- 1Department of Neurology, The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, China
- 2Central Laboratory, The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, China
- 3Department of Neurology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: Impulse control and related disorders (ICRDs) have gained recognition as a severe complication of Parkinson's disease (PD) and are connected to poor quality of life and devastating financial and social problems. This study aimed to evaluate the usefulness of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP) and estimate the risk factors for ICRDs in Chinese patients with PD.
Methods: 207 PD patients were assessed using the QUIP and evaluated for PD motor and nonmotor symptoms. ICRDs were diagnosed via interviews of patients or their caregivers, and the clinical characteristics of patients with and without ICRDs were compared.
Results: The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the C-QUIP were 95.0, 83.4, 38.0, 99.4, and 84.5%. The prevalence of each disorder among participants diagnosed via interview was pathological gambling (0.5%), hypersexuality (1.9%), compulsive shopping (1.0%), binge eating (3.9%), hobbyism (1.9%), punding (0.5%), walkabout (0.5%), and dopamine dysregulation syndrome (2.9%). PD patients with ICRDs had longer PD duration, higher Hoehn and Yahr stage, Non-Motor Symptoms Scale (NMSS), and Hamilton-Depression Rating Scale (HAMD). Also, they received a larger total daily levodopa equivalent dose (LED), levodopa dosage, and dopamine agonist only LED (DA-LED) than did PD patients without ICRDs.
Conclusions: Given its psychometric properties, the C-QUIP is a valid and rapid screening instrument for assessing of ICRDs in PD patients. Higher Hoehn and Yahr staging, NMSS and HAMD scores, a larger mean LED and levodopa dosage are risk factors for ICRDs.
Introduction
In addition to its characteristic motor signs and symptoms, Parkinson's disease (PD), a common degenerative neurological disorder, has many non-motor signs and symptoms, olfactory dysfunction, constipation, depression, apathy, rapid-eye-movement sleep behavior disorder, and sleep disturbances. Impulse control disorders (ICDs) involve repetitive, excessive, and compulsive behaviors driven by intense desire (1). Patients often find it difficult to control themselves even though these behaviors cause harm to themselves or others. ICDs include pathological gambling, hypersexuality, compulsive shopping, and binge eating. As the presence of ICDs in PD has received increasing attention, its clinical symptom spectrum has expanded to include impulse control and related disorders (ICRDs), specifically, dopamine dysregulation syndrome (DDS), hobbyism, and punding. These behaviors are linked by their repetitive nature based on incentives or rewards (2). It is crucial to identify ICRDs because these behaviors can lead to serious financial problems and impair the quality of life of both patients and their caregivers. Early interventions, i.e., medication adjustment, can effectively improve symptoms and prevent severe consequences (3).
To standardize the clinical diagnosis and study of ICRDs in PD, the International Parkinson and Movement Disorder Society recommends using the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP) and Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease Rating Scale (QUIP-RS) for assessments of ICRDs in patients with PD (4). The QUIP is a self-report tool for screening ICRDs (4) but requires a follow-up interview to confirm the diagnosis. The validation of the QUIP has been tested in many languages (5–7) but not in Chinese. Therefore, our study aimed to evaluate the QUIP's usefulness and estimate the risk factors for ICRDs in Chinese patients with PD.
Materials and Methods
Participants
We recruited 207 consecutive patients diagnosed with idiopathic PD in the Department of Neurology at the Fourth Affiliated Hospital Zhejiang University School of Medicine and the Second Affiliated Hospital, Zhejiang University School of Medicine, between October 2018 and January 2021. The clinical diagnosis of PD was based on criteria from the Parkinson's Disease Society Brain Bank in London (8). Patients with secondary Parkinsonism or Parkinson-plus syndrome were excluded from the study. All participants provided written informed consent and the Ethics Committee of The Fourth Affiliated Hospital Zhejiang University School of Medicine approved the study.
Assessments
Participants' demographic and clinical characteristics (gender, age, age at onset of PD, duration of PD, and education) were evaluated. Patients' medications and dosages for PD were recorded at the time of the assessment; the total daily levodopa equivalent dose (total daily LED) (9), levodopa equivalent dose (LED), and dopamine agonist only LED (DA-LED) were calculated. Clinical assessments were performed using the following measures: Parts I, II, and III of the Unified Parkinson's Disease Rating Scale (UPDRS) (10), dyskinesia, the Hoehn and Yahr staging scale (11), the Mini-Mental State Examination (MMSE) (12), the Chinese version of the 39-item Parkinson's Disease Questionnaire (C-PDQ39) (13), the Non-Motor Symptoms Scale (NMSS), the Hamilton-Anxiety Rating Scale (HAMA), and the Hamilton-Depression Rating Scale (HAMD).
