- 1Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, Neuroscience Section, University of Salerno, Fisciano, Italy
- 2Clinical Trial Center Parkinson, San Raffaele Cassino, Cassino, Italy
- 3Section of Medical Statistics and Biometry “GA Maccacaro”, Department of Clinical Science and Community, University of Milan, Milan, Italy
- 4Medical Department, Zambon SpA, Bresso, Italy
Background: There is increasing evidence of gender differences in the epidemiology and clinical manifestation of both motor and non-motor symptoms of Parkinson's disease (PD). Nevertheless, few data are available on gender differences in the response to antiparkinsonian drugs. Safinamide is a multimodal drug with positive effects on motor and non-motor fluctuations that might improve patients' care and quality of life.
Objective: To analyze gender differences on clinical effects of safinamide in PD patients treated in real-life conditions during the SYNAPSES trial.
Methods: SYNAPSES was a multinational, multicenter, observational study. At baseline, patients with PD diagnosis received safinamide as an add-on to levodopa and were followed up for 12 months, with visits performed every 4 months. A new statistical analysis was performed to describe the efficacy of safinamide in men and women on motor complications, motor symptoms, and adverse events.
Results: Six hundred and sixteen (38%) out of 1,610 patients enrolled in the SYNAPSES study were women and 994 (62%) men. Safinamide improved motor symptoms and motor complications (fluctuations and dyskinesia) in both genders, with a good safety profile and without requiring any change in the concomitant dopaminergic therapy. Clinically significant improvements, according to the criteria developed by Shulman et al., were seen in 46% of male and female patients for the UPDRS motor score and 43.5% of men vs. 39.1% of women for the UPDRS total score.
Conclusions: Safinamide was effective in improving motor fluctuations and dyskinesia and proved to be safe in both male and female patients with PD. Further prospective studies, specifically addressing potential gender differences in response to PD therapies, are needed to develop tailored management strategies.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder, affecting about 3% of the population by the age of 65 and up to 5% of the people over 85 years. The prevalence of PD is expected to rise dramatically over the next decades, with an increase in healthcare-related costs (1).
Typical PD motor manifestations include resting tremor, bradykinesia, rigidity, and gait impairment, but there are also several non-motor symptoms such as depression, anxiety, pain, orthostatic hypotension, sleep disorders, and gastrointestinal disturbances, which can precede the motor features by many years (2).
Together with aging, genetics, and environment, biological sex has been recognized as an important factor in the development of PD. Epidemiological studies showed that both incidence and prevalence of PD are 1.5–2 times higher in men than in women (3). Moreover, there are differences in the clinical presentation of both the motor and non-motor features of the disease. Women experience a later onset of motor symptoms and show more frequently a tremor-dominant phenotype associated with a slower disease progression and lower dopaminergic denervation (4), probably due to the neuroprotective effects of estrogens. Stiffness and behavioral and sleep disorders are more common in men (5). Male gender is a risk factor for the development of cognitive impairment and dementia in PD (6). Women more frequently present mood-related non-motor fluctuations (depression, anxiety) and pain, whereas impulse control disorders, such as pathological gambling and hypersexuality, are more common in male patients with PD (7, 8). Levodopa-related motor complications, such as dyskinesia and wearing-off, are more frequent in women, partly due to pharmacokinetic differences beyond a different weight (9). Considering these gender differences may help to tailor treatment, predict outcomes, and meet social needs in men and women with PD (8). In Italy, a law-regulating clinical trial with specific regard to the methodological approach of gender medicine was approved in 2018 (10).
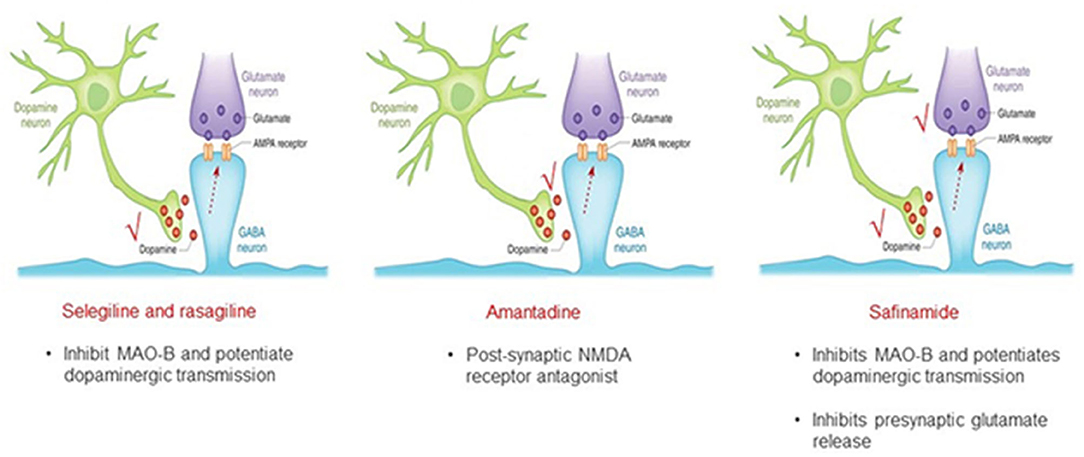
Although the need for personalized medicine according to gender is now generally recognized, to date no gender-oriented advice is available for PD. Current therapies of PD are focused on symptomatic management and dopamine restoration. However, other neurotransmitters, including glutamate, are involved in the neurodegenerative process, clinical symptoms, and fluctuations of PD (11). Targeting a non-dopaminergic system may be an alternative strategy to improve the efficacy of levodopa and avoid its dose increase, with consequent potential worsening of motor and non-motor complications (12). Safinamide has a unique multimodal mechanism of action (MoA), dopaminergic and non-dopaminergic, that includes the reversible inhibition of the monoamine oxidase-B (MAO-B) enzyme and the modulation of excessive glutamate release (13). Safinamide differs from other pure MAO-B inhibitors like selegeline and rasagiline, and also from amantadine (Figure 1). This dual MoA might be important in terms of effect not only on motor symptoms, but also on motor complications, such as wearing-off and dyskinesia, and non-motor symptoms like pain and mood deterioration (14, 15).
