
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 02 December 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.722924
This article is part of the Research TopicPrecision of Minimally Invasive Surgery for Intracerebral Hemorrhage TreatmentView all 13 articles
Objective: This study aimed to investigate the clinical efficacy of robot-assisted neuroendoscopic hematoma evacuation combined intracranial pressure (ICP) monitoring for the treatment of hypertensive intracerebral hemorrhage (HICH).
Patients and Methods: A retrospective analysis of 53 patients with HICH undergoing neuroendoscopic hematoma evacuation in our department from January 2016 to December 2020 was performed. We divided the patients into two groups: the neuroendoscopic group (n = 32) and the robot-assisted neuroendoscopic combined ICP monitoring group (n = 21). Data on clinical characteristics, treatment effects, and outcomes were retrospectively reviewed and analyzed between these two groups.
Results: The operation time of the procedure of the neuroendoscopic group was significantly longer than that of the robot-assisted neuroendoscopic combined ICP-monitoring group (mean time 153.8 ± 16.8 vs. 132.8 ± 15.7 min, P < 0.001). The intraoperative blood loss was significantly less in the robot-assisted neuroendoscopic combined ICP-monitoring group than in the neuroendoscopic group (215.4 ± 28.3 vs. 190.1 ± 25.6 ml, P = 0.001). However, the patients undergoing neuroendoscopic had a comparable hematoma clearance rate with those undergoing robot-assisted neuroendoscopic combined ICP monitoring (85.2 ± 4.8 vs. 89.2 ± 5.4%, P = 0.997). The complications rate was greater in the endoscopic group (25%) than in the robot-assisted neuroendoscopic combined ICP-monitoring group (9.5%) but without significant difference (P = 0.159). We also found that the dose of used mannitol was significantly less in the ICP monitoring group (615.2 ± 63.8 vs. 547.8 ± 65.3 ml, P < 0.001) and there was a significant difference in modified Rankin scale (mRS) score at discharge, patients with less mRS score in the robot-assisted neuroendoscopic combined ICP monitoring group than in the neuroendoscopic group (3.0 ± 1.0 vs. 3.8 ± 0.8, p = 0.011). Patients undergoing robot-assisted neuroendoscopic combined ICP monitoring had better 6-month functional outcomes, and there was a significant difference between the two groups (p = 0.004). Besides, multivariable analysis shows younger age, no complication, and robot-assisted neuroendoscopic combined ICP monitoring were predictors of 6-month favorable outcomes for the patients with HICH.
Conclusion: Robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring appears to be safer and more effective as compared to the neuroendoscopic hematoma evacuation in the treatment of HICH. Robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring might improve the clinical effect and treatment outcomes of the patients with HICH.
Hypertensive intracerebral hemorrhage (HICH) is a common serious cerebrovascular disease with high mortality and morbidity rate, which frequently occurs in the middle-aged and elderly population, with a peak incidence in winter and spring (1–3). Due to the influence of long-term hypertension, it can cause arteriosclerosis in the brain, decrease the elasticity of the blood vessel wall, increase the brittleness, cause cellulose necrosis, and promote the formation of a miliary aneurysm. Once the blood pressure rises, the aneurysm will rupture and cause cerebral hemorrhage. Owing to the harmfulness and the risks of HICH, the patients should get timely and effective treatment (4, 5).
At present, surgical treatment and conservative medical methods are the two main options for patients with HICH, but there is no uniform standardized treatment method. The surgical methods mainly include conventional craniotomy, small-bone window craniotomy, stereotactic aspiration, neuroendoscopic hematoma evacuation, and so on (6, 7). The principle of operation is to clear the hematoma and promote the recovery of neurological function. With the recent advances in endoscopic technique, neuroendoscopes have been used for the treatment of intracranial cysts and hydrocephalus and as an adjunct during microsurgical tumor removal. Meanwhile, neuroendoscopic hematoma evacuation has been applied widely in the treatment of patients with HICH, which is less invasive than craniotomy and can also easily achieve hemostasis of the bleeding vessels (8, 9). However, neuroendoscopic hematoma evacuation also has its inherent shortcomings such as a narrow surgical window results in a low-removal rate, sometimes cannot reach the designated position accurately and rapidly, and should be performed by a skilled surgeon. To the best of our knowledge, there have been several reports on the use of robotic devices in neuroendoscopic surgery. Zimmermann et al. (10) demonstrated their preliminary experience with robot-assisted navigated endoscopic third ventriculostomies, the authors proved for the first time that robot assistance allows highly exact and repeatable positioning of a rigid endoscope with an accuracy of 50 mm, a very smooth and slow insertion of the endoscope within the brain tissue. According to this, neuro-navigation and robotic technologies have been developed and used in surgical procedures to reduce brain injury and improve clinical outcomes and prognosis of patients with HICH (11).