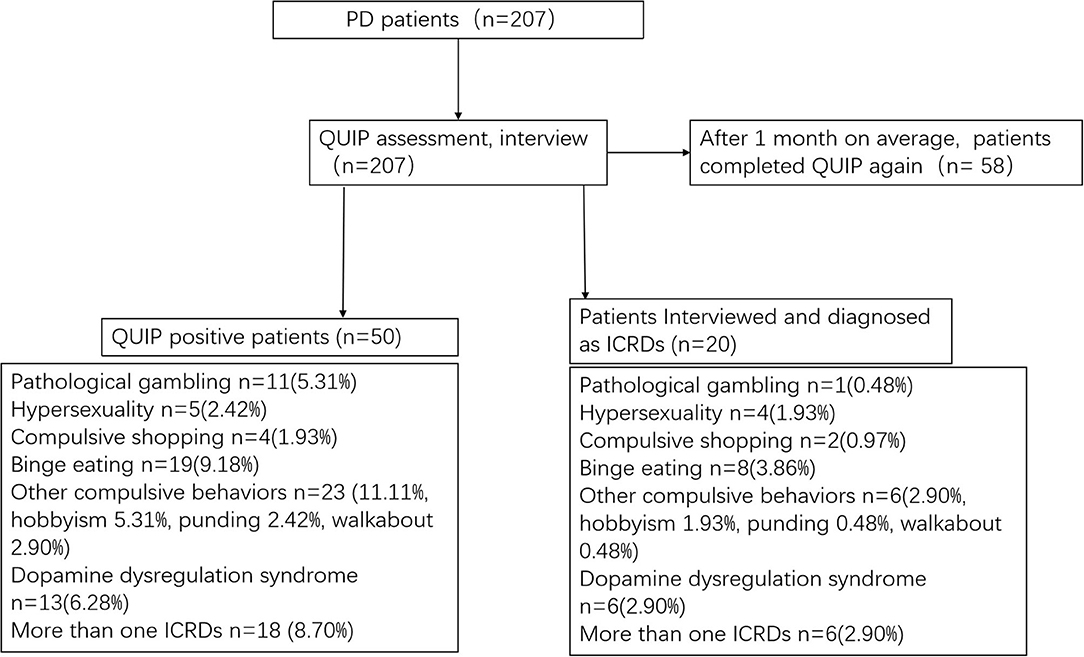
The original QUIP includes a full and short version, and both assess ICRDs currently or anytime during PD (QUIP-Current-Full, QUIP-Anytime During PD-Full, QUIP-Current-Short, QUIP-Anytime During PD-Short) (6). The QUIP contains 30 questions and its shortened version (QUIP-S) contains 13 questions, and the specific questions and overall structure of QUIP-S were not modified. The reason for choosing the time frame of “anytime during PD” is that plenty of PD patients experienced ICRDs during PD and are currently asymptomatic due to clinical management, but may be at increased risk of developing ICRDs in the future. As no differences were found in the sensitivity between these versions (6), they were all recommended for routine use, and we used only the QUIP-Anytime During PD-Short. All patients were interviewed by an experienced movement disorder neurologist, who was blind to patients' QUIP results and their final diagnoses. We diagnosed pathological gambling, hypersexuality, compulsive shopping, binge eating, hobbyism, punding, and DDS using the various diagnostic criteria listed in a review by Voon and Fox (2). We constructed the C-QUIP using forward and backward translations of the original QUIP, and the primary developer of the original questionnaire (DW) proofread the final version. We also obtained permission from the developer to translate the QUIP to Chinese. The specific questions and structure of C-QUIP are the same as the Short-Anytime During PD version of the original QUIP (6). The patient is screened as positive for each ICD if ≥1 affirmative answer to any question (The optimal cutoff point). The test-retest reliability of the C-QUIP was evaluated by performing assessments using the questionnaire a second time within 1 month after the first assessment. Figure 1 demonstrates the diagnostic flow chart for this study.
