- 1Department of Neurology, Dell Medical School, University of Texas at Austin, Austin, TX, United States
- 2Department of Psychology, Behavioral Neuroscience, College of Liberal Arts, University of Texas at Austin, Austin, TX, United States
Despite the high incidence of brain injuries in children, we have yet to fully understand the unique vulnerability of a young brain to an injury and key determinants of long-term recovery. Here we consider how early life stress may influence recovery after an early age brain injury. Studies of early life stress alone reveal persistent structural and functional impairments at adulthood. We consider the interacting pathologies imposed by early life stress and subsequent brain injuries during early brain development as well as at adulthood. This review outlines how early life stress primes the immune cells of the brain and periphery to elicit a heightened response to injury. While the focus of this review is on early age traumatic brain injuries, there is also a consideration of preclinical models of neonatal hypoxia and stroke, as each further speaks to the vulnerability of the brain and reinforces those characteristics that are common across each of these injuries. Lastly, we identify a common mechanistic trend; namely, early life stress worsens outcomes independent of its temporal proximity to a brain injury.
Introduction
According to the Centers for Disease Control (1), children (age 0–17) are more likely to sustain a traumatic brain injury (TBI), with those 4 years and under at highest risk. Here we focus on the young brain, due to the high prevalence of TBIs in this age group and address how early life stress (ELS) may alter recovery after an early brain injury.
Evolution of the Injury
TBI results from both a primary insult, due to the direct tearing and shearing of brain structures, and a secondary cascade of adverse events that begins within minutes post injury and includes disruption of the blood-brain barrier, vasogenic and cytotoxic edema, excitotoxicity, neuroinflammation, dysregulation of metabolism, and cell death [see reviews, Simon et al. (2) and Potts et al. (3)]. With low antioxidant reserves, the developing brain is rendered more vulnerable to these adverse secondary events (4–7). Moreover, injury to the developing brain disrupts normal developmental processes, including myelination, synaptogenesis, synaptic pruning, and gliogenesis, each of which contribute to long-term brain function [(8–12) and see review, Semple et al. (13)]. These disruptions and subsequent progressive neurodegeneration adversely affect normal progression of age-dependent behaviors, such as social cognition, social play, social interaction, working memory, and skill acquisition. When these key stages are disrupted during early childhood, risk-taking tendencies, increased social interactions, novelty seeking, emotional instability, and impulsivity may emerge during adolescence (14–19).
The Developing Brain and TBIs
Children and TBIs
A child is more vulnerable to a TBI than an adult due to unique physical attributes of the young brain and body. With a larger head to body ratio and weak musculature of the neck (20), the child's brain is more likely to be exposed to greater acceleration/deceleration forces, resulting in a higher incidence of diffuse axonal injury and cerebral edema (21–23). Additionally, the young brain may sustain greater damage from an impact due to a thin calvarium (24, 25). Beyond these general physical features, recovery after an early age TBI is also influenced by characteristics of the lesion, such as severity, location, focal or diffuse patterns of damage, and laterality of injury, each of which may impact outcomes (16, 26–28). Children with large, more diffuse, and/or bilateral injuries show the poorest performance across cognitive domains (15, 17, 18, 26, 29, 30).
Biological sex is also a determinant of recovery after an early age TBI. Beyond genetic and endocrine differences (31), sex differences also manifest in the timing of the closure of sensitive developmental periods, which occurs earlier in males than in females (32). Clinical studies of brain-injured children likewise identify differences between sexes. For example, females who sustain a TBI during childhood are more likely to internalize emotional problems such as depression and anxiety, whereas males may display emotional problems in the form of substance abuse and criminal behaviors (33–36). Similarly, other clinical studies have reported that females have an increased risk for developing emotional and psychiatric disorders after injury, while males present an increased risk for social and behavioral problems (i.e., communication, social cognition, attention/executive function) within the first year following an early age TBI (26, 36, 37).
Critical Periods of Brain Development
A TBI during the early postnatal period adversely affects maturation of key developmental processes. Brain development spans early gestation to early adulthood (38). During early postnatal development, the brain's acquisition of new functions and capabilities is highly dependent upon experiential and environmental influences (38). Critical periods of brain development are characterized by robust synaptic pruning, myelination, programmed cell death, alterations in density of neurotransmitters, gliogenesis, and white/gray matter differentiation (16, 39–44). While some developmental processes, including the maturation of the immune system and the blood-brain barrier, are mostly complete by birth (45), others, including synaptogenesis, myelination, and programmed cell death, extend well-beyond the postnatal period, and into adulthood (42). In the human brain, synaptogenesis begins before birth and peaks around the age of 3 (40). A subsequent decrease in synaptogenesis coincides with increased synaptic pruning, which continues over the next several decades (42). Programmed cell death peaks during gestation (40) and also extends into adulthood (40). While myelination is most prominent during years 2–3, this process also continues into early adulthood (40, 46). Importantly, each of these developmental processes are critical for normal brain function at adulthood (40).
