
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 04 November 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.712024
This article is part of the Research Topic Challenges in Acute Minor Ischemic Stroke View all 14 articles
Objectives: Diabetes mellitus (DM) is a significant risk factor for ischemic stroke and associated with platelet reactivity. We aim to evaluate the effect of DM on platelet function in acute ischemic stroke patients taking dual antiplatelet therapy (DAPT).
Methods: We consecutively included patients with acute ischemic stroke taking DAPT. Platelet function was assessed by thromboelastography and the arachidonic acid (AA) or adenosine diphosphate (ADP) induced platelet inhibition rate were used to confirmed the high-residual on-treatment platelet reactivity (HRPR) to aspirin or clopidogrel. We classified patients into DM and non-DM groups. The association between DM and platelet function was assessed and the confounding factors were adjusted by propensity score matching (PSM) analysis. The independent risk factors of HRPR were determined by multivariate logistic regression analysis.
Results: A total of 1,071 acute ischemic stroke patients, 712 in the non-DM group and 359 in the DM group, were included. Patients with DM had a significantly higher maximum amplitude (63.0 vs. 62.0 mm, P < 0.01), ADP-induced clot strength (34.6 vs. 30.3 mm, P < 0.01) and clopidogrel HRPR rate (22.6% vs. 17.3%, P = 0.038) than those without DM. Among 662 patients after PSM, the maximum amplitude (63.1 vs. 62.5 mm, P = 0.032), ADP-induced clot strength (34.6 vs. 29.3 mm, P < 0.01) and clopidogrel HRPR rate (23.0% vs. 15.7%, P = 0.018) is still higher in the DM group. DM was an independent factor of clopidogrel HRPR (OR = 1.48, 95% CI: 1.03–2.07, P < 0.05).
Conclusions: In acute ischemic stroke patients taking DAPT, DM is associated with increased platelet reactivity and higher prevalence of clopidogrel HRPR.
It is well-known that diabetes mellitus (DM) could increase the risk for cerebrovascular diseases. It has been reported that 25–45% of ischemic stroke patients have confirmed DM (1–3). Previous studies have suggested that DM substantially increases the risk for first ischemic stroke (4–6). In addition, DM is associated with increased mortality (7) and recurrence rate of ischemic stroke (8–10).
In the acute treatment and secondary prevention of ischemic stroke, aspirin and clopidogrel are the most widely used antiplatelet drugs in clinical practice. They not only significantly reduce the mortality and disability rate, but also effectively prevent stroke recurrence (11). However, 10–20% of ischemic stroke patients will have new vascular events in the first 3 months despite have received antiplatelet therapy (12–14). One possible reason is the high residual on-treatment platelet reactivity (HRPR), which means the reduced platelet inhibition rate and absence of antiplatelet effect (15).
Studies have found that HRPR may increase the prevalence of ischemic events in coronary heart disease and diabetes patients (16, 17), which may be due to generally higher aggregation activity of platelet and less responsiveness to antiplatelet drugs (18) in patients with DM. Most previous studies on platelet function have focused on individuals with cardiovascular disease. However, studies on the relationship between DM and platelet function in acute ischemic stroke patients taking dual antiplatelet therapy (DAPT) remain scarce. The present study aimed to evaluate whether DM had an effect on platelet function in acute ischemic stroke patients taking DAPT.
This study retrospectively included consecutive patients with acute ischemic stroke hospitalized in the Department of Neurology, Tongji Hospital, from September 2013 and May 2019. The inclusion criteria included: (1) ≥18 years old; (2) diagnosis of acute ischemic stroke on the basis of clinical symptoms and magnetic resonance imaging or computer tomography; (3) having received treatment of clopidogrel (75 mg/day) plus aspirin (100 mg/day) without a loading dose for at least 7 days before platelet function testing. Patients would be excluded if: (1) having medications within the past 3 months affecting blood coagulation function, such as cilostazol, warfarin, dabigatran, heparin, and factor Xa inhibitors (such as rivaroxaban); (2) a history of malignant tumors, digestive diseases, or severe liver, kidney, or blood-related diseases.
This study has been approved by the Tongji Hospital Ethics Committee (No. TJ-IRB20210107). The requirement of informed consent was waived since all data analyzed in this study were anonymized and cannot do any harm to the subjects.
