
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 28 October 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.742899
This article is part of the Research Topic Clinical Applications of Susceptibility Weighted Imaging in Cerebrovascular Diseases View all 4 articles
Objective: This investigation aimed at studying the prevalence of cerebral microbleeds (CMBs), including risk factors and the correlation of CMBs to ischemic stroke (IS) patient end results.
Methods: Four hundred and fifty-nine acute IS cases were recruited between April 2014 and December 2016. Cerebral microbleeds were analyzed using susceptibility-weighted imaging (SWI) brain MRI scan. The enrolled patients with acute IS were followed up for 12–24 months, with a median follow-up time of 19 months. The follow-up endpoint events including recurrent ischemic stroke (RIS), intracranial hemorrhage (ICH), transient ischemic attack (TIA), mortality, and cardiovascular events. The associations between vascular risk factors and CMBs in IS patients were analyzed using univariate and multivariate logistic regression analysis. Cox regression model was employed for evaluating CMB impact on clinical outcome.
Results: Among 459 enrolled patients, 187 (40.7%) had CMBs and 272 (59.2%) had no CMB. In comparison with patients with no CMBs, age was higher and hypertension was more frequent in patients with CMBs. Multivariate logistic regression analyses revealed age and hypertension were independently associated with the presence of CMBs. Among the patient cohort, 450 cases completed the follow-up. During the follow-up period, 22 (4.9%) of patients developed ICH, 12 (2.7%) developed TIA, 68 (15.1%) developed RIS, cardiovascular events occurred in 20 (4.44%), and 13 (2.89%) cases were mortalities. Compared with patients without CMBs, IS patients with CMBs have an increased prevalence of ICH (p < 0.05). However, no statistically valid variations regarding other outcome incidences between both groups was identified (p > 0.05). The incidence of ICH was elevated in tandem with elevations in number of CMBs. Following adjusting for age, multivariate Cox proportional-hazards regression analysis revealed that CMBs ≥10 were independent predictors of ICH in acute IS patients.
Conclusion: Age and hypertension are independently associated with the presence of CMBs. Intracranial hemorrhage incidence rate was increased with the number of CMBs, and the number of CMBs ≥10 were independent predictors of ICH in acute stroke patients.
The concept of cerebral microbleeds (CMBs) was first proposed in the mid-1990s, and it is caused by the deposition of hemosiderin in the brain caused by the leakage of red blood cells from small blood vessels (1). It is one of the typical imaging manifestations of cerebral small vessel diseases (2). Risk factors for the presence of CMBs remain unclear. Investigations demonstrated that CMBs, caused by varying mechanisms, can occur in any location of the brain (3). Recent systematic reviews demonstrated that increased number of CMBs are linked to exacerbated risks for intracranial hemorrhage/ischemic stroke (ICH/IS), and the presence of CMBs may play a key role in vascular cognitive impairment (4, 5).
Magnetic resonance gradient echo T2-weighted imaging (T2* WI) (GE-T2* WI) is a highly sensitive technique for detecting CMBs, which can detect hemosiderin deposition (of several millimeters in size) within the brain parenchyma. Recently, studies have shown that three-dimensional fast field echo susceptibility-weighted imaging (3D-FFESWI), susceptibility-weighted imaging (SWI) demonstrates superior sensitivity and specificity in detecting CMB regions, in comparison with GE-T2* WI (5, 6). Cerebral microbleed anatomical scoring scale (7) and CMB observation scale (8) are often used to evaluate CMB distribution and size. According to the number of detected CMBs, they are divided into grade 0 (none), grade 1 (CMBs: 1–4), grade 2 (CMBs: 5–9), and grade 3 (CMs ≥10) (9).
It is established that CMBs are widespread in the elderly population (10), being linked to age, hypertension, stroke history, fibrinogen, and blood pressure (11). The incidence of presence of CMBs is increasing with age, and it is obviously correlated with IS (11, 12). There are also reports that CMBs occur in coagulopathy and anticoagulation therapy (13, 14).
