- 1Graduate School, Qinghai University, Xining, China
- 2Biomedical Engineering Research Center, Kunming Medical University, Kunming, China
- 3Neurological Intensive Care Department, Shengli Oilfield Central Hospital, Dongying, China
- 4Department of Neurosurgery, Qinghai Provincial People's Hospital, Xining, China
Background: The role of tranexamic acid (TXA) in preventing hematoma expansion (HE) in patients with acute spontaneous intracerebral hemorrhage (ICH) remains unclear. We aim to investigate the efficacy and safety of TXA in acute spontaneous ICH with a particular focus on subgroups.
Methods: Randomized controlled trials (RCTs) were retrieved from CENTRAL, Clinicaltrials.gov, EMBASE, PubMed, and WHO ICTRP. The primary outcome measurement was HE. The secondary outcome measurements included 3-month poor functional outcome (PFO), 3-month mortality, and major thromboembolic events (MTE). We conducted subgroup analysis according to the CT markers of HE (standard-risk population and high-risk population) and the time from onset to randomization (>4.5 and ≤4.5 h).
Results: We identified seven studies (representing five RCTs) involving 2,650 participants. Compared with placebo, TXA may reduce HE on subsequent imaging (odd ratio [OR] 0.825; 95% confidence interval [CI] 0.692–0.984; p = 0.033; I2 = 0%; GRADE: moderate certainty). TXA and placebo arms did not differ in the rates of 3-month PFO, 3-month mortality, and MTE. Subgroup analysis indicated that TXA reduced the risk of HE in the high-risk population with CT markers of HE (OR 0.646; 95% CI 0.503–0.829; p = 0.001; I2 = 0 %) and in patients who were treated within 4.5 h of symptom onset (OR 0.823; 95% CI 0.690–0.980; p = 0.029; I2 = 0%), but this protective effect was not observed in the standard-risk population and patients who were treated over 4.5 h of symptom onset.
Conclusions: Tranexamic acid (TXA) may decrease the risk of HE in patients with acute spontaneous ICH. Importantly, the decreased risk was observed in patients who were treatable within 4.5 h and with a high risk of HE, but not in those who were treatable over 4.5 h and in standard-risk population. However, PFO or mortality at 3 months did not significantly differ between patients who received TXA and those who received placebo. TXA is safe for acute spontaneous ICH without increasing MTE.
Introduction
Spontaneous intracerebral hemorrhage (ICH) is one of the leading causes of disability and death worldwide, and is one of the serious global public health and socioeconomic burdens. Globally, up to 3 million people die from ICH each year, accounting for 5% of all human deaths, while an estimated 18 million people suffer from the sequelae of ICH (1, 2). Although an organized in-patient (stroke unit) care has been shown to contribute in reducing disability and mortality, there is no strong evidence-based acute therapy that is specific to acute spontaneous ICH (3, 4).
Age, neurological deficit, hemorrhage cause, location, and hematoma volume are the main determinants of clinical outcomes in patients with ICH (5, 6). Among them, hematoma volume is the most important factor. Roughly one-third of acute spontaneous ICH are complicated by hematoma expansion (HE), which most often occurs within the first few hours, but could also occur at up to 24 h, presenting a target window for intervention (7, 8). Emergency medical services are traditionally established to bring in patients with acute stroke with haste for diagnosis and treatment within 4.5 h of symptom onset. In addition, tranexamic acid (TXA) therapy is theoretically more suitable for patients with high-risk for ICH growth, such as patients with early computed tomography (CT) markers of HE. CT markers, including black hole sign, blend sign, island sign, and spot sign, have been used as reliable predictors for early HE in patients with acute spontaneous ICH (9–14). Therefore, it seems feasible to identify patients at high risk for HE early, and then for early therapeutic intervention to improve the clinical outcomes of patients with acute spontaneous ICH.
The researchers are looking for a safe acute therapy to reduce the risk of HE and improve the outcome for acute spontaneous ICH patients. TXA is an antifibrinolytic agent that reduces bleeding by inhibiting plasminogen activation and fibrinolysis. It has been shown that it can reduce perioperative blood loss and risk of blood transfusion (15, 16). In addition, trauma guidelines have recommended the early use of TXA in patients with traumatic ICH, as TXA can provide a survival benefit without increasing its adverse events (17, 18). However, the role of TXA in preventing HE and in improving outcome in patients with acute spontaneous ICH remains unclear.
Several randomized controlled trials (RCTs) evaluating the efficacy and safety of TXA in patients with acute spontaneous ICH have been performed in recent years. However, the existing RCTs and previously published systematic reviews have reported fragmentary and conflicting results. Some studies have shown a protective association between TXA use and ICH growth. Other studies have found no such relationship. These studies differed in their study populations. Herein, we performed meta-analysis on the available RCTs to determine the following: (1) the effectiveness and safety of TXA administration, compared against placebo or open control, in adults with acute spontaneous ICH; (2) how this differs between the standard-risk population and the high-risk population (those with ICH with CT markers of HE); and (3) how this differs between the range within 4.5 h and over 4.5 h of the time from onset to randomization.
Methods
Guidance and Protocol
This systematic review and meta-analysis were performed in compliance with the Cochrane Handbook for Systematic Reviews of Interventions (19) and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20). The review protocol was prospectively registered in International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) under number INPLASY202170032. The PRISMA 2020 checklist is available in the Supplementary Table 1.
