
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 22 September 2021
Sec. Neuro-Ophthalmology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.724946
This article is part of the Research Topic Application of Optical Coherence Tomography Angiography in Retinal and Optic Nerve Disorders View all 5 articles
Purpose: We examined the macular microvascular changes of the macula in neuromyelitis optica spectrum disorder (NMOSD) patients and its association with their disability and other clinical variables.
Methods: Thirty-four NMOSD (13 patients without optic neuritis, NMOSD-NON, and 21 patients with a history of optic neuritis, NMOSD-ON) and 44 healthy controls (HCs) were included in the study. Optical coherence tomographic angiography (OCTA) was used to image the superficial (SCP), deep (DCP), and whole capillary plexus (WCP) in a 2.5-mm-diameter concentric circle [excluding the foveal avascular zone (FAZ)]. An algorithm (Dbox) was used to quantify the complexity of the three capillary layers by fractal analysis. We also evaluated the expanded disability scale status (EDSS).
Results: Dbox values were significantly reduced in SCP (p < 0.001), DCP (p < 0.001), and WCP (p = 0.003) of NMOSD when compared with HCs. Dbox values were significantly reduced in NMOSD eyes with optic neuritis when compared with healthy controls (p < 0.001) and eyes without optic neuritis (p = 0.004) in the SCP. In the DCP, eyes with optic neuritis showed significantly reduced Dbox values when compared with eyes without optic neuritis (p = 0.016) and healthy controls (p < 0.001); eyes without optic neuritis showed significantly reduced Dbox values (p = 0.007) in the DCP when compared with healthy controls. A significant negative correlation (Rho = −0.475, p = 0.005) was shown between the superficial macula Dbox values and the EDSS in NMOSD patients. Additionally, a negative correlation (Rho = −0.715, p = 0.006) was seen in the superficial Dbox values in [e]eyes without optic neuritis and EDSS.
Conclusions: Macular microvascular damage in the superficial plexus is associated with disability in NMOSD. Macular microvascular alterations arise independently of the occurrence of ON in NMOSD.
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune inflammatory disorder of the central nervous system (CNS). NMOSD may target the optic nerve and spinal cord resulting in blindness and paralysis (1). Occasionally, NMOSD may target the brainstem or brain involvement (2–4), which may result in different symptoms of which severe pain (5), cognitive impairment (6), and depression (7) are common. The discovery of aquaporin-4 (AQP4) has enabled a deeper understanding of the pathological process in NMOSD and made its diagnosis much easier (8); additionally, the discovery of AQP4 has helped to distinguish NMOSD from multiple sclerosis (MS) (9). Previous reports have shown that NMOSD causes neurodegeneration and destruction of astrocytes, which leads to severe disability (10, 11).
Optic neuritis is repeatedly severe, and recovery is generally poor in NMOSD patients; as such, permanent damage increases with each inflammatory attack leading to increasing disability (12). Visual impairment, a foremost clinical manifestation in NMOSD, is responsible for severe disability and reduced quality of life (13). The retinal microvasculature is an early and prevalent target of inflammatory attack in NMOSD (14). The alterations in the retinal microvascular network of NMOSD are seen as an indicator of retinopathy onset and development (14–16). Fractal analysis has been reported to provide insight into the development of retinal vascular changes during the disease mechanism (17, 18). The emergence of optical coherence tomography angiography (OCTA) has enabled the non-invasive imaging and visualization of the retinal microvasculature in different layers. Previous reports have shown the retinal changes associated with NMOSD (14, 16, 19), and some have suggested that the retinal damage is associated with its cerebral damage (20). Recent reports (14, 15, 19) have shown that the retinal vasculature is sensitive to the inflammatory attack, and some have suggested it to be a reliable structural biomarker.
Disability has been reported to be an independent predictor of poor quality of life (QOL) in NMOSD (21); however, the macular microvascular changes associated with a disability are underexplored. Our current study aimed to explore macular microvascular changes in NMOSD and its association with the disability in NMOSD patients. These discoveries could be diagnostically beneficial and could have inferences for understanding the pathogenesis of NMOSD.
