
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 17 February 2022
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.743023
 Ronil V. Chandra1,2*
Ronil V. Chandra1,2* Julian Maingard1
Julian Maingard1 Lee-Anne Slater1,2
Lee-Anne Slater1,2 Nicholas K. Cheung1
Nicholas K. Cheung1 Leon T. Lai2,3
Leon T. Lai2,3 Seana L. Gall4,5
Seana L. Gall4,5 Amanda G. Thrift5
Amanda G. Thrift5 Thanh G. Phan5,6
Thanh G. Phan5,6Background: Small unruptured intracranial aneurysms (UIAs) are considered to have low risk of rupture. The proportion of UIAs measuring 10 mm or less in size that rupture when selected for conservative management without repair is not well known. The aim of this study is to determine the proportion of UIAs that rupture by size threshold from ≤10 to ≤3 mm when selected for management without repair and to determine the level of precision and sources of heterogeneity in the rupture risk estimate.
Methods: This study was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019121522). The Ovid MEDLINE, EMBASE, Web of Science Core Collection, and the Cochrane Central Register of Controlled Trials were searched (inception to August 2020). Studies with longitudinal follow-up of patients with UIAs ( ≤10 mm to ≤3 mm) without endovascular or neurosurgical repair were eligible. We included studies, which provided details of aneurysm size and in which UIA rupture was reported as an outcome. The primary outcome of the pooled proportion of UIA rupture during follow-up was synthesized with random-effects meta-analysis; heterogeneity was explored using meta-regression.
Results: A total of 31 studies that included 13,800 UIAs ≤10 mm in size were eligible for data synthesis. The pooled proportion of ≤10 mm UIAs that ruptured when managed without repair was 1.1% (95% CI 0.8–1.5; I2 = 52.9%) over 3.7 years. Findings were consistent in sensitivity analyses at all the size stratified thresholds including ≤5 and ≤3 mm; rupture occurred in 1.0% (95% CI 0.8–1.3; I2 = 0%) of 7,280 ≤5 mm UIAs and 0.8% (95% CI 0.4–1.5; I2 = 0%) of 1,228 ≤3 mm UIAs managed without repair. In higher quality studies with lower risk of bias, rupture occurred in 1.8% (95% CI 1.5–2.0; I2 = 0%) over 3.9 years. In meta-regression, aneurysm size, shape, anatomical location, and exposure to prior subarachnoid hemorrhage were not identified as sources of heterogeneity.
Conclusion: For every 1,000 UIAs that are 10 mm or less in size and selected for conservative management without repair, between 8 and 15 UIAs are estimated to rupture over 3.7 years. When stratified by size, these pooled rupture risk estimates are consistent and clinically applicable for ≤5 mm UIAs selected for management without repair.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42019121522.
Incidental small unruptured intracranial aneurysms (ISUIA) are being increasingly discovered with greater utilization of neuroimaging. This creates significant management dilemma for both the physicians and patients, since future aneurysmal rupture causing subarachnoid hemorrhage (SAH) is associated with a risk of death, and in those who survive, a risk of cognitive impairment and disability (1, 2). This requires physicians to balance the risk of harm from preemptive UIA repair to prevent SAH and rupture risk associated with follow-up without repair.
Overall, small UIAs are considered to be at low risk of rupture based on large prior studies. The International Study of Unruptured Intracranial Aneurysms (ISUIA) (3) and the Unruptured Cerebral Aneurysm Study (UCAS) Japan (4) provide insight into understanding the future rupture risk. However, aneurysm repair in these studies was not performed at random, but targeted to UIAs that considered at greater risk of SAH. This treatment selection bias may contribute to underestimation of rupture risk during follow-up without aneurysm repair (5). In addition, aneurysm rupture risk scoring systems such as the Population, Hypertension, Age, Size, Earlier subarachnoid haemorrhage, and Site (PHASES) (6) that utilize this data are subjected to the limitations of the underlying studies.
In clinical practice, physicians consider aneurysm size as a key factor in predicting future UIA rupture (6) and commonly extrapolate a predicted annualized rupture rate across the remaining healthy lifetime of the patient when considering risk and benefit of UIA repair (7). This pragmatic approach is supported by a recent rigorous systematic review of UIAs, which included data from the ISUIA and the UCAS (8). However, mean follow-up in the included studies was <5 years and, therefore, utilization of this methodology extrapolates future rupture risk beyond the duration of observed data. In addition, extrapolation of an annualized rupture rate assumes a constant rate of rupture across the lifetime time horizon. This is also unlikely to be accurate due to potential aneurysm growth or morphological changes over the lifespan of the patient.
Therefore, we aimed to use meta-analytic methods to synthesize the proportion of rupture of ≤10 mm UIAs when selected by physicians for management without aneurysm repair. We aimed to stratify results by size threshold and also aimed to determine the level of precision in the risk estimate and to explore heterogeneity using meta-regression.
This study protocol was prospectively registered with the PROSPERO (CRD42019121522). Search strategy, study selection, data extraction, risk of bias assessment, and data analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The prepiloted search strategy was designed with an experienced medical librarian with an aim of high sensitivity (Supplementary Material). We searched the Ovid MEDLINE, EMBASE, Web of Science Core Collection, and the Cochrane Central Register of Controlled Trials from inception to August 2020. Reference lists of previous systematic reviews and included studies were reviewed, but gray literature sources were not included. Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was utilized during eligibility assessment. Microsoft Excel and Endnote were utilized for data collection, data checking, and reference management.