The demographic and clinical information included: age, sex, smoking (defined as a history of smoking ≥1 cigarette per day for 1 year or more), alcohol intake (defined as weekly alcohol intake exceeding 200 g for 1 year or more), a history of ischemic stroke/transient ischemic attack, hypertension, hyperlipidemia, coronary heart disease (defined as a history of myocardial infarction or angina pectoris) and DM (defined as a HbA1c ≥ 6.5%, or a 2 h plasma glucose ≥ 11.1 mmol/L during an oral glucose tolerance test, or self-reported history of DM) (8, 19, 20). Laboratory test were conducted within 24 h of admission, included creatinine, eGFR, platelet indexes, fasting glucose, and glycosylated hemoglobin A1c (HbA1c). These data were obtained from the hospital medical records.
The platelet function was assessed using a thromboelastography (TEG) Analyzer 5000 (Haemonetics Corporation, USA) with assays taken within 1 h following blood sample collection. Peripheral venous whole blood was collected by venipuncture in vacutainer tubes containing 3.2% sodium citrate and sodium heparin (Becton–Dickinson, San Jose, CA) at least 7 days after patients had received DAPT and 12 h after the last dose in our study. The TEG was performed using standard methods in hospital laboratories according to manufacturers' instructions. Four channels were used to detect the effects of antiplatelet therapy with arachidonic acid (AA) and adenosine diphosphate (ADP) activators at 37°C. The maximum amplitude (MA) was designated as the maximum intensity obtained from a clot, which represented the maximum platelet function that observed in a blood sample. MAADP represented the ADP-induced clot strength, MAAA was the AA-induced clot strength, MAfibrin was the activator-induced clot strength (measurement of fibrin contribution), and MAthrombin was the thrombin-induced clot strength. The platelet inhibition rate induced by AA or ADP was calculated by computer software according to the following formula: Inhibition rate (%) = [(MAthrombin – MAADP or MAAA)/(MAthrombin – MAfibrin)] × 100%, which represented the responsiveness to aspirin or clopidogrel. AA% < 50% can be considered as aspirin HRPR and ADP% < 30% or MAADP > 47 mm can be considered as clopidogrel HRPR (21, 22).
Statistical analysis was performed using IBM SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Categorical variables were presented as percentages and frequencies, whereas continuous data were presented as the mean ± the standard deviation (SD), or median [interquartile range] for data having a skewed distribution. To detect deviations from a normal distribution the Kolmogorov–Smirnov test was used. Comparisons between groups were evaluated using two-sided t-tests, the Mann–Whitney U test, or the Chi-square test according to the respective distribution. A P-value < 0.05 was considered statistically significant.
We classified all patients into DM and non-DM groups. Propensity score matching (PSM) analysis applied logistic regression to balance the distribution of possible confounding variables between groups, including age, hypertension, dyslipidemia, and coronary heart disease. Patients were matched between the groups using the nearest logit of the propensity score and a PSM ratio of 1:1. For further analysis, patients were classified into HRPR and non-HRPR groups with respect to clopidogrel responsiveness. Different variables between the two groups (P < 0.05) were conducted multivariate logistic regression analysis to identify independent risk factors of clopidogrel HRPR. The results were expressed as odd ratios (ORs) with 95% confidence intervals (95% CI).
We have included 1,071 patients with acute ischemic stroke in this study (Figure 1). The demographic and clinical data of DM group (n = 712) and non-DM group (n = 359) are presented in Table 1. No statistical differences were found in sex, cerebrovascular risk factors (smoking, alcohol intake, history of stroke/TIA), and platelet indices between the two groups. In addition, age, prevalence of cerebrovascular risk factors (hypertension, hyperlipidemia, coronary heart disease), creatinine, fasting glucose and HbA1c were significantly different between the DM and non-DM group (P < 0.05).
To reduce differences of baseline characteristics, the PSM was further analyzed to balance the distribution of variables between the groups (Table 1). After PSM, the total number of patients in each study group was 331. There were no significant differences in baseline characteristics between the two groups, except for creatinine, fasting glucose and HbA1c.
Before PSM, we found that the median of MA (63.0 vs. 62.0 mm, P < 0.05) and MAADP (34.6 vs. 30.3 mm, P < 0.05) was significantly higher in the DM group and the ADP% (55.7% vs. 60.3%, P < 0.05) was significantly lower in the DM group compared with the non-DM group. No significant differences in the median levels of AA% were observed between the two groups (96.0% vs. 97.0%, P > 0.05).