Despite being the most frequently studied movement disorder, studies investigating the impact of gender on medical treatments in PD are still scarce. The aim of this study is to investigate the effects of safinamide according to gender in PD patients treated in real-life conditions during the SYNAPSES trial.
Materials and Methods
Study Design
This report describes the results of new additional analyses of the SYNAPSES trial (EU PAS Register Number EUPAS13745), a European multicentre, retrospective–prospective cohort observational study. The study was designed, in agreement with the European Agency (EMA), to investigate how safinamide is prescribed and used in routine clinical practice and to collect safety and efficacy data. The countries involved were Belgium, Germany, Italy, Spain, Switzerland, and United Kingdom, and the study was conducted in 128 Neurology and Geriatric centers specialized in PD treatment. A total of 1,610 patients with PD already receiving levodopa were enrolled. Patients were treated with safinamide as add-on therapy, according to the summary of product characteristics (SmPC), and followed up for 12 months after the start of treatment, with visits every 4 months. Male and female patients with PD were eligible, provided they were aged ≥18 years and started treatment with safinamide according to routine clinical practice. Patients were excluded if they were participating in any other clinical trial at study inclusion or in case of contraindications to safinamide as listed in the SmPC. According to the non-interventional study type, the Investigators were not given any guidelines regarding patient selection and treatment administration (16).
Both protocol and patient materials were approved by Independent Ethics Committees and Health Authorities of the participating countries. All patients signed informed and privacy consent forms and the study was conducted according to the ethical standards of the institutional and/or national research committee and according to the Declaration of Helsinki. Personal data were collected, stored, and processed exclusively in pseudoanymized form and in compliance with the regulatory requirements for the protection of confidentiality of patients.
Data Source and Measurements
All patients' data were recorded by the Investigators in electronic standardized case report forms (eCRFs). Data on demography, adverse events (AEs), and motor complications were collected from the medical charts and by interviewing the patients at each study visit.
Age at onset and PD symptoms were retrieved from the patient's history, taken during the baseline visit. Age at onset was determined as the age at which the patient had first noticed any parkinsonian motor symptoms. Adverse event terms were coded with the Medical Dictionary for Regulatory Activities (MedDRA) version 21.1 (17). Seriousness, severity, and relation with safinamide were entered according to the clinicians' judgment.
Motor complications were recorded using a menu already available in the eCRF; based on the data derived from the SYNAPSES study, the Investigators, in agreement with the protocol and the statistical analysis plan approved by the European Agency (EMA), considered the following motor complications: any fluctuations, wearing-off, early morning fluctuations, unpredictable fluctuations, delayed on, and dyskinesia (16).
As requested by EMA, given the observational nature of the trial, the study was not designed to collect patients' diaries for ON and OFF periods or perform specific scales for motor complications that are not common in routine clinical practice.
Activities of daily living (ADL), motor symptoms, and complications of therapy were evaluated during ON time with the Unified Parkinson's Disease Rating Scale (UPDRS) (18), measuring the changes from baseline to each follow-up visit. The UPDRS part I score is not shown because, as described by Abbruzzese et al. (16), it was stable during the whole study. The UPDRS is a scale widely used by routine to follow the longitudinal clinical course of PD. The UPDRS total scores and the UPDRS part III (motor) scores were also evaluated following the criteria developed by Shulman et al. (19) for clinical significance. Moreover, the effects of safinamide on the cardinal motor symptoms were investigated separately with this questionnaire, specifically bradykinesia (UPDRS items 23,24,25,26,31), rigidity (UPDRS item 22), tremor (UPDRS items 16,20,21), and postural instability and gait disorder (PIGD, UPDRS items 13,14,15,29,30) (20).
Statistical Methods
The statistical analyses were done on all “evaluable patients for the full analysis set” defined as the patients satisfying all inclusion criteria and not violating any exclusion criteria. Categorical variables were described by means of absolute and relative frequencies, while continuous variables by means of mean, standard deviation, quartiles, minimum, and maximum. Response variables recorded at baseline and each follow-up visit were analyzed using generalized estimating equation (GEE) models with identity link function and Gaussian distribution for UPDRS total score, UPDRS subscales scores, and cardinal motor symptoms scores, and also binomial distribution for the rate of motor complications (fluctuations and dyskinesia). Baseline, gender, visit, and gender-by-visit interaction were applied as model fixed effects. The variance-covariance matrix of the GEE model, which takes into account correlation across repeated measures, was parameterized using the first-order autoregressive form. The estimates of changes from baseline at each follow-up visit were computed using proper contrasts applied on the gender-by-visit interaction and were used to test the differences between the genders. Quasi-maximum likelihood estimates of the model parameters were obtained with the GEE procedure of SAS software Version 9.4. Results are reported as model-based estimates [least squares (LS) mean scores or LS proportions depending on the response variable] with standard errors. Conventional Chi-square test or Fisher's exact test, if deemed more appropriate, were used to test gender differences regarding levodopa assumption (increase/decrease) and the incidence of AEs.
