
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 29 October 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.744909
This article is part of the Research Topic Challenges in Acute Minor Ischemic Stroke View all 14 articles
Objective: This study assessed the characteristics of intravenous thrombolysis (IVT) with respect to early neurological deterioration (END) and functional outcome in mild ischemic stroke patients.
Methods: Data were obtained from acute mild ischemic stroke patients (defined as having a National Institute of Health Stroke Score (NIHSS) ≤ 5) treated with IVT in our hospital from July 2017 to December 2020. END was defined as the NIHSS increased ≥1 over the baseline at 24 h after IVT. A modified Rankin scale (mRS) ≤ 1 at 3 months was considered as a favorable outcome, and an mRS ≥2 at 3 months was an unfavorable outcome.
Results: Two hundred thirty-three acute mild ischemic stroke patients (all patients underwent MRI and DWI restriction) with IVT were included in this study. Thirty-one patients experienced END, and 57 patients experienced an unfavorable outcome at 3 months. With multivariate analysis, END was associated with an elevated baseline systolic blood pressure (SBP) (OR = 1.324, 95% CI, 1.053–1.664, p = 0.016) and coronary heart disease (OR = 4.933, 95% CI, 1.249–19.482, p = 0.023). An unfavorable outcome at 3 months after IVT was independently associated with a baseline elevated SBP (OR = 1.213, 95% CI, 1.005–1.465, p = 0.045), baseline NIHSS (OR = 1.515, 95% CI, 1.186–1.935, p = 0.001), prior hyperlipemia (OR = 3.065, 95% CI, 1.107–8.482, p = 0.031), cardioembolic stroke (OR = 0.323, 95% CI, 0.120–0.871, p = 0.025), and END at 24 h (OR = 4.531, 95% CI, 1.950–10.533, p < 0.001) in mild ischemic stroke patients.
Conclusion: In mild ischemic stroke patients with IVT, an elevated baseline SBP and coronary heart disease were associated with END. The elevated baseline SBP, baseline NIHSS, a history of prior hyperlipemia, cardioembolic stroke, and END at 24 h after IVT were useful in predicting an unfavorable outcome at 3 months.
Intravenous thrombolysis (IVT) has been proven to be an effective treatment for acute ischemic stroke patients when it is given within 4.5 h of stroke onset (1). More than half of acute ischemic stroke patients exhibit mild symptoms, including neurological deficits (2, 3). Currently, mild ischemic stroke has no uniform definition, but most studies define mild ischemic stroke as presenting a National Institute of Health Stroke Score (NIHSS) ≤ 5 (4, 5). Due to the risk of hemorrhagic transformation and not increasing the likelihood of a favorable outcome at 90 days (5), IVT is not recommended for mild non-disabled ischemic stroke patients within 4.5 h (6). However, approximately one-third of mild ischemic stroke patients without IVT have unfavorable outcome due to mild stroke (7). Some non-disabled patients who did not receive IVT in the appropriate time frame go on to develop early neurological deterioration (END) (8). Currently, there are no better-accepted treatments that can be given to lengthen the time window of IVT. However, previous research has found that mild ischemic stroke patients could be benefit from IVT (9–11). Although thrombolysis might increase the risk of hemorrhagic transformation, but its occurrence was statically insignificant and did not increase the mortality rate (9, 11). Therefore, this study was designed to identify factors that impacted END and the functional outcome of mild ischemic stroke patients after IVT, which could be useful in predicting a possible unfavorable outcome at 3 months.