After PSM, the difference in the median levels of MA (63.1 vs. 62.5 mm, P < 0.05), MAADP (34.6 vs. 29.3 mm, P < 0.05) and ADP% (55.8 vs. 67.4%, P < 0.05) between the DM and non-DM group (Table 2) remained. However, the median levels of AA% were significantly higher in the DM-group than those without (96.1% vs. 93.0%, P < 0.05).
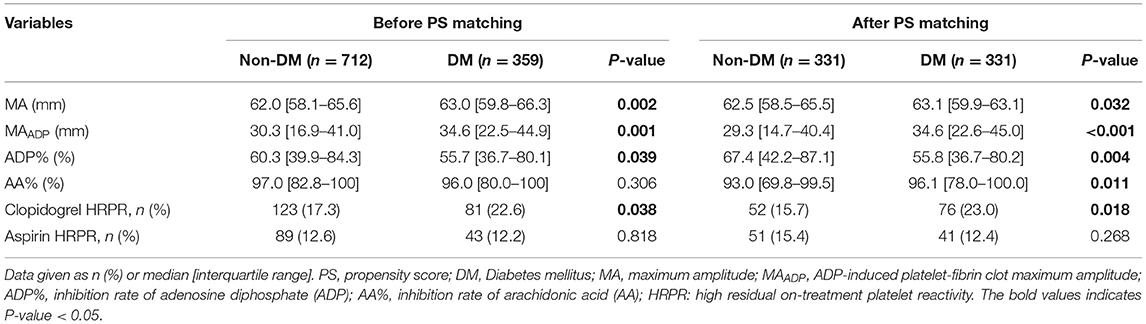
Table 2. Platelet reactivity, high residual on-treatment platelet reactivity (HRPR) before and after PS matching.
Among all study populations 19.0% of patients exhibited clopidogrel HRPR. The prevalence rate of clopidogrel HRPR was significantly lower in the non-DM group compared with the DM group (17.3% vs. 22.6%, P < 0.05), but no difference in aspirin HRPR was found between the two groups (Table 2). The results were the same before and after PSM.
Previous studies have demonstrated that clopidogrel HRPR is affected by many factors. Accordingly, we classified the patients into two groups (non-HRPR and HRPR). The demographic and clinical data of the two groups were compared. Age, sex, smoking, alcohol intake, DM, creatinine and HbA1c were significantly different between the groups (Table 3). After adjustment for potential confounders (age, sex, smoking, alcohol intake, creatinine and HbA1c), the DM (OR = 1.48, 95% CI: 1.03–2.07, P < 0.05) remained independent of the clopidogrel HRPR.
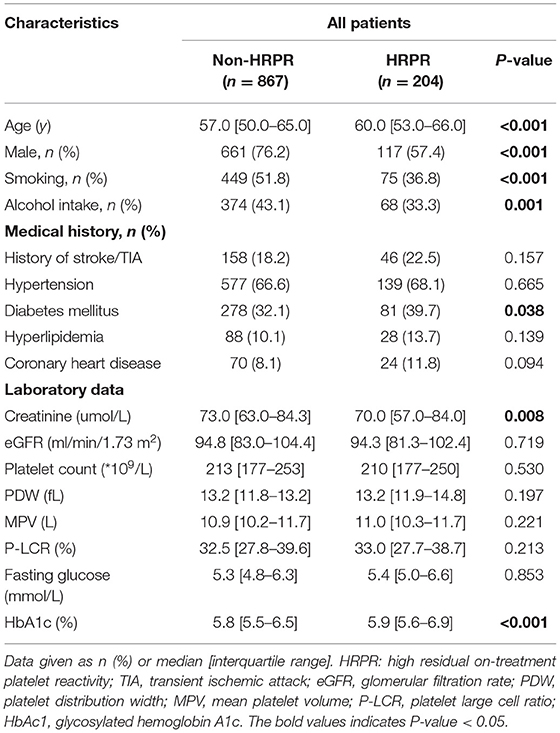
Table 3. Risk factors for clopidogrel high residual on-treatment platelet reactivity (clopidogrel HRPR) in all patients.
In this study, we found that patients with DM had higher levels of MA, MAADP and lower levels of ADP%. The clopidogrel HRPR rate was also higher in DM group than non-DM group. Additionally, after adjustment for potential confounders, DM remained an independent risk factor of HRPR following clopidogrel therapy. These results suggested that in acute ischemic stroke patients taking DAPT, DM is associated not only with an increase of platelet reactivity but also with a low responsiveness to clopidogrel.