Studies that included participants with ≤10 mm UIAs in both the anterior and posterior circulation that did not have neurosurgical or endovascular repair and underwent follow-up were evaluated for inclusion. Randomized and non-randomized clinical trials, prospective and retrospective cohort studies, and case series with >10 patients published in English were evaluated. We included studies that reported aneurysm rupture as an outcome. When aneurysm rupture was reported as a clinical outcome in a mixed cohort of unruptured intradural and cavernous internal carotid aneurysms, we only included data if > 85% of the cohort harbored intradural aneurysms. For studies that included data published more than once, the study report with the most informative dataset was included. Two investigators independently evaluated studies for eligibility against the pre-specified inclusion criteria. Initially, titles and abstracts were screened. Full-text reports were obtained for all the potentially relevant reports and when there was ambiguity on whether or not the articles met inclusion criteria, disagreements were resolved by consensus.
Two investigators performed independent data extraction using a pre-piloted standardized electronic datasheet that included: study author, publication year, study start and end years, country of source population, study design, number of patients, mean or median age, number of aneurysms, number of patients with multiple aneurysms, number of patients with prior SAH, mean or median follow-up, and number of aneurysms that ruptured during follow-up. When aneurysm data were stratified by size, data were extracted under the following size thresholds commonly reported in the literature: ≤10, ≤7, ≤5, and ≤3 mm. Aneurysm shape and anatomical location data were also extracted where available. Irregular aneurysms were defined as UIAs that were reported as irregular, multilobular, or with a daughter sac or aneurysm bleb. For anatomical location analysis, the total number of anterior and posterior circulation UIAs of ≤10 mm was extracted. If specific anatomical location was reported, the total number of anterior communicating artery and anterior cerebral artery aneurysms for UIAs of ≤10 mm was extracted. Discrepancies were resolved by consensus. To improve accuracy of analyses for size, we included details from additional publications of the same study cohort and emailed the corresponding authors of original publications. A separate investigator independently checked the data for consistency and completeness following double data extraction and receipt of unpublished data. An experienced investigator performed all the data analyses, which were checked independently by a separate experienced investigator.
To assess the quality of the included non-randomized studies, the pre-piloted modified Newcastle–Ottawa Scale (NOS) (9) was used (Supplementary Material). Two investigators independently rated included studies on selection, comparability, and outcome and recorded information on each study to justify the judgment made. Any disagreements were resolved by consensus. The NOS ratings were categorized as good quality, fair quality, and poor quality based on the Agency for Healthcare Research and Quality (AHRQ) standards (10).
The pre-specified outcome was rupture of the index UIA included at study entry. The proportion of UIAs that ruptured was extracted from each study. Aneurysmal SAH from de novo, dissecting, or fusiform aneurysms was excluded. A post-hoc decision was made to perform a per aneurysm analysis, since one aneurysm in one patient ruptures at one time, while a patient may harbor multiple aneurysms. Aneurysm multiplicity was taken into account by extracting the total number of aneurysms for UIAs of ≤10 mm. If this was not reported, the total number of aneurysms in the ≤10 mm cohort was derived by applying the proportion of multiple aneurysms in the total observation cohort to the number of patients. If this was not reported, then patients with multiple aneurysms were assumed to harbor two UIAs. For aneurysm shape analysis, if the total number of irregular aneurysms for UIAs of ≤10 mm was not reported, the total number of aneurysms in the ≤10 mm cohort was derived by applying the same proportion of irregular aneurysms in the total observation cohort. For anatomical location analysis, if anatomical location data were not reported for UIAs of ≤10 mm, the number of aneurysms in the ≤10 mm cohort was derived by applying the same proportion of the anatomical location information in the total observation cohort. Median follow-up values were used as best estimates when mean values were missing (11). If mean follow-up was not specifically reported for the ≤10 mm cohort, the mean follow-up of the total observation cohort for all the aneurysm sizes was utilized.
The I2 statistic was used to identify heterogeneity, which was categorized as low (<25%), moderate (25–75%), or significant (>75%) (12). Heterogeneity was explored using outlier and influence analyses. If a reasonable rationale due to clinical and/or methodological diversity was evident, the outlier study was excluded from data synthesis to reduce bias in the pooled proportion estimate and additional sensitivity analyses were performed with and without outlier studies (13).
Random-effects data synthesis was carried out using a random intercept logistic regression model for meta-analysis of proportions. The Wilson procedure was utilized for the 95% CIs. Random-effects meta-regression was used to explore categorical or continuous covariates for residual between-study heterogeneity. Additional information is given in the Supplementary Materials. Prespecified covariates included aneurysm size and exposure to prior SAH. Additional covariates included aneurysm shape and anatomical location. Residual heterogeneity was identified using the I2 statistic. p-values were two-sided with values <0.05 that are considered as statistically significant. Statistical analyses and graphical output were performed in R (version 3.6.3) using the following packages: dmetar (14), meta (15), and metafor (16).