Statistical Analysis
Data were analyzed using SPSS for Windows version 19 (IBM Corp, Armonk, NY). The results are presented as mean ± standard deviation. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the QUIP for the detection of symptoms were calculated. Between-group differences (positive vs. negative diagnosed as ICRDs) were analyzed using the Wilcoxon-Mann-Whitney test for continuous variables and the χ2 test or Fisher's Exact test for categorical variables. P < 0.05 was considered statistically significant. The Guttman split-half coefficient was used to evaluate the test-retest reliability of the C-QUIP.
Results
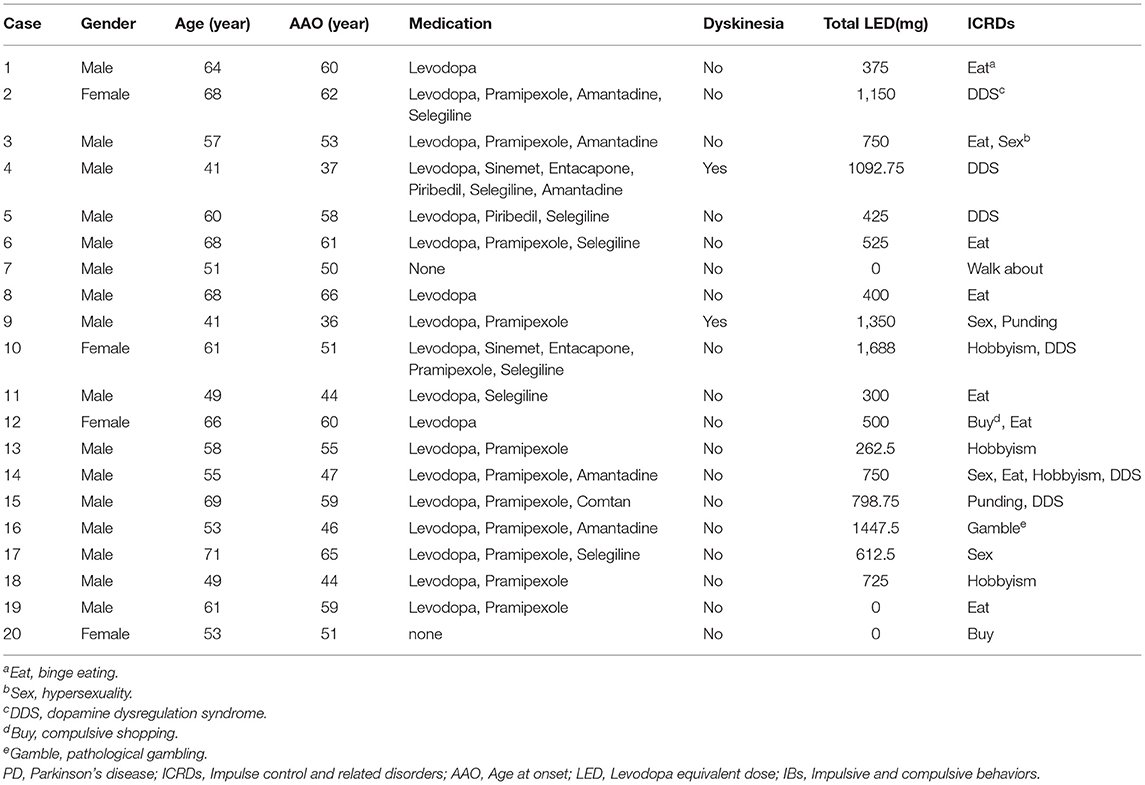
This study analyzed data from 207 patients (63.8% males) aged 60.8 ± 8.7 years with a PD duration of 4.1 ± 3.7 years. Half of the patients or their caregivers take <2 min to complete the questionnaire. Among the 207 patients, 24.2% screened positive on the C-QUIP, but only 9.7% were diagnosed eventually with ICRDs. Their behaviors and medications are presented in Table 1. Binge eating was the most common symptom with a frequency of 3.9%, followed by DDS (2.9%), hypersexuality (1.9%), and hobbyism (1.9%). Approximately 5.3% of the patients screened positive for compulsive gambling, but only one person met the diagnostic criteria, and 2.9% had more than one type of ICRDs (Table 1).