The first several years of life are considered a sensitive period of growth where key developmental processes shape brain function and behavior at adulthood. The importance of this period of development has been demonstrated in studies of social behavior, sensory experience and cognition. Toddler-aged children are characterized by a high level of activity and sociability (47). Early age brain injuries may alter the shaping and maturation of these behaviors. As sociability continues to develop into adolescence [(48, 49) and see review, Blakemore (50)], a disruption in the toddler aged child may interfere with the proper sequence of age-appropriate social behaviors and increase the risk of psychiatric disorders (51). Children, during this critical period, are also particularly sensitive to sensory experience as it shapes neural circuits involved in basic sensory processes. For example, light and sound shape the formation of the visual and auditory cortices, respectively, and dictate visual and auditory processing (52, 53). Prolonged deprivation of either stimulus during this period results in an impairment in sensory processing later on in life (52–55). Similarly, early age TBI may also result in poorer cognitive outcomes (16, 56–61). This relationship between early age TBI and cognitive abilities is considered non-linear and is likely sensitive to injury at critical periods of plasticity and behavioral development (62, 63). The earlier the age of a TBI, the higher the risk for delayed or arrested development of cognitive and higher-level executive functioning (18, 59, 61).
Early Life Stress
Children who are exposed to early life stress are at risk for developing long-term psychosocial impairments and chronic illnesses at adulthood (64–69). ELS may encompass a variety of scenarios including extreme poverty, parental loss, malnutrition, domestic/school/community violence, trauma, child neglect, and/or abuse, altered parental behavior (70–80), and institutional rearing (81). ELS impacts many aspects of brain health and development, including metabolism, circadian rhythms, neuroendocrine function, neuro-immune interactions, and oxidative stress (82–87). Children who experience ELS also have a greater risk for diabetes, obesity-related problems, cardiovascular diseases, autoimmune disease, cancer, and depression at adulthood as well as early mortality (64–69, 88, 89).
The Social Environment and TBI
In a seminal paper, Fletcher et al. (90) questioned why antecedent psychosocial behavior traits, such as adaptive behavior, communication, daily living, and socialization were not considered in studies of brain-injured children. Such questioning has served as a catalyst for subsequent research to examine the moderating role of the social environment before or shortly after an early age TBI. In long term clinical studies of sociocognitive functioning after childhood TBI (18, 19), it was found that, at adulthood, individuals showed poorer emotional perception, as evidenced by deficits in both recognizing and interpreting emotions based upon facial and vocal cues (19). These findings are thought to reflect vulnerability of the immature social brain to this insult, with sociocognitive deficits resulting from disrupted brain development and inability to acquire social skills at the appropriate developmental time (91). Importantly, long term deficits in emotional perception may be linked to a child's socioeconomic status and levels of family intimacy at the time of injury (18). Catroppa et al. (18) reported the first prospective study that compared pre-injury and 6 months post-injury behavioral outcomes with social participation being predicted by both the severity of the TBI and pre-injury deficits, including lower social participation. Subsequent longitudinal studies support these results; children, exposed to a poor social environment prior to a TBI, have greater impairments in psychosocial outcomes, including social cognition and communication compared to brain-injured children with higher socioeconomic status and optimal home environments prior to their injury (14, 91, 92). The results of these early studies indicate that pre-injury demographics such as socioeconomic status and social environment are likely determinants of behavioral recovery after a TBI.
Pre-clinical Models of Early Age Brain Injuries
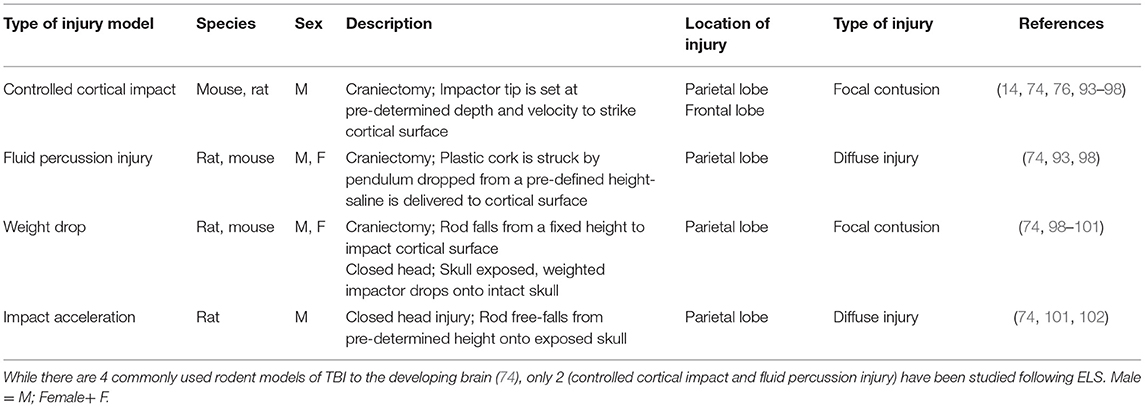
Currently, there are two models of TBIs in rodents that have been used to study the consequences of ELS; namely, a focal cortical injury produced by a controlled cortical impactor device, and a more diffuse injury, produced by a fluid percussion device [Table 1, see reviews, Kochanek et al. (103) and Thompson et al. (104)]. Each of these models involves a craniectomy and exposure of the brain. A focal cortical injury is produced by a pneumatically or electronically driven piston that impacts the exposed dura with tightly controlled velocity, depth of penetration and dwell time, producing a consistent injury to proximal cortical and subcortical areas The fluid percussion model is based upon the delivery of a defined pulse of fluid against the intact dura, resulting in brief deformation of the brain (104). Severity of the injury is dependent upon the strength of the pressure wave, which is generated when a pendulum swings from a variable height to strike a plunger in a saline-filled reservoir. This results in delivery of a pulse of saline against the intact dura. Depending upon the severity of the injury, each of these models may result in deficits in learning and memory, social behaviors, hyperactivity, and anxiety- and depression-like behaviors (56, 103, 105–117).