Results
Demography
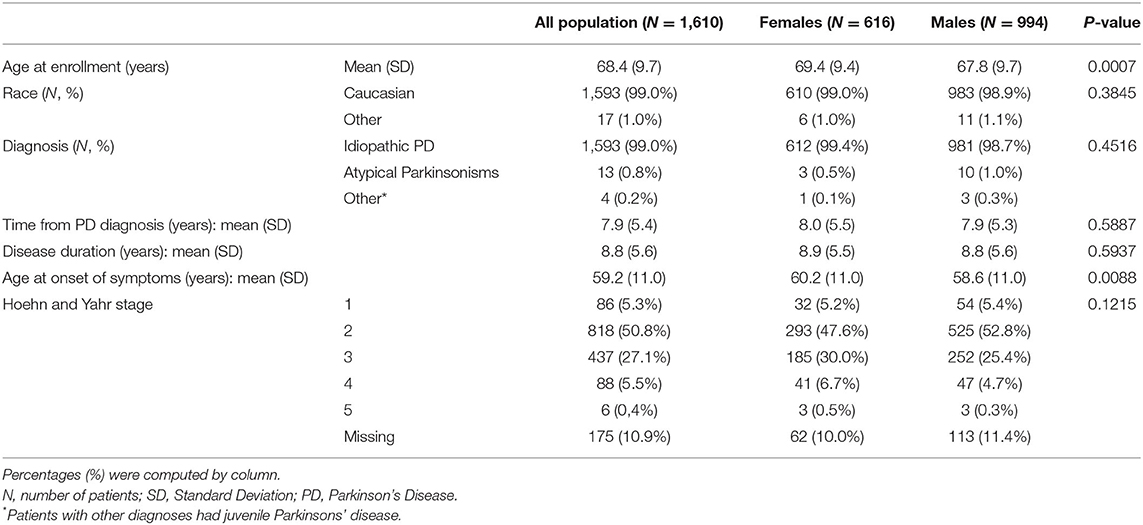
The patients' overview by gender is shown in Table 1. Out of the 1,610 patients enrolled in the SYNAPSES study, 616 (38%) were women and 994 (62%) men. There were no differences as for “time to PD diagnosis” (about 8 years), “disease duration” (about 9 years), “race” (99% were Caucasian), and “Hoehn and Yahr (H&Y) stage” (21) (about 95% of patients had H&Y stage ≥ 2). Women were older than men at enrollment (69.4 years women vs. 67.8 years men, p = 0.0007) and the onset of symptoms (60.2 years women vs. 58.6 men, p = 0.0088).
UPDRS Scores
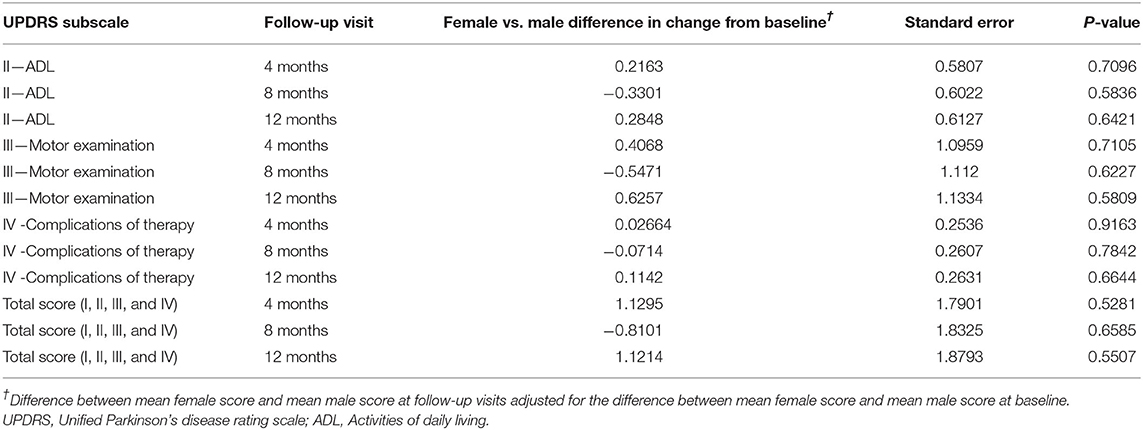
Changes from baseline at each follow-up visit in UPDRS total score and UPDRS subscales scores (part II ADL, part III Motor Examination, part IV Complications of Therapy) are presented in Figure 2 (women and men). At baseline, women presented higher UPDRS scores compared to men, particularly for the total score (44.79 vs. 42.35, respectively) and the part IV, complications of therapy (4.92 vs. 4.08, respectively). Nevertheless, these differences were not statistically significant (Table 2). During the study, improvements were seen in all follow-up visits for both genders, starting already from the 4-month visit. The UPDRS total score, the UPDRS part II (ADL), and the UPDRS part III (motor examination) improved up to 10%, while the highest improvement (up to 20%) was detected in UPDRS part IV (complications of therapy), but did not show significant differences between men and women (Table 2).