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the C-QUIP to detect each of the behaviors are presented in Table 2. We also compared C-QUIP with the original version and Japanese version of QUIP. The sensitivity and specificity for most of the measures on the C-QUIP were above 90%, and the NPV for each impulsive and compulsive behavior was close to 100%. The sensitivity of the C-QUIP for the detection of hypersexuality was 80% because one patient concealed this symptom, and it was not reported until a follow-up interview with his wife. The test-retest reliability assessment was performed with 58 patients, and the Guttman split-half coefficient for the total C-QUIP was 0.869.
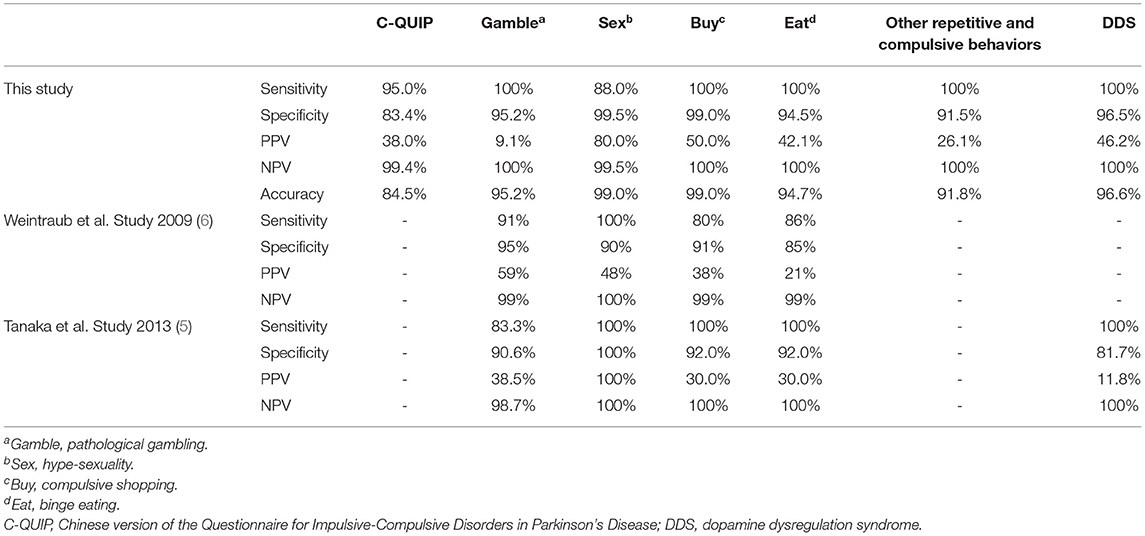
Table 2. The sensitivity, specificity, positive predictive value, negative predictive value of C-QUIP.
The clinical characteristics of PD patients with ICRDs (PD-ICRDs) and PD patients without ICRDs (PD-nonICRDs) are shown in Table 3. The mean scores on Parts I, II, and III of the UPDRS were 2.0, 9.4, and 23.4, respectively. Thirteen patients (6.3%) had dyskinesia; the mean Hoehn and Yahr stage was 2.0; the mean PDQ39 score was 17.1; the mean NMSS score was 22.0; the mean MMSE score was 25.4; and the mean HAMD and HAMA scores were 6.9 and 9.4, respectively. Levodopa usage was reported in 79.2% of the patients (LED 283.4 ± 220.7 mg/d), DA was used by 49.3% of them (DA LED 45.6 mg/d), and the total daily LED was 410.9 mg/d.

Table 3. Comparisons of demographic factors, clinical symptoms, and medications between patients with and without ICRDs.
These patients were then divided into two groups based on whether they had been diagnosed with an ICRD to compare differences in their demographic characteristics, clinical symptoms, and medications. We found that PD-ICRDs had a longer duration of PD (P = 0.040), higher Hoehn and Yahr stage (P = 0.018), higher NMSS (P = 0.013) and HAMD (P = 0.039) scores; and a larger total LED (P = 0.011), levodopa dosage (P = 0.005) and DA LEDs (P = 0.020), compared with PD-nonICRDs. The mean scores on the UPDRS-II (P = 0.094) and the PDQ-39 (P = 0.060) of PD-ICRDs were higher than those of PD-nonICRDs, but their differences were not statistically significant.