Several investigations highlighted that CMBs are linked to exacerbated risks for imminent ICH, particularly patients treated with aspirin (15). On the other hand, CMBs could additionally be highly correlated to recurrence of IS and poor functional prognosis post-acute IS (4). However, there are some inconsistent results. Fiehler et al. (16) showed that the presence of CMBs increased the incidence of post-IS ICH, although ICH was a low-probability event. Another study revealed that the risk of ICH did not increase within aspirin-treated patients, in comparison with IS/CMB positive patients with no aspirin treatments (17). However, Soo et al. (18) reported that IS/CMB positive patients do incur additional risks in developing sICH following antiplatelet aggregation drugs. So whether ischemic stroke (IS) patients with high burden of CMBs receiving aspirin treatment lead to poor outcomes remains controversial.
So in general, within IS patients, high burden of CMBs (CMB number ≥5) tend to predict ICH (9) and IS (19), although the risk/benefit ratio of antiplatelet treatments within IS cases bearing CMBs remains unclear.
Consequently, this study focuses on those IS patients receiving antiplatelet therapy and this investigation consisted of two phases. Initial focus was set on the prevalence and risk factors for the presence of CMBs in IS clinical cases. Second, a prospective cohort study was performed to clarify the IS recurrence rate, ICH incidence rate, together with the incidence rate for other vascular events throughout the follow-up timeframe. Evaluation of risk/benefit ratio for antiplatelet treatments in IS/CMB positive cases was also performed.
Between April 2014 and December 2016, 459 patients with IS were recruited. Patients with IS were validated through brain MRI scans, followed by admittance to the People's Hospital of Deyang City and Second People's Hospital of Deyang City within 48 h following the onset of symptoms. Inclusion criteria: (1) patients with IS confirmed by symptoms and imaging findings; (2) patients who have passed the thrombolytic time window or choose to refuse thrombolytic therapy. Exclusion criteria were as follows: (1) patients contraindicated for MRI scan; (2) brain tumors, arteriovenous malformations, and ruptured hemangioma; (3) thrombolytic therapy; (4) unwillingness to participate in this study. This study was accepted by the hospitals' Ethics Committee. Informed consent was obtained for all patient cases.
Patients with IS were scanned using a 3-T MRI scanner. Cerebral microbleeds were detected using SWI (20). Cerebral microbleeds were classified as aged, silent foci of signal absence/hypo-intensity on SWI, measuring approximately 2–10 mm diameter. According to previous studies, the location of CMB events was scored through employment of the microbleed Anatomical Rating Scale (1, 7), with burden (numbers) stratified as grade 0 (none), grade 1 (1–4), grade 2 (5–9), and grade 3 (≥10).
Demographics (age, sex) and relevant vascular-based risk parameter data were collected, including atrial fibrillation, coronary heart conditions, history of stroke hypertension, smoking, and diabetes mellitus. Fasting blood samples were measured for glucose, total plasma cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and homocysteine blood levels. Hyperlipidemia was deemed to be TC >200 mg/dl, TG >180 mg/dl, or use of lipid-lowering drugs. Stroke severity evaluation was performed by a certified member of the stroke team, using the National Institutes of Health Stroke Scale (NIHSS) once the individual patient was admitted.
Following hospital discharge, all patients were followed up by a research nurse or physician on a quarterly basis, for 12–24 months (median = 19 months). Clinical cases within this study all received standardized medical care according to guidelines for the prevention of stroke in stroke/transient ischemic attack (TIA) patients (21). During the follow-up period, the entire cohort was assessed for multiple clinical outcomes: (1) recurring stroke (ischemic, hemorrhagic, transient TIA); (2) mortality (vascular and nonvascular); (3) cardiovascular events (myocardial infarction, angina). The definition of recurrent stroke required a sudden new neurological deficit fitting the definition of IS or ICH by the physical and imaging examinations.
SPSS V.21.0 software was employed, with continuous variables expressed as mean ± SD. Normality status of numerical variables was assessed through the Kolmogorov–Smirnov test. Normal-distribution variables were compared through single-pair two-tailed independent group t-test. Non-normal-distribution variables were comparatively analyzed through a Mann–Whitney test. Categorical variables were expressed as percentage rates and compared by χ2-test, or (if predicted variable frequencies were minimal) Fisher's exact tests were performed. The clinical data and baseline characteristics for patients with/without CMBs were investigated through univariate analysis methodologies. Multivariate logistic stepwise regression analysis was employed for exploring risk factors of CMBs, and multivariate Cox regression model was employed for evaluating the impact of CMBs on clinical outcome. The variables inputted for multivariate logistic stepwise regression and Cox regression model were variables with a p-value <0.1 (by univariate analysis). Data outcomes of the Cox model were listed as the hazard ratio (HR), with a 95% CI. Survival function estimates for ICH, according to the quantities of CMB (no CMBs, 1–4 CMBs, 5–9 CMBs, ≥10 CMBs), were analyzed through Kaplan–Meier curve/log-rank test analyses. Survival curves were truncated at 24 months. Datasets with a p-value of <0.05 were deemed to be statistically significant.