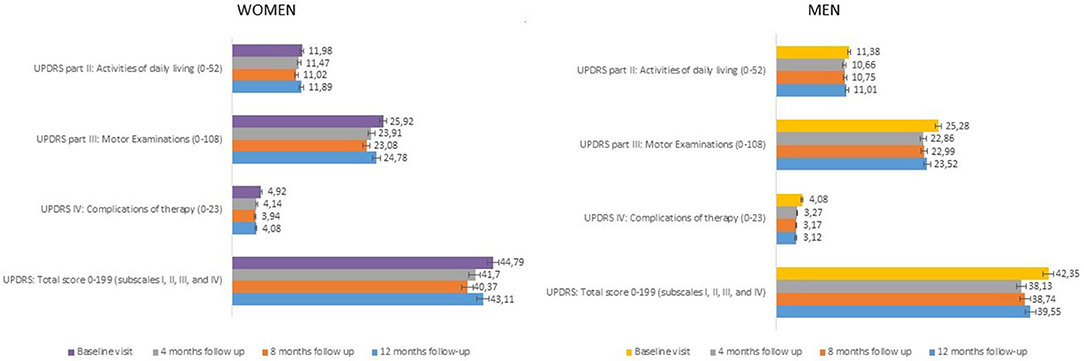
Figure 2. UPDRS scores (part II, III, IV, total scores) at each study visit (± SE). UPDRS, Unified Parkinson's Disease Rating Scale; SE, Standard Error.
The percentage of patients with clinically relevant differences, according to Shulman et al. (19), in the UPDRS part III score (>2.5 points) and UPDRS total score (> 4.3 points) is reported in Figure 3. At the end of the study, the same percentage of subjects showed a clinically significant improvement in UPDRS part III scores (men: 46.6%; women: 46.1%, p: 0.90266), while the clinical improvement on the UPDRS total score was slightly higher in men than women (43.5 vs. 39.1%, respectively), even if there was no statistical difference between genders (p: 0.29831).
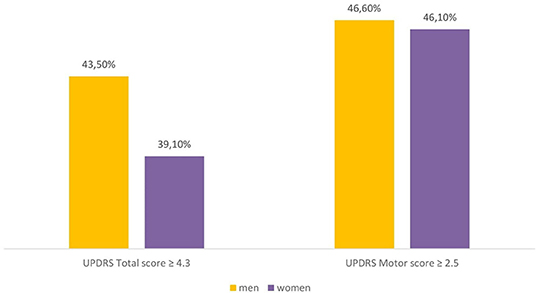
Figure 3. Percentage of male and female patients with clinically important difference (improvement) in the UPDRS scores (difference between 12-months follow-up and baseline). UPDRS, Unified Parkinson's Disease Rating Scale.
Cardinal Motor Symptoms
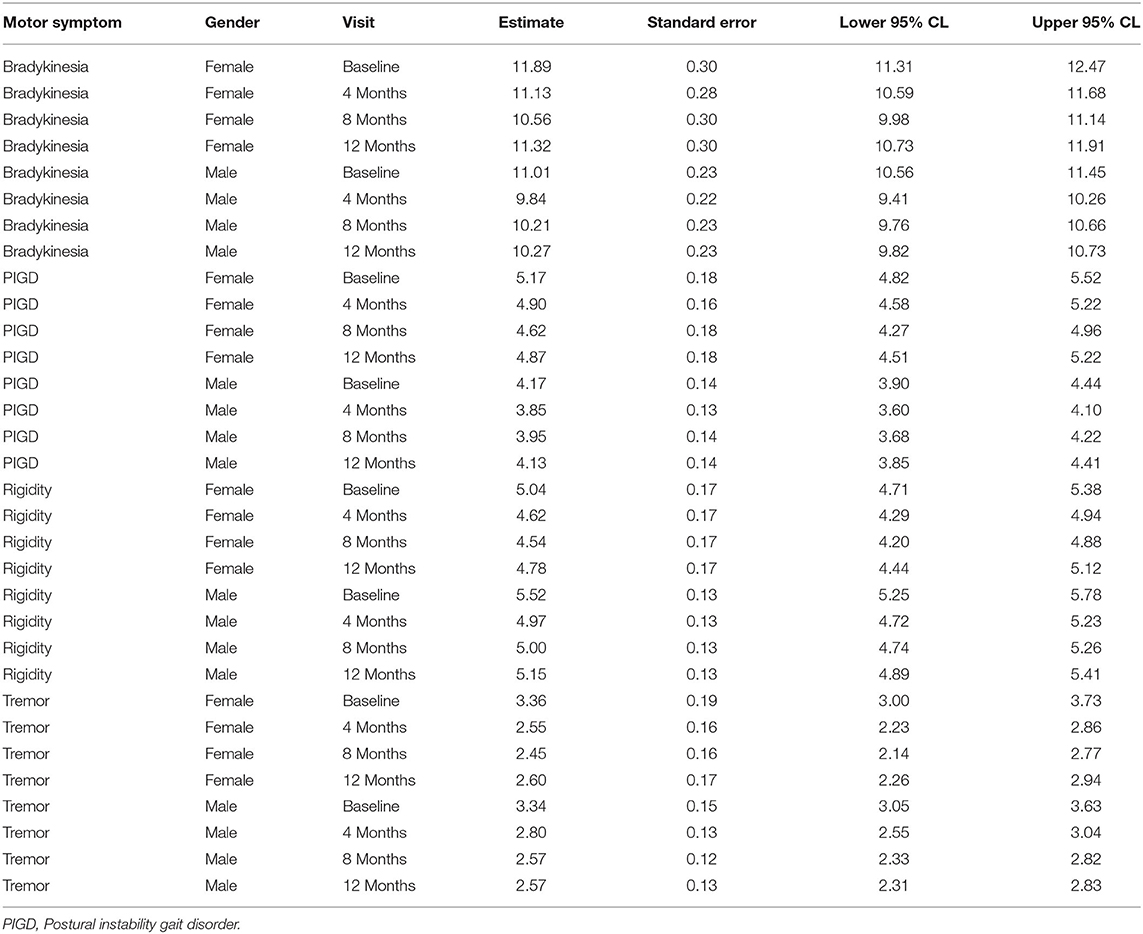
At baseline, bradykinesia and PIGD were more severe in women, while rigidity was more severe in men. These differences were not statistically significant. Tremor showed the same severity in both genders. All cardinal symptoms improved since the first follow-up visit. Tremor showed the highest improvement (up to 23% in men and up to 24% in women). Bradykinesia improved up to 11% and rigidity up to 10% in both genders. PIGD improved up to 8% in men and up to 11% in women (Table 3). As shown in Table 4, no gender differences were detected.