Eligibility Criteria
We considered studies to be eligible if they met the following criteria: (i) Types of studies: RCT published in peer-reviewed medical journals; (ii) Types of participants: Adult patients (aged ≥18) with spontaneous ICH and are treatable within 24 h of symptom onset; (iii) Types of interventions: TXA at any dose versus placebo; (iv) Types of outcome measures: The outcome measurements were HE (defined as hematoma growth >33% and/or >6 ml), 3-month poor functional outcome (PFO; defined as modified Rankin Scale score 4–6), 3-month mortality, and major thromboembolic events (MTE; according to the definition provided in each study).
Search Strategy
We conducted a comprehensive literature search on Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, Embase, PubMed, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) from inception until July 3, 2021 with no language restriction. RCTs investigating the effects of TXA in adult patients with acute spontaneous ICH were included. Controlled vocabulary (i.e., MeSH and Emtree) and keywords were used. Search terms included TXA, ICH, RCT, and their variants. The complete search strategy is available in the Supplementary Table 2. We manually screened the reference lists of eligible trials and previous relevant reviews for additional studies.
Study Selection
After records were imported into the Zotero reference management software (www.zotero.org), duplicate records were manually removed. Two reviewers independently screened the titles and abstracts for relevance, and labeled records as probably included and excluded in duplicates. If records are deemed potentially relevant by either reviewer, the full-text articles were retrieved to assess its eligibility. Disagreements were resolved by discussion, and by a third-party adjudication if required.
Data Extraction
Two reviewers independently extracted data and in duplicate using a standardized form. We extracted the following information from included trials: (i) trial characteristics: study title, fist author name, year of publication, country of origin, study design, and number of participants; (ii) patient characteristics: CT markers of HE, age, sex, and baseline National Institutes of Health Stroke Scale (NIHSS) score; (iii) intervention characteristics: treatment administration time and TXA dose; and (iv) data on outcomes of interest, etc.
Risk of Bias Assessment