Prespecified sensitivity analysis was performed using size thresholds of ≤7, ≤5, and ≤3 mm. Additional post-hoc sensitivity analyses were performed including outlier studies, for high-quality studies, and using the leave-one-out method to confirm that the pooled rupture proportion was not driven by a single study. Reporting bias and small-study effects were examined by visual assessment of a funnel plot, calculation of Egger's regression intercept, and examination of the random-effects and fixed-effects estimates (17).
We identified 21,753 citations through database searches. After removing duplicates, we screened 12,265 citations for eligibility (Figure 1). After full-text review of 270 studies judged to be potentially eligible, we included 32 studies. Five study authors contributed additional unpublished data for sensitivity analyses (18–22).
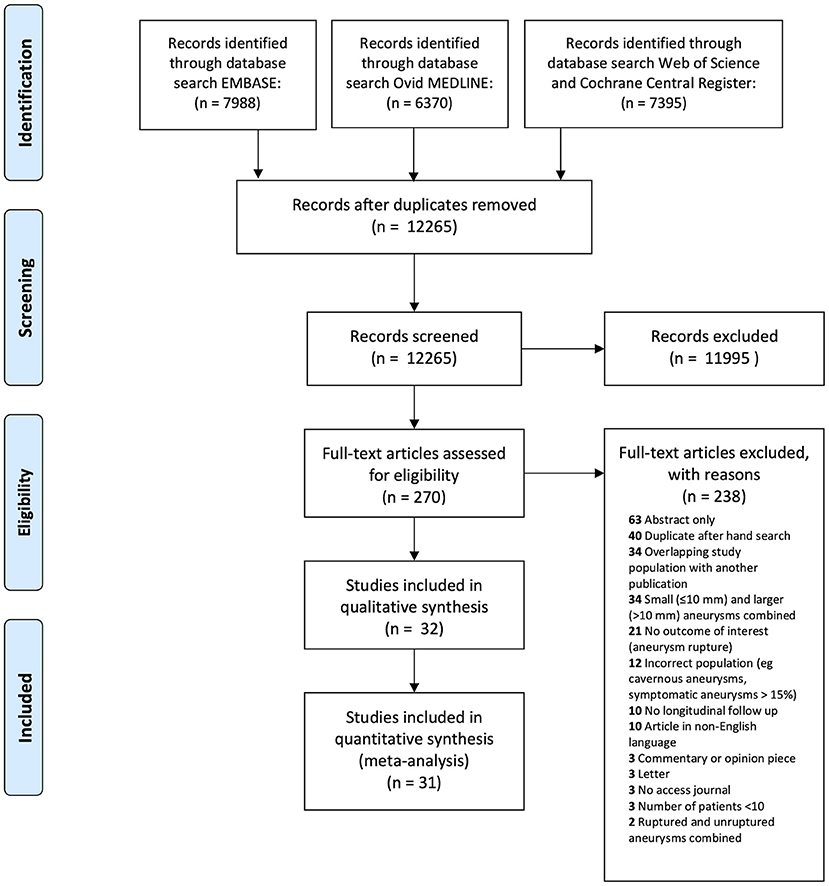
Figure 1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram detailing systematic search.
Baseline study characteristics are given in Table 1. In the 32 studies, source populations were Japan (7 studies) (23–29) United States (7 studies) (20, 22, 30–34), international (5 studies) (3, 35–38), Korea (4 studies) (39–42), Australia (2 studies) (43, 44), and one each from China (45), Netherlands (46), United Kingdom (47), Switzerland (18), Germany (48), Poland (21), and Finland (19). Majority (23 studies) were retrospective and of the prospective studies, two of the three largest studies were from Japan (26, 27). There were no randomized controlled trials. Participants were included in studies from 1956 (19) to 2019 (44). Study publication occurred between 1995 and 2020, with majority (21 studies) published in the last decade.
Across the 32 studies, a total of 12,214 participants with 16,615 UIAs were managed without repair across all the size strata. Of these, 13,966 were ≤10 mm UIAs with mean follow-up of 4.3 years (range 1.4–21 years). Overall, 70.8% of participants were female; mean age was 58.3 ± 7.1 years. The mean proportion of included patients included with prior SAH was 14.5% (range 0–92.3%).
Twelve studies were judged of the good AHRQ standard with lower risk of bias (Table 2). Only one study scored three stars in the outcome domains having complete follow-up (19).
Preliminary data synthesis included 32 studies with 229 rupture events in 13,966 ≤10 mm UIAs over a mean of 4.3 years with significant heterogeneity (I2 = 85%) (Supplementary Figure 1). Outlier analysis identified an outlier (19), with a study rupture proportion of 18.7% (95% CI 13.5–25.3) compared to the pooled proportion of 1.1% (95% CI 0.7–1.7). With leave-one-out analysis, this was the only study that contributed greatly to heterogeneity, with I2 reduction from 85 to 53% (Supplementary Figure 2). Further outlier and influence diagnostics also confirmed these findings (Supplementary Figures 3, 4).
