
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 16 November 2021
Sec. Neurocritical and Neurohospitalist Care
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.732176
This article is part of the Research TopicCerebral Autoregulation and Neurovascular Coupling in Brain DisordersView all 25 articles
 Yaroslava Longhitano1*†
Yaroslava Longhitano1*† Francesca Iannuzzi2†
Francesca Iannuzzi2† Giulia Bonatti3
Giulia Bonatti3 Christian Zanza4
Christian Zanza4 Antonio Messina5,6
Antonio Messina5,6 Daniel Godoy7
Daniel Godoy7 Wojciech Dabrowski8
Wojciech Dabrowski8 Li Xiuyun9
Li Xiuyun9 Marek Czosnyka10
Marek Czosnyka10 Paolo Pelosi2,11
Paolo Pelosi2,11 Rafael Badenes12‡
Rafael Badenes12‡ Chiara Robba2,11‡
Chiara Robba2,11‡Introduction: Cerebral autoregulation (CA) plays a fundamental role in the maintenance of adequate cerebral blood flow (CBF). CA monitoring, through direct and indirect techniques, may guide an appropriate therapeutic approach aimed at improving CBF and reducing neurological complications; so far, the role of CA has been investigated mainly in brain-injured patients. The aim of this study is to investigate the role of CA in non-brain injured patients.
Methods: A systematic consultation of literature was carried out. Search terms included: “CA and sepsis,” “CA and surgery,” and “CA and non-brain injury.”
Results: Our research individualized 294 studies and after screening, 22 studies were analyzed in this study. Studies were divided in three groups: CA in sepsis and septic shock, CA during surgery, and CA in the pediatric population. Studies in sepsis and intraoperative setting highlighted a relationship between the incidence of sepsis-associated delirium and impaired CA. The most investigated setting in the pediatric population is cardiac surgery, but the role and measurement of CA need to be further elucidated.
Conclusion: In non-brain injured patients, impaired CA may result in cognitive dysfunction, neurological damage, worst outcome, and increased mortality. Monitoring CA might be a useful tool for the bedside optimization and individualization of the clinical management in this group of patients.
Cerebral autoregulation (CA) is a complex mechanism of brain protection against changes in cerebral perfusion pressure (CPP) in order to maintain an adequate cerebral blood flow (CBF) (1). CA works by balancing vasoconstriction and vasodilation of the cerebral vessels regulating CBF with the aim to maintain a constant CBF (1). The concept of CA was introduced approximately half a century ago and afterward it was progressively used as a parameter to help in the management of mean arterial blood pressure in relationship with CBF in the neuro-intensive care unit (neuro-ICU) settings. Indeed, monitoring CA has shown to be particularly useful in the context of acute subarachnoid hemorrhage, traumatic brain injury (TBI), and acute stroke (2–5). Although no randomized controlled trials (RCTs) assessing the effect of autoregulation on outcome are available at present, it is accepted among experts that impairment of CA can lead to secondary cerebral insult and poor outcomes also in patients without primary brain injury (6–10).
The latest guidelines from the Brain Trauma Foundation suggest to maintain a CPP of 60–70 mm Hg in patients with TBI (11); however, the concept of “one-size-fits-all” may not be appropriate, as it does not represent the physiological needs of an individual and changes of CA; more recently, experts suggest a personalized management of CA based on the intrinsic autoregulatory state and function of single patients (12). In this context, several methods aimed to individualize the assessment of CA status have been proposed (13). The gold standard for CA measurement is represented by the neuroimaging studies, which allow to obtain a direct visualization of CBF such as PET, single-photon emission CT (SPECT), and CT perfusion. However, these methods are expensive, time-consuming, and have a very limited role in the clinical context. Therefore, a number of indirect techniques aimed at CA monitoring by the bedside have been proposed and evaluated including invasive methods (based on intracranial pressure and invasive cerebral oxygenation monitoring) and non-invasive methods [based on transcranial Doppler (TCD) and near-IR spectroscopy (NIRS)]; both of them are widely adopted in the neuro-ICU population.
The aim of this study is to systematically review the role of CA in non-brain injured patients, in different clinical settings, such as surgery, sepsis, and septic shock and in the pediatric population.
In this study, we adhered to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines (14). A systematic literature search was performed by using the following databases to identify relevant studies in indexed scientific journals: the PubMed, the Medical Literature Analysis and Retrieval System Online (MEDLINE) (via Ovid), the Excerpta Medica dataBASE (EMBASE) (via Ovid), and the Cochrane Central Register of Controlled Trials by using the terms: cerebral autoregulation and sepsis, cerebral autoregulation and surgery and/or perioperative, and cerebral autoregulation and non-brain injured patients with filters for humans, language (English), and time of publication (January 1, 2010 to February 28, 2021). Inclusion criteria were studies describing the measurement of CA in non-brain injured patients and its effect on outcome of patients (evaluated as mortality and/or neurological outcome). This study was limited to clinical trials, meta-analysis, RCTs, review, and systematic review. Search criteria included “CA and surgery,” “CA and sepsis and/or metabolic coma,” “CA and general intensive care,” and “CA and non-brain injured patients.” A specific literature search was then conducted for the pediatric population, with the same search strategy, but including patients <18 years. We excluded literature concerning brain injury population and not concerning CA specifically or not mentioning the effect of CA measurement on outcome. We also excluded editorials, commentaries, letters to the editor, opinion articles, reviews, meeting abstracts, and original articles lacking abstract.
