepileptic drugs on cognition. Epilepsia. (2001) 42:46–49. doi: 10.1046/j.1528-1157.2001.00516.x
36. Helmstaedter C, Elger CE, Witt J-A. The effect of quantitative and qualitative antiepileptic drug changes on cognitive recovery after epilepsy surgery. Seizure. (2016) 36:63–9. doi: 10.1016/j.seizure.2016.02.001
37. Tatsumi IF, Watanabe M. Verbal Memory. In: Encyclopedia of Neuroscience. Binder MD, Hirokawa N, Windhorst U, editors. Berlin, Heidelberg: Springer. (2009) p. 4176–4178. doi: 10.1007/978-3-540-29678-2_6266
38. Schmidt M. Rey auditory verbal learning test: A handbook. Los Angeles, CA: Western Psychological Services. (1996).
39. Saury J-M, Emanuelson I. Neuropsychological assessment of hippocampal integrity. Appl Neuropsychol Adult. (2017) 24:140–51. doi: 10.1080/23279095.2015.1113536
40. Folstein MF, Folstein SE, White T, Messer MA, Munteanu C, Iliescu D, et al. Mini-Mental State Examination, Second Edition. User's Manual (Romanian adaptation). Bucharest: OS Romania. (2013).
41. Miclea M. Platforma computerizata de evaluare psihologica (CAS++) [Measurement Instrument]. (2009). Available online at: https://www.cognitrom.ro/produs/evaluare-psihologica/
42. Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition. San Antonio, TX: Pearson Assessment. (2008).
43. Egeland J. Measuring working memory with digit span and the letter-number sequencing subtests from the WAIS-IV: too low manipulation load and risk for underestimating modality effects. Applied Neuropsychology: Adult. (2015) 22:445–51. doi: 10.1080/23279095.2014.992069
44. Hill B, Elliott E, Shelton J, Pella R, O'Jile J, Gouvier W. Can we improve the clinical assessment of working memory? An evaluation of the WAISIII using a working memory criterion construct. J Clin Exp Neuropsychol. (2010) 32:315–23. doi: 10.1080/13803390903032529
45. Spezzano LC, Radanovic M. Naming abilities: Differentiation between objects and verbs in aphasia. Dement Neuropsychol. (2010) 4:287–92. doi: 10.1590/S1980-57642010DN40400006
46. Stern RA, White T. NAB Naming Test: Professional manual, 2009. Lutz, FL: Psychological Assessment Resources. (2009).
47. Patterson J. Verbal Fluency. In: Encyclopedia of clinical neuropsychology. Kreutzer JS, DeLuca J, Caplan B, editors. New York, NY: Springer. (2008) p 2603–2606. doi: 10.1007/978-0-387-79948-3_1423
48. Raven J, Raven J. Raven Progressive Matrices. In: Handbook of nonverbal assessment. New York, NY, US: Kluwer Academic/Plenum Publishers. (2003) p. 223–237. doi: 10.1007/978-1-4615-0153-4_11
49. Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. (2nd. Ed.). Sydney: Psychology Foundation. (1995).
50. Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. (1986) 51:1173–82. doi: 10.1037/0022-3514.51.6.1173
51. Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers. (1988).
52. Intellectus Statistics. Intellectus Statistics [Online computer software]. (2019). Available online at: http://analyze.intellectusstatistics.com/
54. Faul F, Erdfelder E, Buchner A. Lang A-G. Statistical power analyses using G*Power 31: Tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
55. Tavakoli M, Barekatain M, Doust HTN, Molavi H, Nouri RK, Moradi A, et al. Cognitive impairments in patients with intractable temporal lobe epilepsy. J Res Med Sci. (2011) 16:1466–72.