Examination of the outlier study (19) revealed underlying clinical and methodological diversity responsible for between-study heterogeneity. All the patients in this study were managed without aneurysm repair due to lack of treatment availability. In the remaining 31 studies, treatment was considered and a clinical decision made to manage the patient without aneurysm repair. In addition, the outlier study had the longest follow-up (21 vs. mean 3.7 years), the highest proportion of exposure to prior SAH (92.3 vs. mean 11.9%) and an earlier recruitment period (1956–1978 vs. median midpoint 2006). A post-hoc decision was made to exclude this outlier study (19) from the main analysis because of lack of availability of aneurysm repair at the time of the study and to appropriately address heterogeneity (13).
The meta-analytic proportion of ruptures without aneurysm repair was synthesized across the remaining 31 studies and included 198 rupture events in 13,800 ≤10 mm UIAs (Figure 2). Aneurysm rupture occurred in 1.1% of ≤10 mm UIAs (95% CI 0.8–1.5; I2 = 53%) over mean 3.7 years of follow-up.
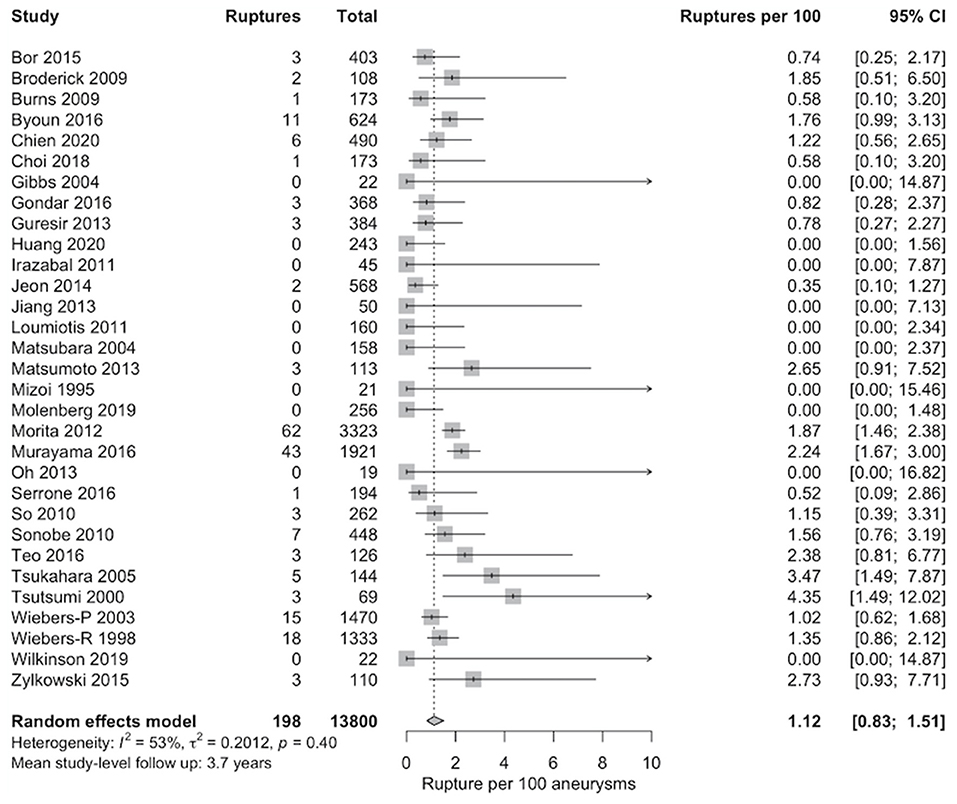
Figure 2. Pooled proportion of rupture per 100 unruptured intracranial aneurysms (UIAs) ≤10 mm managed without aneurysm repair. Random-effects data synthesis was carried out using a random intercept logistic regression model. The Wilson procedure was utilized for calculation of the 95% CIs.
Meta-regression included the following study-level covariates: proportion of patients with exposure to prior SAH, proportion with aneurysm size ≤5 mm, proportion with multiple aneurysms, proportion with irregular aneurysms, proportion with posterior circulation aneurysms, proportion with anterior circulation aneurysms, proportion with anterior communicating artery or anterior cerebral aneurysms, mean age, source population categorized as Japanese or non-Japanese, study type categorized as prospective or retrospective, and length of follow-up.
In subgroup analysis according to whether or not the source population was Japanese, a larger proportion of ruptures was identified in Japanese compared to non-Japanese populations during follow-up [test for subgroup differences, p <0.001, residual I2 = 0% (95% CI 0.0–19.4%)] (Supplementary Figure 5). However, the mean age in Japanese samples (64.5 years) was greater than in non-Japanese samples (57.4 years, p = 0.001).
Associations between other covariates in the remaining meta-regression analyses including proportion with prior SAH, proportion with ≤5 mm aneurysms, proportion with multiple aneurysms, proportion with irregular aneurysms, proportion with posterior circulation aneurysms, proportion with anterior circulation aneurysms, proportion with anterior communicating artery or anterior cerebral aneurysms, study type, study-level age as a continuous variable, and follow-up time as a continuous variable were not detected (Supplementary Figures 6–15).
In leave-one-out sensitivity analysis, the meta-analytic proportion of ruptures remained consistent with no clinically relevant effect (Supplementary Figure 16). Heterogeneity could be further reduced by exclusion of Murayama et al. (27) (residual I2 = 42%) or Jeon et al. (41) (residual I2 = 44%), but these levels of reduction in heterogeneity were considered unimportant.