Two authors (FI and GB) selected the articles independently screening titles, abstracts, and full texts. The standardized data extraction form included aim and study design. The articles were then subdivided into three subgroups: “included” and “excluded” (if the two examiners agreed with the selection) or “uncertain” (in case of disagreement). In the case of “uncertain” classification, discrepancies were resolved by further examination performed by the two expert authors (CR and YL). We used a standardized electronic spreadsheet (Microsoft Excel, version 14.4.1; Microsoft, Redmond, Washington, USA) to extract the data from all the included studies and recording trial characteristics.
The primary outcome of this study is to investigate the CA in patients without brain injury. Secondary outcome is to assess whether alteration in CA in different settings such as surgery, sepsis, and septic shock and in the pediatric population can have an effect on morbidity and mortality.
Two examiners (FI and YL) independently assessed the internal validity of the included studies and discrepancies were resolved by a third senior author (AM or CR) by using the version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2). The RoB 2 considers five bias domains: (1) the randomization process, (2) the deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. Finally, the overall risk of bias was calculated and, accordingly, studies were included in either high-risk/unclear risk/low-risk groups.
After general screening, initial results from this search lead to a number of 40 studies (Figure 1, Flowchart). Among them, 22 studies were further selected, which were divided in three sections (CA in sepsis and septic shock, CA in surgery, and CA in the pediatric population) (Tables 1, 2). Specific reasons for exclusion of the studies are presented in Table 3. The risk of bias assessment reported: “low risk” for 13 papers (59%), “unclear risk” for 9 articles (41%), and none of them reported high risk of bias. The bias was mostly related to the randomization process selection of the reported results (Table 4).
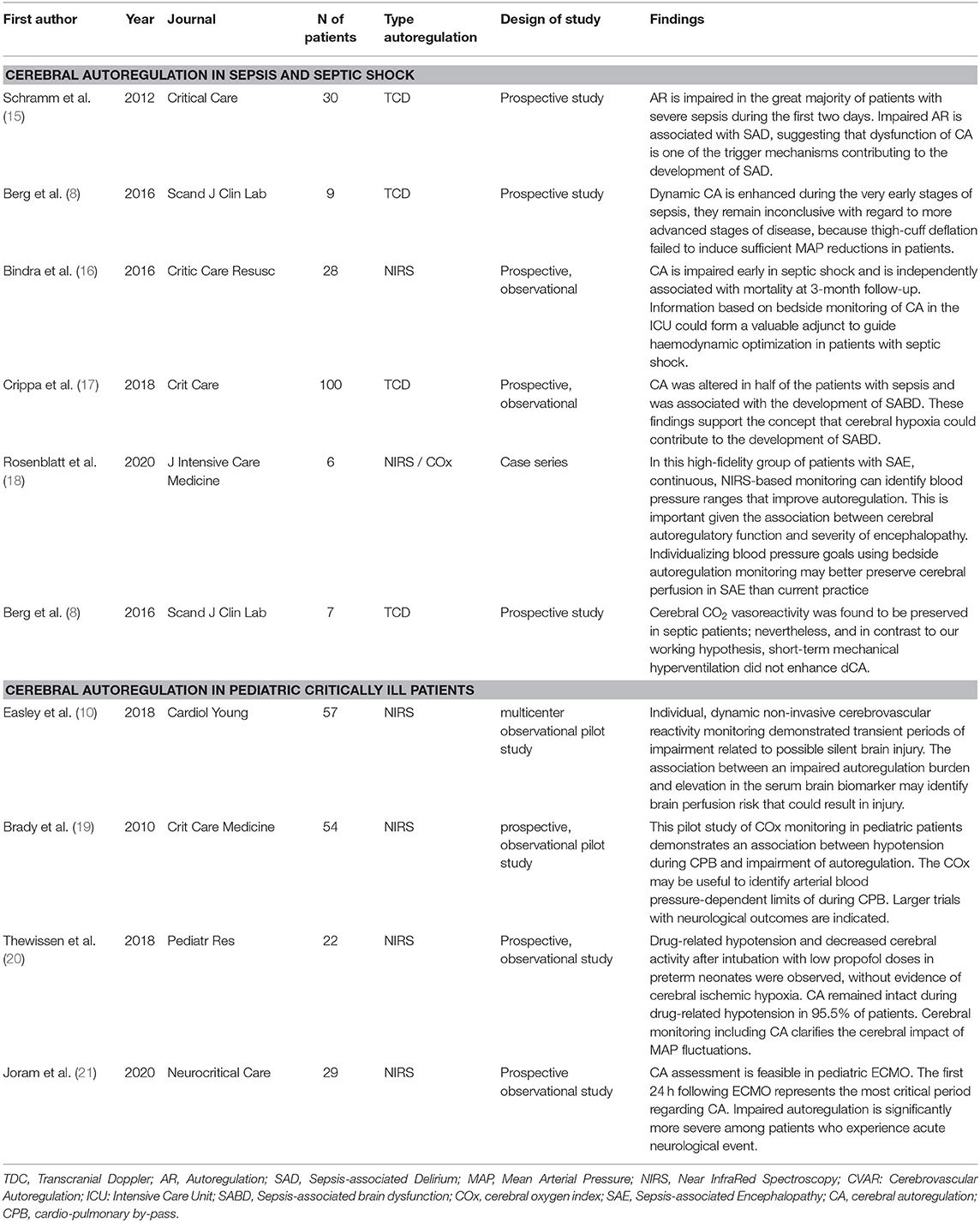
Table 1. Summary of the included studies about CA in sepsis and pediatric population: primary findings, type of method to assess autoregulation, number of patients evaluated.