Although it is widely recognized that DM could influence the platelet reactivity in patients following percutaneous transluminal coronary intervention (23, 24), the data examining the DM influences on platelet function in acute ischemic stroke patients taking DAPT have been sparse. Previous studies have found that in ischemic stroke patients receiving clopidogrel (75 mg) daily for 1 week, clopidogrel resistance is associated with DM (25). In a study involving 237 patients following recent ischemic stroke or TIA treated receiving DAPT, DM was found to be associated with HRPR defined as aspirin resistance or clopidogrel resistance (26), which was different from our study. Our study provides further evidence that DM is associated with clopidogrel HRPR but not with aspirin HRPR.
In our study, AA-mediated platelet reactivity and aspirin HRPR were not affected by DM. The relationship between DM and aspirin HRPR remained controversies. As reported by previous studies (27), despite a high risk of aspirin HRPR in DM patients, DM itself does not contribute to a higher risk of aspirin HRPR and is more likely to be related to insulin resistance. In another study, it is found that aspirin resistance was more common among participants treated with low dose aspirin compared with higher doses (28). We speculate that different methods and time of platelet function tested, differences in aspirin dosing and frequency, and characteristics of the patient populations studied would affect the results of platelet function test. In future studies, we will try to explore which factors have effects on the relationship between DM and aspirin HRPR.
Studies have shown that clopidogrel HRPR is 4–30% in patients who use conventional doses of clopidogrel (29) and rises to 28% in ischemic cerebrovascular disease (30–32). Moreover, up to 40% of patients exhibiting clopidogrel HRPR may have recurring thrombotic events (33, 34). Therefore, the prediction and identification of patients with HRPR is a significant issue, as they may benefit from other antiplatelet drugs for the prevention of ischemic events and improvement of clinical outcomes. In our study, ADP-mediated platelet reactivity and clopidogrel HRPR was associated with DM. Therefore, the presence of DM should be considered in developing strategies for anti-platelet treatment of acute ischemic stroke patients.
The mechanism underlying increased platelet reactivity and clopidogrel HRPR in DM remains unclear. One possible reason is that chronic inflammatory reaction accompanies DM, which can upregulate the expression of cyclooxygenase-2 (COX-2) in vascular endothelial cells, monocyte-macrophages and other cells, leading to a significant increase in platelet activity (35, 36). In addition, DM patients have a high level of glycosylation of platelet surface membrane proteins, which competitively inhibits their acetylation and weakens the anti-platelet aggregation effect of clopidogrel (37).
Some limitations should be addressed. First, in this retrospective study, although most of the measured confounders were balanced between the two groups by PSM, bias may still exist. Confounding factors such as the use of proton pump inhibitors and the types of use, the use of statin, liver function or heterogeneity of metabolic genes (e.g., CYP2C19 gene type) may affect the responsiveness of clopidogrel drugs. However, we have included as many confounding factors as possible. Second, the degree of platelet aggregation and its response also depends on the type of diabetes. Type 2 diabetes has a higher ADP-induced aggregation rate than type 1, although due to the current sparsity of data conclusions are inconsistent. Third, our research subjects are Chinese patients, so generalizing these results to non-Asian patients may need careful interpretation and further research.
In conclusion, we found that in acute ischemic stroke patients taking DAPT, DM was associated with increased platelet activity and a greater prevalence of clopidogrel HRPR. Hence, it is necessary for us to stratify patients according to the presence or absence of DM and to choose a personalized treatment strategy to reduce the risk of ischemic events.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Board of Tongji Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YG, YZ, and LW collected the clinical data. YG, DH, and JZ processed statistical data. YG, ZY, and HH drafted and revised the manuscript. XL and HH designed and guided the study. All authors contributed to the article and approved the submitted version.