Findings were consistent in prespecified sensitivity analyses at all the size thresholds (Supplementary Figures 17–19). For ≤7 mm UIAs managed without repair, data were synthesized across 28 studies, 134 rupture events, and 11,371 UIAs: rupture occurred in 0.9% UIAs (95% CI 0.7–1.3; I2 = 49.1%) over 3.6 years of follow-up. For ≤5 mm UIAs managed without repair, data synthesis occurred across 24 studies, 74 rupture events, and 7,280 UIAs: rupture occurred in 1.0% UIAs (95% CI 0.8–1.3; I2 = 0%) over 3.5 years of follow-up. For ≤3 mm UIAs managed without repair, data synthesis occurred across 18 studies, 10 rupture events, and 1,228 UIAs: rupture occurred in 0.8% UIAs (95% CI 0.4–1.5; I2 = 0%) over 3.8 years of follow-up.
There were also consistent results in post-hoc sensitivity analyses that included the outlier study (Supplementary Figure 20). Notably, in sensitivity analyses limited to high-quality studies, the pooled proportion of rupture was greater, 1.8% (95% CI 1.5–2.0; I2 = 0%) over 3.9 years (Supplementary Figure 21).
Funnel plot distribution was asymmetrical on visual assessment and confirmed on Egger's test of the intercept (Supplementary Figure 22). Multiple possible sources of asymmetry other than random chance were identifiable. These include non-reporting bias, selective reporting bias, small-study effects, and residual heterogeneity. The presence of small-study effects was investigated with additional post-hoc sensitivity analysis considering a fixed-effects model estimate with no clinically relevant effect on the meta-analytic proportion of ruptures (Supplementary Figure 23).
In this meta-analysis of 31 studies with 13,800 UIAs 10 mm and less in size managed conservatively without repair, 0.8 to 1.5% UIAs ruptured over 3.7 years. This is an evidence-based clinically relevant pooled risk estimate with consideration of the level of precision over the observed follow-up time. Importantly, the risk estimates were consistent across studies when stratified by size for 2–5 mm UIAs. In higher quality studies, a greater proportion (1.5 to 2.0%) of ruptures were identified over a similar time period, indicating that inclusion of studies with higher risk of bias leads to underestimation of the proportion of rupture.
In addition, in our analysis of over 1,200 ≤3 mm UIAs managed conservatively without repair, we have found a rupture risk of between 0.4 and 1.5% over 3.8 years. This is similar to 0.8 to 1.3% rupture risk over 3.5 years for over 7,000 ≤5 mm UIAs. These findings are in contrast to the conclusions of ISUIA that the 5-year cumulative rupture rate of anterior circulation aneurysms <7 mm was 0% (3) and the prior systematic review and narrative synthesis (8), which analyzed 7 studies and concluded that the estimated annualized rupture rate was 0% for ≤3 mm UIAs. Subgroup analysis in this ≤3 mm UIA cohort is limited due to lack of reporting of aneurysm-related characteristics such as morphology in all the included studies. However, anatomical location for ≤3 mm ruptured aneurysm was reported for 5 of ten patients. Four ≤3 mm UIAs that ruptured were in the anterior circulation, with 3 at the anterior communicating artery or distal anterior cerebral artery. These findings are concordant with expert physician experience and recent literature that <5 mm UIAs represent a large proportion of all the ruptured aneurysms (49).
For ≤10 mm UIAs managed conservatively without repair, we did not identify anatomical location in the anterior or posterior circulation or involving the anterior communicating artery or anterior cerebral artery as sources of heterogeneity modifying the rupture risk. Majority of UIAs included were 2–5 mm and these findings are similar to the prior prospective Small Unruptured Intracranial Aneurysm Verification Study that was limited to < 5 mm UIAs (28). A minority of studies reported data on aneurysm shape and our aggregate data meta-analysis did not identify irregular aneurysm shape as a source of heterogeneity. Individual participant data meta-analysis would reduce heterogeneity and increase the statistical power to better explore these associations of rupture risk with aneurysm shape and anatomical location. This is particularly important for expert physicians to consider, as irregular UIAs and anterior communicating artery and posterior communicating artery location SIUAs were associated with a higher risk of rupture compared to regular UIA shape and alternate anatomical locations in a large prospective cohort (4).
Compared to the prior systematic review, we have synthesized a meta-analytic rupture risk estimate and explored heterogeneity. To improve clinical applicability compared to the prior systematic review, studies with a high proportion of cavernous UIAs (50, 51) were excluded from this study, since they are known to have negligible risk of SAH. In addition, our broader search strategy design yielded 15 additional studies (20, 22, 30–34, 37, 38, 40, 42–46), a third of which have published (20, 22, 40, 44, 46) since the prior systematic review.
This primary study outcome of a cumulative incidence of UIA rupture over the included follow-up time period is a pragmatic method to communicate rupture risk for both the physicians and patients. This is an alternate approach to extrapolating an annualized rupture rate (8, 26) over the remaining lifetime when considering risk and benefit of UIA repair (5). Extrapolation of an annualized rupture rate is unlikely to be accurate due to multiple assumptions. First, extrapolation assumes a constant rupture rate of UIAs, which is unlikely to be plausible, since UIA growth or morphological change over time is associated with rupture (52, 53) and the risk of rupture may decrease after a certain follow-up time within the lifetime (19). Moreover, additional competing risks such as death from causes other than SAH need to be considered (5) and appropriate external data sources to assess validity of such extrapolation are lacking (54).