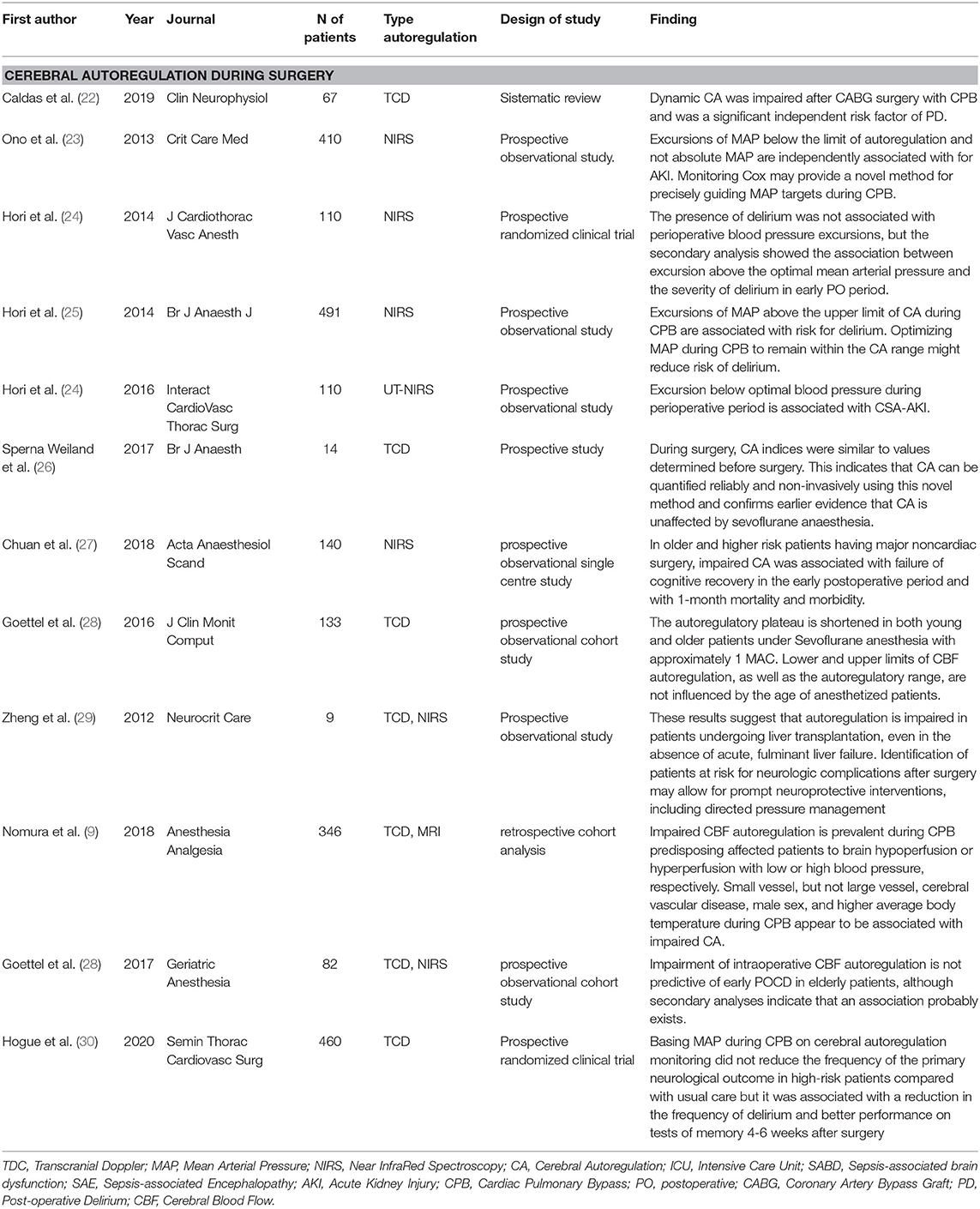
Table 2. Summary of the included studies about CA in surgery: primary findings, type of method to assess autoregulation, number of patients evaluated.
