
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 16 September 2021
Sec. Neuroinfectious Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.738405
This article is part of the Research TopicNeuro-Covid: Neuropsychological Implications of the PandemicView all 9 articles
Background and Objectives: Neurological and psychiatric symptoms are frequent in patients with post-COVID-19 syndrome (PCS). Here, we report on the clinical presentation of the first 100 patients who presented to our PCS Neurology outpatient clinic ≥12 weeks after the acute infection with SARS-CoV-2. To date, PCS is only defined by temporal connection to SARS-CoV-2 infection. Identification of clinical phenotypes and subgroups of PCS is urgently needed.
Design: We assessed clinical data of our first 100 ambulatory patients regarding clinical presentations; self-questionnaires focusing on daytime sleepiness, mood, and fatigue; and a screening assessment for detecting cognitive impairment.
Results: A total of 89% of the patients presenting to the Neurology outpatient clinic had an initially mild course of COVID-19 and had not been hospitalized. The majority of the patients were female (67 vs. 33% male). The most frequent symptom reported was cognitive impairment (72%). There were 30% of patients who reported cognitive deficits and scored below 26 points on the Montreal Cognitive Assessment Scale. Fatigue (67%), headache (36%), and persisting hyposmia (36%) were also frequently reported; 5.5% of all patients showed signs of severe depression.
Discussion: To our knowledge, this is the first report of patient data of a PCS Neurology outpatient clinic. Neurological sequelae also exist for more than 3 months after mainly mild SARS-CoV-2 acute infections. The reported symptoms are in accordance with recently published data of hospitalized patients.
Signs and symptoms that develop during or after an infection with SARS-CoV-2 and continue for more than 12 weeks and are not explained by an alternative diagnosis are defined as post-COVID-19 syndrome (PCS) (1). Recent publications report a number of neurological and psychiatric symptoms including fatigue, cognitive impairment, insomnia, myalgia, headache, vertigo, anxiety, and depression (2–5). The most frequently reported PCS symptoms, analyzed in a Swedish cohort study in health care professionals with mild SARS-CoV-2 acute infection, were of neurological nature and led to continuous functional impairment in work, social, and home life (6). A retrospective cohort study showed that neurological and psychiatric disorders were more common after COVID-19 compared to other respiratory tract infections and influenza (7). Here, we present clinical data of ambulatory patients from a specialized PCS outpatient clinic for patients with neurological manifestations.
In September 2020, we established a PCS outpatient clinic focusing on neurological symptoms in the aftermath of COVID-19. Patients presenting to our outpatient clinic did not suffer from neurological diseases prior to the infection with SARS-CoV-2 and were referred from general practitioners or neurologists working in the outpatient setting. Between September 2020 and April 2021, patients admitted to our outpatient clinic were interviewed and examined by a physician who specialized in Neurology. All patients had a confirmed diagnosis of SARS-Cov-2 infection (either positive PCR testing for SARS-CoV-2-RNA during the acute infection or positive testing for SARS-CoV-2 antibodies). Self-questionnaires for further assessment of daytime sleepiness (Epworth Sleepiness Scale, ESS), mood (Beck Depression Inventory Version I, BDI), and fatigue (Fatigue Severity Scale, FSS) were applied. The Montreal Cognitive Assessment Scale (MoCA) was used to detect cognitive impairment. Tables 1, 2 show an overview of patient characteristics, clinical symptoms, and results of self-questionnaires and cognitive testing of our cohort.