Pre-Clinical Models of ELS
There are two common models of ELS in rodents, the maternal separation model and the limited bedding nestlet model. These models target early brain development that spans birth to postnatal day 21 with notable variations that include the timing and duration of exposure to an impoverished environment and/or maternal separation.
One of the earliest accounts of the maternal separation paradigm used handling or non-handling of rat pups to invoke an early stress (stimulation) response (118). This foundational model examined how neonatal handling affected plasma corticosterone levels and emotionality later on in life (118, 119). The maternal separation model subsequently evolved into the more modern paradigm of physically separating the pups from the mom, resulting in a more pronounced response of the HPA axis (118–126). While maternal separation is suitable for an examination of acute or repeated stressors, the model is not typically applied to chronic stress, which may result in pup exhaustion due to malnutrition and hypothermia (87). Additionally, the maternal separation model may result in inconsistent results and includes many variations of the paradigm (i.e., timing of separation, duration of separation, measure of stress response). The Limited Bedding Nestlet (LBN) model was developed to examine the effects of chronic ELS, in which rodent pups and the nursing dam are exposed to a metal mesh cage bottom and a reduced nestlet square (87). The LBN model produces a robust activation of the HPA axis as a result of erratic and unpredictable maternal care with minimal observer handling (87, 127–132).
Maternal Separation Model
In this rodent model of childhood neglect (133, 134), the mother is separated from her pups for a defined period of time each day during the postnatal period. The MS model is used by a number of groups (118, 119, 121–126, 135, 136). It results in activation of the hypothalamic-pituitary-adrenal (HPA) axis, as evidenced by elevated corticosterone and altered expression of corticotropin releasing-hormone (CRH) (118, 120, 123, 136, 137). The MS model also results in long-term changes in psychosocial behaviors, including anxiety- and depression-related behaviors. Importantly, there are several variations of this model, including the daily duration of MS (brief vs. prolonged), the timing of the first day of separation, the number of days of separation, if the mother remains in the same room as the pups, and if the pups are maintained on a warming pad while separated from the mother. In some cases, there seems to be habituation to the handling by the observer over an extended period of time (87). In other cases, a brief separation may actually produce positive physiological and behavioral effects later in development, presumably because it replicates the repeated, short periods of separation between mom and pups in the wild, in which the nursing dam leaves her nest to forage for food (135, 138). The desired adverse effects of MS seem to emerge when periods of separation exceed 15 min (139–141). While variation in MS methods may produce some variability in outcomes, there are some key behaviors at adulthood that are common to most, including anxiety- and depression-like behaviors (51, 142–145). Moreover, these models typically show an exaggerated response of the HPA axis, a hallmark of ELS, immediately after the separation period that extends well into adulthood (51, 146–150).
Limited Bedding Nestlet Model
In the LBN model, the mother rears her pups on an altered cage bottom, typically metal mesh, with a reduced amount of a nesting material during the first week of postnatal life. This model creates a stressful environment, resulting in altered maternal behavior toward her pups (neglect, abuse, and hypervigilance) (87, 89, 127, 129, 131, 132, 151–159) and an exaggerated response by the HPA axis of the pups, based on changes in CRH and elevated corticosterone levels, that extends into adulthood (87, 129, 130, 155, 160). This paradigm, usually applied from P2-P9, produces long-term behavioral impairments such as anxiety, fear learning, depression, anxiety, reduced sociality (play behavior), and deficits in spatial learning and memory later in life (87, 89, 129, 131, 132, 151, 153–155, 157–159). A key strength of this model is that there is opportunity to continuously monitor maternal care and interaction with her pups without any confounding effects, resulting from handling by the experimenter.
There is reduced pup weight during and after the period of LBN (129, 130, 161, 162), which in some cases persists into adulthood (132). Although the LBN model shows variability in body development, it consistently results in altered metabolism, as evidenced by changes in brown adipose tissue and in circulating leptin and glucose levels. The lasting metabolic effects of LBN may be a result of the combination of the quality and quantity of nutrition, stress hormones, and sensory stimuli from the mother (163).
ELS and Immune Priming
While the immune response to a TBI contributes to secondary damage (113, 164–167), we have yet to fully understand the interaction between ELS and TBI in this context. ELS may prime the immune system, leaving it sensitized to inflammatory reactions later in life.
Causes and Effects of Immune Priming
Exposure to a wide variety of early-life insults may elicit a persistent immune-sensitized condition in the brain, such that a subsequent insult produces a heightened inflammatory response. This phenomenon is referred to as “immune priming.” Early life insults that have been shown to cause immune priming include infections (168, 169), seizures (170), early postnatal alcohol exposure (171), in utero stress (172), and as discussed in detail below, ELS (99, 173–175). Insults in the early period of life may produce life-long sensitization, creating immune cells that remain primed for many months in rodents and decades in humans (99, 176). Immune priming typically involves circulating immune cells, peripheral macrophages, astrocytes, or even neurons, but the most heavily implicated cells in immune priming of the CNS are the brain's resident immune cells, microglia, which undergo a phenotypic shift, producing much faster and more robust responses to subsequent immune signals (177–179).