Two reviewers have assessed the risk of bias of each trial independently and in duplicates using the Cochrane Collaboration's tool based on the recommendations of the Cochrane Handbook for Systematic Reviews of Intervention (21). The following items for risk of bias were examined: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete data outcome (attrition bias), selective reporting (reporting bias), and, other biases (such as stopping early and funding source). The risk of bias was determined as “high risk,” “unclear risk,” or “low risk.” Disagreements were resolved by discussion, and third-party adjudication if required.
Quality of Evidence
Two reviewers assessed the overall certainty of evidence for each outcome using the Grading Recommendations Assessment, Development and Evaluation (GRADE) system (22). The items assessed included risk of bias, inconsistency, indirectness, imprecision, and publication bias. The evidence quality was classified as very low, low, moderate, and high. Disagreements were resolved by discussion, and third-party adjudication if required.
Statistical Analysis
We calculated odds ratios (ORs) and their corresponding 95% CIs to measure the effect size while comparing TXA vs. control among patients with acute spontaneous ICH. Meta-analyses were performed using DerSimonian and also Laird random-effects models accounting for clinical heterogeneity (23). A p-value of < 0.05 was considered statistically significant. The heterogeneity between trials was assessed using the Cochran Q test (with p < 0.1 indicating significance) and was quantified by the I2 statistic (for which a value of 50% or greater was considered to represent significant heterogeneity) (24). Publication bias across individual trials was graphically evaluated using a funnel plot and also with the Egger's test at a significance level of p < 0.05 (25). We performed one post hoc sensitivity analysis, restricting to trials with a low risk of bias, to test the robustness of our findings. We also conducted subgroup analysis according to different subjects (high-risk population or standard-risk population) and the time from onset to randomization ( ≤ 4.5 or >4.5 h). High-risk population was defined as patients with CT markers of HE in patients with ICH. Standard-risk population was defined as general ICH population, without further screening of CT markers of HE. Specific data of the intervention and control groups were extracted from publications. If the original data were not available, the adjusted ORs, as the indicators of the outcomes, were alternatively extracted. All statistical analyses were conducted with Comprehensive Meta-Analysis software (version 3) and Stata software (version 15).