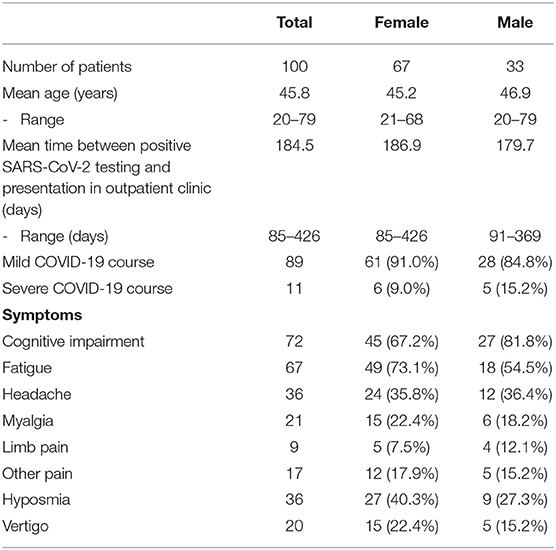
Table 1. Characteristics of patients presenting to the neurological Post-COVID-19 outpatient clinic.
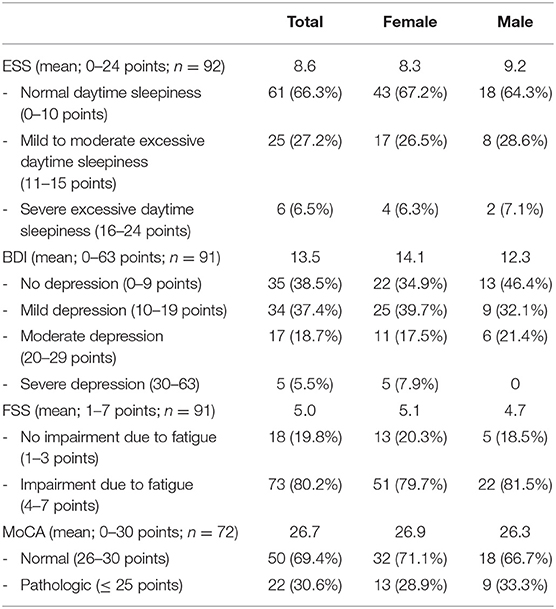
Table 2. Evaluation of self-questionnaires and results of MoCA of patients presenting to the neurological post-COVID-19 outpatient clinic.
The mean age was 45.8 years (range: 20–79 years). Time between positive testing for SARS-CoV-2 and first presentation in our outpatient clinic varied from 85 to 426 days (mean, 184.5 days). Considerably more female (67%) than male (33%) patients were referred. The majority of the patients (89%) reported a mild course of acute SARS-CoV-2 infection with no need of oxygen supplementation or admission to a hospital. The most frequent symptoms were cognitive impairment (72%), fatigue (67%), headache (36%), and persisting hyposmia (36%). Further symptoms were myalgia (21%), vertigo (20%), and other pain syndromes (17%) including limb pain (9%). A total of 30% of patients with cognitive deficits showed pathologic MoCA scores (≤ 25/30 points). In 6.5%, the ESS signified a severe excessive daytime sleepiness. Female patients tended to have a higher BDI score than male patients (14.1 vs. 12.3 points); 5.5% of the patients had severe depression, and 38.5% showed scores compatible with no depression. There were 80.2% of patients who reported significant impairment of their daily life due to fatigue measured by the FSS.
We report data of patients who presented to our PCS Neurology outpatient clinic due to persisting neurological symptoms ≥12 weeks after the acute infection with SARS-CoV-2. To our knowledge, this is the first report of patient data of a PCS Neurology outpatient clinic. The percentage of patients who had an initial mild clinical course of the disease was slightly higher in comparison to the overall reported percentage of 81% mild disease courses in COVID-19 (8). Interestingly and according to recently published data of hospitalized patients, residual neurological complaints expressed by our cohort predominantly involved cognitive impairment, fatigue, and headache (2–5, 9). Thirty percent of patients reporting cognitive deficits showed pathologic MoCA scores resulting in further diagnostics including imaging, cerebrospinal fluid diagnostic, and neuropsychological assessment (10, 11). These additional data need evaluation in a sufficient number of patients with PCS to draw further conclusions.