A total of six studies were screened for septic shock and sepsis (Table 1). A disruption of CA prolonged over time can lead to cerebral hypoperfusion and consequently neuronal ischemia. In a prospective, observational study, 100 adult patients with sepsis were evaluated with the hypothesis that impaired CA may lead to brain hypoperfusion and neuronal damage (17). Crippa et al. registered impaired CA in 50 patients (50%) and this represented one of the independent predictors of sepsis-associated brain dysfunction (SABD), which was diagnosed in 57 patients (57%). SABD was defined as the Glasgow Coma Scale (GCS) score <15 or when disorientation, altered thinking, or agitation was reported. In case of continuous sedation (n = 6), patients were considered as having SABD. The CA was evaluated by using the mean flow index (Mxa) assessed by TCD (17). The authors concluded that in SABD, brain hypoperfusion and neuronal damage were probably caused by impaired CA and this could lead to a higher morbidity and mortality in these patients. The findings of this prospective observational study support the concept that cerebral hypoxia could contribute to the development of SABD, despite its multifactorial pathophysiology. Schramm et al. also found a relationship between impaired CA and sepsis-associated delirium (SAD). Indeed, in the majority of septic patients, CA is impaired, in particular the first 2 days, suggesting an important contribution of CA in the development of SAD (15). In this study (15), 30 patients were evaluated with the average Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) score of 32 ± 6. The CA was estimated by using TCD and the Confusion Assessment Method for the ICU (CAM-ICU) was assessed at day 4 when sedative medications were temporarily reduced to reach the Richmond Agitation and Sedation Scale (RASS) score greater than−2. SAD was detected in 76% of patients. This study concluded that impaired CA at day 1 was associated with the incidence of SAD at day 4 (p = 0.035) (15).
Cerebral autoregulation can be also evaluated to guide optimal blood pressure. Indeed, in a study including six patients (18), pharmacological sedation free with extracranial sepsis and cerebral oximetry was measured with NIRS to identify blood pressure range, demonstrating feasibility to optimize autoregulation maintaining adequate blood pressure range. In this case series, Rosenblatt et al. found an association between cerebral autoregulatory function and the development and severity of encephalopathy defined as GCS <15 but ≥ 13 (mild encephalopathy), whereas values <13 were defined as moderate or severe impairment (18). The authors concluded that individualizing blood pressure goals by using bedside autoregulation monitoring may preserve cerebral perfusion by individualizing blood pressure range in Sepsis-associated encephalopathy (SAE) (18). In another prospective observational study, Bindra et al. investigated 28 patients with early septic shock in the ICU to understand if impaired CA was associated with neurological outcome and mortality (16). This study concluded that the mortality at 3-month follow-up was independently associated with impaired CA in early septic shock. Thus, bedside monitoring of CA could adjunct important information for hemodynamic optimization of patients with septic shock. Other two studies discussed the management of CA in the early stages of septic shock (8, 31). Both were based on small groups of patients (seven and nine patients, respectively). The first study analyzed seven critically ill patients, who underwent hyperventilation (8) and the second one described healthy volunteers, who underwent lipopolysaccharide (LPS) infusion to reproduce early sepsis (31). The CA was evaluated by TCD and the authors concluded that dynamic CA (dCA) to spontaneous fluctuations in blood pressure was enhanced and cerebral carbon dioxide vasoreactivity was preserved in the early phases of sepsis, but this regulation disappeared in the late stages (8, 31).
A total of 12 studies were selected in the literature about CA in surgery (Table 2). A high number of articles about CA during surgery were performed in cardiac surgery, where a possible association between impaired CA and postoperative delirium (PD) was evaluated. Caldas et al. in his single-center observational and prospective study analyzed 67 patients undergoing cardiopulmonary bypass. All the enrolled subjects underwent TCD and the CAM-ICU assessment preoperatively at 24 h and 7 days after the procedure to evaluate CA and PD, respectively. Impaired CA was found in 55% of patients 24 h postoperatively and in 20% after 7 days. In addition, the authors concluded that impaired CA during coronary artery bypass graft (CABG) surgery is a significant independent risk factor for PD (p = 0.003) (22). Another study analyzed the use of CA with NIRS. In the prospective observational study, 110 patients who underwent cardiac surgery were analyzed (24). They were monitored with NIRS during surgery and in the first 3 h postoperatively and their CAM-ICU score was calculated on postoperative (PO) days 1 and 3. A total of 42.7% of patients presented delirium. Hori et al. observed no association between the perioperative blood pressure excursion and PD, but a secondary analysis showed higher blood pressure excursions above the optimal medial arterial pressure in the group who developed delirium postoperatively (r = 0.27, p = 0.011) (24). These data were also confirmed by a large prospective study, which analyzed 491 patients who were monitored with NIRS during surgery and the PD was assessed by the CAM-ICU. In this case, delirium was diagnosed in 9.2% of cases and the excursion of mean arterial pressure (MAP) above the upper limit of CA during cardiopulmonary bypass was independently associated with PO delirium [odds ratio (OR), 1.09; 95% CI, 1.03–1.15] (25).