This study enrolled 36 NMOSD patients and 44 healthy controls. NMOSD patients were enrolled from the Neurology Department of the First Affiliated Hospital, College of Medicine, Zhejiang University, China. The diagnosis of NMOSD patients was based on the revised diagnostic criteria of Wingerchuk et al. (1). All NMOSD patients enrolled in our study were positive for the anti-AQP4 antibody and did not have optic neuritis in the last 6 months. Clinical data, such as disease duration, frequency of optic neuritis, expanded disability status scale (EDSS) and visual acuity, were obtained. Ethical approval for the study was obtained from the institutional Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China. Written informed consent was obtained from each participant, and the protocols of the study were under the Declaration of Helsinki.
Sex- and age-matched healthy controls were recruited from participants who had their annual health checkup at our hospital. Subjects were excluded if they had a systemic or neurological disease that could affect the brain and retina.
Snellen charts at a 2.5-m distance were used as the measurement of visual acuity of each participant, and finger counting [later transformed as visual acuity (22)] was done for patients with poor vision. Visual acuity was transformed to the logarithm of the minimum angle of resolution (LogMAR) for statistical analyses.
Imaging of the macular microvascular was done with the OCTA tool (Cirrus 5000, V.10.0; Zeiss Meditec, CA, USA). The OCTA used a wavelength of 840 nm and an A-scan rate of 68,000 scans per second. Participants were asked to focus on the center of the target in the machine to allow imaging. Imaging of the macula microvasculature was done at the macula with a 3 × 3-mm scan pattern.
For the inclusion criteria in our present study, high-quality images with a signal strength index ≥6 were accepted according to the OSCAR-IB criteria (23).
Quantitative analysis of the macular microvascular fractal dimension (FD) at the superficial capillary plexus (SCP), deep capillary plexus (DCP), and whole capillary plexus (WCP) was done using the en face OCTA images (PNG format) as shown in Figure 1. The superficial retinal capillary plexus (SCP) extended from 3 μm below the internal limiting membrane (ILM) to 15 μm below the inner plexiform layer (IPL); the deep retinal capillary plexus (DCP) extended from 15 to 70 μm below the IPL. The WCP was 3 μm below the internal limiting membrane to the base of the outer plexiform layer (OPL). A parafoveal capillary network was acquired through 3 × 3-mm2 scans within the annular zone of 0.6–2.5-mm diameter around the foveal center. FD measurement was fully automated and implemented on a vasculature segmentation map. To obtain the binary microvasculature network, the U-net framework, which contains an encoder and a decoder component was applied on each two-dimensional OCT-A grayscale image. The used U-net model was pretrained in the ImageNet and then adjusted through our OCTA dataset where the vessel standard was annotated by image experts in SCP, DCP, and WCP layers, respectively (http://www.image-net.org/download-images). The boundary foveal avascular zone (FAZ) was detected in all three capillary plexus layers by the U-net model and later generated binary images of the microvasculature. Based on these binary images, a skeletonized image was created.
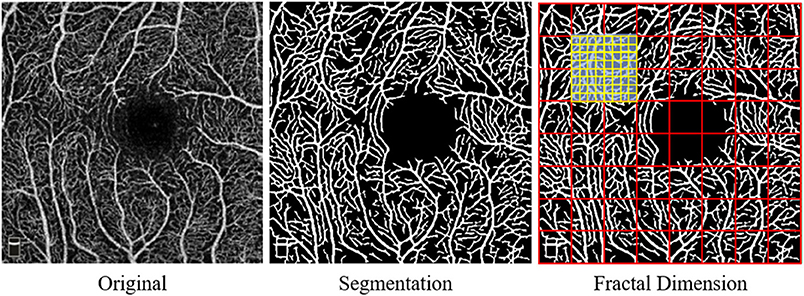
Figure 1. Display of the macular capillary plexus and its quantitative analysis using fractal dimension (FD).
After that, the fractal analysis was done on skeletonized images of the superficial, deep, and whole capillary plexus. The box-counting method was used on each macular microvascular segmentation map to calculate the fractal dimension (FD). Box counting (Dbox values) from Fraclab (https://www.project.inria.fr/fraclab/) was applied to quantify the complexity in all three capillary plexuses (i.e., SCP, DCP, and WCP) in the 2.5-mm diameter of the fovea. The methods above were implemented using MATLAB 2016b (Mathworks, Inc., Natick, MA, USA) (24).