The HPA Axis and Inflammation
In response to a stressor, the body activates the HPA axis. The hypothalamus, initially stimulated by the sympathetic nervous system, releases corticotropin-releasing hormone into the nearby pituitary gland, which in turn releases adrenocorticotropic hormone (ACTH) into the blood stream. Upon reaching the adrenal glands, ACTH causes release of glucocorticoids (GC), namely corticosterone in rodents and cortisol in humans. GCs then act on cells expressing glucocorticoid receptors throughout the body including the brain. In this way the stress signal is amplified and extended to enable a whole-animal response in the minutes and hours following a stressor. In general, GCs have an anti-inflammatory effect, inhibiting lymphocyte proliferation, reducing expression of pro-inflammatory cytokines and inhibiting production of anti-inflammatory cytokines (93, 180–183). This is especially true when GC levels are high, since, of the two GC receptors, the one that predominates in response to elevated levels of GC has a distinctly more anti-inflammatory signaling profile (94, 95). How then, does ELS lead to chronic inflammation and immune priming? One part of the puzzle may be that GCs elicit responses in the brain that are quite different than the primarily anti-inflammatory effect in the periphery. In addition to microglia, neurons and astrocytes in the brain also express GC receptors and elevated GCs can weaken these cells, compromising their ability to withstand further insult (96–98, 100, 101). Frank et al. have recently demonstrated that either stress or exogenous GCs produces immune-primed hippocampal microglia that, when challenged with lipopolysaccharide (LPS) ex vivo, secrete increased proinflammatory cytokines (102, 184). Furthermore, this effect is long-lasting, with these microglia still exhibiting a primed phenotype 28 days after a single stressor. One intriguing potential mechanism for GC-mediated priming of microglia is the nod-like receptor protein 3 (NLRP3) inflammasome. This protein complex is induced by GCs, is capable of regulating proinflammatory cytokine release, and has been implicated in microglial immune priming (102, 184–187).
The HPA Axis and TBI
TBI results in a suppression of the HPA axis [see review, Tapp et al. (188)]. As described above, the HPA axis responds to stressors by releasing corticotropin-releasing hormone (CRH) to the pituitary gland, which releases ACTH into the bloodstream. ACTH causes a release in glucocorticoids, like corticosterone (CORT). Under normal conditions, HPA axis activity is regulated by glucocorticoid receptors (GR) in the hypothalamus, pituitary, and adrenal glands. In addition to damage to subcortical areas (189), TBI causes a release of CORT in the brain. GR involved in the HPA axis negative feedback loop also become damaged from TBI, resulting in an excess of CORT. The pituitary is particularly vulnerable to injury-induced dysfunction, which results in a decreased release of ACTH and cannot stimulate the adrenal glands. The lack of stimulation results in decreased CORT release from the adrenal glands, resulting in an aberrant altered stress response. Experimental models of TBI have examined HPA axis suppression in rats, in which CORT was diminished in injured mice at 7 and 21 days after injury (190, 191). Excessive glucocorticoid release and a suppressed HPA axis response after TBI causes microglial priming and increases inflammatory cytokine expression, resulting in neuronal death (192, 193). This maladaptive chronic inflammatory response contributes to the development or worsening of psychiatric disorders later in life, such as depression (194, 195). The aberrant interaction between the persistent neuroendocrine response and compromised psychiatric behavior illustrates the importance of HPA axis dysfunction and long-term TBI recovery.
ELS Animal Models and Immune Priming
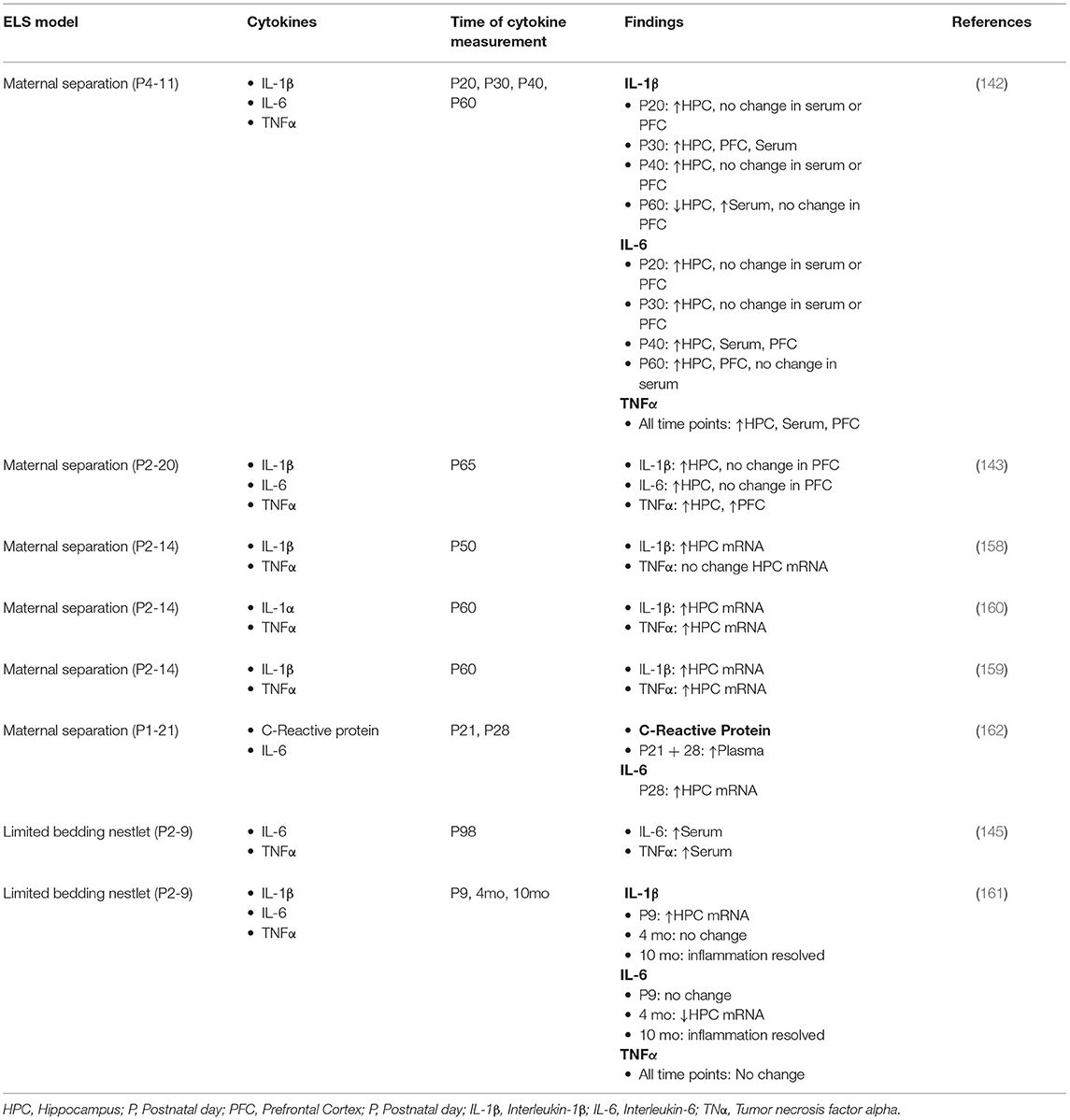