Discussion
The QUIP is a self-administered questionnaire to assess a comprehensive range of ICRDs in PD patients. Its usefulness has been verified in different countries, such as Japan and Germany (5–7). Several studies have examined ICDs or ICRDs in Chinese patients with PD (14–18), but they used different assessment instruments. Hence, the between-study results could not be compared. The QUIP was designed as a suitable screening tool rather than a diagnostic or rating tool for ICRDs in Parkinson's disease. A high NPV is crucial for a screening tool and this study's NPVs for most subtypes of ICRDs were 100%, with an accuracy >90%, which are similar detection rates as those of previous studies, indicating that the possibility of ICRDs would be extremely low in a patient with PD with a negative QUIP screening. As a brief, comprehensive and self-rated screening questionnaire, QUIP-S consumes only a very short time to complete without sacrificing diagnostic capability. There are several cons to note. First, it is necessary to confirm the diagnosis through a follow-up interview for the low PPVs and high false-positive rates. Concealment of one's symptoms, such as hypersexuality, whether intentional or not, should be considered, as it can be expected to be detected via a subsequent interview, especially one with the patient's spouse or caregivers. Second, it does not assess the severity of these behaviors, which is important for monitoring changes in these behaviors. Overall, the C-QUIP is practical as a questionnaire for rapid screening for ICRDs in clinical care and research.
Studies of different populations have reported prevalence rates of ICRDs in PD, ranging from 10.1 to 34.2% in countries outside of China (19, 20). The largest study to date has estimated that ICDs affect 13.6% of patients with PD (21), although the number of patients varies widely across samples. A multi-center longitudinal study found the 5-year cumulative incidence of ICDs was 46.1% (22). The percentage of positive responses for any of the ICRDs in our study was 9.15%, much lower than the rates in Western countries but similar to the rates reported in other studies conducted in China (15, 16). Another Chinese study identified ICDRs in 31.0% of patients with PD, but it used only the QUIP (without a follow-up interview) to clarify the diagnosis, which resulted in higher scores. The lower percentage of ICRDs in Chinese patients with PD than in Western countries may be attributable to lifestyle, cultural background, and medication differences. For instance, patients who self-reported pathological gambling in the QUIP did not meet the diagnostic criteria. Gambling is illegal in China; thus, the prevalence of pathological gambling is lower to some extent.
Previous studies have indicated that numerous controllable and uncontrollable factors are related to the development of ICRDs in PD. Uncontrollable risk factors include male gender (14, 18, 20, 23), young age (5, 20, 23, 24), early age at onset of the disease (5, 20, 23, 24), disease duration (5, 14, 20), and family history of impulsivity (21). Generally speaking, the positive rate of ICDRs in patients with PD is higher in males than in females (14, 18, 20, 23). A multi-center study that assessed Italian patients treated for PD for more than 2 years showed that 32.5% (223/686) of the males and 21.7% (83/383) of the females screened positive for ICDs (20). The gender difference was more pronounced in the analysis of specific ICRDs; hypersexuality was more common among male patients, while compulsive shopping and binge eating were more common among women. Controllable risk factors include smoking (20, 21), alcohol consumption (15, 20), depression (14, 20, 23, 25, 26), the use of DA (15, 21, 23, 25, 26) and levodopa (21), and the dosages of DA (14–17) and levodopa (5, 18). Chinese patients with PD are usually treated with lower dosages of medications. Zhang et al. found a DA dosage >1 mg/d was independently associated with ICRDs in PD (17). Dopamine agonists, such as pramipexole and ropinirole, have a higher affinity for D3 receptors than D1 or D2 receptors (27). D3 receptors are concentrated in the ventral striatum, ventral putamen, globus pallidus, and the medial dorsal nucleus of the thalamus, where they play a role in the mesolimbic reward pathways (28). We found an association of ICRDs with the total daily LED and levodopa dosage. The DA LED in PD-ICRDs was higher than in PD-nonICRDs, but the difference was not statistically significant. The total LED (410.9 ± 318.2 mg/d) and DA LED (45.6 ± 58.0 mg/d) in our study were much lower than those reported in studies conducted in countries outside of China.
Several previous studies found a close relationship between ICRDs and dyskinesia (14, 17). The ICARUS study found more than half of patients with PD and dyskinesia had ICRDs (20), and both ICRDs and dyskinesia were associated with dopaminergic drug therapy. Furthermore, they share common risk factors, including early age at onset of the disease, duration of the disease, and polymorphism of dopamine receptor genes, suggesting they might have similar pathogenic mechanisms (29). However, our study did not find a relationship between ICRDs and dyskinesia.