Statistical analyses were done using SPSS software (SPSS, version 24). Student's t-test or Chi-square test was performed to compare groups depending on the normality and properties of the variables. A generalized estimation equation was used for all analyses between and among groups while adjusting for age, gender, signal quality (SQ) of the angiogram, and inter-eye dependencies from the same participant. Pearson's correlation was used to assess correlations between OCTA measures and clinical parameters and after GEE was used while adjusting for signal quality of OCTA images. A value of p < 0.05 represents statistical significance.
We initially enrolled 46 NMOSD patients, but 12 were excluded due to poor image quality (SQ < 6). Thirty-four NMOSD patients (mean age: 52.48 ± 12.99 years) and 44 healthy controls (mean age: 53.11 ± 11.83 years) who were sex- and age-matched were included for data analyses. Out of the 34 NMOSD patients, 13 (38.2%, Table 1) had no history of optic neuritis, 10 (26.3%, Table 1) had experienced optic neuritis once, while 11 (35.5%, Table 1) had experienced optic neuritis twice or more. The mean disease duration of the NMOSD patients was 7.2 ± 5.3 months, while the frequency of optic neuritis was 2.48 ± 1.91 times. Our NMOSD patients were divided into two groups according to the occurrence of optic neuritis: NMOSD without optic neuritis, NMOSD-NON, and NMOSD without optic neuritis, NMOSD-ON. The clinical variable of our participants is shown in Table 1.
VA did not differ (p = 0.063) in NMOSD patients without optic neuritis when compared with healthy controls, but a significant difference was seen in NMOSD patients with optic neuritis when compared with healthy controls (p < 0.001).
Dbox values were significantly reduced in the SCP (p < 0.001, Table 2), DCP (p < 0.001, Table 2), and WCP (p = 0.003, Table 2) of NMOSD patients when compared with healthy controls.
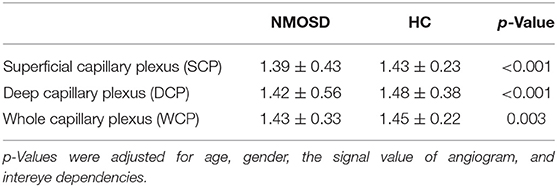
Table 2. Comparison of macula microvascular fractal dimension (FD) between NMOSD and healthy controls (HCs).
Dbox values were significantly reduced in the SCP of NMOSD eyes with optic neuritis when compared with healthy controls (p < 0.001, Figure 2) and NMOSD eyes without optic neuritis (p = 0.004, Figure 2). In the DCP, eyes with optic neuritis showed significantly reduced Dbox values when compared with eyes without optic neuritis (p = 0.016, Figure 2) and healthy controls (p < 0.001, Figure 2); NMOSD eyes without optic neuritis showed significantly reduced Dbox values (p = 0.007, Figure 2) in the DCP when compared with healthy controls. In the WCP, eyes with optic neuritis showed significantly reduced Dbox values when compared with healthy controls (p < 0.001, Figure 2) and eyes without optic neuritis (p = 0.017, Figure 2).
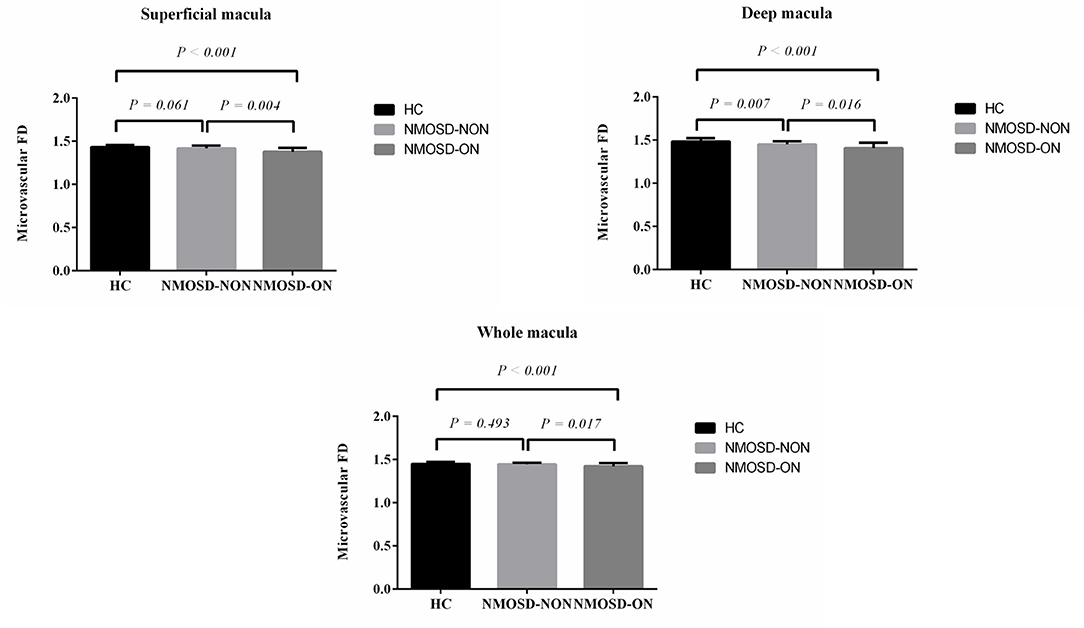
Figure 2. Comparison of macular FD among neuromyelitis optica spectrum disorder (NMOSD) eyes with and without optic neuritis and healthy controls (HC).