Of the 459 patients with acute IS, 187 (40.7%) had CMBs and 272 (59.2%) had no CMBs (Table 1). The age was older and hypertension was more frequent in IS patients with CMBs than those without CMBs (Table 1). Multivariate logistic stepwise regression analyses revealed age and hypertension to be independently associated with the presence of CMBs in IS patients.
Stemming from 459 patients, 173 cases were managed using aspirin alone, 3 were managed using clopidogrel therapy alone, and 283 cases were treated with aspirin and clopidogrel within 21 days from onset of symptoms, followed by aspirin thereafter. Almost all patients were treated with aspirin (456 cases−99%).
Stemming from the 459-patient cohort, 9 cases were lost from follow-up due to incorrect patient contact details although 450 cases completed the follow-up. The rate of loss to follow-up was 2.0%.
During the follow-up period, 22 (4.9%) patients developed ICH, 12 (2.7%) patients developed TIA, 68 (15.1%) patients developed recurrent ischemic stroke (RIS), cardiovascular events occurred in 20 (4.44%), and 13 (2.89%) cases led to a mortality due to cardiovascular or cerebrovascular event. Outcomes in patients with/without CMBs are displayed in Table 2. The incidence of ICH was higher in patients with CMBs than those without CMBs [8.70% (16/184) vs. 2.26% (6/266), p = 0.003] (Table 2; Figure 1). Notwithstanding, there were no significant differences in incidence of RIS, cardiovascular events, TIA, and mortality between patients with and without CMBs (Table 2; Figure 1).
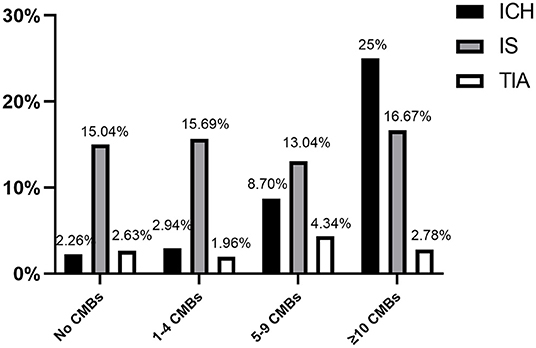
Figure 1. Main study endpoints, separated based on number of CMB events (ICH, intracerebral hemorrhage; IS, ischemic stroke recurrence; TIA, transient ischemic attack).
Outcomes such as ICH, IS, and TIA were stratified according to the numbers of CMBs (i.e., 1–4 CMBs, 5–9 CMBs, and ≥10 CMBs) and patients with no CMBs (Figure 1). Intracranial hemorrhage events had a directly proportional correlation with frequency of the number of CMBs: 2.9% in patients with 1–4 CMBs, 8.7% with 5–9 CMBs, and 25% with ≥10 CMBs. However, the incidence of RIS and TIA did not increase with the number of CMBs (Figure 1).
Univariate Cox proportional-hazards regression showed that age, CMB occurrence, and the CMBs ≥10 were significantly associated with ICH (p < 0.05, Table 3). According to previous research (18) and our study results, age deemed to be linked with CMBs and additional risk for ICH. Multivariate Cox proportional-hazards regression analysis revealed that CMB events ≥10 were independent predictors of ICH post-acute IS, after adjusting for age (Table 3). The Kaplan–Meier curve revealed intimate correlation between the number of CMBs and ICH development risks (Figure 2).