Results
Result of Literature Search
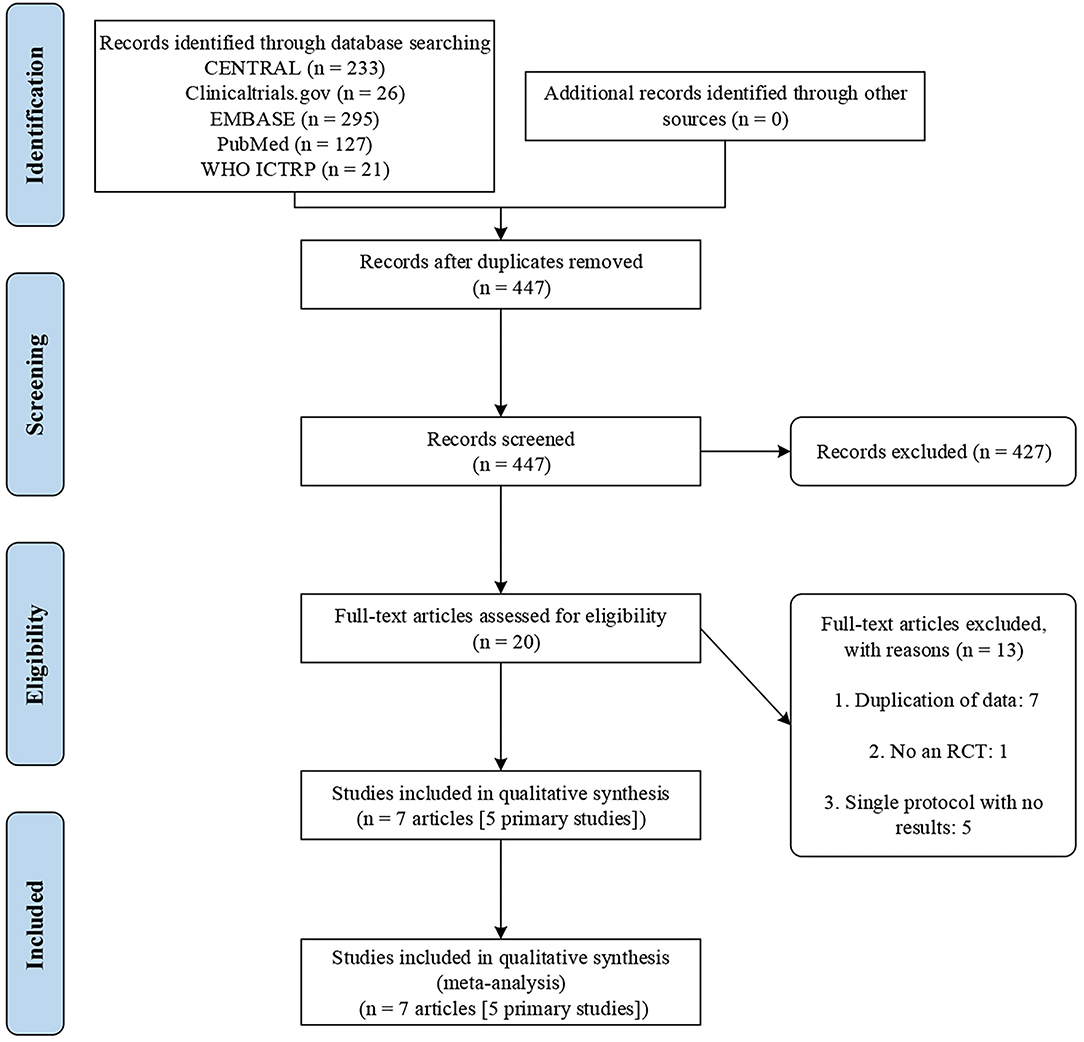
The initial search yielded 702 records. We excluded 275 duplicates and a further 427 records after title and abstract screening. Then, 20 potentially eligible articles were retained for full-text evaluation. Finally, seven trial reports (26–32) representing 5 RCTs were included according to the inclusion criteria. Figure 1 illustrates the study selection process.
Characteristics of Eligible Studies
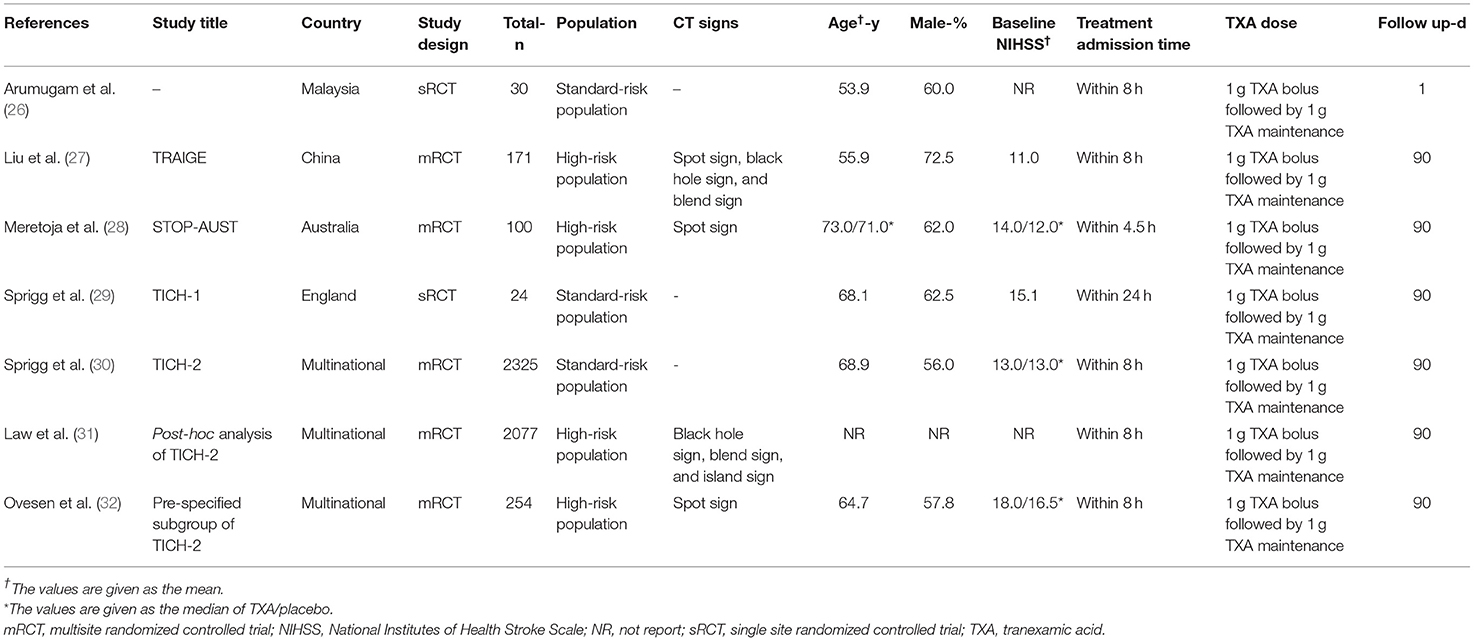
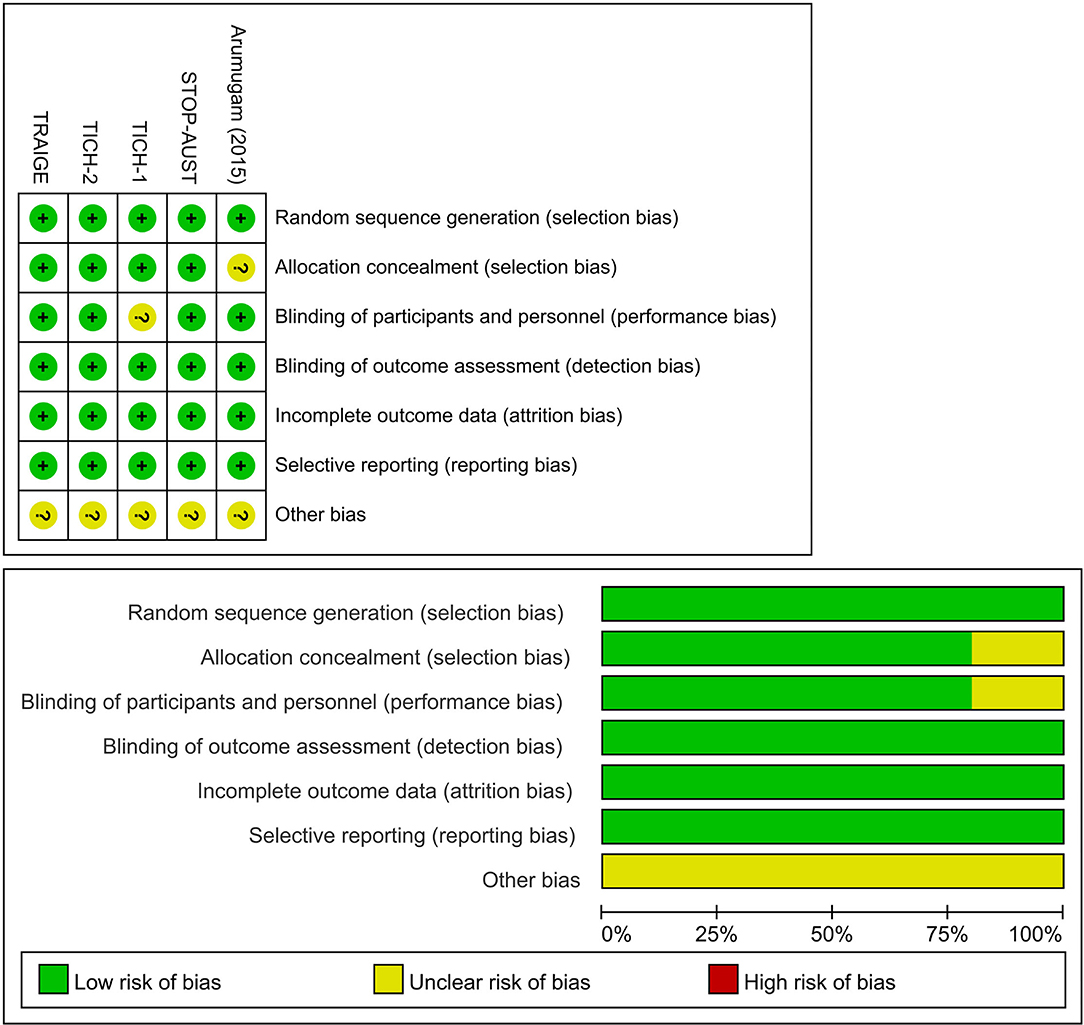
Baseline characteristics of the included seven trial reports are summarized in Table 1. Of the two additional trial reports, one was a post hoc analysis (31), and the other was a pre-specified subgroup analysis (32) of a previously published trial (30). The 7 trial reports were published from 2014 to 2021, with sample sizes ranging from 24 to 2,325 subjects, and a total of 2,650 subjects. Three trials were multicenter studies (27, 28, 30), while two were conducted at a single center (26, 29). The subjects were mainly men and the mean age of subjects ranged from 53 to 73 years. The baseline NIHSS scores ranged from 12 to 18. Two trials (27, 28) enrolled acute spontaneous ICH patients susceptible to HE based on imaging assessment; however, the majority of patients included were general acute spontaneous ICH patients. The TXA administration time was varied among studies: within 4.5 h in one trial (28), within 8 h in three trials (26, 27, 30), and, up to 24 h in one trial (29). The dosage of TXA was consistent across included trials with the most common regimen being a loading dose of 1 g, followed by a maintenance dose of 1 g over 8 h. Overall, three trials (27, 28, 30) were categorized as being at low risk of bias and 2 (26, 29), as being unclear. Details of the risk of bias are presented in Figure 2 and Supplementary Table 3.
Association Between TXA and Outcomes
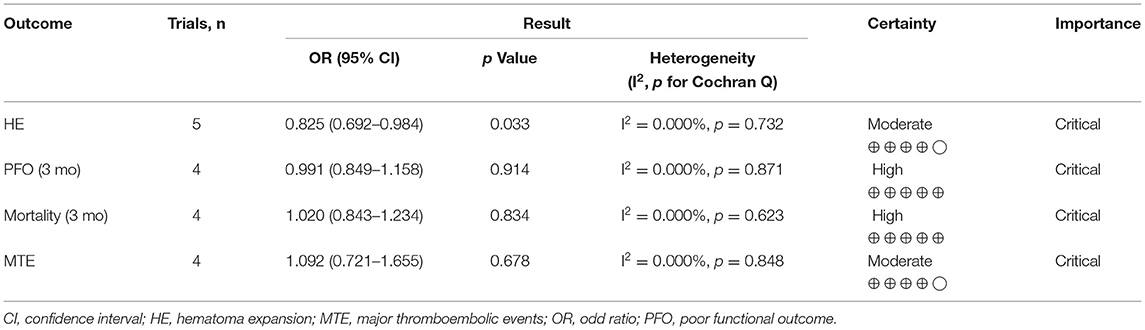
Table 2 summarizes the overview of the association between TXA and various clinical outcomes. GRADE summary of findings is available in the Table 2 and Supplementary Table 4.