To date, only a handful of studies have examined immune priming or markers of chronic inflammation in the context of either the MS or LBN model of ELS (Table 2). Most of these have reported robust and long-lasting effects of ELS on cytokine expression. Reus et al. used an MS model in rats (P1-P10, 3 h/day), and quantified multiple cytokines at P20, P30, P40, and P60 in 3 different brain regions (99). They found persistently increased levels of the proinflammatory cytokines IL-1β, IL-6, and TNFα, as well as decreased levels of anti-inflammatory cytokine IL-10 (see Table 2 for details). Wang et al. employed a rat MS model (P2-P20, 4 h/day) and reported elevated pro-inflammatory IL-1β, IL-6, and TNFα protein in the hippocampus and elevated TNFα protein in the prefrontal cortex at P60 (173). Three studies, all from the same group and using an MS model in mice (P2-P14), reported similar results at between P50 and P60; that is, elevated hippocampal mRNA for pro-inflammatory cytokines IL-1β and TNFα, as well as for the inflammasome protein NLRP3 (196–198). Saavedra et al., using a rat MS model (P1-P14, 3 h/day) did not examine cytokines but found an increased proportion of hippocampal microglia that maintained an activated phenotype when examined long after ELS, at between P140 and P170 (174). Sagae et al. utilizing an LBN model (P3-P9) in rats, also reported elevation in circulating pro-inflammatory cytokines TNFα and IL-6 at P98 (175).
Other studies have found smaller or more subtle impacts of ELS models on cytokines. Hoejimakers et al. used a LBN model from P2-P9 in mice and reported increased hippocampal expression of IL-1β at P9, immediately after stress, but decreased hippocampal IL-6 mRNA at 4 months and no differences in any pro-inflammatory cytokines at 10 months (199). Additionally, these investigators reported an increase in CD68 immunoreactivity, characteristic of activated microglia, at 4 months after stress, but not at 10 months. Delpech et al. (200) used a brief MS model (P1-P21, 15 min/day) in mice, following ELS at P21 and at P28 and demonstrated an elevation of serum c-reactive protein, a marker of immune activation. At P28 however, there was no effect of ELS on the number and morphology of hippocampal microglia, that had been seen at P21. They also reported elevated IL-6 mRNA from microglia isolated from the hippocampus at P28.
Perhaps the variability of results from ELS models is not surprising given the differences both in the details of the stress paradigms and in the methodology employed to measure cytokines and other features of immune priming. In explaining the differences between the MS studies (99, 173, 174, 196–198), it seems that the duration of the separation may underly the stark differences in results between Delpech et al. (200) (15 min/day) and the rest (3–4 h/day). In the case of the two LBN studies (175, 199), differences may arise from the quite disparate means of cytokine quantification [protein in serum for Sagae et al. (175) vs. hippocampal mRNA for Hoeijmakers et al. (199)]. There may also be species differences in how the immune systems of mice and rats respond to ELS, as several of the studies that found the most robust signs of immune priming were in rats (99, 173–175), while the two with the weakest evidence of immune priming were both evident in mice (199, 200).
Immune Priming by ELS in Humans
In humans, childhood adversity has been linked to a chronic inflammatory state (201–204), as well as to diseases associated with inflammation, such as cancer, cardiovascular disease, diabetes, and arthritis (64–69, 88, 89). Many studies have examined the relationship between socioeconomic status during childhood and inflammation, typically measured by plasma c-reactive protein (205). Such studies may be complicated by controlling for covariates, such as adult socioeconomic status. A recent meta-analysis examined 35 such studies and found a significant relationship between childhood socioeconomic status and the profile of adult inflammation, but this relationship did not survive when adjusted to factor out adult socioeconomic status (205). Ehrlich et al. (201) examined whether teens' early-life adversity scores, generated from interviews, were associated with differences in their inflammatory profiles. Rather than rely on cytokine or c-reactive protein expression, inflammation was quantified by ex vivo challenge of monocytes, obtained from blood samples, with either lipopolysaccharide alone or with lipopolysaccharide in combination with varying concentrations of GC. IL-6 secreted into the culture media was quantified, and a cluster analysis was performed. ELS was associated with higher inflammation clusters, indicating persistent immune priming by early life adversity in this population.