A significant negative correlation (Rho = −0.475, p = 0.005; Figure 3) was shown between the SCP Dbox values and the EDSS in all NMOSD patients. Additionally, a negative correlation (Rho = −0.715, p = 0.006; Figure 3) was seen in the SCP Dbox values in eyes without optic neuritis and EDSS. However, no significant correlation was seen between EDSS and the SCP (Rho = −0.221, p = 0.336), DCP (Rho = −0.085, p = 0.714), and WCP (Rho = −0.001, p = 995) in NMOSD eyes with optic neuritis.

Figure 3. Correlation between expanded disability scale status (EDSS) and macula FD in NMOSD patients.
A significant positive correlation (Rho = 0.378, p = 0.011) was seen between the visual acuity and Dbox values in the WCP of healthy controls. A significant negative correlation (Rho = −0.445, p = 0.008) was seen between the visual acuity and Dbox values in the SCP of NMOSD patients.
There was no significant correlation between EDSS and visual acuity in the entire NMOSD group (Rho = 0.477, p = 0.053). No significant correlation (p > 0.05) was seen between the EDSS and visual acuity in the NMOSD subgroups.
To our knowledge, this is the first study to use fractal analysis of OCTA images to evaluate the macular microvasculature complexity in NMOSD and explore the association between the macular microvascular changes and their clinical implication. Our study showed that Dbox values were significantly reduced in the SCP, DCP, and WCP in the eyes with optic neuritis when compared with eyes without optic neuritis. Additionally, we found that the reduced Dbox value in the SCP was significantly associated with the EDSS in NMOSD patients. Our current study suggests that fractal analysis of OCTA images can help in identifying the subclinical vasculopathy in NMOSD before the occurrence of the clinically apparent optic neuritis.
Macular microvascular changes seen in our NMOSD patients when compared with healthy controls are congruent with previous OCTA reports (14–16). A noteworthy finding was that eyes without optic neuritis showed a significantly reduced Dbox value in the DCP when compared with healthy controls. This finding suggests that early macular microvascular alterations associated with NMOSD may first occur in the DCP because this capillary plexus contains capillaries that are smaller and prone to slight changes (25). The Dbox values in eyes with ON were significantly reduced compared with eyes without ON in the superficial, deep, and whole macular microvascular plexus. This may suggest that the amount of dropoff in macular microvascular complexity is linked with and foretells the development of retinopathy in NMOSD after the inflammatory attack(s).
A novel finding in our current report is the association between EDSS and macular microvascular damage in the SCP. EDSS is a method of quantifying disability in NMOSD; furthermore, it has been reported that disability is an independent predictor of poor QOL in NMOSD (21). Previous reports (26–28) on multiple sclerosis (MS) found an inverse correlation between ganglion cell layer–inner plexiform layer (GCIP) thickness and EDSS; these reports suggested that neuronal damage in this portion of the retina reflect the disability of patients in MS. Likewise, the significant inverse correlation between EDSS and macular microvascular damage in NMOSD patients could suggest that the microvascular damage in the SCP, which is found within the retinal nerve fiber layer (RNFL), ganglion cell layer–inner plexiform layer, may affect the disability in NMOSD patients. EDSS scores are rough estimates of clinical status and are weighted toward locomotor incapacity, which is likely dependent on the damage of the spinal cord as previously reported (29). Our report suggests that macular microvascular damage in the SCP may be associated with damage in the spinal cord, and further studies are needed to validate our hypothesis. Besides, the significant inverse correlation between the EDSS and SCP microvascular damage in NMOSD without optic neuritis could also suggest the same hypothesis stated above.