Primary Outcome Measurement
Pooled analysis found that TXA administration may reduce HE on subsequent neuroimaging (OR 0.825; 95 % CI 0.692–0.984; p = 0.033; I2 = 0 % and chi-square p = 0.732; Figure 3A). The GRADE certainty of the evidence was moderate. Sensitivity analysis showed similar results when limited to trials with low risk of bias (OR 0.828; 95% CI 0.693–0.988; p = 0.036; I2 = 0 % and chi-square p = 0.935; Supplementary Appendix 1). Subgroup analyses indicated that TXA reduced the risk of HE in the high-risk population (OR 0.646; 95% CI 0.503–0.829; p = 0.001; I2 = 0 % and chi-square p = 0.886; Figure 4B) and patients who were treatable within 4.5 h of symptom onset (OR 0.823; 95% CI 0.690–0.980; p = 0.029; I2 = 0% and chi-square p = 0.690; Figure 5A), but not in the standard-risk population (OR 0.834; 95% CI 0.690–1.007; p = 0.059; I2 = 0 % and chi-square p = 0.388; Figure 4A), and patients who were treatable over 4.5 h of symptom onset (OR 1.026; 95% CI 0.795–1.324; p = 0.844; I2 = 0 % and chi-square p = 0.399; Figure 5B).
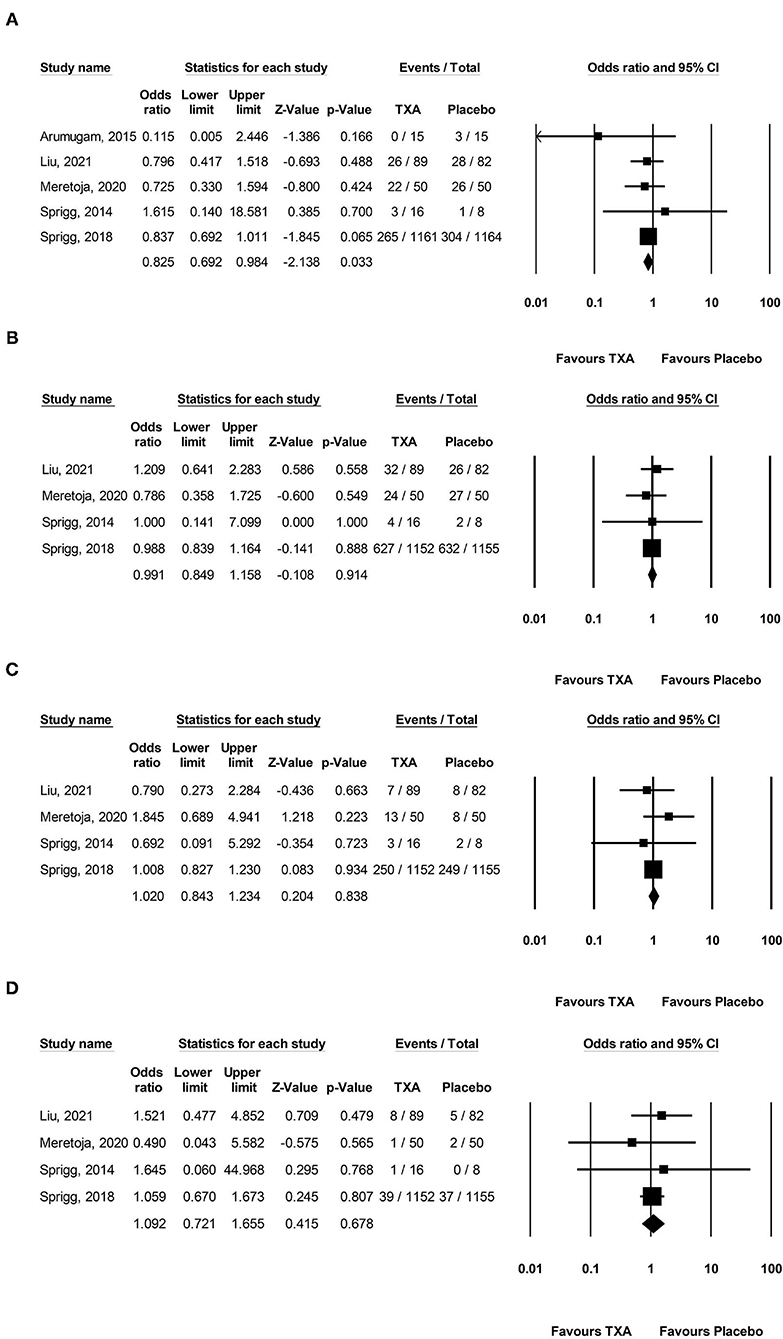
Figure 3. Forest plot comparing the risk of (A) hematoma expansion, (B) 3-month poor functional outcome, (C) 3-month mortality, and (D) major thromboembolic events between the TXA and placebo groups. TXA, tranexamic acid.