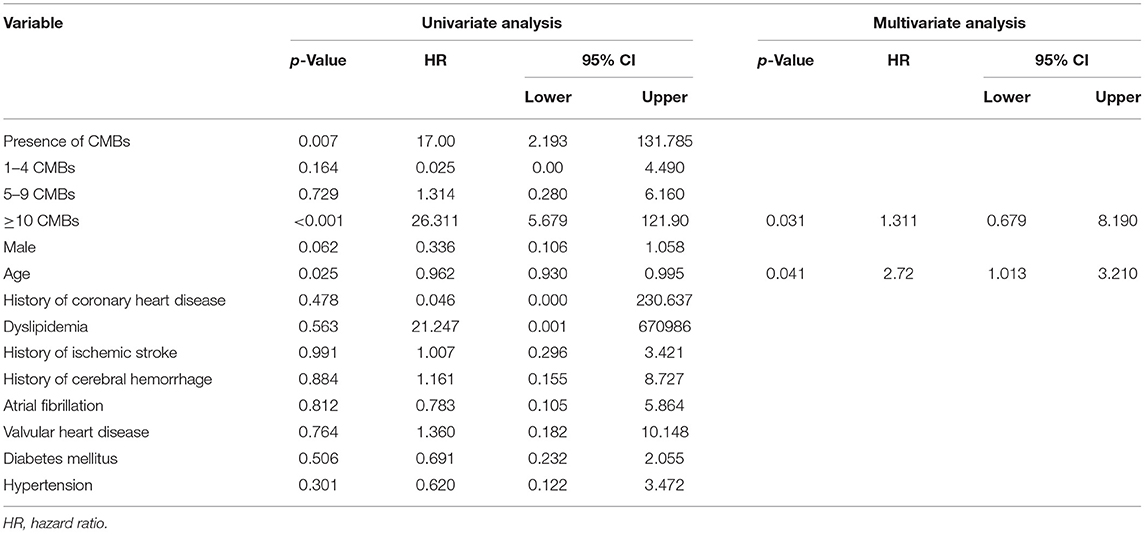
Table 3. Univariate Cox regression analyses for consequent ICH and multivariate Cox regression analyses for ≥10 CMB events in predicting subsequent ICH (adjusted by age).
Univariate Cox proportional-hazards regression revealed that age, smoking, and hypertension were significantly associated with RIS (Table 4). Multiple Cox regression analysis showed that age, smoking, and hypertension were also independent predictors for RIS. However, CMBs were not significantly linked to RIS (Table 4).
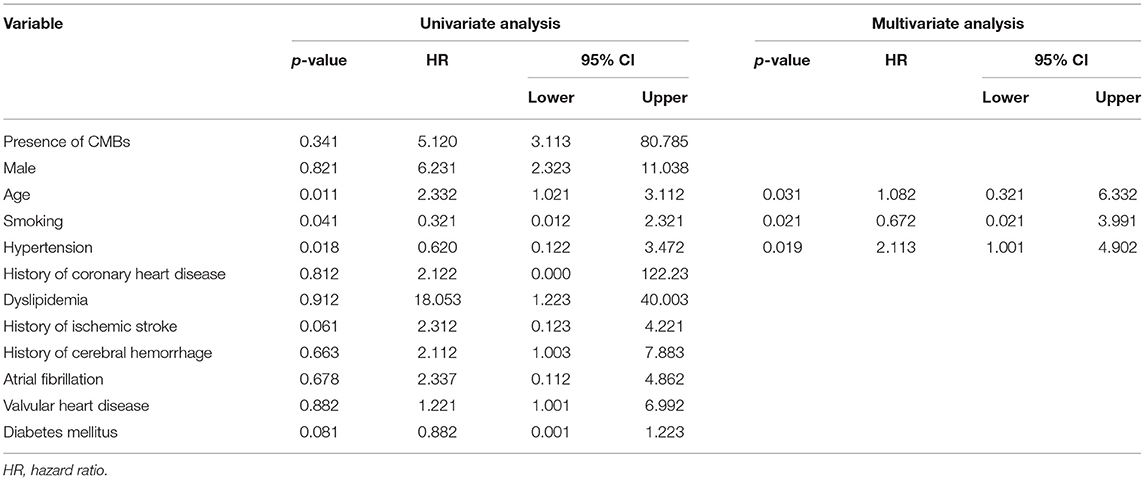
Table 4. Univariate Cox regression analyses and multivariate Cox regression analyses for recurrence of ischemic stroke.
The pathogenesis of occurrence of CMBs is not yet fully understood. Blood–brain barrier damage, endothelial cell dysfunction, neovascularization, apolipoprotein E (apoE) ε4 genotype, and inflammation are intimately correlated to CMB etiology (22, 23). The incidence, location, and number of CMBs are affected by many parameters, including hypertension, diabetes, hypercholesterolemia, smoking, and obesity (11). Such risk factors are highly related to micro-circulatory vascular disease, consequently exacerbating CMB occurrence risks. Presently, research on CMBs are mainly based on the links between cerebral microcirculatory vascular disease and stroke, particularly regarding IS. Poels et al. (12) demonstrated CMB incidence in acute IS patients aged >80 years was as high as 35.7%, which was significantly higher than within patients aged ≤ 80 years. These results were consistent with our current results.