The limitations of our study include its cross-sectional design, lack of a longitudinal follow-up, and the small sample size of participants. As our study population consisted of only patients who could visit our hospital, we did not analyze data from a random sample. Besides, it is inadequate to complete multiple regression analysis to clarify the risk factors related to ICBs.
Conclusions
In conclusion, we verified the usefulness of the C-QUIP as a rapid screening questionnaire for ICRDs, reported the prevalence and assessed possible risk factors for ICRDs in Chinese patients with PD. Case reports have suggested that reducing or discontinuing dopamine agonists often relieves symptoms of ICRDs (30). However, this medical adjustment might be difficult for some patients; thus, identifying ICRDs in patients with PD will help clinicians make therapeutic decisions and avoid severe consequences.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
TX: data acquisition for the work and original draft preparation. LC: statistical analysis and interpretation of data for the work. WL: revising the work critically for important intellectual content. GZ: design of the work and revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Research Foundation of Zhejiang Health (2020RC061) and the Jinhua Science and Technology Bureau Project (2020–3–004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all of the patients for their participation in this study.
References
1. Weintraub D, David AS, Evans AH, Grant JE, Stacy M. Clinical spectrum of impulse control disorders in Parkinson's disease. Mov Disord. (2015) 30:121–7. doi: 10.1002/mds.26016
2. Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. (2007) 64:1089–96. doi: 10.1001/archneur.64.8.1089
3. Mestre TA, Strafella AP, Thomsen T, Voon V, Miyasaki J. Diagnosis and treatment of impulse control disorders in patients with movement disorders. Ther Adv Neurol Disord. (2013) 6:175–88. doi: 10.1177/1756285613476127
4. Evans AH, Okai D, Weintraub D, Lim SY, O'Sullivan SS, Voon V, et al. Scales to assess impulsive and compulsive behaviors in Parkinson's disease: critique and recommendations. Mov Disord. (2019) 34:791–8. doi: 10.1002/mds.27689
5. Tanaka K, Wada-Isoe K, Nakashita S, Yamamoto M, Nakashima K. Impulsive compulsive behaviors in Japanese Parkinson's disease patients and utility of the Japanese version of the questionnaire for impulsive–compulsive disorders in Parkinson's disease. J Neurol Sci. (2013) 331:76–80. doi: 10.1016/j.jns.2013.05.013
6. Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease. Mov Disord. (2009) 24:1461–7 doi: 10.1002/mds.22571
7. Probst CC, Winter LM, Möller B, Weber H, Weintraub D, Witt K, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease (QUIP) and the QUIP-rating scale in a German speaking sample. J Neurol. (2014) 261:936–42. doi: 10.1007/s00415-014-7299-6
8. Daniel SE, Lees AJ. Parkinson's Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. (1993) 39:165–72
9. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
10. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. (2003) 18:738-50. doi: 10.1002/mds.10473
11. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. (1967) 17:427–42. doi: 10.1212/WNL.17.5.427
12. Qiao J, Wang X, Lu W, Cao H, Qin X. Validation of neuropsychological tests to screen for dementia in Chinese patients. Am J Alzheimers Dis Other Demen. (2016) 31:368–74 doi: 10.1177/1533317515619478
13. Luo W, Gui X, Wang B, Zhang W, Ouyang Z, Guo Y, et al. Validity and reliability testing of the Chinese (mainland) version of the 39-item Parkinson's Disease Questionnaire (PDQ-39). J Zhejiang Univ Sci B. (2010) 11:531–8. doi: 10.1631/jzus.B0900380
14. Auyeung M, Tsoi TH, Tang WK, Cheung CM, Lee CN, Li R, et al. Impulse control disorders in Chinese Parkinson's disease patients: the effect of ergot derived dopamine agonist. Parkinsonism Relat Disord. (2011) 17:635–7. doi: 10.1016/j.parkreldis.2011.06.001