In the meantime, patients still have many complications after the operation, including rehemorrhage, brain swelling, and cerebral ischemia that can cause increased intracranial pressure (ICP), thus, ICP monitoring in the treatment of patients with HICH is of great importance for preventing the occurrence of complications. Many scholars have reported that ICP monitoring was associated with a good prognosis (12, 13). In our study, we retrospectively reviewed 53 patients with HICH undergoing neuroendoscopic hematoma evacuation in the Tongji Hospital, the purpose of this study was to investigate the clinical efficacy of robot-assisted neuroendoscopic hematoma evacuation and ICP monitoring for the treatment of HICH.
This retrospective study was permitted and sponsored by the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. January 2016 to December 2020, we reviewed 53 patients who underwent neuroendoscopic hematoma evacuation at the Department of Neurosurgery, Tongji Hospital. The patients were divided into two groups according to the surgical strategy: the neuroendoscopic group (n = 32) and the robot-assisted neuroendoscopic combined ICP monitoring group (n = 21). The clinical data regarding patient age, sex, neuroimaging features, outcomes, postoperative complications, and the duration of the operation procedure and hospitalization were retrospectively analyzed. The inclusion criteria were as follows: patients with HICH were confirmed by CT scan with hematoma volume > 30 ml; the past medical history of hypertension; disease onset within 24 h and GCS score ≥ 4. The exclusion criteria were as follows: hemorrhage caused by aneurysm, trauma, tumor, arteriovenous malformation, venous sinus thrombosis; preoperative administration of antiplatelet or anticoagulant drugs; patients with severe systematic comorbidities.
All the surgeries were performed by the same neurosurgeon, Professor Kai SHU, Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, who performed 300 operations of robotic surgery every year. Thirty-two patients underwent neuroendoscopic hematoma evacuation, and 21 patients underwent robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring. All the patients underwent CT angiography to exclude arteriovenous malformation and aneurysm before surgery. We used the Glasgow Coma Scale (GCS) and the National Institutes of Health Stroke Scale (NIHSS) to assess the neurological function of patients with HICH at admission. The Coniglobus formula was used to estimate the hematoma volumes, that is, volume = (length × width × thickness)/2. And the hematoma clearance rate =100- (postoperative volume)/(preoperative volume) × 100. All the patients were followed up for 6 months.
For the neuroendoscopic hematoma evacuation procedures, after skin disinfection, a burr hole was made on the skull nearest to the hematoma for patients with lobar hemorrhage or the forehead at the site 1.5 cm inside the hairline and 2.5–3.5 cm from the midline for the patients with basal ganglia hemorrhage. A small-bone window was opened with a size of 3 cm diameter by using the milling cutter. Then, a sheath with stylet inside was inserted after the dura matter was incised. Based on the location and volume of the hematoma, the depth and orientation of the sheath were determined by the experience of the surgeon. The stylet was removed and an endoscope with 0 or 30 degrees was inserted once the sheath reached the hematoma. The hematoma was removed under neuroendoscope. The hematoma cavity was filled with absorbable hemostatic gauze, and a monopolar electrocautery probe was used for hemostasis when artery bleeding was seen. After the hematoma was evacuated, the drainage tube was inserted into the hematoma cavity and we sutured and disinfected the wound.