ELS and Brain Injury
Despite the clinical relevance, there are few preclinical studies that have examined brain injuries after exposure to LBN, brief maternal stress (BMS) or prolonged maternal stress (PMS) [Table 3, (133, 134, 206–210)]. Thus, there is substantial opportunity to build upon what has been reported, focusing on the unanswered questions, with the end goal of optimizing recovery in brain-injured children who have experienced prior ELS.
ELS + Stroke
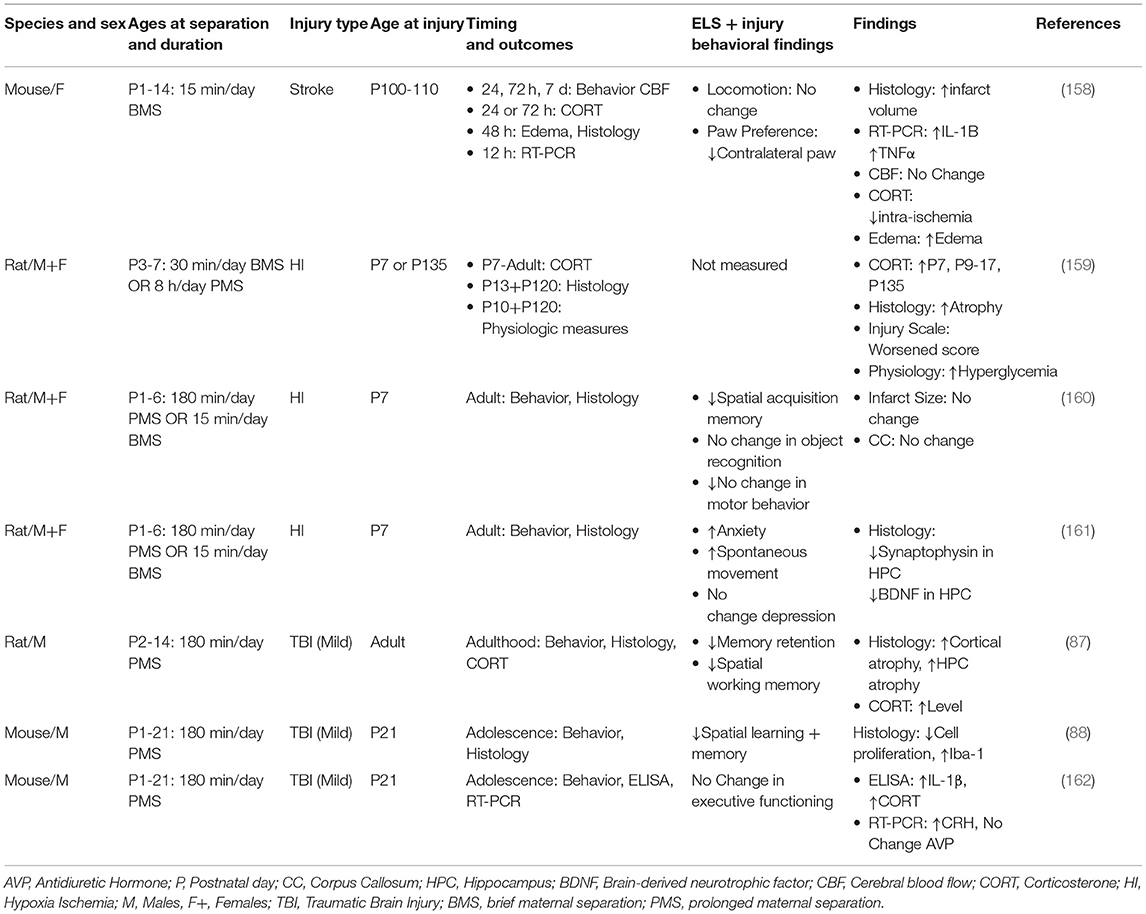
Although risk of stroke increases with age, incidence of stroke may occur at any age, including children (211). To date there is only one preclinical study that has examined the relationship between ELS and stroke [Table 3, (206)]. In this study, mothers were briefly separated (BMS) from their pups on a daily basis from P1-P14, a sensitive period of brain development. After reaching adulthood, animals were subjected to an occlusion of the middle cerebral artery followed by reperfusion. There were several findings that distinguished BMS in combination with stroke from controls. These animals showed a pronounced elevation of proinflammatory cytokines IL-1β and TNFα, vasogenic edema, and higher mortality compared to BMS alone. Such findings build upon other studies showing enhanced expression of pro-inflammatory cytokines IL-1β, TNFα, and IL-6 as a result of ELS exposure (99, 175, 199, 200). BMS in combination with stroke also resulted in an impairment of sensorimotor function compared to controls, based upon paw preference using the cylinder test (212, 213). It is noteworthy that there were no changes in corticosterone, either pre- or post-injury compared to relevant controls. While others have reported elevated levels of corticosterone at adulthood after BMS alone (87, 99, 175, 199, 200), the duration of maternal separation may, at least in part, account for these differences. In this stroke study, the duration of BMS was 15 min/day over a period of 2 weeks. In contrast, those studies that detected elevated levels of corticosterone at adulthood after BMS alone (146–150, 214–221), reported a duration of 180 min/day or longer. Collectively, these findings provide the first evidence that ELS in combination with stroke at adulthood elicits a pronounced immune response and adversely affects post-stroke sensorimotor recovery.