In addition to a possible neuronal damage, an acute kidney injury (AKI) related to CA was investigated. Ono et al. in a large (410 patients) prospective study monitored CBF intraoperatively by using NIRS and evaluated the incidence of AKI by using the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) criteria during the first 7 days postoperatively. The results showed that MAP excursions below the lower limit of CBF autoregulation during cardiopulmonary bypass were independently associated with AKI [relative risk (RR), 1.02; 95% CI, 1.01 to 1.03; p < 0.0001] (23). These results were confirmed by another prospective study, which included 110 patients who underwent cardiopulmonary bypass. The CBF was monitored by NIRS during the surgery and the monitoring of AKI was performed for 2 days after surgery. A total of 27.3% of subjects developed AKI and the excursion below optimal blood pressure values during the perioperative period was associated with the development of AKI postoperatively (p = 0.008) (32). Other studies aimed to evaluate the principal risk factors associated to CA alteration. In a large prospective randomized clinical trial, 346 patients which underwent cardiac surgery and performed standardized general anesthesia were evaluated with preoperative TCD and cerebral MRI performed between days 3 and 5 after surgery to assess the preoperative factors, which can be associated with impaired CA in cardiac surgery. The presence of small vessel cerebral vascular disease was strongly associated with impaired CBF autoregulation (OR, 3.25; 95% CI, 1.21–8.71; p = 0.019). Other risk factors were male sex, high body temperature during surgery, elderly, and large vessel disease (10). Based on these evidences, it is possible to affirm that changes in CA during cardiac surgery influence clinical outcomes as rated by Caldas et al. in a recently published study (33). Also, Hogue et al. investigated the neurological impairment related to CA during cardiac surgery. A total of 460 patients were analyzed by TCD to correlate CA impairment with ischemic injury, PD, and performance on memory test 4–6 weeks after surgery. The presence of ischemic injuries was not related to intraoperative CA impairment (p = 0.752), instead both the delirium and memory test performance were strongly related to CA (p = 0.035 and p = 0.019, respectively) (30). However, further studies are needed to develop the therapeutic approaches in order to better understand the prognostic implications of this evidence.
In non-cardiac surgery, the role of CA was less frequently investigated and the results are discordant. Two large studies are available in scientific literature. The first one (27) is prospective and it is focused on PD and CA in major non-cardiac surgery. A total of 110 patients were included, who were intraoperatively monitored by NIRS and PO cognitive disorders were recorded. Chuan et al. concluded that impaired CA in these patients was associated with failure of cognitive recovery in the early PO period (p = 0.02) with 1-month mortality and morbidity (p = 0.04) (27). The second one (28) also investigated an association between impaired CA and PO cognitive dysfunction (POCD). Indeed, Goettel et al. recruited 86 patients who underwent non-cardiac surgery and standardized anesthesia and were intraoperatively evaluated with both the TCD and NIRS. In addition, C-reactive protein (CRP) and two biomarkers of neuronal injury neuron-specific enolase (NSE) and S100β protein were dosed on PO days 2 and 7. The study participants completed a battery of neuropsychological tests at baseline and 1 week after surgery and 3 months postoperatively. Authors concluded that dysregulation of CBF was not predictive of cognitive dysfunction after surgery, but there may be an association not yet fully explained (28). The risk factors associated with impaired CA in non-cardiac surgery were also evaluated in a study including 133 patients who underwent sevoflurane anesthesia during non-cardiac major surgery and subsequently they were divided in two groups depending on age. CBF thresholds were not influenced by the age of patients (p = 0.075) and autoregulatory plateau was shortened in both the groups during anesthesia with sevoflurane (34). In contrast, Sperna et al. evaluated CA in general anesthesia with TCD before surgery during 3 min of paced breathing at 6, 10, and 15 beats per min (bpm) and during surgery. The authors concluded that sevoflurane did not affect CA because preoperative and intraoperative values were superimposable (26). Finally, one study investigated the role of CA in liver transplant surgery. In this study, six patients were retrospectively analyzed with TCD to evaluate CA and its changes in perioperative period. Authors revealed marked alterations of CBF and they concluded that direct pressure management and others neuroprotective measures may reduce the risk for neurological complications in this group of patients (29).