For the robot-assisted neuroendoscopic hematoma evacuation combined ICP monitoring procedures, markers were attached to the temple and forehead of the patient, and then CT scans were performed before operation. All images were copied to the Remebot robot system (developed by Beijing Baihuiweikang Technology Company, and approved by the National Medical Products Administrations, China), and then the entry point, the range of hematoma, and optimal trajectory were carefully planned by the surgeon. The head of the patient was immobilized in a Mayfield clamp after general anesthesia. After accurate registration, a burr hole was drilled based on preoperative planning. After the dura matter was incised, a sheath with stylet inside was inserted into the hematoma cavity with the assist of a Remebot robot. The methods of removing and aspirating hematoma, hemostasis, and suturing were the same as the treatment in the neuroendoscopic hematoma evacuation group. And we inserted a drainage tube with the Codman ICP monitoring probe to the ventricle or hematoma cavity (Figure 1). The drainage tube with the Codman ICP monitoring probe was inserted into the ventricle for patients who were complicated with ventricular hemorrhage. The drainage tube with the Codman ICP monitoring probe was inserted into the hematoma cavity for patients with HICH with no involvement of the ventricular system. Management of different dosages of mannitol was guided by ICP changes: for the patients with ICP higher than 25 mmHg, 125 ml of 20% mannitol every 6 h was used to decrease ICP to maintain ICP lower than 15 mmHg, also emergent CT scan would be performed if necessary; for ICP between 15 and 25 mmHg, 125 ml of 20% mannitol compound was intravenously injected every 8–12 h; and for ICP between 0 and 15 mmHg, it would be considered as normal.
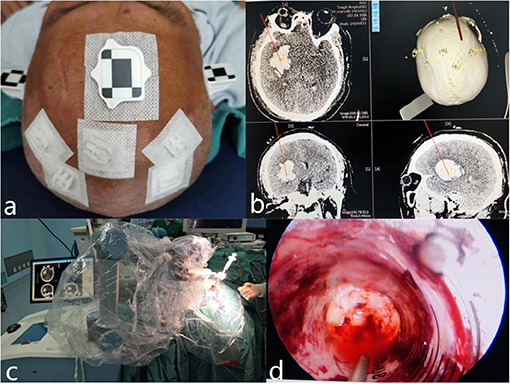
Figure 1. Representative images of robot-assisted neuroendoscopic hematoma evacuation combined ICP monitoring surgical workflow. (a) Markers were attached to the temple and forehead of the patient, and then CT scans were performed before the operation. (b) The entry point, the range of hematoma, and optimal trajectory were carefully planned by the surgeon. (c) After the dura matter was incised, a sheath with stylet inside was inserted into the hematoma cavity with the assist of a Remebot robot. (d) The hematoma was removed under neuroendoscope.
Statistical analysis was performed using SPSS Statistics 22.0 (IBM Corporation, USA). Data are described as ± s. The intergroup comparison was performed using the Student's t-test and the chi-squared test. Predictors of 6-month favorable outcomes were identified by using multiple-variable logistic regression (variables with statistical significance in univariate analysis were entered into a multivariate analysis). p < 0.05 was considered to be statistically significant.
Baseline characteristics are all shown in Table 1. A total of 53 patients were identified, with 34 men (64.2%) and 19 women (35.8%). The mean age of the population was 56.5 ± 9.1 years. There were no significant differences in patient age, sex, the GCS score, systolic pressure admission, the NIHSS score, localization of hematoma, hematoma volume, or midline shift between the neuroendoscopic group, and the robot-assisted neuroendoscopic hematoma evacuation combined ICP monitoring group.
The operation time of the procedure of the neuroendoscopic group was significantly longer than that of the robot-assisted neuroendoscopic combined ICP-monitoring group (mean time 153.8 ± 16.8 vs. 132.8 ± 15.7 min, P < 0.001). The intraoperative blood loss was significantly less in the robot-assisted neuroendoscopic combined ICP-monitoring group than in the neuroendoscopic group (215.4 ± 28.3 vs. 190.1 ± 25.6 ml, P = 0.001). However, the patients undergoing neuroendoscopic had a comparable hematoma clearance rate with those undergoing robot-assisted neuroendoscopic combined ICP monitoring (85.2 ± 4.8 vs. 89.2 ± 5.4%, P = 0.997). The complications rate was greater in the endoscopic group (25%) than in the robot-assisted neuroendoscopic combined ICP-monitoring group (9.5%) but without significant difference (p = 0.159). Complications occurred in eight cases of the neuroendoscopic group, including four patients with pulmonary infection, two with digestive tract hemorrhage, one with bleeding recurrence, and one who experienced a seizure. In the robot-assisted neuroendoscopic combined ICP monitoring group, one patient experienced pulmonary infection and one patient with digestive tract hemorrhage. The postoperative duration of hospitalization of the neuroendoscopic group was significantly longer than the robot-assisted neuroendoscopic combined ICP monitoring group (mean 13.8 ± 3.3 vs. 11.1 ± 2.8 days, P = 0.016).