The study was carried out between July 2017 and December 2020 in Shaoxing People's Hospital (Shaoxing Hospital, Zhejiang University School of Medicine). Initially, there were 282 acute, mild, and ischemic stroke patients receiving intravenous recombinant tissue plasminogen activator (alteplase 0.9 mg/kg up to a maximum of 90 mg, 10% of the total dosage as a bolus and the rest over 1 h) therapy in our hospital. We eliminated 49 patients (25 patients were stroke mimic, 2 patients were newly diagnosed lung cancer, 18 patients lacked follow-up data, and 4 patients lacked MRI images). Finally, 233 patients were enrolled in this study. Patients were selected using the following criteria: (1) is aged >18 years; (2) diagnosed with acute ischemic stroke (AIS) according to clinical symptoms, MRI, and DWI restriction; (3) had a baseline NIHSS ≤ 5; (4) received IVT within 4.5 h of AIS onset; and (5) underwent CT scans at 24 h after IVT. The exclusion criteria included the following: (1) the presence of existing contraindications for intravenous thrombolysis according to the standard IVT guidelines (12); (2) prior stroke or the presence of other diseases that resulted in a baseline mRS ≥1; (3) a long-term life expectancy of 3 months or less; (4) necessity of daily life need care because of other chronic system diseases, such as chronic heart failure, chronic obstructive pulmonary disease, and end-stage renal disease; (5) limb fracture affecting movement; and (6) lack of follow-up data.
A neurologist who was blinded to the patient's outcome reviewed the medical records to collect the following data: demographic characteristics, baseline NIHSS, baseline SBP, baseline DBP, history of smoking, hypertension, atrial fibrillation, coronary heart disease, diabetes, prior hyperlipemia, and prior stroke or transient ischemic attack (TIA). A history of prior hyperlipemia disease included hypertriglyceridemia and hypercholesterolemia. Coronary heart disease is defined as a patient having a history of acute coronary syndrome or angina pectoralis. Acute ischemic stroke subtypes were determined by using the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification (13). This study was approved by the Shaoxing Hospital, Zhejiang University School of Medicine Sciences Ethics Committee.
END was defined as an NIHSS at 24 h after IVT that was increased ≥1 over the baseline (14). There was a neurologist in charge of the follow-up. The mRS score at 3 months was all collected by phone. An mRS score ≤ 1 at 3 months was determined to be a favorable outcome after IVT, and an mRS score ≥2 at 3 months was an unfavorable outcome. Hemorrhage transformation was classified as hemorrhagic infarction types I and II and parenchymal hemorrhage types I and II according to the definition provided by the European Cooperative Acute Stroke Study (ECASS) (15). Symptomatic intracerebral hemorrhage (SICH) was defined as the presence of a neurological decline attributed to parenchymal hemorrhage type II with an NIHSS score increase of ≥4 after IVT (16).
Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, Illinois, USA). Fisher's exact test was used to compare the dichotomous variables between groups, while the Mann–Whitney U-test was used for the continuous variables. Variables with a two-tailed p-value of < 0.1 in univariate regression analyses were included in the binary multivariate logistic regression model to determine the independent risk factors of END at 24 h and functional outcome at 3 months after IVT. A two-tailed p-value < 0.05 was considered statistically significant.
Two hundred thirty-three mild AIS with IVT were included. No patients underwent thrombectomy. The mean age (±SD) was 60.08 ± 10.87 years. Eighty-four (36.1%) patients were female. The median baseline NIHSS was 2 (interquartile range, IQR, 1–4) among all patients: 31 (13.3%) patients experienced END, 9 (3.9%) patients experienced hemorrhagic transformation, and 2 (0.9%) of the nine exhibited SICH. Nine patients experienced stroke recurrence in 3 months. Fifty-seven (24.5%) patients experienced an unfavorable outcome at 3 months after IVT. The demographics and baseline characteristics are shown in Table 1.