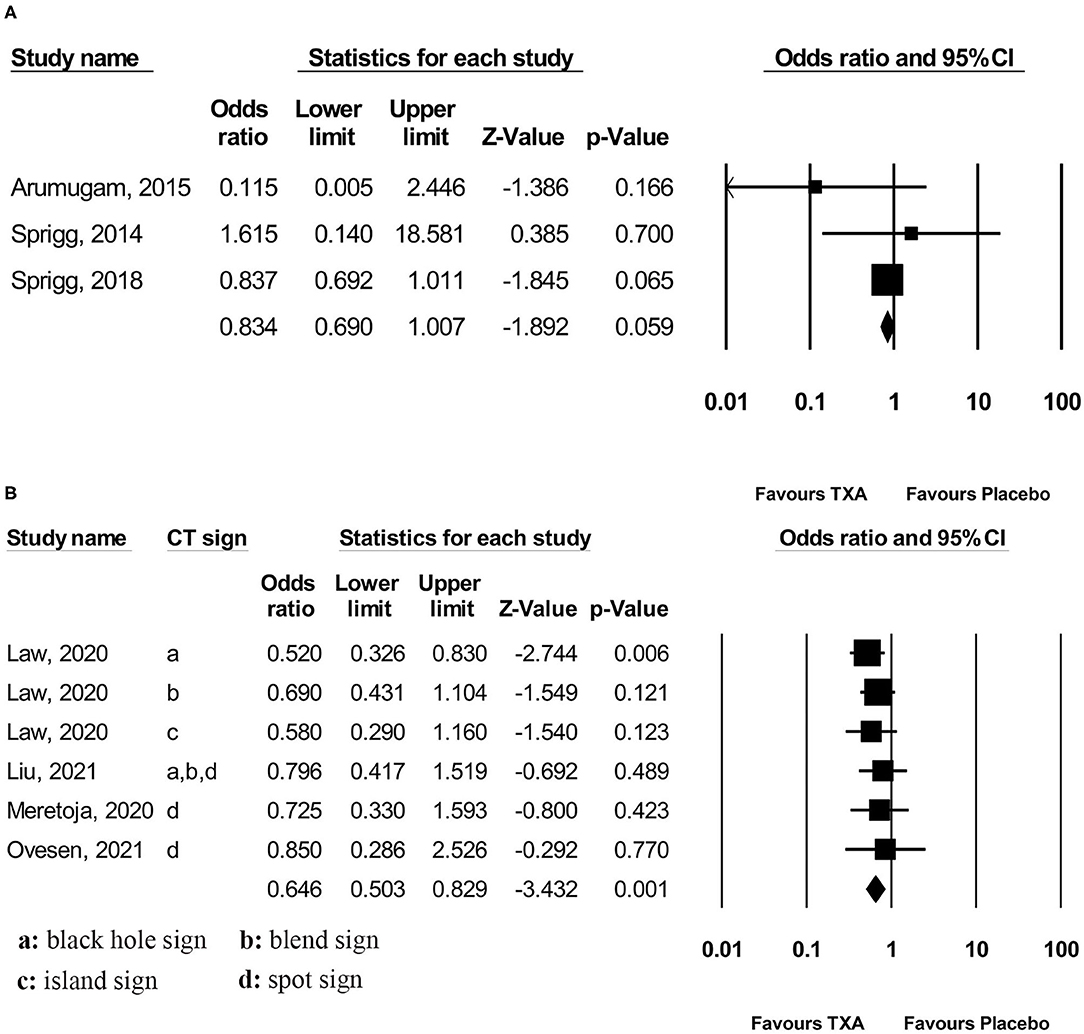
Figure 4. Subgroup analysis of primary outcome measurement: (A) standard-risk population and (B) high-risk population. TXA, tranexamic acid.
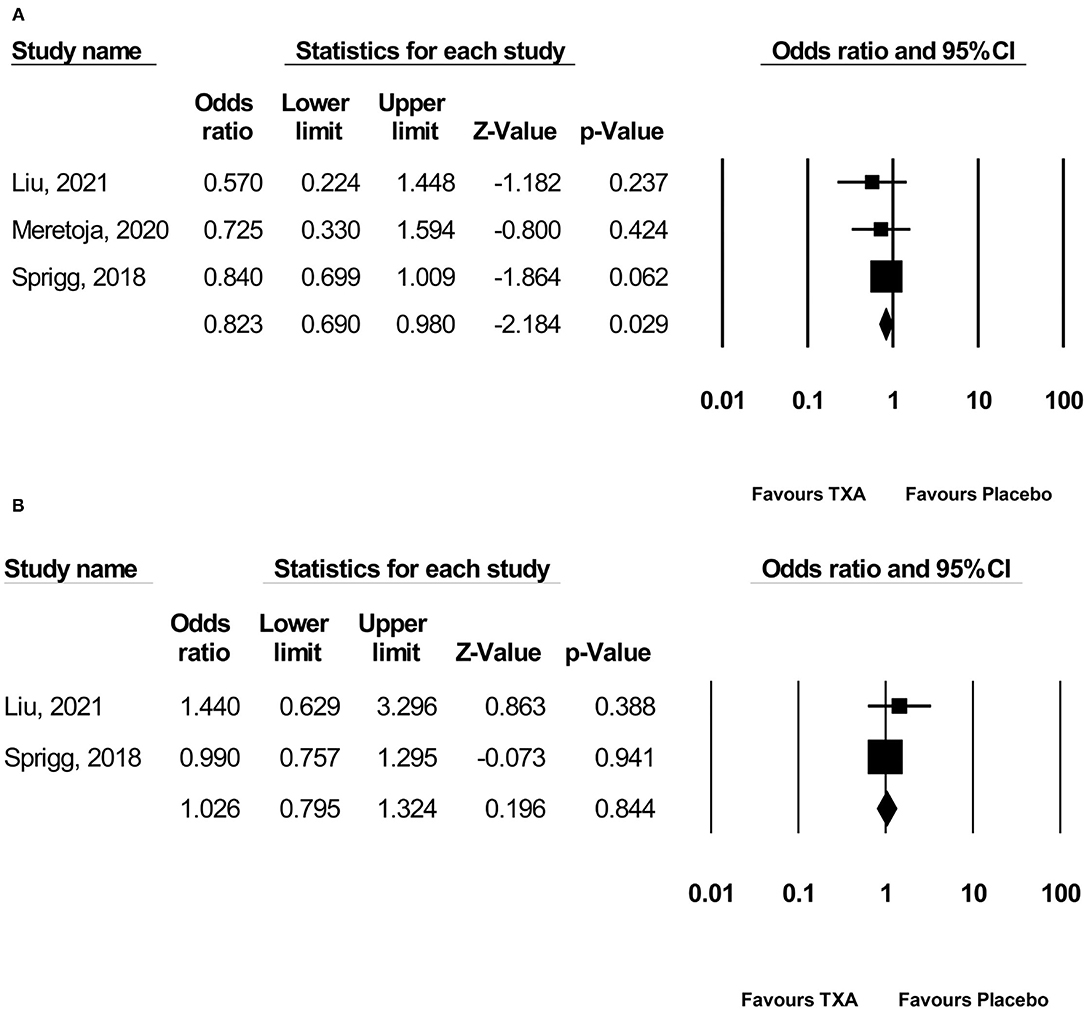
Figure 5. Subgroup analysis of primary outcome measurement: (A) ≤ 4.5 h and (B) > 4.5 h. TXA, tranexamic acid.