We also used the modified Rankin scale (mRs) to evaluate the neurological function recovery state of the patients. We found that the dose of used mannitol was significantly less in the ICP monitoring group (615.2 ± 63.8 vs. 547.8 ± 65.3 ml, P < 0.001), and there was a significant difference in mRS score at discharge, patients with less mRS score in the robot-assisted neuroendoscopic combined ICP monitoring group than in the neuroendoscopic group (3.0 ± 1.0 vs. 3.8 ± 0.8, p = 0.011). Moreover, there was no significant difference in mortality rate between the two groups. The patients were followed up for 6 months, and we utilized Glasgow Outcome Scale to assess the clinical outcome of the patients. In the endoscopic group, 16 patients had a good recovery, 13 patients had moderate disability, one patient was with a vegetative state, and two patients died. In the robot-assisted neuroendoscopic combined ICP-monitoring group, 15 patients had a good recovery, four patients had moderate disability, one patient was with a vegetative state, and one patient died. Patients undergoing robot-assisted neuroendoscopic combined ICP monitoring had better 6-month functional outcomes, and there was a significant difference between the groups (P = 0.004). These results are all summarized in Table 2. Table 3 summarizes the results from single and multiple logistic regression of Predictors of 6-month favorable outcomes. On single-variable logistic regression, younger age, less hematoma volume, not deep location, no complication, and robot-assisted neuroendoscopic hematoma combined with ICP monitoring were independent predictors of 6-month favorable outcomes. Sex, GCS score, systolic pressure admission, NIHSS score, the bleeding side of brain hemisphere, midline shift, operation time, intraoperative blood loss, hematoma clearance rate, a dose of mannitol, and postoperative duration of hospitalization were not significant prognostic factors for survival (P > 0.05). Multivariable analysis identified younger age, no complication and robot-assisted neuroendoscopic hematoma combined with ICP monitoring were predictors of 6-month favorable outcomes. There are four typical examples of the robot-assisted neuroendoscopic combined ICP-monitoring group in Figure 2.
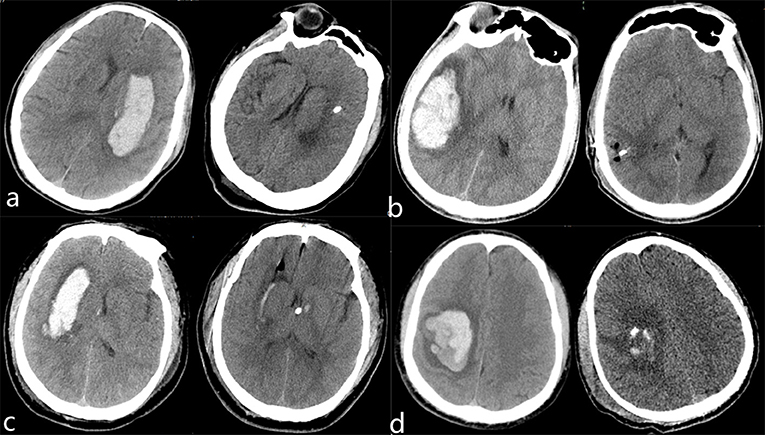
Figure 2. Preoperative and postoperative CT images in robot-assisted neuroendoscopic hematoma evacuation combined ICP monitoring of representative patients with HICH (a–d). (a) A 62-year-old female was presented with a headache and right hemiplegia for 4 h. The hematoma was removed completely and the symptoms of headache were improved obviously. Continuous rehabilitation training was given to the patient after discharge. (b) A 54-year-old male presented with a headache for 10 h. Preoperative CT shows right temporal lobe hemorrhage that has been evacuated totally. (c) A 65-year-old male was presented with consciousness disturbance for 5 h. Preoperative CT scan shows right basal ganglia hemorrhage, which has been evacuated and we inserted a drainage tube with the Codman ICP monitoring probe to the ventricle. (d) A 73-year-old male was presented with left hemiplegia for 3 h. Preoperative CT shows right parietal lobe hemorrhage, which has been evacuated and we inserted a drainage tube with the Codman ICP monitoring probe to the hematoma cavity. Continuous rehabilitation training was given to the patient after they were discharged.