This study was supported by the National Nature Science Foundation of China (81771341 to XL), the Flagship Program of Tongji Hospital (2019CR106 to XL), the second batch of clinician research projects of HUST to XL, and the Natural Science Foundation of Guangdong Province (2018A030313820 to DH). Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease, Guangdong Provincial Clinical Research Center for Neurological Diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.712024/full#supplementary-material
1. Jia Q, Zheng H, Zhao X, Wang C, Liu G, Wang Y, et al. Abnormal glucose regulation in patients with acute stroke across China: prevalence and baseline patient characteristics. Stroke. (2012) 43:650–7. doi: 10.1161/STROKEAHA.111.633784
2. Zhang X, Shi Q, Zheng H, Jia Q, Zhao X, Liu L, et al. Prevalence of abnormal glucose regulation according to different diagnostic criteria in ischaemic stroke without a history of diabetes. Biomed Res Int. (2018) 2018:8358724. doi: 10.1155/2018/8358724
3. Cho KH, Kwon SU, Lee JS Yu S, Cho AH. Newly diagnosed diabetes has high risk for cardiovascular outcome in ischemic stroke patients. Sci Rep. (2021) 11:12929. doi: 10.1038/s41598-021-92349-y
4. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:517–84. doi: 10.1161/STR.0b013e3181fcb238
5. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. (2010) 376:112–23. doi: 10.1016/S0140-6736(10)60834-3
6. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. (2010) 375:2215–22. doi: 10.1016/S0140-6736(10)60484-9
7. Chen M, Luo W, Li J, Cao K, Li X, Huang H, et al. Clinical characteristics and outcomes of acute ischemic stroke in patients with type 2 diabetes: a single-center, retrospective study in Southern China. Int J Endocrinol. (2021) 2021:5517228. doi: 10.1155/2021/5517228
8. Chen W, Pan Y, Jing J, Zhao X, Liu L, Meng X, et al. Recurrent stroke in minor ischemic stroke or transient ischemic attack with metabolic syndrome and/or diabetes mellitus. J Am Heart Assoc. (2017) 6:e005446. doi: 10.1161/JAHA.116.005446
9. Callahan A, Amarenco P, Goldstein LB, Sillesen H, Messig M, Samsa GP, et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch Neurol. (2011) 68:1245–51. doi: 10.1001/archneurol.2011.146
10. Kaplan RC, Tirschwell DL, Longstreth WT, Manolio TA, Heckbert SR, Lefkowitz D, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology. (2005) 65:835–42. doi: 10.1212/01.wnl.0000176058.09848.bb
11. Oza R, Rundell K, Garcellano M. Recurrent ischemic stroke: strategies for prevention. Am Fam Physician. (2017) 96:436–40.
12. Arsava EM, Kim GM, Oliveira-Filho J, Gungor L, Noh HJ, Lordelo Mde J, et al. Prediction of early recurrence after acute ischemic stroke. JAMA Neurol. (2016) 73:396–401. doi: 10.1001/jamaneurol.2015.4949
13. Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhao P, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. (2016) 374:1533–42. doi: 10.1056/NEJMoa1412981
14. Lovett JK, Dennis MS, Sandercock PA, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke. (2003) 34:e138–140. doi: 10.1161/01.STR.0000080935.01264.91
15. Wiśniewski A, Filipska K. The phenomenon of clopidogrel high on-treatment platelet reactivity in ischemic stroke subjects: a comprehensive review. Int J Mol Sci. (2020) 21:6408. doi: 10.3390/ijms21176408
16. Angiolillo DJ, Bernardo E, Sabate M, Jimenez-Quevedo P, Costa MA, Palazuelos J, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. (2007) 50:1541–7. doi: 10.1016/j.jacc.2007.05.049
17. Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. (2007) 115:708–16. doi: 10.1161/CIRCULATIONAHA.106.667741
18. Saucedo JF. Antiplatelet therapy for patients with diabetes mellitus and acute coronary syndrome. Prim Care Diabetes. (2012) 6:167–77. doi: 10.1016/j.pcd.2012.02.001
19. Lo JW, Crawford JD, Samaras K, Desmond DW, Kohler S, Staals J, et al. Association of prediabetes and type 2 diabetes with cognitive function after stroke: a STROKOG collaboration study. Stroke. (2020) 51:1640–6. doi: 10.1161/STROKEAHA.119.028428
20. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
21. Rao Z, Zheng H, Wang F, Wang A, Liu L, Dong K, et al. The association between high on-treatment platelet reactivity and early recurrence of ischemic events after minor stroke or TIA. Neurol Res. (2017) 39:719–26. doi: 10.1080/01616412.2017.1312793