Pathophysiological mechanisms of the described neurological deficits remain largely unknown for PCS. To date, biomarkers and imaging findings are not identified. For severe COVID-19 courses, inflammation, hypoxemia, and vascular mechanisms might contribute to etiopathogenesis. Additionally, an autoantibody production triggered by SARS-CoV-2 is postulated (12, 13). While male sex is proven to be a risk factor for severe COVID-19 acute disease course, women seem to be more vulnerable to develop PCS. This finding is in line with previously published data (14). This might be due to reporting bias, since women tend to seek medical advice more commonly. Another possible explanation is that women are more frequently prone to autoimmune disorders (15).
Anxiety and depression have been reported in patients with PCS; however, severe depression was only presented in 5% in our cohort. Overall prevalence of depressive symptoms in the German population is reported to be 8.1% compared to 61.5% in our cohort (16). The etiology of these neuropsychiatric symptoms needs further evaluation, but might be partially attributed to reactive mood alterations suitable for the prolonged impairment. As previously reported, fatigue in PCS has a high impact on the daily life of patients (14). Prevalence of fatigue in our patients was over four times higher than in a reported cohort of healthy individuals (18%) and higher than in a cohort of patients with multiple sclerosis (69 vs. 80.2%) (17). Distinguishing fatigue from excessive daytime sleepiness, the latter was only present in one third of our patients. This is a markedly higher prevalence than that reported of the general populations of several countries (18–21). Pathophysiological mechanisms of fatigue and sleep disorders including daytime sleepiness are under current investigation. Mannose-binding lectin deficiency and elevated interleukin-8 (IL-8) levels have been reported in patients suffering from fatigue and might serve as potential biomarkers (22).
We see a high demand of neurological PCS outpatient care, and rising numbers of PCS patients are to be expected. For the best medical care of these patients, thoroughly assessed data of patients in the outpatient setting are needed. Close interdisciplinary collaboration between pneumologists, cardiologists, psychiatrist, psychosomatic physicians, and neurologists should be established for the most beneficial diagnostic workup and the development of management plans for PCS patients, which are currently unavailable. In the absence of medical therapeutic options, the valuable expertise of physiotherapists, occupational therapist, psychotherapists, and neuropsychologists is crucial in the treatment of PCS. Availability of specialized PCS rehabilitation centers and interdisciplinary treatment options is strongly needed. A broad organized network of structured patient education might prevent misinformation of affected patients in need. Further evaluation and in-depth studies of the pathophysiological mechanisms of PCS conditions are urgently needed to offer specific therapeutic regimens in the future.
This report is providing data of a single neurological outpatient clinic that was established to offer specialized medical care to patients with neurological manifestations of PCS. Therefore, comparison to a cohort of PCS patients without neurological manifestations was not possible. Comparison of our data with data of other neurological PCS outpatient clinics is needed to validate our data and allow generalization.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Charité's Ethics Committee Chairperson: Ms. Dr. Orzechowski Number: EA2/066/20 Charité–Universtity Medicine Berlin. The patients/participants provided their written informed consent to participate in this study.
FB and CF conceptualized the project, had a major role in data collection, and wrote the manuscript. HA, ME, and HP conceptualized the project. All authors critically revised the manuscript for important intellectual content and approved the final draft.