Presently, the prevention and treatment of CMBs is still under exploration. The occurrence of CMBs is closely related to the risk of cardiovascular and cerebrovascular diseases. Therefore, this is crucial to the prevention and treatment of occurrence of CMBs. This investigation revealed that age was higher within IS patients with CMBs. Hypertension was also more prevalent within the CMB positive group. Multivariable logistic regression analysis results demonstrated the occurrence of CMBs to be intimately correlated to age/hypertension. Therefore, for acute IS patients with increased age or hypertension, imaging examinations should be performed in a timely manner for awareness of CMB occurrence.
Lee et al. (9) speculate that the presence of CMBs is highly related to ICH. Also, the presence of CMBs exacerbates ICH incidence within IS patients undergoing thrombolytic therapy (24). However, whether IS patients with high burden of CMBs (CMBs ≥10) receiving aspirin treatment lead to poor outcomes remains controversial. It is known that oral antiplatelet drugs in IS cases could effectively lower the possibility for IS recurrence. However, selected studies have shown that antiplatelet drugs (aspirin alone or the combination of two antiplatelet drugs) increases the incidence of CMBs (25). A systematic review and meta-analysis showed that risk of ICH was higher in IS patients with CMBs who are receiving antiplatelet therapy compared with the patients without CMBs, and the number of CMBs was significantly associated with subsequent ICH after the use of antiplatelet agents, especially when CMBs >5 (26).
In comparison with other antiplatelet drugs such as clopidogrel, cilostazol, and ticlopidine, aspirin has the highest correlation with antiplatelet-associated hemorrhages (27). Also, research showed that there is no difference between the use of aspirin alone or the combination of two antiplatelet drugs for increasing the incidence of CMB-related ICH (28). Regarding IS patients with CMBs, the current guidelines do not provide opinions on the safety of antiplatelet therapy. Lau et al. (15) suggested that antiplatelet drugs cannot be stopped within 1 year of onset for these patients. However, they also pointed out that the risk of ICH may outweigh any benefit thereafter for IS patients with CMBs ≥5. Therefore, whether post-acute phase gradual discontinuation of antiplatelet drugs can bring benefits or otherwise still requires further research.
As shown in this study, in the case of almost all patients (99%) receiving aspirin treatment, 68 (15.11%) patients developed RIS and 22 patients developed subsequent ICH (4.89%). The risk of RIS is higher than the incidence of subsequent ICH during the follow-up period. There was no significant difference in incidence of RIS between patients with and without CMBs. Smoking, hypertension, and age were independent predictors for RIS. CMBs ≥10 were independent predictors of subsequent ICH after adjusting for age. Our current results were consistent with other previous studies, which is that CMBs is a relevant and accurate predictor of hemorrhagic transformations in acute IS (15, 18, 29). Our study also showed that although IS patients with high burden of CMBs have a higher probability of ICH, the recurrence rate of IS is still much higher than the probability of cerebral hemorrhage in all patients regardless of whether they have CMBs, and this result is also consistent with previous studies (30). It is indicating that antiplatelet treatment is still necessary for these patients. However, the ICH occurrence risks were highly exacerbated for IS patients bearing CMBs ≥10, which means that the ICH occurrence risks overwhelmed benefits for these patients treated with antiplatelet drugs. However, further well-designed studies are warranted to replicate this finding.
This study does have a set of limitations. First, this investigation did not include patients on thrombolytic therapy, and we did not know the occurrence of ICH post-thrombolytic therapy in patients with CMBs. According to Fiehler's research (16), IS cases having CMBs tend not to raise the possibility of ICH occurrence post-thrombolysis. However, the study by Tsivgoulis indicated that acute IS patients with CMBs have an increased danger of incurring sICH post-intravenous thrombolysis, in comparison with CMB-free cases, which means that CMBs are being intimately correlated to the possibility of incurring post-thrombolytic ICH. Moreover, they also indicated that high CMB burden (CMB number ≥10) may be included in individual risk stratification scores predicting sICH risk following IVT for AIS (24). So the rapid progression of CMBs post-thrombolysis can be one of the causes of ICH. Second, due to condition requirements, most of our patients were treated with aspirin, and there was no comparison of the prognosis for various antiplatelet drugs. Third, due to the limited sample size and two-center design of this study, our findings must be confirmed with larger, multi-center studies.