With respect to CA in the pediatric population, four studies were included (Table 1). Even in the pediatric population, the most studied field for CA use is the cardiac surgery. In the first study included (19), the lower limit of autoregulation during cardiac surgery was measured; hypotensive events were associated with increased values of the cerebral oximetry index (Cox) (p < 0.0001) and the mean lower limits of pressure autoregulation were 42 ± 7 mm Hg. Authors concluded that despite larger studies must confirm these findings, COx can be useful to determine the arterial blood pressure-dependent limits of autoregulation in the pediatric population (19). Another study (10) conducted in cardiac surgery aimed to detect brain hypoperfusion injuries by elevation in serum glial fibrillary acidic protein levels due to impaired cerebrovascular reactivity. A total of 57 children were analyzed and transient period of compromised CA was detected with dynamic noninvasive cerebrovascular reactivity monitoring that showed transient periods of impairment related to possible silent brain injury (10). Out of cardiac surgery settings, CA after propofol infusion in the pediatric population was investigated. During general anesthesia, hypotension due to low dose of propofol infusion before endotracheal intubation did not lead to cerebral hypoxia and with stable levels of regional cerebral oxygen saturation. Unharmed CA drug-related hypotension was detected, underlining its importance during MAP fluctuations (20).
Another study evaluated CA during Extracorporeal Membrane Oxygenation (ECMO) treatment. This prospective observational study analyzed 29 children, who required ECMO support; in particular, the CA was evaluated with NIRS (21). In this study, 34.5% experienced acute neurological events; it concluded that the first 24 h following ECMO is the most critical period regarding CA and altered CA is associated with acute neurological events (p = 0.04) (21).
In this study, we set out to describe how CA can be modified in patients without brain injury in sepsis/septic shock during surgery and in pediatric critically ill patients. Impaired autoregulation in these pathological situations can result in cognitive dysfunction, neurological damage, increased morbidity, and mortality even in non-brain injured patient (6–9). These pathological situations can alter the autoregulatory mechanisms and values, thus modifying the ability of CA to preserve good CBF and prevent possible secondary neuronal injury (8, 17, 24).
In sepsis and septic shock, the systemic alterations that involve compromise in CA have been studied by several authors for whom the condition of SABD has also been defined (24). Despite this, the timing at which impaired CA begins with respect to the onset of the disease still needs further investigations (33). Furthermore, a correlation was found between impaired CA and outcome and mortality of septic patients independently from baseline septic disease severity (16, 18). The lack of homogeneous outcomes found in the studies included can be explained by the different settings applied and makes difficult to draw any conclusion regarding the possible effects of brain monitoring on outcome. The presence of sedation during neurological evaluation, the use of different scores for neurological impairment, and CA evaluation methods can also impact on the reproducibility of the results; nevertheless, all the studies suggest that measuring CA is safe and feasible and can potentially provide important information regarding intracerebral changes during the ICU stay and possibly improve outcomes (16–18). The analyzed studies confirm the presence of frequently altered CA in sepsis or septic shock, mostly during latest phases of sepsis and altered CA is the important risk factor for cognitive dysfunction (15, 17).
Important consequences of impaired CA were also found in the PO period. The studies included were mainly conducted in the context of cardiac surgery, in particular during cardiopulmonary bypass in which the cause of PD must be sought together with other risk factors (22, 24, 25). In support of this theory, an increased level of neuronal biomarkers was found in the serum of patients with impaired CA in the PO period, but further studies are needed to fully understand the association between POCD and altered brain flow as a consequence of the loss of CA (28). Besides cardiac surgery, in a study conducted in liver transplant surgery, we found that blood pressure management and neuroprotective measures may help in reducing the neurological complications (29). In this pathological setting, the alteration of CA is related to PO neurological and cognitive impairment (22, 27, 34), but it is not the only one comorbidity related to altered CBF. Indeed, also AKI was associated with altered CA (23, 32).
In the pediatric population, CA was evaluated mainly during cardiopulmonary bypass surgery (10, 19). An increase in PO serum neurological biomarkers following impaired CA was also detected in pediatric patients. This has been related to major fluctuations in MAP and intraoperative hypotension (10). Although evidence is limited in this setting, even in this population, the use of CA monitoring seems to be beneficial to early detect the intracerebral complications (10).
The CBF was evaluated mostly by NIRS and TCD. In case of TCD, the correlation between systemic MAP and mean flow velocity (FVm) in the middle cerebral artery was used to evaluate CA (Mx index) (15, 22, 33). Instead, in case of NIRS, the CA can be monitored by the Pearson's correlation coefficient between MAP and NIRS signals to generate the variable COx. When MAP is within the limits of CBF autoregulation, Mx and Cox approach zero or are negative, but when MAP is outside the limits of autoregulation, Mx and Cox have positive values, indicating that CBF is blood pressure passive (16, 25). Sperna et al. evaluated CA with an innovative method by using paced breathing. Indeed, the blood pressure and CBF velocity fluctuate spontaneously around the two predominant frequencies. The low frequency is due to baroreflex-mediated sympathetic nervous system activity and the high frequency is due to respiration. By using “frequency domain” analysis, the power of these fluctuations can determine the efficacy of CA (26).
This study has some limitations. First, this study summarizes the available evidence. There are still inadequate data describing the impact of CA on outcome and its clinical use in both the adult and pediatric population without brain injury. Second, the studies described in our review included a small number of patients and are extremely heterogeneous with often important methodological limitations. This makes difficult to draw any conclusion and makes a meta-analysis of the available evidence unviable.