15. Fan W, Ding H, Ma J, Chan P. Impulse control disorders in Parkinson's disease in a Chinese population. Neurosci Lett. (2009) 465:6–9. doi: 10.1016/j.neulet.2009.06.074
16. Wang X, Wei M, Xiao Q. A survey of impulse control disorders in Parkinson's disease patients in Shanghai area and literature review. Transl Neurodegener. (2016) 5:4. doi: 10.1186/s40035-016-0051-7
17. Zhang Y, He AQ, Li L, Chen W, Liu ZG. Clinical characteristics of impulse control and related disorders in Chinese Parkinson's disease patients. BMC Neurol. (2017) 17:98. doi: 10.1186/s12883-017-0874-6
18. Chiang H, Huang Y, Chen S, Wu Y. Are there ethnic differences in impulsive/compulsive behaviors in Parkinson's disease? Eur J Neurol. (2012) 19:494–500. doi: 10.1111/j.1468-1331.2011.03571.x
19. Lee J, Kim J, Kim JW, Cho J, Lee WY, Kim H, et al. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinsonism Relat Disord. (2010) 16:202–7. doi: 10.1016/j.parkreldis.2009.12.002
20. Antonini A, Barone P, Bonuccelli U, Annoni K, Asgharnejad M, Stanzione P. ICARUS study: prevalence and clinical features of impulse control disorders in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2017) 88:317–24. doi: 10.1136/jnnp-2016-315277
21. Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. (2010) 67:589–95. doi: 10.1001/archneurol.2010.65
22. Corvol J, Artaud F, Cormier-Dequaire F, Rascol O, Durif F, Derkinderen P, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology. (2018) 91:e189–201. doi: 10.1212/WNL.0000000000005816
23. Joutsa J, Martikainen K, Vahlberg T, Voon V, Kaasinen V. Impulse control disorders and depression in Finnish patients with Parkinson's disease. Parkinsonism Relat Disord. (2012) 18:155–60. doi: 10.1016/j.parkreldis.2011.09.007
24. Guerra DF, Lemos SA, Paz T, Filho L, Vasconcellos LF, de Britto V, et al. Measurement properties from the Brazilian Portuguese version of the QUIP-RS. NPJ Parkinsons Dis. (2020) 6:6. doi: 10.1038/s41531-020-0108-2
25. Vela L, Martínez Castrillo JC, García Ruiz P, Gasca-Salas C, Macías Macías Y, Pérez Fernández E, et al. The high prevalence of impulse control behaviors in patients with early-onset Parkinson's disease: a cross-sectional multicenter study. J Neurol Sci. (2016) 368:150–4. doi: 10.1016/j.jns.2016.07.003
26. Erga AH, Alves G, Larsen JP, Tysnes OBR, Pedersen KF. Impulsive and compulsive behaviors in Parkinson's disease: the Norwegian ParkWest study. J Parkinsons Dis. (2017) 7:183–91. doi: 10.3233/JPD-160977
27. Seeman P. Parkinson's disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse. (2015) 69:183–9. doi: 10.1002/syn.21805
28. Weintraub D, Mamikonyan E. Impulse control disorders in Parkinson's disease. Am J Psychiatry. (2019) 176:5–11. doi: 10.1176/appi.ajp.2018.18040465
29. Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, et al. Impulse control disorders and levodopa-induced dyskinesias in Parkinson's disease: an update. Lancet Neurol. (2017) 16:238–50. doi: 10.1016/S1474-4422(17)30004-2
Keywords: Parkinson's disease, impulsive-compulsive disorders, impulse control and related disorders, validity, questionnaire
Citation: Xu T, Cao L, Long W and Zhao G (2021) Validation of the Chinese Version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease. Front. Neurol. 12:731552. doi: 10.3389/fneur.2021.731552
Received: 27 June 2021; Accepted: 15 November 2021;
Published: 07 December 2021.
Edited by:
Yih-Ru Wu, Chang Gung Memorial Hospital, TaiwanReviewed by:
Jong-Min Kim, Seoul National University Bundang Hospital, South KoreaJong-Ling Fuh, Taipei Veterans General Hospital, Taiwan
Copyright © 2021 Xu, Cao, Long and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Zhao, Z3poYW8mI3gwMDA0MDt6anUuZWR1LmNu; Wenying Long, MTEzMTgwMzAmI3gwMDA0MDt6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Tian Xu
Tian Xu Lanxiao Cao
Lanxiao Cao Wenying Long
Wenying Long Guohua Zhao
Guohua Zhao