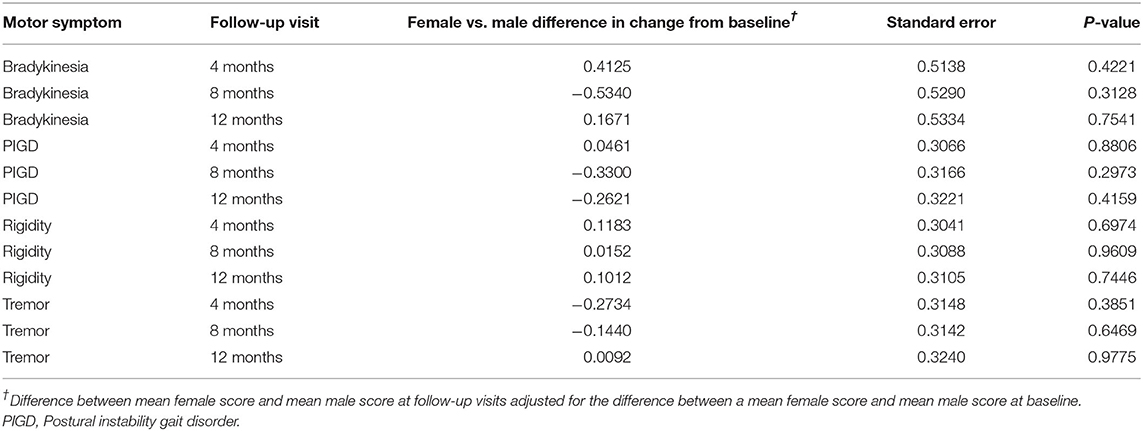
Table 4. Changes of cardinal motor symptoms scores from baseline at each follow-up visit in female vs. male patients.
Levodopa Dose
During the study, the overall mean dose of levodopa (alone or associated with catechol-O-methyltransferase inhibitors) did not change significantly. The mean levodopa (L-dopa) daily dose at baseline was 425 mg (300–550 mg) and the mean daily dosage at the end of the study was 450 mg (300–600 mg). There were no differences between the genders both for baseline and follow-up L-dopa daily doses.
Motor Complications (Fluctuations and Dyskinesia)
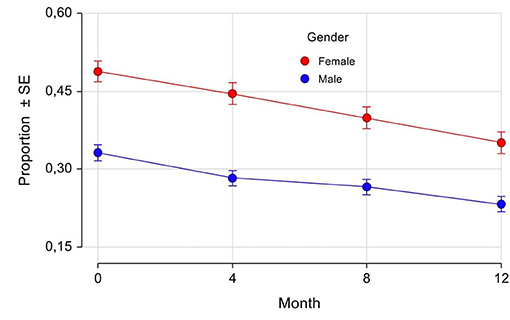
Fluctuations have been recorded in the eCRF by the Investigators as “any fluctuations,” “wearing off,” “early morning fluctuations,” “unpredictable fluctuations,” and “delayed ON.” As shown in Table 5, at baseline the majority of patients had motor fluctuations, with a slightly higher percentage in women (93.6%) compared with men (91.6%). The most frequent one was “wearing off,” again with a mild prevalence in women (76.5 vs. 73.5% in men). Also “early morning fluctuations” were more frequent in women (25.7 vs. 21.5% in men), while there was the same prevalence between the genders for “unpredictable fluctuations” and “delayed ON.” The prevalences of different fluctuations at baseline were not significantly different between genders. The percentage of patients with motor fluctuations decreased in both genders during the study since the 4-month follow-up visit, thus indicating a rapid effect of safinamide. At 12 months, the percentage decreased to 24% (women) and 28% (men) for “any fluctuations”; 27% (women) and 30% (men) for “wearing off”; 44% (women) and 40% (men) for “early morning fluctuations”; 37% (women) and 43% (men) for “unpredictable fluctuations”; and 32% (women) and 22% (men) for “delayed ON.” In Figure 4 the proportion of patients at each study visit for “any fluctuations” (A) and “wearing off” (B) are shown graphically. There were no statistically significant differences in changes from baseline except for “any fluctuations” at 4-month follow-up, where there was a higher percentage reduction of prevalence in men as compared with women (22 vs. 16%, respectively, p = 0.0210).