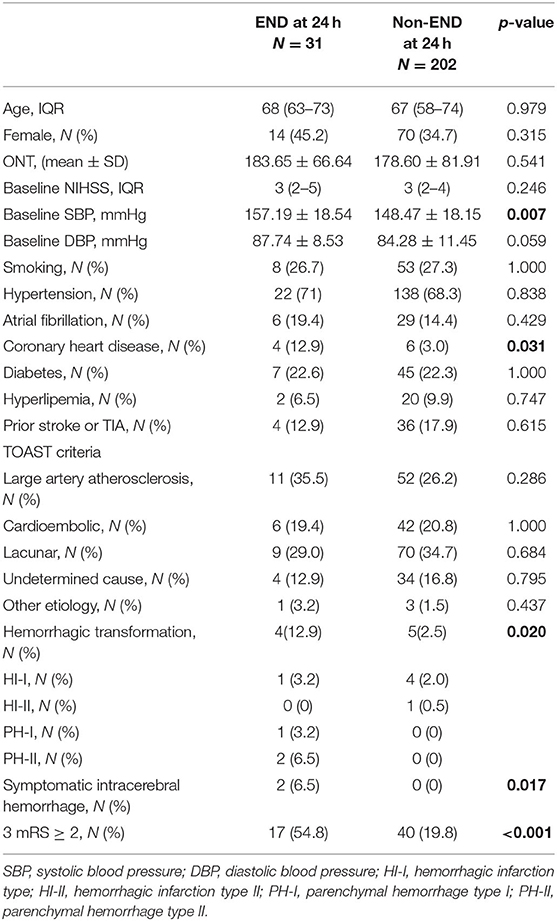
Table 1. Characteristics of mild acute ischemic stroke patients with IVT with or without END at 24 h.
After univariate analysis is carried out, the mild AIS patients with END exhibited a higher baseline SBP (157.19 ± 18.54 vs. 148.47 ± 18.15, p = 0.007) and a higher baseline DBP (87.74 ± 8.53 vs. 84.28 ± 11.45, p = 0.059) compared with non-END patients. Moreover, the mild AIS patients with END presented a higher rate of coronary heart disease compared with the non-END group (12.9% vs. 3.0%, p = 0.031). Baseline SBP, DBP, and coronary heart disease were included in the binary logistic multivariate analysis. The results revealed that an elevated baseline SBP (OR = 1.324, 95% CI, 1.053–1.664, p = 0.016) and coronary heart disease (OR = 4.933, 95% CI, 1.249–19.482, p = 0.023) were independently associated with END at 24 h after IVT (Table 2).
In this study, 57 (24.5%) mild AIS patients experienced an unfavorable outcome. Two patients experienced SICH and had an unfavorable outcome at 3 months. Based on univariate analysis, we observed that patients with unfavorable outcomes at 3 months had a higher baseline NIHSS (IQR, 3 (2–4) vs. 3 (2–5), p = 0.001), a higher baseline SBP (154.68 ± 17.27 vs. 147.99 ± 18.51, p = 0.016), and increased END (29.8% vs. 8.0%, p < 0.001) after IVT. In the TOAST classification, the unfavorable outcome group had a higher rate of large artery atherosclerosis (40.4% vs. 23.3%, p = 0.017) and a lower rate of cardioembolic stroke (10.5 vs. 23.9%, p = 0.037). However, there was no difference in the incidence of hemorrhagic transformation (5.3 vs. 3.4%, p = 0.692) between the two groups (Table 3). The variables with p-values M < 0.1 in the univariate analysis underwent binary logistic multivariate analysis. After large artery atherosclerosis of stroke and the presence of diabetes were adjusted for, the results demonstrated that the baseline elevated SBP (OR = 1.213, 95% CI, 1.005–1.465, p = 0.045), baseline NIHSS (OR = 1.515, 95% CI, 1.186–1.935, p = 0.001), prior hyperlipemia (OR = 3.065, 95% CI, 1.107–8.482, p = 0.031), cardioembolic stroke (OR = 0.323, 95% CI, 0.120–0.871, p = 0.025), and END at 24 h (OR = 4.531, 95% CI, 1.950–10.533, p < 0.001) after IVT were independently associated with unfavorable outcomes at 3 months in mild AIS patients (Table 4). SICH could not be included in the logistic analysis due to the small number of patients.