With the current social improvement of living standards, more and more people are suffering from hypertension, and there are ~200 million patients with hypertension in China according to the latest epidemiological data (14). HICH is one of the most serious complications of hypertension because of its high morbidity and mortality rate, which accounts for 70–80% of a spontaneous cerebral hemorrhage. The mass effect of hematoma can cause primary brain injury in a short time and perihematoma edema is the main source of secondary cerebral damage (15). Therefore, surgical treatment has become an effective treatment for HICH patients because it can release the compression of the hematoma rapidly and reduce ICP at an early stage. A traditional craniotomy is not widely applied because of its disadvantages of severe trauma and postoperative complications. Currently, minimally invasive puncture drainage, small bone-window craniotomy, and neuroendoscopic hematoma evacuation are widely recognized by neurosurgeons. Because they have their advantages and limitations, experts and scholars have not reached a unified consensus about which of these surgical treatments is the most effective until now (16–18).
Nowadays, neuroendoscopic hematoma evacuation has been widely applied in the treatment of patients with HICH owing to its advantages of a clear operative field, short time, less bleeding, and injury (8, 9, 11, 19). However, there are also some limitations of the neuroendoscopic procedures, such as a narrow surgical window results in a low removal rate, sometimes cannot reach the designated position accurately and rapidly, and should be performed by a skilled surgeon. Hayashi et al. (11) reported that the surgeons should have experience doing the endoscopic procedure to achieve a good removal rate. Atsumi et al. (20) showed that the navigation system is beneficial for avoiding a burr hole exactly above the transverse and sigmoid sinus confirming the direction of hematoma extension in the neuroendoscopic surgery. For these reasons, we introduced the Remebot robot to improve clinical outcomes and prognosis of patients with HICH. The Remebot robot system has been successfully used in a variety of surgical methods that were designed and produced in China. We have examined the accuracy of the Remebot robot in several applications such as stereotactic brain biopsy and have shown it to be accurate (21). In addition, Wang et al. (14) showed that robot-assisted surgery using a Remebot is a safe and effective treatment method for hematoma removal and tube drainage in patients with HICH, and the target error is <1 mm. To the best of our knowledge, this is the first report to perform robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring for patients with HICH. In this study, we found that the operation time of the procedure of the neuroendoscopic group was significantly longer than that of the robot-assisted neuroendoscopic combined ICP monitoring group (mean time 153.8 ± 16.8 vs. 132.8 ± 15.7 min, P < 0.001) and the intraoperative blood loss was significantly less in the robot-assisted neuroendoscopic combined ICP monitoring group than in the neuroendoscopic group (215.4 ± 28.3 vs. 190.1 ± 25.6 ml, p = 0.001). The Remebot robot navigation system has several benefits. First, because a supine lateral position and other positions can lead to disorientation, the robot system could help us to confirm whether the selection of the site of the burr hole was satisfactory. Second, it could help us better confirm the direction of hematoma extension and the depth to complete the hematoma extraction, which can reduce the number of punctures and trauma of most puncture wounds.
Moreover, postoperative management has important value for the recovery of patients with HICH. As is well-known, ICP monitoring has been widely used in the field of head trauma and neuro-critical care. MacLaughlin et al. (22) found that ICP monitoring was associated with a significant decrease in mortality rate through a retrospective analysis of 123 patients with severe traumatic brain injury. Many researchers also showed that ICP monitoring is crucial in the postoperative management of patients with HICH because it can improve postoperative treatment and prognosis by detecting the information of any abnormal increase of ICP in time (13). The application of mannitol drugs could reduce ICP of the patients with HICH after surgery and reduce the risk of rehemorrhage and brain edema. However, excessive use of mannitol drugs increases the risk of complications such as electrolyte disturbances, the imbalance of body fluid, and acute renal failure that can result in rapid clinical deterioration and increase the length of recovery. Without ICP monitoring, we made the postoperative treatment decisions that depends on clinical signs and imaging methods. In our study, we found that the postoperative duration of hospitalization of the neuroendoscopic group was significantly longer than the robot-assisted neuroendoscopic combined ICP-monitoring group (mean 13.8 ± 3.3 vs. 11.1 ± 2.8 days, p = 0.016), the dose of used mannitol was significantly less in the ICP monitoring group (615.2 ± 63.8 vs. 547.8 ± 65.3 ml, p < 0.001). In the meantime, there was no significant difference in mortality rate between the two groups, but there was a significant difference in mRS score at discharge, patients with less mRS score in the robot-assisted neuroendoscopic combined ICP-monitoring group than in the neuroendoscopic group (3.0 ± 1.0 vs. 3.8 ± 0.8, p = 0.011). The results showed that ICP monitoring could significantly reduce the postoperative duration of hospitalization and mannitol use because it can help the clinicians to initiate intervention timely before the irreversible cerebral damage. The complications rate was greater in the endoscopic group (25%) as compared with the robot-assisted neuroendoscopic combined ICP-monitoring group (9.5%) but without significant difference (p = 0.159). This could be explained by the limited number of patients.