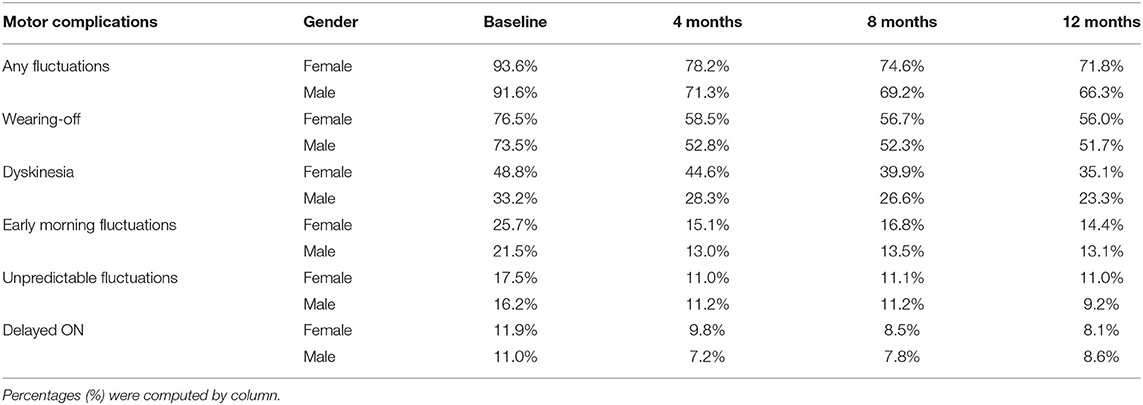
Table 5. Prevalence of fluctuations and dyskinesia according to gender at the start of treatment with safinamide and during the follow-up.

Figure 4. Any fluctuations (A) and wearing-off (B): proportion of patients by gender and visits. SE, Standard Error. *p = 0.0210 (difference between female and male proportions at follow-up visits adjusted for the difference between female and male proportions at baseline).
At baseline, dyskinesia had the highest prevalence in women compared with males (48.8 vs. 33.2%, respectively, Table 5), even if not statistically significant. Safinamide reduced the percentage of patients with dyskinesia in both genders up to 30%, with noticeable effects already at 4 months follow-up (refer to Table 5, Figure 5). No gender differences were detected at any study visit.
Adverse Events and Serious AEs
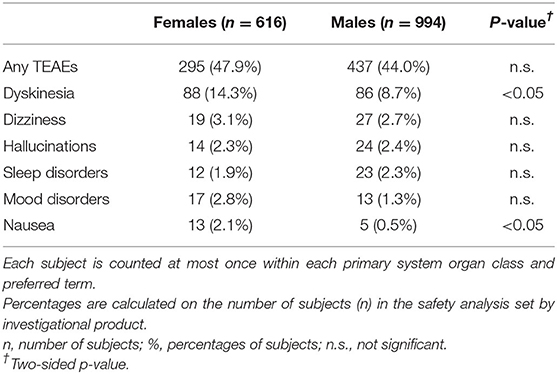
As reported in Table 6, during observation 295 (47.9%) female patients and 437 (44.0%) male patients experienced at least one AE. This difference was not statistically significant. The majority of AEs were rated as mild or moderate and were those already described in the patients' leaflet (16). The most frequent AE was dyskinesia, with a higher prevalence in women (14.3%) compared with men (8.7%; p < 0.05). Dyskinesia was generally transient and did not lead to drug discontinuations. Other AEs with a frequency ≤ 3% of the total number of events were hallucinations (3.1% in women vs. 2.7% in men), dizziness (2.3% in women vs. 2.4% in men), sleep disorders (1.9% in women vs. 2.3% in men), mood disorders (2.8% in women vs. 1.3% in men), and nausea (2.1% in women vs. 0.5% in men; p < 0.05). There were no statically significant differences between genders except for dyskinesia and nausea, but none of the above AEs was considered related to safinamide treatment by the clinicians.
Serious adverse events (SAEs) occurred in 9.6% of women and 8.9% of men. This difference was not statistically significant. The reason for reporting SAEs in these patients was a new or prolonged disability. The most frequently reported SAE was dyskinesia, with the same percentage in both genders (0.2%). As already reported by Abbruzzese et al. (16), the majority of SAEs were completely resolved at follow-up, and no SAEs were considered related to safinamide treatment.
Discussion
Increased recognition of PD sex-based differences could help to develop tailored approaches for patients' care (22). These additional analyses of the SYNAPSES study enable for the first time the assessment of the effects of safinamide across genders.
The observed 2-year difference in age at PD onset and enrollment between men and women is consistent with most epidemiological studies, suggesting that the development of symptomatic PD is slightly delayed in women compared with men (3). This could be explained by higher initial striatal dopamine levels in women that could delay the dopamine depletion and hence postpone the development of parkinsonian symptoms. Moreover, estrogens are supposed to play an important role in differences between male and female patients (4, 23).
At baseline, women showed a more severe postural instability and bradykinesia compared to men; rigidity was more severe in men, whereas the severity of tremor was similar. It is acknowledged that bradykinesia and rigidity are most responsive to levodopa, which has a limited effect on postural stability, gait, and tremor (24). The addition of safinamide improved all cardinal symptoms in both genders. Consistent with these benefits, UPDRS scores improved with safinamide with a clinically important difference (CID) in the motor scores (19) in 46% of men and women. A CID is the change that patients recognize as clinically valuable and is one of the most important tools for patient-centered trials (25). A similar improvement was seen in the activities of daily living, measured by the UPDRS II. These benefits are particularly important for women because they are less likely to have informal caregiver support (26). Overall, these results are noteworthy because patients were already receiving a concomitant dopaminergic therapy, and the improvement was not related to changes in the levodopa dose that was found to be stable during the study.
The occurrence of dyskinesia and motor fluctuations, such as wearing-off (WO), is a fundamental problem in the long-term management of patients with PD. A higher prevalence of levodopa-induced dyskinesia and WO has been reported in women vs. men with PD (6, 26) and is confirmed also in our study. WO is associated with a poorer quality of life and frequently extends beyond motor impairment to non-motor domains (27). Safinamide, as adjunct therapy, significantly reduced motor fluctuations, particularly WO, and dyskinesia in both genders, with a rapid-onset effect that may be explained by glutamate modulation. Glutamate and other neurotransmitters, in addition to dopamine, are known to contribute to the appearance of motor complications (28). The statistical difference between genders at month 4 for “any fluctuations” was not confirmed in the later visits and the clinical significance is not clear. Evidence reports a shorter time to develop WO in women compared with men (29, 30). We could speculate that a longer presence of motor fluctuations in women can account for this difference in time to get a similar benefit from safinamide on the reduction of motor fluctuations. Unfortunately, we have no data about the time from PD onset to the occurrence of motor fluctuations in our population.