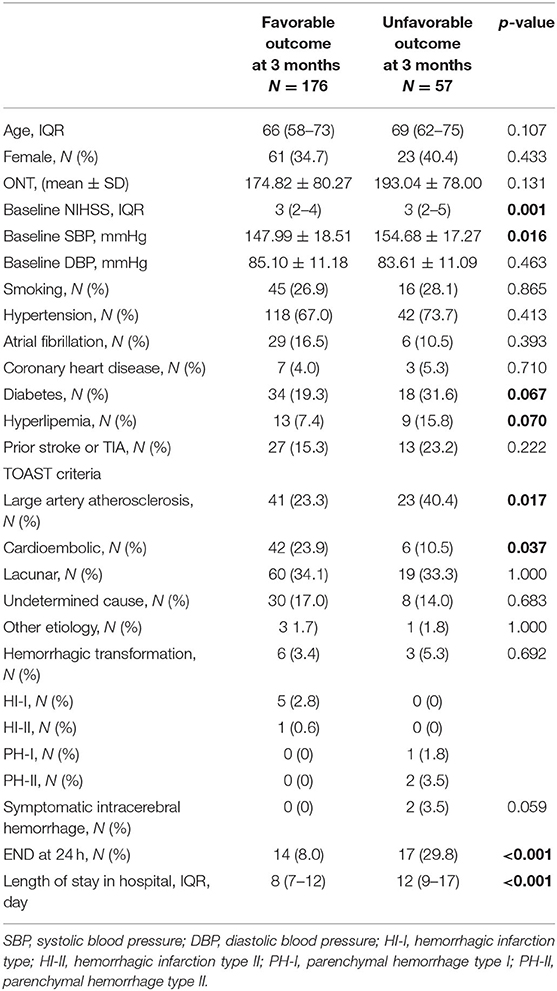
Table 3. Characteristics of mild acute ischemic stroke patients with IVT with or without favorable outcomes at 3 months.
In this study, we demonstrated that an elevated baseline SBP and coronary heart disease were independently associated with END. In addition, an elevated baseline SBP, baseline NIHSS, prior hyperlipemia, cardioembolic stroke, and END at 24 h after IVT were useful for predicting an unfavorable outcome in mild AIS patients.
An elevated baseline SBP was an independent predictor of END and unfavorable outcomes at 3 months after IVT in mild AIS patients. After the occurrence of ischemic stroke, SBP might remain elevated to maintain constant cerebral perfusion (17). Current guidelines (12) recommend that blood pressure should be controlled (<180/105 mmHg) in the first 24 h after IVT. However, the relationship between blood pressure and functional outcome exhibits a U-shape (18, 19). Both high SBP and low SBP are correlated with unfavorable outcomes in ischemic stroke patients (18, 20, 21). On one hand, lower SBP might lead to unfavorable outcome by reducing cerebral hemodynamic reserve and cerebral hypoperfusion (22). On the other hand, higher SBP might be associated with unfavorable outcome due to cerebral edema, stroke recurrence, and hemorrhagic transformation (18). Yan et al. found that when the SBP was maintained within a range of 140–149 mmHg for the first 24 h after IVT, neurological deterioration was the lowest (19). In our study, the mean SBP in non-END patients was 148.47 ± 18.15 mmHg, which fell within the recommended 140–149 mmHg range.
The mechanism by which SBP contributes to END is unclear. Yan He et al. found that blood pressure was directly proportional to serum levels of MMP-9 and AQP-4 at 24 h after thrombolysis (19). Thus, they presumed that the SBP that occurred with END might be associated with oxidative stress-induced blood–brain barrier disruption and AQP-4 upregulation (19). Also, high SBP could increase the risk of cerebral edema, hemorrhagic transformation, and stroke recurrence, which are associated with unfavorable outcomes in ischemic stroke patients. We observed that END in mild AIS patients directly impacted the unfavorable outcome at 3 months after IVT. This result was consistent with a prior study (23).