ELS + Perinatal Brain Injury
Neonatal hypoxia ischemia (HI), the most common form of perinatal brain injury, results in neonatal encephalopathy and long-term disabilities (222).
Several preclinical studies have examined the consequences of ELS in combination with HI [Table 3, (207–209)]. Early studies evaluated ELS using BMS (15 min/day) or PMS (180 min/day) exposure to MS on P3-P7, followed immediately by HI, and then studied shortly after HI or at adulthood (207). Prior exposure to PMS and neonatal HI resulted in elevated levels of corticosterone shortly after the time of injury. Histological findings, based upon pathological scoring of hematoxylin stained sections, suggested enhanced damage to white matter in the thalamus and internal capsule. Studies of HI at adulthood showed altered metabolism, as evidenced by elevated levels of glucose and insulin compared to BMS or PMS alone.
A later study focused on the long-term consequences of BMS or PMS in combination with HI on hippocampal functioning at adulthood [Table 3, (208)]. After BMS or PMS from P1-P6, animals were exposed to HI shortly thereafter and then were evaluated at adulthood. While ELS in combination with HI showed no differences in non-spatial recognition (novel object recognition and novel placement test), there were impairments in spatial learning and memory, as measured by the Morris Water Maze, compared to either insult alone.
Lastly, a follow up study focused on the interaction of ELS and HI in the context of synaptic integrity in the hippocampus and metrics of emotionality [Table 3, (209)]. Animals were exposed to PMS and subsequent HI and thereafter evaluated at adulthood for anxiety- and depressive-like behaviors, based upon performance in the elevated plus maze and the forced swim test, respectively. While HI followed by PMS resulted in a more pronounced anxiety-like phenotype, compared to either insult alone, there was no evidence of depressive-like behavior across any groups. Subsequent histological analyses of the dentate gyrus revealed altered long-term synaptic plasticity as evidenced by a reduction in levels of brain-derived neurotrophic factor and synaptophysin in the hippocampus compared to either PMS or HI alone. These results indicate that cell survival and synaptic density in the hippocampus are particularly vulnerable to the additive effect of MS and HI (209).
ELS + TBI
ELS has been evaluated in pre-clinical models of TBI with variables that include the type of injury (focal vs. diffuse), the age at time of injury, and the timing of outcomes. Sanchez et al. (133) (Table 3), examined how prolonged ELS influences hippocampal-related function after a TBI at adulthood. Animals were exposed to daily PMS (180 min/day) from P2-P14 followed by a mild fluid percussion injury at adulthood. Behavioral assessments were conducted 2, 3, and 4 weeks after injury. Based on contextual fear learning (2 weeks post injury), brain-injured animals, reared in PMS, showed less freezing after the cue compared to controls. Animals were subsequently tested using the Morris Water Maze at 3–4 weeks post injury. The group with PMS in combination with TBI showed deficits in spatial learning as well as greater cortical and hippocampal atrophy compared to other conditions. At 8 weeks post injury, corticosterone levels were highest in PMS in combination with TBI.
An alternative approach examined how PMS (P1-P21) is influenced by a mild TBI at P21 [Table 3, (134)]. In these experiments, a mild focal injury was produced by a controlled cortical impact. Deficits in spatial learning and memory were most pronounced in brain-injured adolescent rodents exposed to both PMS and TBI. Although there was no difference in the lesion volumes across all groups, PMS prior to TBI resulted in an increase in activated microglia and a reduction in proliferation of the markers bromodexoyuridine and the nuclear protein Ki67 in the hippocampus. Taken together, these findings suggest that PMS prior to an early age mild TBI, results in more profound activation of microglia, which, in turn, adversely affects neurogenesis and hippocampal-dependent behaviors (223).
A follow-up study, using the same model of PMS and TBI, examined cognitive flexibility and thereafter measured pro-inflammatory cytokines, IL-1β, TNFα, and IL-6 in the prefrontal cortex and hippocampus. Cognitive flexibility was measured using the attentional shift task in early adolescence, whereby mice learned how to discriminate between positive odors and associate this experience with a cue (210, 224, 225). Mild injury had a significant impact on the first reversal of the attentional shift task. However, this was not worsened by prior exposure to MS. IL-1β, elevated in the hippocampus, was highest in those animals exposed to both PMS and TBI compared to controls. These findings suggest that PMS in combination with a mild TBI results in a heightened inflammatory response compared to either condition alone. Although there was no additive effect seen on cognitive flexibility or in IL-1β in the prefrontal cortex, the authors suggest that IL-1β may be involved in crosstalk between hippocampal and cortical-related cognitive impairments seen after an early age mild TBI.
Where Do We go From Here?
There are a number of research opportunities that could build upon our current knowledge of the interactions between ELS and recovery after a brain injury. Here we address several basic directions.
Consider Alternative Models of ELS
ELS has profound adverse effects on brain development and results in both physical and psychological sequelae at adulthood. Few preclinical studies have addressed how ELS may influence recovery after brain injury. And, of these studies, ELS has only been studied in the context of MS (133, 134, 206–210). As ELS represents a broad spectrum of adverse conditions including physical, sexual and emotional forms of abuse and neglect (226), there is a need to address alternative models of ELS, including LBN, as well as others that capture a broader range of adverse exposures.