22. Yang Y, Chen W, Pan Y, Yan H, Meng X, Liu L, et al. Effect of ticagrelor versus clopidogrel on platelet reactivity measured by thrombelastography in patients with minor stroke or TIA. Aging. (2020) 12:20085–94. doi: 10.18632/aging.103452
23. Zou X, Deng XL, Wang YM, Li JH, Liu L, Huang X, et al. Genetic polymorphisms of high platelet reactivity in Chinese patients with coronary heart disease under clopidogrel therapy. Int J Clin Pharm. (2020) 42:158–66. doi: 10.1007/s11096-019-00953-w
24. Hackeng CM, ten Berg JM, Verheugt FWA, Bouman HJ, Zomer CA, van de Wal RMA, et al. The influence of clinical characteristics, laboratory and inflammatory markers on ‘high on-treatment platelet reactivity’ as measured with different platelet function tests. Thromb Haemostasis. (2017) 102:719–27. doi: 10.1160/TH09-05-0285
25. Su JF, Hu XH, Li CY. Risk factors for clopidogrel resistance in patients with ischemic cerebral infarction and the correlation with ABCB1 gene rs1045642 polymorphism. Exp Ther Med. (2015) 9:267–71. doi: 10.3892/etm.2014.2058
26. Jia W, Jia Q, Zhang Y, Zhao X, Wang Y. Effect of prediabetes on asprin or clopidogrel resistance in patients with recent ischemic stroke/TIA. Neurol Sci. (2020) 42:2829–283. doi: 10.1007/s10072-020-04881-w
27. Kumbhani DJ, Marso SP, Alvarez CA, McGuire DK. State-of-the-Art: Hypo-responsiveness to oral antiplatelet therapy in patients with type 2 diabetes mellitus. Curr Cardiovasc Risk Rep. (2015) 9:4. doi: 10.1007/s12170-014-0430-5
28. Ertugrul DT, Tutal E, Yildiz M, Akin O, Yalcin AA, Ure OS, et al. Aspirin resistance is associated with glycemic control, the dose of aspirin, and obesity in type 2 diabetes mellitus. J Clin Endocrinol Metab. (2010) 95:2897–901. doi: 10.1210/jc.2009-2392
29. Ben-Dor I, Kleiman NS, Lev E. Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy. Am J Cardiol. (2009) 104:227–33. doi: 10.1016/j.amjcard.2009.03.022
30. Moukarbel GV, Bhatt DL. Antiplatelet therapy and proton pump inhibition: clinician update. Circulation. (2012) 125:375–80. doi: 10.1161/CIRCULATIONAHA.111.019745
31. Mijajlovic MD, Shulga O, Bloch S, Covickovic-Sternic N, Aleksic V, Bornstein NM. Clinical consequences of aspirin and clopidogrel resistance: an overview. Acta Neurol Scand. (2013) 128:213–9. doi: 10.1111/ane.12111
32. Fong J, Cheng-Ching E, Hussain MS, Katzan I, Gupta R. Predictors of biochemical aspirin and clopidogrel resistance in patients with ischemic stroke. J Stroke Cerebrovasc Dis. (2011) 20:227–30. doi: 10.1016/j.jstrokecerebrovasdis.2009.12.004
33. Fifi JT, Brockington C, Narang J, Leesch W, Ewing SL, Bennet H, et al. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol. (2013) 34:716–20. doi: 10.3174/ajnr.A3405
34. Fu Z, Dong W, Shen M, Xue H, Guo J, Jing J, et al. Relationship between hyporesponsiveness to clopidogrel measured by thrombelastography and in stent restenosis in patients undergoing percutaneous coronary intervention. Clin Biochem. (2014) 47:197–202. doi: 10.1016/j.clinbiochem.2014.08.009
35. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. (2018) 17:121. doi: 10.1186/s12933-018-0763-3
36. Gaiz A, Mosawy S, Colson N, Singh I. Thrombotic and cardiovascular risks in type two diabetes; Role of platelet hyperactivity. Biomed Pharmacother. (2017) 94:679–86. doi: 10.1016/j.biopha.2017.07.121
Keywords: diabetes mellitus, dual antiplatelet therapy (DAPT), ischemic stroke, platelet function, thromboelastography (TEG)
Citation: Guo Y, Zhang Y, Zhao J, Wu L, Yu Z, He D, Huang H and Luo X (2021) Impact of Diabetes on Platelet Function in Acute Ischemic Stroke Patients Taking Dual Antiplatelet Therapy. Front. Neurol. 12:712024. doi: 10.3389/fneur.2021.712024
Received: 19 May 2021; Accepted: 13 October 2021;
Published: 04 November 2021.
Edited by:
Linxin Li, University of Oxford, United KingdomReviewed by:
Matthew Dean Linden, University of Western Australia, AustraliaCopyright © 2021 Guo, Zhang, Zhao, Wu, Yu, He, Huang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Luo, Zmx5ZG90dGpoQDE2My5jb20=; Hao Huang, aHVhbmdoYW90akBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.