Coronary heart disease is defined as the patient having a history of acute coronary syndrome or angina pectoralis. It is well known that ischemic stroke and coronary heart diseases have the same risk factors. It has been reported that coronary heart disease is correlated with an unfavorable outcome in AIS patients (24). Our study demonstrated that coronary heart disease was an independent predictor of END after IVT in mild AIS patients, but the underlying mechanism is unknown. In fact, there was no difference in ejection fraction of coronary heart disease patients between the END group and non-END group (66.00 ± 7.83% vs. 65.17 ± 4.54%, p = 0.967). It is notable that we observed 10 patients with coronary heart disease that included four (12.9%) in the END group and six (3.0%) in the non-END group. In the END group, three of the four coronary heart disease patients concurrently experienced cerebral vascular stenosis. Conversely, no patient experienced cerebral vascular stenosis in the other group. Therefore, we proposed that coronary heart disease associated with END was possibly related to the fact that the patients with coronary heart disease also had a high occurrence of cerebral vascular stenosis. This possibility could be confirmed in a future study.
In this study, the incidence of hemorrhagic transformation was 3.9% (9/233), and SICH was 0.9% (2/233). We found that hemorrhagic transformation did not affect the occurrence of an unfavorable outcome at 3 months after IVT in mild AIS patients. Although two SICH patients experienced an unfavorable outcome at 3 months, the incidence of SICH in mild AIS patients with IVT was not higher than other ischemic stroke patients with IVT. Previous studies demonstrated that IVT did not increase the risk of SICH in mild ischemic stroke patients (25).
Baseline NIHSS has been determined to be an independent predictor of unfavorable outcomes in mild ischemic stroke after IVT (26, 27), and the result in this study was the same as in these prior studies. For cardioembolic stroke, we found those patients had higher rate of favorable outcomes at 3 months after IVT. Similar studies were published (28, 29). It seemed that IVT was more effective in cardioembolic stroke. This result might be associated with the composition of the thrombus. The thrombus of cardioembolic stroke contains more fibrin and platelet, but other thrombi contain more red blood cells (28). Meanwhile, rt-PA has high banding affinity for fibrin and might be more prone to result in thrombus dissolution (28).
We also observed that hyperlipemia was associated with unfavorable outcomes in mild AIS patients. Hypertriglyceridemia and hypercholesterolemia were both included in hyperlipemia. A previous study confirmed that hypertriglyceridemia might be a predictor of END (30). However, high LDC-C early in the course of stroke has been associated with a favorable outcome at 3 months in mild ischemic stroke (31). The specific pathophysiological mechanism underlying this association remains unclear. Because of our retrospective design, we did not subdivide the patients with hypertriglyceridemia and hypercholesterolemia.
Our study is mainly limited by its retrospective design of single center. First, although we collected data using an established prospective stroke registry, a risk of selection bias is possible. Second, we found baseline SBP, baseline NIHSS, a history of prior hyperlipemia, cardioembolic stroke, and END at 24 h after IVT were associated with unfavorable outcome at 3 months in mild stroke patients. But our registry did not collect the outcome data of mild ischemic stroke patients without IVT. There might be other factors associated with unfavorable outcome of mild ischemic stroke patients without IVT, which will be collected and studied in our future work. Third, we found that coronary heart disease had a high occurrence of cerebral vascular stenosis and proposed that coronary heart disease associated with END was possibly related to this fact. But we have no complete data on large vessel stenosis of all included mild stroke patients. We could not get a conclusion on in this study on whether mild stroke patients with large vessel stenosis were more likely to experience END. This possibility could be confirmed in a future study that uses a larger sample size.
In this study, 24.5% of patients with mild ischemic stroke experienced an unfavorable outcome after IVT. The incidence of SICH was low (0.9%). Moreover, elevated baseline SBP was an independent predictor of END at 24 h and an unfavorable outcome at 3 months after IVT. Thus, blood pressure might be rigorously controlled for mild ischemic stroke patients during and after IVT. This conclusion needs to be confirmed with a larger sample size and the inclusion of additional blood pressure-related parameters in future work.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Human Ethics Committee of Shaoxing People's Hospital. The patients/participants provided their written informed consent to participate in this study.