Age and hypertension are independently associated with CMBs in IS patients. Intracranial hemorrhage prevalence was exacerbated within patients with the number of CMBs, while CMBs ≥10 were independent predictors of ICH on follow-up assessments.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Subject of Deyang Science and Technology Bureau, China. The patients/participants provided their written informed consent to participate in this study.
C-xX and HX contributed to the drafting. J-pM, TY, and X-yY contributed to the concept and revision of the article. All authors have read and approved the final version to be published.
This study was supported by the Subject of Deyang Science and Technology Bureau, China (grant no. 2016SE030).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all patients, caregivers, students, and institutions who have contributed and collaborated in this study.
CMB, cerebral microbleed; IS, ischemic stroke; SWI, susceptibility-weighted imaging; RIS, recurrent ischemic stroke; ICH, intracranial hemorrhage; TIA, transient ischemic attack; GE-T2* WI, gradient echo T2-weighted imaging; sICH, symptomatic cerebral hemorrhage; NIHSS, National Institutes of Health Stroke Scale; TG, triglycerides; TC, total plasma cholesterol.
1. Greenberg SM, Vernooij MW, Cordonnier C, Salman RA, Edin F, Warach S, et al. Cerebral microbleeds: a field guide to their detection and interpretation. Lancet Neurol. (2009) 8:165–74. doi: 10.1016/S1474-4422(09)70013-4
2. Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke. (2015) 10:469–78. doi: 10.1111/ijs.12466
3. Ding J, Sigurdsson S, Garcia M, Phillips CL, Eiriksdottir G, Gudnason V, et al. Risk factors associated with incident cerebral microbleeds according to location in older people: the age, gene/environment susceptibility (AGES)-Reykjavik study. JAMA Neurol. (2015) 72:682–8. doi: 10.1001/jamaneurol.2015.0174
4. Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis-the Karolinska imaging dementia study. Am J Neuroradiol. (2015) 36:661–6. doi: 10.3174/ajnr.A4176
5. Uetani H, Hirai T, Hashimoto M, Ikeda M, Kitajima M, Sakamoto F, et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. Am J Neuroradiol. (2013) 34:984–9. doi: 10.3174/ajnr.A3332
6. Bian W, Hess CP, Chang SM, Nelson SJ, Lupo JM. Susceptibility-weighted MR imaging of radiation therapy-induced cerebral microbleeds in patients with glioma: a comparison between 3T and 7T. Neuroradiology. (2014) 56:91–6. doi: 10.1007/s00234-013-1297-8
7. Gregoire S, Chauchary U, Jager R, Brown M, Yousry T, Werring D. The microbleed anatomical rating scale (MARS): a new reliable scale with good intra- and inter-observer agreement for mapping brain microbleeds. Stroke. (2009) 40:227. doi: 10.1212/WNL.0b013e3181c34a7d
8. Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CLM, et al. Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. (2009) 40:94–9. doi: 10.1161/STROKEAHA.108.526996
9. Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. (2004) 62:72–6. doi: 10.1212/01.WNL.0000101463.50798.0D
10. Chung CP, Chou KH, Chen WT, Liu LK, Lee WJ, Chen LK, et al. Cerebral microbleeds are associated with physical frailty: a community-based study. Neurobiol Aging. (2016) 44:143–50. doi: 10.1016/j.neurobiolaging.2016.04.025
11. Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy an imaging-pathologic study of concept validation. JAMA Neurol. (2016) 73:994–1001. doi: 10.1001/jamaneurol.2016.0832
12. Poels MMF, Vernooij MW, Ikram MA, Hofman A, Krestin GP, Van Der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the rotterdam scan study. Stroke. (2010). 41:S103–6. doi: 10.1161/STROKEAHA.110.595181
13. Noorbakhsh-Sabet N, Pulakanti VC, Zand R. Uncommon causes of cerebral microbleeds. J Stroke Cerebrovasc Dis. (2017) 26:2043–9. doi: 10.1016/j.jstrokecerebrovasdis.2017.07.012