We acknowledge support from the German Research Foundation (DFG, FR 4479/1-1) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Overview. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19 | Guidance | NICE. (2021). Available online at: https://www.nice.org.uk/guidance/ng188
2. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
3. Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. (2021) 325:1525–34. doi: 10.1001/jama.2021.3331
4. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. (2020) 324:603–5. doi: 10.1001/jama.2020.12603
5. Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. (2021) 174:576–8. doi: 10.7326/M20-5661
6. Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. (2021) 325:2015–6. doi: 10.1001/jama.2021.5612
7. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. doi: 10.1016/S2215-0366(21)00084-5
8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
9. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. (2021) 11:16144. doi: 10.1038/s41598-021-95565-8
10. Franke C, Warnke C, Gorsler A, Prüß H. Neurologische manifestationen bei patienten mit Post-COVID-19-Syndrom. DGNeurologie. (2021) 4:276–80. doi: 10.1007/s42451-021-00345-3
11. Schweitzer F, Kleineberg NN, Göreci Y, Onur OA, Franke C, Warnke C. Neuro-COVID-19 is more than anosmia: clinical presentation, neurodiagnostics, therapies, and prognosis. Curr Opin Neurol. (2021) 34:423–31 doi: 10.1097/WCO.0000000000000930
12. Kreye J, Reincke SM, Kornau H-C, Sánchez-Sendin E, Corman VM, Liu H, et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell. (2020) 183:1058–69.e19. doi: 10.1016/j.cell.2020.09.049
13. Franke C, Ferse C, Kreye J, Reincke SM, Sanchez-Sendin E, Rocco A, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Beha Immun. (2021) 93:415–9. doi: 10.1016/j.bbi.2020.12.022
14. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. (2021) 6:100122. doi: 10.1016/j.lanepe.2021.100122
15. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. (2014) 35:347–69. doi: 10.1016/j.yfrne.2014.04.004
16. Busch MA, Maske UE, Ryl L, Schlack R, Hapke U. Prävalenz von depressiver symptomatik und diagnostizierter depression bei erwachsenen in deutschland: ergebnisse der studie zur gesundheit erwachsener in deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2013) 56:733–9. doi: 10.1007/s00103-013-1688-3
17. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. (2008) 31:1601–7. doi: 10.1093/sleep/31.11.1601
18. Wu S, Wang R, Ma X, Zhao Y, Yan X, He J. Excessive daytime sleepiness assessed by the Epworth Sleepiness Scale and its association with health related quality of life: a population-based study in China. BMC Public Health. (2012) 12:849. doi: 10.1186/1471-2458-12-849
19. Hayley AC, Williams LJ, Kennedy GA, Berk M, Brennan SL, Pasco JA. Prevalence of excessive daytime sleepiness in a sample of the Australian adult population. Sleep Med. (2014) 15:348–54. doi: 10.1016/j.sleep.2013.11.783
20. Thorarinsdottir EH, Bjornsdottir E, Benediktsdottir B, Janson C, Gislason T, Aspelund T, et al. Definition of excessive daytime sleepiness in the general population: feeling sleepy relates better to sleep-related symptoms and quality of life than the epworth sleepiness scale score. Results from an epidemiological study. J Sleep Res. (2019) 28:e12852. doi: 10.1111/jsr.12852
21. Takegami M, Sokejima S, Yamazaki S, Nakayama T, Fukuhara S. An estimation of the prevalence of excessive daytime sleepiness based on age and sex distribution of epworth sleepiness scale scores: a population based survey. Nihon Koshu Eisei Zasshi. (2005) 52:137–45.
22. Kedor C, Freitag H, Meyer-Arndt L, Wittke K, Zoller T, Steinbeis F, et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany - a first analysis of a prospective observational study. medRxiv [Preprint]. (2021). doi: 10.1101/2021.02.06.21249256
Keywords: post-COVID-19, long-COVID-19, neurology, outpatient, SARS-CoV-2
Citation: Boesl F, Audebert H, Endres M, Prüss H and Franke C (2021) A Neurological Outpatient Clinic for Patients With Post-COVID-19 Syndrome — A Report on the Clinical Presentations of the First 100 Patients. Front. Neurol. 12:738405. doi: 10.3389/fneur.2021.738405
Received: 08 July 2021; Accepted: 11 August 2021;
Published: 16 September 2021.
Edited by:
Sara Palermo, Unità di Neuroradiologia, Carlo Besta Neurological Institute (IRCCS), ItalyReviewed by:
Ava Easton, Encephalitis Society, United KingdomCopyright © 2021 Boesl, Audebert, Endres, Prüss and Franke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabian Boesl, ZmFiaWFuLmJvZXNsQGNoYXJpdGUuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.