Secondary Outcome Measurements
However, TXA probably had no effect on a 3-month PFO (OR 0.991; 95 % CI 0.849–1.158; p = 0.914; I2 = 0 % and chi-square p = 0.871; GRADE: high certainty; Figure 3B), 3-month mortality (OR 1.020; 95 % CI 0.843–1.234; p = 0.834; I2 = 0 % and chi-square p = 0.623; GRADE: high certainty; Figure 3C), and MTE (OR 1.092; 95% CI 0.721–1.655; p = 0.678; I2 = 0 % and chi-square p = 0.848; GRADE: high certainty; Figure 3D). To confirm the robustness of our findings, we conducted sensitivity analyses using data from trials categorized as low risk of bias for various clinical outcomes. Sensitivity analysis for the rate of each endpoint showed that the overall effect of TXA therapy was consistent with the overall estimates from all studies (Supplementary Appendix 1). There was no significant difference in the 3-month PFO, 3-month mortality, and MTE in the high-risk population vs. standard-risk population subgroups (Supplementary Appendix 2).
Publication Bias
For the safety and efficacy analyses on different endpoints, funnel plot and the Egger's test revealed no evidence of asymmetry (Supplementary Figure 1). However, the results from such analyses should be treated with considerable caution based on a limited number of trials.
Discussion
Principal Findings
Our meta-analysis has comprehensively and systematically reviewed the current available literature that compared TXA with placebo for treating acute spontaneous ICH, and we obtained three major findings. Firstly, in patients with spontaneous ICH, TXA may decrease HE on subsequent imaging. However, TXA probably had no effect on PFO and on mortality at 3 months. The use of TXA was safe without increasing thromboembolic complications. Secondly, further analyses restricted to patients with spontaneous ICH susceptible to HE based on imaging assessment have showed consistent results, but this protective effect was not found in the standard-risk population. CT markers of HE may be a useful determinant of benefit from TXA administration in acute spontaneous ICH patients. Thirdly, early treatment (within 4.5 h after stroke onset) seemed to show more benefits in preventing HE, but this protective effect was not observed in the patients who were treatable over 4.5 h.
Relation to Other Systematic Reviews
Two previous systematic reviews on the similar topic have been published (33, 34). One of them evaluated the treatment of acute spontaneous ICH with different hemostatic therapies (including TXA) and the results showed that there was no evidence of either benefit or harm from TXA for people with ICH (33). The other one evaluated the effect of TXA on acute spontaneous ICH and found that TXA could reduce HE in ICH (34). In line with the second study (34), our meta-analysis also found that TXA may decrease HE on subsequent imaging. Besides, we found that the decreased risk was observed in patients who were treatable within 4.5 h and in high-risk population, but not in those who were treatable over 4.5 h and in standard-risk population. In summary, our meta-analysis further confirms that TXA is an effective and safe drug for the treatment of acute spontaneous ICH and that CT markers of HE, as well as early time windows, are useful clinical determinants of TXA administration in acute spontaneous ICH patients. However, some differences also should be noted. First, previous meta-analyses included <4 RCTs. Our meta-analysis identified another three recent studies and further reinforced earlier results of previous meta-analyses. Second, we pooled RCTs data with a random-effects model accounting for clinical heterogeneity to ensure a more conservative estimate of the efficacy and safety of TXA for the treatment of acute spontaneous ICH. Third, we evaluated the certainty of evidence using GRADE approach to facilitate clinical decision-making.
Strengths and Weaknesses of the Review
This systematic review and meta-analysis have several strengths including a pre-registered protocol, a comprehensive literature search, a duplicate and independent screening and data extraction, and GRADE assessment of certainty of evidence. However, certain limitations of this meta-analysis need to be acknowledged. First, subgroup analysis for each CT markers of HE was not performed because of the lack of data. Thus, the difference in efficacy for patients with different CT markers of HE was not determined. Second, the inclusion of the post hoc RCT would lead to a declined quality of subgroup analysis that we conducted.
Future Perspectives
The study limitations mentioned above provided several considerations for future studies. In particular, the difference in efficacy for patients with different CT markers of HE should be determined. Subgroup analyses according to time from onset to randomization have found that early treatment (within 4.5 h) seemed to show more benefit. The proposed time window could also be used for hemostatic therapy in future research.