14. Renard D. Cerebral microbleeds: a magnetic resonance imaging review of common and less common causes. Eur J Neurol. (2018) 25:441–50. doi: 10.1111/ene.13544
15. Lau KK, Lovelock CE, Li L, Simoni M, Gutnikov S, Küker W, et al. Antiplatelet treatment after transient ischemic attack and ischemic stroke in patients with cerebral microbleeds in 2 large cohorts and an updated systematic review. Stroke. (2018) 49:1434–42. doi: 10.1161/STROKEAHA.117.020104
16. Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, et al. Bleeding risk analysis in stroke imaging before thrombolysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke. (2007) 38:2738–44. doi: 10.1161/STROKEAHA.106.480848
17. Park HK, Lee JS, Kim BJ, Park JH, Kim YJ, Yu S, et al. Cilostazol versus aspirin in ischemic stroke with cerebral microbleeds versus prior intracerebral hemorrhage. Int J Stroke. (2020) 2020:1–12. doi: 10.1177/1747493020941273
18. Soo YOY, Yang SR, Lam WWM, Wong A, Fan YH, Leung HHW, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. (2008) 255:1679–86. doi: 10.1007/s00415-008-0967-7
19. Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA. Neurology. (2016) 87:1501–10. doi: 10.1212/WNL.0000000000003183
20. Nandigam RNK, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am J Neuroradiol. (2009) 30:338–43. doi: 10.3174/ajnr.A1355
21. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
22. Schilling S, DeStefano AL, Sachdev PS, Choi SH, Mather KA, DeCarli CD, et al. APOE genotype and MRI markers of cerebrovascular disease. Neurology. (2013) 81:292–300. doi: 10.1212/WNL.0b013e31829bfda4
23. Low A, Mak E, Rowe JB, Markus HS, O'Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. (2019) 53:100916. doi: 10.1016/j.arr.2019.100916
24. Tsivgoulis G, Zand R, Katsanos AH, Turc G, Nolte CH, Jung S, et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden ameta-analysis. JAMA Neurol. (2016) 73:675–83. doi: 10.1001/jamaneurol.2016.0292
25. Darweesh SKL, Leening MJG, Akoudad S, Loth DW, Hofman A, Ikram MA, et al. Clopidogrel use is associated with an increased prevalence of cerebral microbleeds in a stroke-free population: the rotterdam study. J Am Heart Assoc. (2013) 2:1–8. doi: 10.1161/JAHA.113.000359
26. Wang DN, Hou XW, Yang BW, Lin Y, Shi JP, Wang N. Quantity of cerebral microbleeds, antiplatelet therapy, and intracerebral hemorrhage outcomes: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2015) 24:2728–37. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.003
27. Naka H, Nomura E, Kitamura J, Imamura E, Wakabayashi S, Matsumoto M. Antiplatelet therapy as a risk factor for microbleeds in intracerebral hemorrhage patients: analysis using specific antiplatelet agents. J Stroke Cerebrovasc Dis. (2013) 22:834–40. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.001
28. Wang Z, Xu C, Wang P, Wang Y, Xin H. Combined clopidogrel–aspirin treatment for high risk TIA or minor stroke does not increase cerebral microbleeds. Neurol Res. (2015) 37:993–7. doi: 10.1179/1743132815Y.0000000087
29. Dar NZ, Ain QU, Nazir R, Ahmad A. Cerebral microbleeds in an acute ischemic stroke as a predictor of hemorrhagic transformation. Cureus. (2018). 10:e3308. doi: 10.7759/cureus.3308
Keywords: cerebral microbleeds, ischemic stroke, intracranial hemorrhage, susceptibility-weighted imaging, aspirin
Citation: Xu C-x, Xu H, Yi T, Yi X-y and Ma J-p (2021) Cerebral Microbleed Burden in Ischemic Stroke Patients on Aspirin: Prospective Cohort of Intracranial Hemorrhage. Front. Neurol. 12:742899. doi: 10.3389/fneur.2021.742899
Received: 19 July 2021; Accepted: 28 September 2021;
Published: 28 October 2021.
Edited by:
Anna Rosell, Vall d'Hebron Research Institute (VHIR), SpainReviewed by:
David Z. Rose, University of South Florida, United StatesCopyright © 2021 Xu, Xu, Yi, Yi and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-peng Ma, bWFqdW5wZW5nbGpAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.