Overall, our results are considered applicable to pooled estimates of rupture risk for 2–5 mm UIAs managed conservatively without repair over mean of 3.7 years. This is based on the characteristics of the included cohorts: UIAs included were mostly ≤5 mm, five largest studies did not include UIAs <2 mm, and majority of studies had <5 years of follow-up. This is a clinically relevant population, since many patients with 2–5 mm UIAs are usually considered for follow-up without repair (5).
To better understand rupture risk beyond the mean of 3.7 years, additional long-term data are required. There remains only one almost lifelong prospective follow-up study of rupture risk not subjected to treatment selection bias (19). However, outcomes from this long-term follow-up cohort are not generalizable to patients with incidental UIAs identified by neuroimaging today. Only 5 of 142 patients included in this long-term follow-up cohort harbored an incidental UIA and the comorbidity profile (70% smokers, 36% hypertensive, and 21% alcohol abuse) reflects the recruitment period (1956 to 1978) in Finland at that time (55).
The main limitation of our systematic review and meta-analysis is the utilization of aggregate data and, thus, adjustment could not be made for individual patient-level factors and aneurysm-level factors, which would only be possible in an individual patient-level meta-analysis.
In addition to UIA anatomical location and shape, there are additional patient-level variables and aneurysm-level variables that would be useful to explore in future studies including smoking status, hypertension, and systolic blood pressure or aneurysm morphological factors such as aspect ratio. Regardless, this study results remain valid, since aneurysm size is a key factor consistently associated with rupture risk (6) and addition of patient-level variables and aneurysm-level variables would help to improve the precision of risk prediction within the upper and lower limits of the 95% CIs that we have already identified.
In addition, functional outcome and mortality after rupture could not be determined due to inconsistent and non-reporting of clinical outcomes. The pooled risk estimate over time was limited by case follow-up in the individual study cohorts. A third of included studies introduced bias due to inadequate follow-up identified during quality assessment.
These limitations were reduced by inclusion of a large number of studies, large number of rupture events, and careful categorization of UIAs.
Our meta-analysis demonstrates that for every 1,000 UIAs that are ≤10 mm in size and selected for conservative management without repair, between 8 and 15 UIAs are estimated to rupture over 3.7 years. Pooled rupture risk estimates stratified by UIA size are consistent and clinically applicable for 2–5 mm UIAs. This is an evidence-based pragmatic method to communicate rupture risk for both the physicians and patients. To better understand and individualize long-term UIA rupture risk with greater precision, additional UIA follow-up data are required.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
RC and TP contributed to the conception and design of the study. RC carried out the systematic search. Data abstraction was performed independently by RC and JM. NC independently checked the data abstraction. NC and L-AS independently carried out the quality and bias assessments. Statistical analysis was performed by RC and TP. RC completed the first draft of the manuscript with further additions by LL and TP. All authors contributed to data interpretation and subsequent revisions and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.743023/full#supplementary-material
1. Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. (2009) 8:635–42. doi: 10.1016/S1474-4422(09)70126-7
2. Wong GK, Lam S, Ngai K, Wong A, Mok V, Poon WS, et al. Evaluation of cognitive impairment by the Montreal cognitive assessment in patients with aneurysmal subarachnoid haemorrhage: prevalence, risk factors and correlations with 3 month outcomes. J Neurol Neurosurg Psychiatry. (2012) 83:1112–7. doi: 10.1136/jnnp-2012-302217
3. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
4. Investigators UJ, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. (2012) 366:2474–82. doi: 10.1056/NEJMoa1113260
5. Kimura T, Ochiai C, Kawai K, Morita A, Saito N. How definitive treatment affects the rupture rate of unruptured cerebral aneurysms: a competing risk survival analysis. J Neurosurg (2019):1–6. doi: 10.3171/2018.11.JNS181781
6. Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. (2014) 13:59–66. doi: 10.1016/S1474-4422(13)70263-1
7. Williams LN, Brown RD. Management of unruptured intracranial aneurysms. Neurol Clin Pract. (2013) 3:99–108. doi: 10.1212/CPJ.0b013e31828d9f6b
8. Malhotra A, Wu X, Forman HP, Grossetta Nardini HK, Matouk CC, Gandhi D, et al. Growth and rupture risk of small unruptured intracranial aneurysms: a systematic review. Ann Intern Med. (2017) 167:26–33. doi: 10.7326/M17-0246
9. Wells GSB, O'Connell, D,. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-analyses. (2019). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.doc.
10. Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. methods guide for effectiveness and comparative effectiveness reviews. AHRQ Methods for Effective Health Care Rockville (MD). (2012).
11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
13. Deeks JJHJ, Altman DG. “Chapter 10: Analysing data and undertaking meta-analyses,” In: Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor. Cochrane Handbook for Systematic Reviews of Interventions version 60 (updated July 2019). Cochrane (2019).