Furthermore, patients undergoing robot-assisted neuroendoscopic combined ICP monitoring had better 6-month functional outcomes, and there was a significant difference between the groups (P = 0.004). Besides, multivariable analysis shows younger age, no complication, and robot-assisted neuroendoscopic combined ICP monitoring were predictors of 6-month favorable outcomes for the patients with HICH. The results are in accordance with those of previous literature. Che et al. (12) reported that a good 6-month functional recovery was associated with ICP monitoring. Ferrete-Araujo et al. (23) performed a prospective observational study of 186 patients with severe ICH, which showed that ICP monitoring and early operation were predictors of longer survival and better functional outcomes. For these reasons, we proposed that robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring should be used for patients with HICH. Because it has the advantages of less operation time and intraoperative blood loss, less mannitol use and postoperative duration of hospitalization, and better functional recovery.
There are several limitations of our study. The primary limitations are its retrospective design and the number of patients enrolled was still not large enough, which limits the power of statistical tests. Moreover, the findings are limited by the presence of selection bias, as described previously, and the lack of multicenter participation. Therefore, a prospective, randomized controlled trial is needed to further evaluate the effect of robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring for the treatment of HICH.
Robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring appears to be safer and more effective than neuroendoscopic hematoma evacuation in the treatment of HICH. Robot-assisted neuroendoscopic hematoma evacuation combined with ICP monitoring might improve the clinical effect and treatment outcomes of the patients with HICH.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethical Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KS contributed to the conception and design of this study. SW and HW contributed to data analysis and wrote the manuscript. WJ and TL contributed to the data analysis and to modify the article. FH and JW contributed to the data collection and data interpretation. All authors read and approved the final manuscript.
This study was funded by the Research-type Clinician funding plan of Tongji Medical College, Huazhong University of Science and Technology (Grant No.5001540025).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the patients and their families.
1. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. (2007) 38:2001–23. doi: 10.1161/STROKEAHA.107.183689
2. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
3. Zurasky JA, Aiyagari V, Zazulia AR, Shackelford A, Diringer MN. Early mortality following spontaneous intracerebral hemorrhage. Neurology. (2005) 64:725–7. doi: 10.1212/01.WNL.0000152045.56837.58
4. Zuccarello M, Brott T, Derex L, Kothari R, Sauerbeck L, Tew J, et al. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. (1999) 30:1833–9. doi: 10.1161/01.STR.30.9.1833
5. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. (2014) 9:840–55. doi: 10.1111/ijs.12309
6. Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. (2016) 15:1228–37. doi: 10.1016/S1474-4422(16)30234-4
7. Tang Y, Yin F, Fu D, Gao X, Lv Z, Li X. Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. BMC Neurol. (2018) 18:136. doi: 10.1186/s12883-018-1138-9
8. Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. J Neurosurg. (2012) 117:767–73. doi: 10.3171/2012.7.JNS111567
9. Nishihara T, Nagata K, Tanaka S, Suzuki Y, Izumi M, Mochizuki Y, et al. Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care. (2005) 2:67–74. doi: 10.1385/NCC:2:1:067