Impaired autoregulation in different pathological conditions can result in cognitive dysfunction, neurological damage, worse outcome, and increased mortality, even in non-brain injured patients. The analyzed studies show an association between alteration in CA and outcome; however, the heterogeneity of the studies and the low level of quality in the study design and methods further suggest that more in-depth investigations are needed, especially considering the different subgroups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
FI, YL, GB, and CZ contributed to the analysis of scientific literature and writing of manuscript. AM, DG, WD, LX, MC, PP, and CR contributed to the revision of manuscript and fundamental conceptual contribution. RB reviewed the manuscript and added fundamental contribution. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.732176/full#supplementary-material
1. Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. (2016) 34:465–77. doi: 10.1016/j.anclin.2016.04.002
2. Robba C, Citerio G. How I manage intracranial hypertension. Crit Care. (2019) 23:243. doi: 10.1186/s13054-019-2529-z
3. Robba C, Bonatti G, Battaglini D, Rocco PRM, Pelosi P. Mechanical ventilation in patients with acute ischaemic stroke: from pathophysiology to clinical practice. Crit Care. (2019) 23:388. doi: 10.1186/s13054-019-2662-8
4. Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, Healy RJ, Ziai W, Mirski MA. Cerebral autoregulation-oriented therapy at the bedside: a comprehensive review. Anesthesiology. (2017) 126:1187–99. doi: 10.1097/ALN.0000000000001625
5. Klein SP, Depreitere B, Meyfroidt G. How I monitor cerebral autoregulation. Crit Care. (2019) 23:160. doi: 10.1186/s13054-019-2454-1
6. Depreitere B, Citerio G, Smith M, Adelson PD, Aries MJ, Bleck TP, et al. Cerebrovascular autoregulation monitoring in the management of adult severe traumatic brain injury: a delphi consensus of clinicians. Neurocrit Care. (2021) 34:731–8. doi: 10.1007/s12028-020-01185-x
7. de Azevedo DS, Salinet ASM, de Lima Oliveira M, Teixeira MJ, Bor-Seng-Shu E, de Carvalho Nogueira R. Cerebral hemodynamics in sepsis assessed by transcranial Doppler: a systematic review and meta-analysis. J Clin MonitComput. (2017) 31:1123–32. doi: 10.1007/s10877-016-9945-2
8. Berg RM, Plovsing RR. Effects of short-term mechanical hyperventilation on cerebral blood flow and dynamic cerebral autoregulation in critically ill patients with sepsis. Scand J Clin Lab Invest. (2016) 76:226–33. doi: 10.3109/00365513.2015.1137350
9. Nomura Y, Faegle R, Hori D, Al-Qamari A, Nemeth AJ, Gottesman R, et al. Cerebral small vessel, but not large vessel disease, is associated with impaired cerebral autoregulation during cardiopulmonary bypass: a retrospective cohort study. Anesth Analg. (2018) 127:1314–22. doi: 10.1213/ANE.0000000000003384
10. Easley RB, Marino BS, Jennings J, Cassedy AE, Kibler KK, Brady KM, et al. Impaired cerebral autoregulation and elevation in plasma glial fibrillary acidic protein level during cardiopulmonary bypass surgery for CHD. Cardiol Young. (2018) 28:55–65. doi: 10.1017/S1047951117001573
11. Hutchinson PJ, Kolias AG, Tajsic T, Adeleye A, Aklilu AT, Apriawan T, et al. Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury: Consensus statement. Acta Neurochir (Wien). (2019) 161:1261–74. doi: 10.1007/s00701-019-03936-y
12. Czosnyka M. In a Search of Pressure Which Optimizes Autoregulation of Cerebral Blood Flow. Crit Care Med. (2019) 47:1472–3. doi: 10.1097/CCM.0000000000003947
13. Donnelly J, Aries MJ, Czosnyka M. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev Neurother. (2015) 15:169–85. doi: 10.1586/14737175.2015.996552
14. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
15. Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. (2012) 16:R181. doi: 10.1186/cc11665
16. Bindra J, Pham P, Chuan A, Jaeger M, Aneman A. Is impaired cerebrovascular autoregulation associated with outcome in patients admitted to the ICU with early septic shock? Crit Care Resusc. (2016) 18:95–101.