Conclusions
In the overall population with acute spontaneous ICH, TXA may decrease the risk of HE on subsequent imaging. Patients with high-risk of HE predicted by CT markers and patients who are treatable within 4.5 h may both have the greatest risk reduction of HE. However, TXA probably has no effect on PFO or mortality at 3 months. The use of TXA probably does not increase the risk of MTE.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YG, X-MG, and R-LL: acquisition of data, analysis and interpretation of data, and drafting the article. KZ, Q-JB, J-CY, and QZ: critical revision of the manuscript for important intellectual content. M-FY: conception and design of the study and critical revision of the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by the Science and Technology Department of Qinghai Province (Nos. 2019-ZJ-7040 and 2020-ZJ-774). The funding had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.761185/full#supplementary-material
References
1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
2. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
3. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. (2014) 9:840–55. doi: 10.1111/ijs.12309
4. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
5. Hemphill JC 3rd, Farrant M, Neill TA Jr. Prospective validation of the ICH score for 12-month functional outcome. Neurology. (2009) 73:1088–94. doi: 10.1212/WNL.0b013e3181b8b332
6. Meretoja A, Strbian D, Putaala J, Curtze S, Haapaniemi E, Mustanoja S, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. (2012) 43:2592–7. doi: 10.1161/STROKEAHA.112.661603
7. Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. (1997) 28:1–5. doi: 10.1161/01.STR.28.1.1
8. Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. (1996) 27:1783–7. doi: 10.1161/01.STR.27.10.1783
9. Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, et al. Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke. (2016) 47:1777–81. doi: 10.1161/STROKEAHA.116.013186
10. Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X., et al. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. (2015) 46:2119–23. doi: 10.1161/STROKEAHA.115.009185
11. Li Q, Liu QJ, Yang WS, Wang XC, Zhao LB, Xiong X, et al. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke. (2017) 48:3019–25. doi: 10.1161/STROKEAHA.117.017985
12. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. (2009) 40:2994–3000. doi: 10.1161/STROKEAHA.109.554667
13. Morotti A, Arba F, Boulouis G, Charidimou A. Noncontrast CT markers of intracerebral hemorrhage expansion and poor outcome: a meta-analysis. Neurology. (2020) 95:632–43. doi: 10.1212/WNL.0000000000010660
14. Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke. (2012) 43:3427–32. doi: 10.1161/STROKEAHA.112.664003
15. Franchini M, Mannucci PM. The never ending success story of tranexamic acid in acquired bleeding. Haematologica. (2020) 105:1201–5. doi: 10.3324/haematol.2020.250720
16. Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. (2012) 17:e3054. doi: 10.1136/bmj.e3054
17. Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
18. Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. (2017) 82:605–17. doi: 10.1097/TA.0000000000001333
19. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. (2011). Available online at: www.handbook.cochrane.org (accessed August 10, 2021).
20. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
21. Higgins JP, Altman DG, Gøtzsche PC., Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Arumugam A, Rahman NAA, Theophilus SC, Shariffudin A, Abdullah JM. Tranexamic acid as antifibrinolytic agent in non traumatic intracerebral hemorrhages. Malays J Med Sci. (2015) 22:62–71.
27. Liu J, Nie X, Gu H, Zhou Q, Sun H, Tan Y, et al. Tranexamic acid for acute intracerebral haemorrhage growth based on imaging assessment (TRAIGE): a multicentre, randomised, placebo-controlled trial. Stroke Vasc Neurol. (2021) 6:160–9. doi: 10.1136/svn-2021-000942
28. Meretoja A, Yassi N, Wu TY, Churilov L, Sibolt G, Jeng JS, et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST): a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. (2020) 19:980–7. doi: 10.1016/S1474-4422(20)30369-0
29. Sprigg N, Renton CJ, Dineen RA, Kwong Y, Bath PMW. Tranexamic acid for spontaneous intracerebral hemorrhage: a randomized controlled pilot trial (ISRCTN50867461). J Stroke Cerebrovasc Dis. (2014) 23:1312–8. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.007
30. Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. (2018) 391:2107–15. doi: 10.1016/S0140-6736(18)31033-X
31. Law ZK, Ali A, Krishnan K, Bischoff A, Appleton JP, Scutt P, et al. Noncontrast computed tomography signs as predictors of hematoma expansion, clinical outcome, and response to tranexamic acid in acute intracerebral hemorrhage. Stroke. (2020) 51:121–8. doi: 10.1161/STROKEAHA.119.026128
32. Ovesen C, Jakobsen JC, Gluud C, Steiner T, Law Z, Flaherty K, et al. Tranexamic acid for prevention of hematoma expansion in intracerebral hemorrhage patients with or without spot sign. Stroke. (2021) 52:2629–36. doi: 10.1161/STROKEAHA.120.032426
33. Salman RAS, Law ZK, Bath PM, Steiner T, Sprigg N. Haemostatic therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev. (2018) 4:CD005951. doi: 10.1002/14651858.CD005951.pub4
Keywords: cerebral hemorrhage, hematoma, tranexamic acid, randomized controlled trial, meta-analysis
Citation: Guo Y, Guo X-M, Li R-L, Zhao K, Bao Q-J, Yang J-C, Zhang Q and Yang M-F (2021) Tranexamic Acid for Acute Spontaneous Intracerebral Hemorrhage: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 12:761185. doi: 10.3389/fneur.2021.761185
Received: 19 August 2021; Accepted: 25 November 2021;
Published: 20 December 2021.
Edited by:
Ashkan Shoamanesh, McMaster University, CanadaReviewed by:
Vignan Yogendrakumar, University of Ottawa, CanadaDavid Howells, University of Tasmania, Australia
Copyright © 2021 Guo, Guo, Li, Zhao, Bao, Yang, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Fei Yang, aWxvdmV5b3VjbXVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yu Guo1†
Yu Guo1† Ming-Fei Yang
Ming-Fei Yang