10. Zimmermann M, Krishnan R, Raabe A, Seifert V. Robot-assisted navigated neuroendoscopy. Neurosurgery. (2002) 51:1446–52. doi: 10.1097/00006123-200212000-00015
11. Hayashi T, Karibe H, Akamatsu Y, Narisawa A, Shoji T, Sasaki T, et al. Endoscopic hematoma evacuation for intracerebral hemorrhage under local anesthesia: factors that affect the hematoma removal rate. World Neurosurg. (2019) 126:e1330–e6. doi: 10.1016/j.wneu.2019.03.089
12. Che XR, Wang YJ, Zheng HY. Prognostic value of intracranial pressure monitoring for the management of hypertensive intracerebral hemorrhage following minimally invasive surgery. World J Emerg Med. (2020) 11:169–73. doi: 10.5847/wjem.j.1920-8642.2020.03.007
13. Men D, Huang Z, Yin Y, Wu W, Li W, Liu H, et al. Advantages of small bone-window craniotomy under microscope combined postoperative intracranial pressure monitoring in the treatment of hypertensive intracerebral hemorrhage. J Craniofac Surg. (2021) 32:e77–e80. doi: 10.1097/SCS.0000000000006986
14. Wang T, Zhao QJ, Gu JW, Shi TJ, Yuan X, Wang J, et al. Neurosurgery medical robot Remebot for the treatment of 17 patients with hypertensive intracerebral hemorrhage. Int J Med Robot. (2019) 15:e2024. doi: 10.1002/rcs.2024
15. Lee S, Cho BK, Kim H. Hypertensive encephalopathy with reversible brainstem edema. J Korean Neurosurg Soc. (2013) 54:139–41. doi: 10.3340/jkns.2013.54.2.139
16. Zhang HT, Chen LH, Xu RX. Portable 3D-Head Computed Tomography (CT) navigation-guided key-hole microsurgery for spontaneous hypertensive hemorrhages. Med Sci Monit. (2019) 25:10095–104. doi: 10.12659/MSM.918815
17. Sun S, Li Y, Zhang H, Gao H, Zhou X, Xu Y, et al. Neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. (2020) 134:477–88. doi: 10.1016/j.wneu.2019.10.115
18. Liu J, Cheng J, Zhou H, Deng C, Wang Z. Efficacy of minimally invasive surgery for the treatment of hypertensive intracerebral hemorrhage: a protocol of randomized controlled trial. Medicine (Baltimore). (2021) 100:e24213. doi: 10.1097/MD.0000000000024213
19. Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G, et al. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy. J Neurosurg. (2018) 128:553–9. doi: 10.3171/2016.10.JNS161589
20. Atsumi H, Baba T, Sunaga A, Sakakibara Y, Nonaka Y, Sorimachi T, et al. Neuroendoscopic evacuation for spontaneous cerebellar hemorrhage is a safe and secure approach and may become a mainstream technique. Neurol Med Chir (Tokyo). (2019) 59:423–9. doi: 10.2176/nmc.oa.2019-0108
21. Wu S, Wang J, Gao P, Liu W, Hu F, Jiang W, et al. A comparison of the efficacy, safety, and duration of frame-based and Remebot robot-assisted frameless stereotactic biopsy. Br J Neurosurg. (2021) 35:319–23. doi: 10.1080/02688697.2020.1812519
22. MacLaughlin BW, Plurad DS, Sheppard W, Bricker S, Bongard F, Neville A, et al. The impact of intracranial pressure monitoring on mortality after severe traumatic brain injury. Am J Surg. (2015) 210:1082–7. doi: 10.1016/j.amjsurg.2015.08.007
Keywords: hypertensive intracerebral hemorrhage, neuroendoscopic hematoma evacuation, intracranial pressure monitoring, Remebot robot, clinical effect
Citation: Wu S, Wang H, Wang J, Hu F, Jiang W, Lei T and Shu K (2021) Effect of Robot-Assisted Neuroendoscopic Hematoma Evacuation Combined Intracranial Pressure Monitoring for the Treatment of Hypertensive Intracerebral Hemorrhage. Front. Neurol. 12:722924. doi: 10.3389/fneur.2021.722924
Received: 09 June 2021; Accepted: 26 October 2021;
Published: 02 December 2021.
Edited by:
Hasegawa Yu, International University of Health and Welfare, JapanReviewed by:
Siying Song, Capital Medical University, ChinaCopyright © 2021 Wu, Wang, Wang, Hu, Jiang, Lei and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Shu, a3NodUB0amgudGltdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.