56. Giovagnoli AR, Erbetta A, Villani F, Avanzini G. Semantic memory in partial epilepsy: verbal and non-verbal deficits and neuroanatomical relationships. Neuropsychologia. (2005) 43:1482–92. doi: 10.1016/j.neuropsychologia.2004.12.010
57. Saling MM, Berkovic SF, O'Shea MF, Kalnins RM, Darby DG, Bladin PF. Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. J Clin Exp Neuropsychol. (1993) 15:608–18. doi: 10.1080/01688639308402582
58. Giovagnoli AR, Avanzini G. Learning and memory impairment in patients with temporal lobe epilepsy: relation to the presence, type, and location of brain lesion. Epilepsia. (1999) 40:904–11. doi: 10.1111/j.1528-1157.1999.tb00797.x
59. Wilde N, Strauss E, Chelune GJ, Loring DW, Martin RC, Hermann BP, et al. WMS–III performance in patients with temporal lobe epilepsy: Group differences and individual classification. J Int Neuropsychol Soc. (2001) 7:881–91. doi: 10.1017/S1355617701777120
60. Atkinson RC, Shiffrin RM. Human Memory: A Proposed System and its Control Processes. In: Psychology of Learning and Motivation. Spence KW, Spence JT, editors. Academic Press. (1968) 89–195. doi: 10.1016/S0079-7421(08)60422-3
61. Jaimes-Bautista AG, Rodríguez-Camacho M, Martínez-Juárez IE, Rodríguez-Agudelo Y. Semantic processing impairment in patients with temporal lobe epilepsy. Epilepsy Res Treat. (2015) 2015: doi: 10.1155/2015/746745
62. Covington NV, Duff MC. Expanding the Language Network: Direct Contributions from the Hippocampus. Trends Cogn Sci (Regul Ed). (2016) 20:869–70. doi: 10.1016/j.tics.2016.10.006
63. Baciu M, Perrone-Bertolotti M. What do patients with epilepsy tell us about language dynamics? A review of fMRI studies. Rev Neurosci. (2015) 26:323–41. doi: 10.1515/revneuro-2014-0074
64. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. (2004) 5:435–9. doi: 10.1016/j.yebeh.2004.03.006
65. Durwen HF, Elger CE. Verbal learning differences in epileptic patients with left and right temporal lobe foci–a pharmacologically induced phenomenon? Acta Neurol Scand. (1993) 87:1–8. doi: 10.1111/j.1600-0404.1993.tb04066.x
66. Durwen HF, Hufnagel A, Elger CE. Anticonvulsant drugs affect particular steps of verbal memory processing–an evaluation of 13 patients with intractable complex partial seizures of left temporal lobe origin. Neuropsychologia. (1992) 30:623–31. doi: 10.1016/0028-3932(92)90067-v
67. Jokeit H, Krämer G, Ebner A. Do antiepileptic drugs accelerate forgetting? Epilepsy Behav. (2005) 6:430–2. doi: 10.1016/j.yebeh.2004.12.012
68. Wang W-H, Liou H-H, Chen C-C, Chiu M-J, Chen T-F, Cheng T-W, et al. Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: a retrospective cross-sectional study. Epilepsy Behav. (2011) 22:728–34. doi: 10.1016/j.yebeh.2011.08.038
69. Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, Mason A, Golder S, O'Meara S, Sculpher M, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess. (2005) 9:1–157. doi: 10.3310/hta9150
70. Rayner G, Tailby C, Jackson G, Wilson S. Looking beyond lesions for causes of neuropsychological impairment in epilepsy. Neurology. (2019) 92:e680. doi: 10.1212/WNL.0000000000006905
71. Jones-Gotman M, Harnadek MC, Kubu CS. Neuropsychological assessment for temporal lobe epilepsy surgery. Can J Neurol Sci. (2000) 27 Suppl 1:S39–43; discussion S50-52. doi: 10.1017/s0317167100000639
72. Mielicki MK, Koppel RH, Valencia G, Wiley J. Measuring Working memory capacity with the letter–number sequencing task: Advantages of visual administration. Appl Cogn Psychol. (2018) 32:805–14. doi: 10.1002/acp.3468
73. Shura RD, Hurley RA, Taber KH. Working memory models: insights from neuroimaging. JNP. (2016) 28:A4–5. doi: 10.1176/appi.neuropsych.15120402
74. Baddeley A. Working memory and language: an overview. J Commun Disord. (2003) 36:189–208. doi: 10.1016/S0021-9924(03)00019-4
75. Baddeley A. Working memory: Theories, Models, and Controversies. New York, NY, US: Clarendon Press/Oxford University Press. (1986).