The positive effects on both motor fluctuations and dyskinesia are in line with previous reports showing that safinamide has stronger efficacy than rasagiline on fluctuations and dyskinesia (16, 31), and are reflected also by the improvement found in both genders in the UPDRS part IV assessing the complications of the therapy.
Safinamide was safe and well-tolerated in both genders. As reported in the literature, women had a higher prevalence of dyskinesia and nausea compared with men, although at a lower frequency than those observed in the interventional clinical trials (9, 32). No gender differences were detected as for serious adverse events nor the causal relationship with safinamide.
We must acknowledge some limitations of our study, such as the retrospective design of the trial and the lack of standardized scales or patients' diaries assessing motor complications.
Moreover, stratifications according to the administration of PD medications other than safinamide in addition to levodopa were not feasible since all patients had ≥ 2 concomitant baseline treatments; thus treatment subgroups partly overlapped. Finally, the study was not powered to discriminate between different doses of safinamide, and for this reason, the data of the patients were pooled irrespective of the dose administered. However, these limitations were counterbalanced by the large sample size of fluctuating patients in all stages of the disease, the real-life evaluation, and the high number of outpatient clinics involved in different European countries.
Conclusions
No studies are yet available about gender differences in the response to anticholinergics, catechol-O-methyltransferase inhibitors, and MAO-B inhibitors, nor recommendations have been formulated about a gender-tailored medical treatment in PD.
Our additional analysis of the SYNAPSES study has shown no significant gender differences on the efficacy of safinamide in fluctuating PD patients, suggesting that safinamide might improve motor complications in both genders with no changes in the concomitant dopaminergic therapy. Moreover, this study confirms the good tolerability of safinamide in both genders, making safinamide a safe and effective option to treat motor complications of PD in both men and women.
Future prospective studies, specifically addressing gender differences in response to antiparkinsonian drugs, are needed to develop tailored-management strategies in PD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was designed, in agreement with the European Agency (EMA), to investigate how safinamide is prescribed and used in routine clinical practice and efficacy data. The countries involved were Belgium, Germany, Italy, Spain, Switzerland, and United Kingdom and the study was conducted in 128 Neurology and Geriatric centers specialized in PD treatment (the list available as a supplementary file). Both protocol and patient materials were approved by Independent Ethics Committees and Health Authorities of the participating countries. All patients signed informed and privacy consent forms and the study was conducted according to the ethical standards of the institutional and/or national research committee and according to the Declaration of Helsinki. Personal data were collected, stored, and processed exclusively in pseudonymized form and in compliance with the regulatory requirements for the protection of confidentiality of patients. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors contributed to writing and reviewing the manuscript and approved the final manuscript.
Conflict of Interest
MTP, MP, MR, and MD are members of the Scientific Advisory Board of Zambon S.p.A. CC and IM are employees at Zambon S.p.A. EB is a statistical consultant for Zambon S.p.A. Zambon S.p.A. was involved in the study design and collection of data for the original study. Zambon S.p.A funded the original study. CC requested approval for publication from Zambon S.p.A as an employee but Zambon S.p.A was not involved in the analysis, interpretation of data, writing of this article, or the decision to submit it for publication.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the investigators and the patients involved in the trial.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.756304/full#supplementary-material
References
1. Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. (2013) 62:132–44. doi: 10.1016/j.freeradbiomed.2013.01.018
2. Postuma RB, Aarlsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord. (2012) 27:617–26. doi: 10.1002/mds.24996
3. Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, et al. Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. (2000) 55:1358–63. doi: 10.1212/WNL.55.9.1358
4. Kaasinen V, Joutsa J, Noponen T, Johansson J, Seppänen M. Effects of aging and gender on striatal and extrastriatal [123I]FP-CIT binding in Parkinson's disease. Neurobiol Aging. (2015) 36:1757–63. doi: 10.1016/j.neurobiolaging.2015.01.016
5. Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2007) 78:819–24. doi: 10.1136/jnnp.2006.103788
6. Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. (2014) 83:1253–560. doi: 10.1212/WNL.0000000000000842
7. Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. J Neurol. (2005) 252:1201–5. doi: 10.1007/s00415-005-0835-7
8. Picillo M, Erro R, Amboni M, Longo K, Vitale C, Moccia M, et al. Gender differences in non-motor symptoms in early Parkinson's disease: a 2-years follow-up study on previously untreated patients. Parkinsonism Relat Disord. (2014) 20:850–4. doi: 10.1016/j.parkreldis.2014.04.023
9. Colombo D, Abbruzzese G, Antonini A, Barone P, Bellia G, Franconi F, et al. The “gender factor” in wearing-off among patients with Parkinson's disease: a post hoc analysis of DEEP study. Sci World J. (2015) 2015:787451. doi: 10.1155/2015/787451
11. Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res. (2003) 5:139–46. doi: 10.1007/BF03033378
12. Fox SH. Non-dopaminergic treatments for motor control in Parkinson's disease. Drugs. (2013) 73:1405–15. doi: 10.1007/s40265-013-0105-4
13. Müller T Foley P. Clinical pharmacokinetics and pharmacodynamics of safinamide. Clin Pharmacokinet. (2017) 56:251–61. doi: 10.1007/s40262-016-0449-5
14. Pagonabarraga J, Tinazzi M, Caccia C, Jost WH. The role of glutamatergic neurotransmission in the motor and non-motor symptoms in Parkinson's disease: clinical cases and a review of the literature. J Clin Neurosci. (2021) 90:178–83. doi: 10.1016/j.jocn.2021.05.056
15. Mancini M, Di Fonzo A, Lazzeri G, Borellini L, Silani V, Lacerenza M, et al. Real life evaluation of safinamide effectiveness in Parkinson's disease. Neurol Sci. (2018) 39:733–9. doi: 10.1007/s10072-018-3272-y
16. Abbruzzese G, Kulisevsky J, Bergmans B, Gomez-Esteban JC, Kägi G, Raw J, et al. A European observational study to evaluate the safety and the effectiveness of safinamide in routine clinical practice: the SYNAPSES trial. J Parkinsons Dis. (2021) 11:187–98. doi: 10.3233/JPD-202224
18. Fahn S Elton R Members Members of the UPDRS Development Committee. The unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease, Vol. 2. Florham Park, NJ: McMellam Health Care Information (1987). p. 153–63.
19. Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the Unified Parkinson's Disease rating scale. Arch Neurol. (2010) 67:64–70. doi: 10.1001/archneurol.2009.295
20. Stebbins GT, Goetz CG, Burn DJ, ankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the Movement Disorder Society Unified Parkinson's disease rating scale: comparison with the Unified Parkinson's disease rating scale. Mov Disord. (2013) 28:668–70. doi: 10.1002/mds.25383
21. Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. (1967) 17:427–42. doi: 10.1212/WNL.17.5.427
22. Meoni S, Macerollo A, Moro E. Sex differences in movement disorders. Nat Rev Neurol. (2020) 16:84–96. doi: 10.1038/s41582-019-0294-x
23. Crispino P, Gino M, Barbagelata E, Ciarambino T, Politi C, Ambrosino I, et al. Gender differences and quality of life in Parkinson's disease. Int Environ Res Public Health. (2021) 18:198. doi: 10.3390/ijerph18010198
24. Navarta-Sánchez MV, Senosiain García JM, Riverol M, Ursúa Sesma ME, Díaz de Cerio Ayesa S, Anaut Bravo S, et al. Factors influencing psychosocial adjustment and quality of life in Parkinson patients and informal caregivers. Qual Life Res. (2016) 25:1959–68. doi: 10.1007/s11136-015-1220-3
25. Barret B, Brown D, Mundt M, Brown R. Sufficiently important difference: expanding the framework of clinical significance. Med Decis Making. (2005) 25:250–61. doi: 10.1177/0272989X05276863
26. Cerri S, Mus L, Blandini F. Parkinson's disease in women and men: what is the difference? J Parkinsons Dis. (2019) 9:501–15. doi: 10.3233/JPD-191683
27. Stocchi F, Antonini A, Barone P, Tinazzi M, Zappia M, Onofrj M, et al. Early DEtection of wEaring-off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord. (2014) 20:204–11. doi: 10.1016/j.parkreldis.2013.10.027
28. Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson's disease. Mol Neurobiol. (1996) 12:73–94. doi: 10.1007/BF02740748
29. Sato K, Hatano T, Yamashiro K, Kagohashi M, Nishioka K, Izawa N, et al. Prognosis of Parkinson's disease: time to stage III, IV, V, and to motor fluctuations. Mov Disord. (2006) 21:1384–95. doi: 10.1002/mds.20993
30. Bjornestad A, Forsaa EB, Pedersen KF, Tysnes OB, Larsen JP, Alves G. Risk and course of motor complications in a population-based incident Parkinson's disease cohort. Parkinsonism Relat Disord. (2016) 22:48–53. doi: 10.1016/j.parkreldis.2015.11.007
31. Bianchini E, Sforza M, Rinaldi D, Alborghetti M, De Carolis L, Della Gatta F, et al. Switch from rasagiline to safinamide in fluctuating Parkinson's disease patients: a retrospective, pilot study. Neurol Res. (2021) 18:1–5. doi: 10.1080/01616412.2021.1942408
Keywords: Parkinson's disease, motor fluctuations, safinamide, gender differences, real-life evaluation
Citation: Pellecchia MT, Picillo M, Russillo MC, De Pandis MF, Bonizzoni E, Marjanovic I and Cattaneo C (2021) Efficacy of Safinamide and Gender Differences During Routine Clinical Practice. Front. Neurol. 12:756304. doi: 10.3389/fneur.2021.756304
Received: 10 August 2021; Accepted: 15 November 2021;
Published: 14 December 2021.
Edited by:
Emilia Mabel Gatto, Sanatorio de la Trinidad Mitre, ArgentinaReviewed by:
Carlos Henrique Ferreira Camargo, Federal University of Paraná, BrazilMarcela Claudia Uribe Roca, Hospital Británico de Buenos Aires, Argentina
Copyright © 2021 Pellecchia, Picillo, Russillo, De Pandis, Bonizzoni, Marjanovic and Cattaneo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Cattaneo, Y2FybG8uY2F0dGFuZW8mI3gwMDA0MDt6YW1ib25ncm91cC5jb20=
 Maria T. Pellecchia
Maria T. Pellecchia Marina Picillo1
Marina Picillo1 Erminio Bonizzoni
Erminio Bonizzoni Ivan Marjanovic
Ivan Marjanovic Carlo Cattaneo
Carlo Cattaneo