14. Harrer M CP, Furukawa, T, Ebert, DD,. dmetar: Companion R Package For The Guide 'Doing Meta-Analysis in R'. (2019) Available online at: http://dmetarprotectlaborg
15. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
16. Retio WV. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
18. Gondar R, Gautschi OP, Cuony J, Perren F, Jagersberg M, Corniola MV, et al. Unruptured intracranial aneurysm follow-up and treatment after morphological change is safe: observational study and systematic review. J Neurol Neurosurg Psychiatry. (2016) 87:1277–82. doi: 10.1136/jnnp-2016-313584
19. Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. (2013) 44:2414–21. doi: 10.1161/STROKEAHA.113.001838
20. Wilkinson DA, Heung M, Deol A, Chaudhary N, Gemmete JJ, Thompson BG, et al. Cerebral aneurysms in autosomal dominant polycystic kidney disease: a comparison of management approaches. Neurosurgery. (2018) 27:27. doi: 10.1093/neuros/nyy336
21. Zylkowski J, Kunert P, Jaworski M, Rosiak G, Marchel A, Rowinski O. Changes of size and shape of small, unruptured intracranial aneurysms in repeated computed tomography angiography studies. Wideochirurgia i Inne Techniki Maloinwazyjne. (2015) 10:178–88. doi: 10.5114/wiitm.2015.52707
22. Chien A, Callender RA, Yokota H, Salamon N, Colby GP, Wang AC, et al. Unruptured intracranial aneurysm growth trajectory: occurrence and rate of enlargement in 520 longitudinally followed cases. J Neurosurg. (2020) 20:1–11. doi: 10.3171/2018.11.JNS181814
23. Matsubara S, Hadeishi H, Suzuki A, Yasui N, Nishimura H. Incidence and risk factors for the growth of unruptured cerebral aneurysms: observation using serial computerized tomography angiography. J Neurosurg. (2004) 101:908–14. doi: 10.3171/jns.2004.101.6.0908
24. Matsumoto K, Oshino S, Sasaki M, Tsuruzono K, Taketsuna S, Yoshimine T. Incidence of growth and rupture of unruptured intracranial aneurysms followed by serial MRA. Acta Neurochir (Wien). (2013) 155:211–6. doi: 10.1007/s00701-012-1566-z
25. Mizoi K, Yoshimoto T, Nagamine Y, Kayama T, Koshu K. How to treat incidental cerebral aneurysms: a review of 139 consecutive cases. Surg Neurol. (1995) 44:114–20. doi: 10.1016/0090-3019(95)00035-6
26. Morita A, Teramoto A, Ucas Japan I. The natural course of unruptured cerebral aneurysms: natural course analysis of the unruptured cerebral aneurysm study in Japan. J Neurosurg. (2012) 117:A386-A.
27. Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I, et al. Risk Analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke. (2016) 47:365–71. doi: 10.1161/STROKEAHA.115.010698
28. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. (2010) 41:1969–77. doi: 10.1161/STROKEAHA.110.585059
29. Tsutsumi K, Ueki K, Morita A, Kirino T. Risk of rupture from incidental cerebral aneurysms. J Neurosurg. (2000) 93(4):550-3. doi: 10.3171/jns.2000.93.4.0550
30. Burns JD, Huston J. 3rd, Layton KF, Piepgras DG, Brown RD. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke. (2009) 40:406–11. doi: 10.1161/STROKEAHA.108.519165
31. Gibbs GF, Huston J, Qian Q, Kubly V, Harris PC, Brown RD, et al. Follow-up of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int. (2004) 65:1621–7. doi: 10.1111/j.1523-1755.2004.00572.x
32. Irazabal MV, Huston J. 3rd, Kubly V, Rossetti S, Sundsbak JL, Hogan MC, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. (2011) 6:1274–85. doi: 10.2215/CJN.09731110
33. Loumiotis I, Brown RD Jr, Vine R, Cloft HJ, Kallmes DF, Lanzino G. Small (<10-mm) incidentally found intracranial aneurysms, Part 2: treatment recommendations, natural history, complications, and short-term outcome in 212 consecutive patients. Neurosurg Focus. (2011) 31:E4. doi: 10.3171/2011.9.FOCUS11237
34. Serrone JC, Tackla RD, Gozal YM, Hanseman DJ, Gogela SL, Vuong SM, et al. Aneurysm growth and de novo aneurysms during aneurysm surveillance. J Neurosurg. (2016) 125:1374–82. doi: 10.3171/2015.12.JNS151552
35. Bor AS, Tiel Groenestege AT. terBrugge KG, Agid R, Velthuis BK, Rinkel GJ, et al. Clinical, radiological, and flow-related risk factors for growth of untreated, unruptured intracranial aneurysms. Stroke. (2015) 46:42–8. doi: 10.1161/STROKEAHA.114.005963
36. Broderick J, Hornung R, Sauerbeck L, Huston J, Woo D, Connolly ES, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. (2009) 40:e136. doi: 10.1161/STROKEAHA.108.542571
37. Tsukahara T, Murakami N, Sakurai Y, Yonekura M, Takahashi T, Inoue T, et al. Treatment of unruptured cerebral aneurysms; a multi-center study at Japanese national hospitals. Acta Neurochir Suppl. (2005) 94:77–85. doi: 10.1007/3-211-27911-3_12