HT was responsible for setting this topic, designing protocol, statistical analysis, and writing papers. SY was responsible for designing protocol and statistical analysis. CW was responsible for data collection and assessing hemorrhagic transformation. YZ was responsible for data collection and follow-up work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services.
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
2. Reeves M, Khoury J, Alwell K, Moomaw C, Flaherty M, Woo D, et al. Distribution of national institutes of health stroke scale in the cincinnati/northern kentucky stroke study. Stroke. (2013) 44:3211–3. doi: 10.1161/STROKEAHA.113.002881
3. Kim BJ, Park JM, Kang K, Lee SJ, Ko Y, Kim JG, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. (2015) 17:38–53. doi: 10.5853/jos.2015.17.1.38
4. Kim DH, Lee DS, Nah HW, Cha JK. Clinical and radiological factors associated with unfavorable outcome after intravenous thrombolysis in patients with mild ischemic stroke. BMC Neurol. (2018) 18:30. doi: 10.1186/s12883-018-1033-4
5. Khatri P, Kleindorfer DO, Devlin T, Sawyer RN. Jr., Starr M, Mejilla J, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor non-disabling neurologic deficits: the prisms randomized clinical trial. JAMA. (2018) 320:156–66. doi: 10.1001/jama.2018.8496
6. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:I–LXII. doi: 10.1177/2396987321989865
7. Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke. (2005) 36:2497–9. doi: 10.1161/01.STR.0000185798.78817.f3
8. Yeo LLL, Ho R, Paliwal P, Rathakrishnan R, Sharma VK. Intravenously administered tissue plasminogen activator useful in milder strokes? A meta-analysis. J Stroke Cerebrovasc Dis. (2014) 23:2156–62. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.008
9. Lan L, Rong X, Li X, Zhang X, Pan J, Wang H, et al. Reperfusion therapy for minor stroke: a systematic review and meta-analysis. Brain Behav. (2019) 9:e01398. doi: 10.1002/brb3.1398
10. Laurencin C, Philippeau F, Blanc-Lasserre K, Vallet AE, Cakmak S, Mechtouff L, et al. Thrombolysis for acute minor stroke: outcome and barriers to management. Results from the resuval stroke network. Cerebrovasc Dis. (2015) 40:3–9. doi: 10.1159/000381866
11. Choi JC, Jang MU, Kang K, Park JM, Ko Y, Lee SJ, et al. Comparative effectiveness of standard care with iv thrombolysis versus without IV thrombolysis for mild ischemic stroke. J Am Heart Assoc. (2015) 4:e001306. doi: 10.1161/JAHA.114.001306
12. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2018) 49:e46–110. doi: 10.1161/STR.0000000000000172
13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
14. Shi Z, Zheng WC, Fu XL, Fang XW, Xia PS, Yuan WJ. Hypercoagulation on thromboelastography predicts early neurological deterioration in patients with acute ischemic stroke. Cerebrovasc Dis. (2018) 46:125–31. doi: 10.1159/000492729
15. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA. (1995) 274:1017–25. doi: 10.1001/jama.1995.03530130023023
16. Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (sits-most) An observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
17. Tucker N, Stoffel JM, Hayes L, Jones GM. Blood pressure management following acute ischemic stroke: a review of primary literature. Crit Care Nurs Q. (2020) 43:109–21. doi: 10.1097/CNQ.0000000000000297
18. Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA, Group ISTC. Blood pressure and clinical outcomes in the international stroke trial. Stroke. (2002) 33:1315–20. doi: 10.1161/01.STR.0000014509.11540.66