17. Crippa IA, Subirà C, Vincent JL, Fernandez RF, Hernandez SC, Cavicchi FZ, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care. (2018) 22:327. doi: 10.1186/s13054-018-2258-8
18. Rosenblatt K, Walker KA, Goodson C, Olson E, Maher D, Brown CH. Cerebral autoregulation-guided optimal blood pressure in sepsis-associated encephalopathy: a case series. J Intensive Care Med. (2020) 35:1453–64. doi: 10.1177/0885066619828293
19. Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. (2010) 41:1957–62. doi: 10.1161/STROKEAHA.109.575167
20. Thewissen L, Caicedo A, Dereymaeker A, Van Huffel S, Naulaers G, Allegaert K, et al. Cerebral autoregulation and activity after propofol for endotracheal intubation in preterm neonates. Pediatr Res. (2018) 84:719–25. doi: 10.1038/s41390-018-0160-3
21. Joram N, Beqiri E, Pezzato S, Andrea M, Robba C, Liet JM, et al. Continuous monitoring of cerebral autoregulation in children supported by extracorporeal membrane oxygenation: a pilot study. Neurocrit Care. (2021) 34:935–45. doi: 10.1007/s12028-020-01111-1
22. Caldas JR, Panerai RB, Bor-Seng-Shu E, Ferreira GSR, Camara L, Passos RH, et al. Dynamic cerebral autoregulation: A marker of post-operative delirium? Clin Neurophysiol. (2019) 130:101–8. doi: 10.1016/j.clinph.2018.11.008
23. Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. (2013) 41:464–71. doi: 10.1097/CCM.0b013e31826ab3a1
24. Hori D, Max L, Laflam A, Brown C, Neufeld KJ, Adachi H, et al. Blood pressure deviations from optimal mean arterial pressure during cardiac surgery measured with a novel monitor of cerebral blood flow and risk for perioperative delirium: a pilot study. J CardiothoracVascAnesth. (2016) 30:606–12. doi: 10.1053/j.jvca.2016.01.012
25. Hori D, Brown C, Ono M, Rappold T, Sieber F, Gottschalk A, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth. (2014) 113:1009–17. doi: 10.1093/bja/aeu319
26. Sperna Weiland NH, Hermanides J, Hollmann MW, Preckel B, Stok WJ, van Lieshout JJ, et al. Novel method for intraoperative assessment of cerebral autoregulation by paced breathing. Br J Anaesth. (2017) 119:1141–9. doi: 10.1093/bja/aex333
27. Chuan A, Short TG, Peng AZY, Wen SYB, Sun AX, Ting TH, et al. Is cerebrovascular autoregulation associated with outcomes after major noncardiac surgery? A prospective observational pilot study. Acta Anaesthesiol Scand. (2019) 63:8–17. doi: 10.1111/aas.13223
28. Goettel N, Burkhart CS, Rossi A, Cabella BC, Berres M, Monsch AU, et al. Associations between impaired cerebral blood flow autoregulation, cerebral oxygenation, and biomarkers of brain injury and postoperative cognitive dysfunction in elderly patients after major noncardiac surgery. Anesth Analg. (2017) 124:934–42. doi: 10.1213/ANE.0000000000001803
29. Zheng Y, Villamayor AJ, Merritt W, Pustavoitau A, Latif A, Bhambhani R, et al. Continuous cerebral blood flow autoregulation monitoring in patients undergoing liver transplantation. Neurocrit Care. (2012) 17:77–84. doi: 10.1007/s12028-012-9721-1
30. Hogue CW, Brown CH, Hori D, Ono M, Nomura Y, Balmert LC, et al. Personalized blood pressure management during cardiac surgery with cerebral autoregulation monitoring: a randomized trial. Semin Thorac Cardiovasc Surg. (2021) 33:429–38. doi: 10.1053/j.semtcvs.2020.09.032
31. Berg RM, Plovsing RR, Bailey DM, Holstein-Rathlou NH, Møller K. Dynamic cerebral autoregulation to induced blood pressure changes in human experimental and clinical sepsis. Clin PhysiolFunct Imaging. (2016) 36:490–6. doi: 10.1111/cpf.12256
32. Hori D, Hogue C, Adachi H, Max L, Price J, Sciortino C, et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact Cardiovasc Thorac Surg. (2016) 22:445–51. doi: 10.1093/icvts/ivv371
33. Caldas JR, Haunton VJ, Panerai RB, Hajjar LA, Robinson TG. Cerebral autoregulation in cardiopulmonary bypass surgery: a systematic review. Interact Cardiovasc Thorac Surg. (2018) 26:494–503. doi: 10.1093/icvts/ivx357
Keywords: cerebral autoregulation, non-brain injury, neurologic outcome, sepsis, perioperative care, pediatric surgery
Citation: Longhitano Y, Iannuzzi F, Bonatti G, Zanza C, Messina A, Godoy D, Dabrowski W, Xiuyun L, Czosnyka M, Pelosi P, Badenes R and Robba C (2021) Cerebral Autoregulation in Non-Brain Injured Patients: A Systematic Review. Front. Neurol. 12:732176. doi: 10.3389/fneur.2021.732176
Received: 28 June 2021; Accepted: 11 October 2021;
Published: 16 November 2021.
Edited by:
Danilo Cardim, University of Texas Southwestern Medical Center, United StatesReviewed by:
Shraddha Mainali, The Ohio State University, United StatesCopyright © 2021 Longhitano, Iannuzzi, Bonatti, Zanza, Messina, Godoy, Dabrowski, Xiuyun, Czosnyka, Pelosi, Badenes and Robba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaroslava Longhitano, bG9uLnlhcm9AZ21haWwuY29t
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.