Our study showed a significant correlation under normal conditions between the whole microvasculature and visual acuity indicating that the integrity of the microvasculature contributes to the visual function in a healthy individual. Remarkably, our data showed that a reduction in the Dbox value in the SCP was associated with poor visual acuity in NMOSD patients. Sotirchos et al. (30) suggested that the anterior visual pathway is significantly involved in the subclinical phase of NMOSD; since SCP consists of the sublayers of the retina (retinal ganglion cells, RGCs), we suggest that our current data are congruent with the aforementioned report.
Our study had some limitations. First, the cross-sectional study does not yet prove that microvascular changes seen on the OCTA are necessarily associated with disability and prognosis; longer-term follow-up is needed to demonstrate the correlation between disability progression and retinal microvasculature damage. Also, the cross-sectional study limits our understanding on whether these microvascular changes are comprehensive ischemia or due to changes in the retinal structure. Thus, longitudinal studies are required to warrant our hypotheses on the microvascular changes.
In conclusion, our results indicated that fractal dimensions decrease in NMOSD patients with or without optic neuritis when compared with healthy controls. A significant decrease in the deep capillary plexus in the eyes without optic neuritis shows that macular microvascular damage in the deep plexus may occur before optic neuritis. A novel finding in our report was the possible association between macula microvascular damage in the superficial plexus and disability in NMOSD. We suggest that macular microvascular alterations arise independently of the occurrence of ON in NMOSD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by First Affiliated Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was financially supported by Administration of traditional Chinese medicine project of Zhejiang Province (2018ZA067).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Neurology Department staff for their contribution to this work.
1. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
2. Sato DK, Lana-Peixoto MA, Fujihara K, de Seze J. Clinical spectrum and treatment of neuromyelitis optica spectrum disorders: evolution and current status. Brain Pathol. (2013) 23:647–60. doi: 10.1111/bpa.12087
3. Li Y, Jiang B, Chen B, Zhao M, Zhou C, Wang S, et al. Neuromyelitis optica spectrum disorders with multiple brainstem manifestations: a case report. Neurol Sci. (2016) 37:309–13. doi: 10.1007/s10072-015-2196-z
4. Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. (2014) 20:843–7. doi: 10.1177/1352458513507822
5. Kanamori Y, Nakashima I, Takai Y, Nishiyama S, Kuroda H, Takahashi T, et al. Pain in neuromyelitis optica and its effect on quality of life: a cross-sectional study. Neurology. (2011) 77:652–8. doi: 10.1212/WNL.0b013e318229e694
6. Oertel FC, Schliesseit J, Brandt AU, Paul F. Cognitive impairment in neuromyelitis optica spectrum disorders: a review of clinical and neuroradiological features. Front Neurol. (2019) 10:608. doi: 10.3389/fneur.2019.00608
7. Chavarro VS, Mealy MA, Simpson A, Lacheta A, Pache F, Ruprecht K, et al. Insufficient treatment of severe depression in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e286. doi: 10.1212/NXI.0000000000000286
8. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. (2004) 364:2106–12. doi: 10.1016/S0140-6736(04)17551-X
9. Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler J. (2015) 21:678–88. doi: 10.1177/1352458514567216
10. Popescu BF, Lucchinetti CF. Immunopathology: autoimmune glial diseases and differentiation from multiple sclerosis. Handb Clin Neurol. (2016) 133:95–106. doi: 10.1016/B978-0-444-63432-0.00006-2
11. Wingerchuk DM, Lucchinetti CF. Comparative immunopathogenesis of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol. (2007) 20:343–50. doi: 10.1097/WCO.0b013e3280be58d8
12. Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. (2007) 68:603–5. doi: 10.1212/01.wnl.0000254502.87233.9a
13. Schmidt F, Zimmermann H, Mikolajczak J, Oertel FC, Pache F, Weinhold M, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. (2017) 11:45–50. doi: 10.1016/j.msard.2016.11.008
14. Huang Y, Zhou L, ZhangBao J, Cai T, Wang B, Li X, et al. Peripapillary and parafoveal vascular network assessment by optical coherence tomography angiography in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders. Br J Ophthalmol. (2019) 103:789–96. doi: 10.1136/bjophthalmol-2018-312231
15. Chen Y, Shi C, Zhou L, Huang S, Shen M, He Z. The detection of retina microvascular density in subclinical aquaporin-4 antibody seropositive neuromyelitis optica spectrum disorders. Front Neurol. (2020) 11:35. doi: 10.3389/fneur.2020.00217
16. Kwapong WR, Peng C, He Z, Zhuang X, Shen M, Lu F. Altered macular microvasculature in neuromyelitis optica spectrum disorders. Am J Ophthalmol. (2018) 192:47–55. doi: 10.1016/j.ajo.2018.04.026
17. Moss HE. Retinal vascular changes are a marker for cerebral vascular diseases. Curr Neurol Neurosci Rep. (2015) 15:40. doi: 10.1007/s11910-015-0561-1
18. Li L-J, Ikram MK, Wong TY. Retinal vascular imaging in early life: insights into processes and risk of cardiovascular disease. J Physiol. (2016) 594:2175–203. doi: 10.1113/JP270947
19. Oertel FC, Zimmermann H, Mikolajczak J, Weinhold M, Kadas EM, Oberwahrenbrock T, et al. Contribution of blood vessels to retinal nerve fiber layer thickness in NMOSD. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e338. doi: 10.1212/NXI.0000000000000338
20. Finke C, Zimmermann H, Pache F, Oertel FC, Chavarro VS, Kramarenko Y, et al. Association of visual impairment in neuromyelitis optica spectrum disorder with visual network reorganization. Jama Neurol. (2018) 75:296–303. doi: 10.1001/jamaneurol.2017.3890
21. Chanson JB, Zephir H, Collongues N, Outteryck O, Blanc F, Fleury M, et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol. (2011) 18:836–41. doi: 10.1111/j.1468-1331.2010.03252.x
22. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophth Vis Sci. (2006) 47:1236–40. doi: 10.1167/iovs.05-0981
23. Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PloS ONE. (2012) 7:e34823. doi: 10.1371/journal.pone.0034823
24. Ma Y, Hao H, Xie J, Fu H, Zhang J, Yang J, et al. ROSE: a retinal OCT-angiography vessel segmentation dataset and new model. IEEE Trans Med Imaging. (2021) 40:928–39. doi: 10.1109/TMI.2020.3042802
25. Wang L, Murphy O, Caldito NG, Calabresi PA, Saidha S. Emerging applications of Optical Coherence Tomography Angiography (OCTA) in neurological research. Eye Vis. (2018) 5:11. doi: 10.1186/s40662-018-0104-3
26. Garcia-Martin E, Polo V, Larrosa JM, Marques ML, Herrero R, Martin J, et al. Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology. (2014) 121:573–9. doi: 10.1016/j.ophtha.2013.09.035
27. Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. (2013) 70:34–43. doi: 10.1001/jamaneurol.2013.573
28. Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler J. (2011) 17:1449–63. doi: 10.1177/1352458511418630
29. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/WNL.33.11.1444
Keywords: neuromyelitis optica spectrum disorders, optic neuritis, capillaris, optical coherence tomography angiography, expanded disability status scale
Citation: Wei R, Xie J, Wu H, He F, Meng F, Liu J, Liang H and Zhao Y (2021) Superficial Macula Capillary Complexity Changes Are Associated With Disability in Neuromyelitis Optica Spectrum Disorders. Front. Neurol. 12:724946. doi: 10.3389/fneur.2021.724946
Received: 14 June 2021; Accepted: 03 August 2021;
Published: 22 September 2021.
Edited by:
Rosa Dolz Marco, Catholic University of Valencia San Vicente Mártir, SpainReviewed by:
Itay Lotan, Rabin Medical Center, IsraelCopyright © 2021 Wei, Xie, Wu, He, Meng, Liu, Liang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yitian Zhao, eWl0aWFuLnpoYW9AbmltdGUuYWMuY24=; Ruili Wei, d2VsbHlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.