19. He Y, Yang Q, Liu H, Jiang L, Liu Q, Lian W, et al. Effect of blood pressure on early neurological deterioration of acute ischemic stroke patients with intravenous rt-pa thrombolysis may be mediated through oxidative stress induced blood-brain barrier disruption and aqp4 upregulation. J Stroke Cerebrovasc Dis. (2020) 29:104997. doi: 10.1016/j.jstrokecerebrovasdis.2020.104997
20. Teng RSY, Tan BYQ, Miny S, Syn NL, Ho AFW, Ngiam NJH, et al. Effect of pretreatment blood pressure on outcomes in thrombolysed acute ischemic stroke patients: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2019) 28:906–19. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.008
21. Gill D, Cox T, Aravind A, Wilding P, Korompoki E, Veltkamp R, et al. A fall in systolic blood pressure 24 hours after thrombolysis for acute ischemic stroke is associated with early neurological recovery. J Stroke Cerebrovasc Dis. (2016) 25:1539–43. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.002
22. Alvarez FJ, Segura T, Castellanos M, Leira R, Blanco M, Castillo J, et al. Cerebral hemodynamic reserve and early neurologic deterioration in acute ischemic stroke. J Cereb Blood Flow Metab. (2004) 24:1267–71. doi: 10.1097/01.WCB.0000139370.93203.4A
23. Coutts SB, Modi J, Patel SK, Aram H, Demchuk AM, Goyal M, et al. What causes disability after transient ischemic attack and minor stroke? Results from the CT and MRI in the triage of TIA and minor cerebrovascular events to identify high risk patients (catch) study. Stroke. (2012) 43:3018–22. doi: 10.1161/STROKEAHA.112.665141
24. Mehrpour M, Afrakhte M, Shojaei SF, Sohrabi A, Ashayeri R, Esmaeili S, et al. Factors predicting the outcome of intravenous thrombolysis in stroke patients before RT-pa administration. Caspian J Intern Med. (2019) 10:424–30. doi: 10.22088/cjim.10.4.424
25. Tsivgoulis G, Goyal N, Katsanos AH, Malhotra K, Ishfaq MF, Pandhi A, et al. Intravenous thrombolysis for large vessel or distal occlusions presenting with mild stroke severity. Eur J Neurol. (2020) 27:1039–47. doi: 10.1111/ene.14199
26. Strambo D, Zambon AA, Roveri L, Giacalone G, Di Maggio G, Peruzzotti-Jametti L, et al. Defining minor symptoms in acute ischemic stroke. Cerebrovasc Dis. (2015) 39:209–15. doi: 10.1159/000375151
27. Romano JG, Smith EE, Liang L, Gardener H, Camp S, Shuey L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the get with the guidelines-stroke registry. JAMA Neurol. (2015) 72:423–31. doi: 10.1001/jamaneurol.2014.4354
28. Vaclavik D, Vilionskis A, Jatuzis D, Karlinski MA, Gdovinova Z, Korv J, et al. Clinical outcome of cardioembolic stroke treated by intravenous thrombolysis. Acta Neurol Scand. (2018) 137:347–55. doi: 10.1111/ane.12880
29. Anticoli S, Bravi MC, Perillo G, Siniscalchi A, Pozzessere C, Pezzella FR, et al. Effect of cardioembolic etiology on intravenous thrombolysis efficacy for acute ischemic stroke. Curr Neurovasc Res. (2016) 13:193–8. doi: 10.2174/1567202613666160506125426
30. Kwon HM, Lim JS, Park HK, Lee YS. Hypertriglyceridemia as a possible predictor of early neurological deterioration in acute lacunar stroke. J Neurol Sci. (2011) 309:128–30. doi: 10.1016/j.jns.2011.06.057
Keywords: mild ischemic stroke, thrombolysis, early neurological deterioration, unfavorable outcome, systolic blood pressure (SBP)
Citation: Tang H, Yan S, Wu C and Zhang Y (2021) Characteristics and Outcomes of Intravenous Thrombolysis in Mild Ischemic Stroke Patients. Front. Neurol. 12:744909. doi: 10.3389/fneur.2021.744909
Received: 21 July 2021; Accepted: 28 September 2021;
Published: 29 October 2021.
Edited by:
Linxin Li, University of Oxford, United KingdomReviewed by:
Leonard Yeo, National University Health System, SingaporeCopyright © 2021 Tang, Yan, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanxing Zhang, YXV3eGYwMUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.