76. Metternich B, Buschmann F, Wagner K, Schulze-Bonhage A, Kriston L. Verbal fluency in focal epilepsy: a systematic review and meta-analysis. Neuropsychol Rev. (2014) 24:200–18. doi: 10.1007/s11065-014-9255-8
77. Hamberger MJ. Object naming in epilepsy and epilepsy surgery. Epilepsy Behav. (2015) 46:27–33. doi: 10.1016/j.yebeh.2014.12.019
78. Bartha-Doering L, Trinka E. The interictal language profile in adult epilepsy. Epilepsia. (2014) 55:1512–25. doi: 10.1111/epi.12743
79. Drane DL, Pedersen NP. Knowledge of language function and underlying neural networks gained from focal seizures and epilepsy surgery. Brain Lang. (2019) 189:20–33. doi: 10.1016/j.bandl.2018.12.007
80. Dutta M, Murray L, Miller W, Groves D. Effects of epilepsy on language functions: scoping review and data mining findings. Am J Speech Lang Pathol. (2018) 27:350–78. doi: 10.1044/2017_AJSLP-16-0195
81. Yurchenko A, Golovteev A, Dragoy O. Single-word, sentence, and discourse comprehension in individuals with temporal lobe epilepsy. Epilepsy Behav. (2020) 110: doi: 10.1016/j.yebeh.2020.107140
82. Bell B, Dow C, Watson ER, Woodard A, Hermann B, Seidenberg M. Narrative and procedural discourse in temporal lobe epilepsy. J Int Neuropsychol Soc. (2003) 9:733–9. doi: 10.1017/S1355617703950065
83. Antonsson M, Longoni F, Einald C, Hallberg L, Kurt G, Larsson K, et al. High-level language ability in healthy individuals and its relationship with verbal working memory. Clin Linguist Phon. (2016) 30:944–58. doi: 10.1080/02699206.2016.1205664
84. Acheson DJ, Hamidi M, Binder JR, Postle BR, A. common neural substrate for language production and verbal working memory. J Cogn Neurosci. (2011) 23:1358–67. doi: 10.1162/jocn.2010.21519
85. Schwering SC, MacDonald MC. Verbal working memory as emergent from language comprehension and production. Front Hum Neurosci. (2020) 14:66. doi: 10.3389/fnhum.2020.00068
86. Acheson DJ, MacDonald MC. Verbal working memory and language production: common approaches to the serial ordering of verbal information. Psychol Bull. (2009) 135:50–68. doi: 10.1037/a0014411
87. Field SJ, Saling MM, Berkovic SF. Interictal discourse production in temporal lobe epilepsy. Brain Lang. (2000) 74:213–22. doi: 10.1006/brln.2000.2335
88. Bartha L, Benke T, Bauer G, Trinka E. Interictal language functions in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. (2005) 76:808–14. doi: 10.1136/jnnp.2004.045385
89. Frisk V, Milner B. The relationship of Working memory to the immediate recall of stories following unilateral temporal or frontal lobectomy. Neuropsychologia. (1990) 28:121–35. doi: 10.1016/0028-3932(90)90095-6
90. Joo EY, Han HJ, Lee EK, Choi S, Jin JH, Kim JH, et al. Resection extent versus postoperative outcomes of seizure and memory in mesial temporal lobe epilepsy. Seizure. (2005) 14:541–51. doi: 10.1016/j.seizure.2005.08.011
91. Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, et al. Cerebral language lateralization: Evidence from intracarotid amobarbital testing. Neuropsychologia. (1990) 28:831–8. doi: 10.1016/0028-3932(90)90007-B
Keywords: drug-resistant temporal lobe epilepsy, working memory, verbal memory and learning, language, picture naming
Citation: Bolocan M, Iacob CI and Avram E (2021) Working Memory and Language Contribution to Verbal Learning and Memory in Drug-Resistant Unilateral Focal Temporal Lobe Epilepsy. Front. Neurol. 12:780086. doi: 10.3389/fneur.2021.780086
Received: 20 September 2021; Accepted: 09 November 2021;
Published: 08 December 2021.
Edited by:
Yvonne Höller, University of Akureyri, IcelandReviewed by:
Frank Van Schalkwijk, University of Tübingen, GermanyPatricia Rzezak, University of São Paulo, Brazil
Copyright © 2021 Bolocan, Iacob and Avram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica Bolocan, bW9uaWNhLmJvbG9jYW4yMDEzQGdtYWlsLmNvbQ==
 Monica Bolocan
Monica Bolocan Claudia I. Iacob
Claudia I. Iacob Eugen Avram
Eugen Avram