38. Wiebers D, Whisnant J, Forbes G, Meissner I, Brown R, Piepgras D, et al. Unruptured intracranial aneurysms - Risk of rupture and risks of surgical intervention. New Engl J Med. (1998) 339:1725–33. doi: 10.1056/NEJM199812103392401
39. Byoun HS, Huh W, Oh CW, Bang JS, Hwang G, Kwon OK. Natural history of unruptured intracranial aneurysms : a retrospective single center analysis. J Korean Neurosurg Soc. (2016) 59:11–6. doi: 10.3340/jkns.2016.59.1.11
40. Choi HH, Cho YD, Jeon JP, Yoo DH, Moon J, Lee J, et al. Growth of Untreated unruptured small-sized aneurysms (7mm): incidence and related factors. Clin Neuroradiol. (2018) 28:183–9. doi: 10.1007/s00062-017-0559-y
41. Jeon JS, Ahn JH, Huh W, Son YJ, Bang JS, Kang HS, et al. A retrospective analysis on the natural history of incidental small paraclinoid unruptured aneurysm. J Neurol Neurosurg Psychiatry. (2014) 85:289–94. doi: 10.1136/jnnp-2013-305019
42. Oh YS, Shon YM, Kim BS, Cho AH. Long-term follow-up of incidental intracranial aneurysms in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:329–33. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.011
43. So TY, Dowling R, Mitchell PJ, Laidlaw J, Yan B. Risk of growth in unruptured intracranial aneurysms: a retrospective analysis. J Clin Neurosci. (2010) 17:29–33. doi: 10.1016/j.jocn.2009.04.010
44. Huang H, O'Neill AH, Chandra RV, Lai LT. Asymptomatic intracranial aneurysms in the elderly: long-term clinical and radiologic follow-up of 193 consecutive patients. World Neurosurg. (2020) 133:e600–e8. doi: 10.1016/j.wneu.2019.09.103
45. Jiang T, Wang P, Qian Y, Zheng X, Xiao L, Yu S. et al. A follow-up study of autosomal dominant polycystic kidney disease with intracranial aneurysms using 30 T three-dimensional time-of-flight magnetic resonance angiography. Eur J Radiol. (2013) 82:1840–5. doi: 10.1016/j.ejrad.2013.01.024
46. Molenberg R, Aalbers MW, Metzemaekers JDM, Mazuri A, Luijckx GJ, Groen RJM, et al. Clinical relevance of short-term follow-up of unruptured intracranial aneurysms. Neurosurg Focus. (2019) 47:E7. doi: 10.3171/2019.4.FOCUS1995
47. Teo M, St George EJ. Radiologic surveillance of untreated unruptured intracranial aneurysms: a single surgeon's experience. World Neurosurg. (2016) 90:20–8. doi: 10.1016/j.wneu.2016.02.008
48. Guresir E, Vatter H, Schuss P, Platz J, Konczalla J, de Rochement Rdu M, et al. Natural history of small unruptured anterior circulation aneurysms: a prospective cohort study. Stroke. (2013) 44:3027–31. doi: 10.1161/STROKEAHA.113.001107
49. Bender MT, Wendt H, Monarch T, Beaty N, Lin LM, Huang J, et al. Small aneurysms account for the majority and increasing percentage of aneurysmal subarachnoid hemorrhage: a 25-year, single institution study. Neurosurgery. (2018) 83:692–9. doi: 10.1093/neuros/nyx484
50. Inagawa T. Follow-up study of unruptured aneurysms arising from the C3 and C4 segments of the internal carotid artery. Surg Neurol. (1991) 36:99–105. doi: 10.1016/0090-3019(91)90225-X
51. Thien A, See AA, Ang SY, Primalani NK, Lim MJ, Ng YP, et al. Prevalence of Asymptomatic Unruptured Intracranial Aneurysms in a Southeast Asian Population. World Neurosurg. (2017) 97:326–32. doi: 10.1016/j.wneu.2016.09.118
52. Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ, Murad MH, Wang Z, et al. Risk Factors for Growth of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. (2016) 37:615–20. doi: 10.3174/ajnr.A4575
53. Juvela S. Growth and rupture of unruptured intracranial aneurysms. J Neurosurg. (2019) 131:843–51. doi: 10.3171/2018.4.JNS18687
54. Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. (2013) 33:743–54. doi: 10.1177/0272989X12472398
Keywords: evidence based medicine (EBM), Systematic Reviews and Meta-Analyses, subarachnoid hemorrhage, cerebral aneurysm, intracranial aneurysm
Citation: Chandra RV, Maingard J, Slater L-A, Cheung NK, Lai LT, Gall SL, Thrift AG and Phan TG (2022) A Meta-Analysis of Rupture Risk for Intracranial Aneurysms 10 mm or Less in Size Selected for Conservative Management Without Repair. Front. Neurol. 12:743023. doi: 10.3389/fneur.2021.743023
Received: 17 July 2021; Accepted: 28 December 2021;
Published: 17 February 2022.
Edited by:
Jean-Marc Olivot, Centre Hospitalier Universitaire de Toulouse, FranceReviewed by:
Athanasios Petridis, University Hospital of Düsseldorf, GermanyCopyright © 2022 Chandra, Maingard, Slater, Cheung, Lai, Gall, Thrift and Phan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronil V. Chandra, cm9uaWwuY2hhbmRyYUBtb25hc2guZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.