Injury Severity as a Modifier of Recovery After ELS
Two of the most commonly used rodent models of TBI, controlled cortical impact and fluid percussion injury, have been studied in the context of ELS (133, 134, 210). The severity of the injury likely influences recovery after ELS. This raises the possibility that very mild forms of TBIs, such as concussions, which present with nominal changes at structural and behavioral levels, may, in fact, be sensitive to prior ELS and, as such, result in broader pathological and behavioral findings. Understanding the relationships between ELS and mild TBIs has broad implications, including how we manage concussions in youth sports.
Sex as a Biological Variable
There are few studies of ELS in combination with TBI that include both males and females in the experimental design (Table 3). There is evidence that speaks to the complexities of TBIs, where variables such as the severity and type of insult may be differential modifiers between sexes. Thus, from simply the perspective of TBI alone, sex as a biological variable should be a key element in the experimental design [see review, Gupte et al. (227)]. Importantly, in a scoping review of both clinical and preclinical studies, Gupte et al. (227) have indicated that variables such as injury severity and nature of the injury interact differently based upon sex and that these differences influence long-term outcomes.
Genetics and Epigenetics
Genetics, including both gene variants and epigenetics, play a central role in how a brain recovers after ELS [see review, Fogelman and Canli (226)]. Similarly, genetics, and in particular epigenetics, also contribute to heterogeneity in recovery after a TBI, as evidenced in both preclinical models and in human studies [see reviews, Bennett et al. (228), Cortes and Pera (229), Treble-Barna et al. (230), and Kurowski et al. (231)].
Immune Function
ELS results in persistent immune priming (201) [and see reviews, Neher and Cunningham (232), and Fagundes et al. (233), von Leden et al. (234)]. We have yet to address how this priming may alter the immune response after a TBI. We and others have reported that the developing brain is sensitive to early cytokine exposure and, in fact, an early age TBI results in an enhanced immune response that is, in part, related to the prolonged recruitment of leukocytes to the injured brain (234). Thus, these collective findings support a further investigation into inflammatory responses, mediated by ELS, that may be magnified after a subsequent TBI.
ELS, TBI, and Plasticity
There are varying thoughts regarding plasticity after an early age lesion [see review, Giza and Prins (235)]. One viewpoint is that a younger brain has the ability to undergo significant reorganization and recovery after an injury and that ongoing brain development may support recovery processes. This is in contrast to others who consider the vulnerability of the young brain, where growth and formation of circuitry may be compromised by injury during critical periods of brain development. To address these differing viewpoints, further studies are needed to address factors that may influence outcomes, including age at time of injury in the context of brain development, severity and location of the injury, and the type of injury (focal and/or diffuse), as well as a broader viewpoint on plasticity that takes into account both its beneficial and adverse consequences.
Author Contributions
KP, MD, and LN-H contributed equally to writing and editing of this manuscript. KS contributed to formatting of tables, interpretation of findings, and fact checking of references. All authors have contributed to read and approved the final version of the manuscript.
Funding
This work was supported by the Eunice Kennedy Shriver Institute of Child Health and Human Development at the National Institute of Health (NIH) 1F31HD104491-01 that was awarded to KP and NIH/NINDS RO1NS077767 that was awarded to LN-H.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. CDC. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, Deaths—United States, 2014. Centers for Disease Control Prevention, U.S. Department of Health and Human Services (2019).
2. Simon DW, McGeachy MJ, Bayir H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. (2017) 13:572. doi: 10.1038/nrneurol.2017.116
3. Potts MB, Koh SE, Whetstone WD, Walker BA, Yoneyama T, Claus CP, et al. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx. (2006) 3:143–53. doi: 10.1016/j.nurx.2006.01.006
4. Fan P, Yamauchi T, Noble LJ, Ferriero DM. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J Neurotrauma. (2003) 20:437–45. doi: 10.1089/089771503765355513
5. Tsuru-Aoyagi K, Potts MB, Trivedi A, Pfankuch T, Raber J, Wendland M, et al. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol. (2009) 65:540–9. doi: 10.1002/ana.21600
6. Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. (2002) 51:571–8. doi: 10.1203/00006450-200205000-00005
7. Bayir H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. (2006) 28:420–31. doi: 10.1159/000094168
8. Tasker RC. Changes in white matter late after severe traumatic brain injury in childhood. Dev Neurosci. (2006) 28:302–8. doi: 10.1159/000094156
9. Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. (2006) 23:1412–26. doi: 10.1089/neu.2006.23.1412
10. Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. (2005) 22:719–32. doi: 10.1089/neu.2005.22.719
11. Domowicz M, Wadlington NL, Henry JG, Diaz K, Munoz MJ, Schwartz NB. Glial cell responses in a murine multifactorial perinatal brain injury model. Brain Res. (2018) 1681:52–63. doi: 10.1016/j.brainres.2017.12.020
12. Threlkeld SW, Rosen GD, Fitch RH. Age at developmental cortical injury differentially alters corpus callosum volume in the rat. BMC Neurosci. (2007) 8:94. doi: 10.1186/1471-2202-8-94
13. Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. (2013) 106–7:1–16. doi: 10.1016/j.pneurobio.2013.04.001
14. Anderson V, Beauchamp MH, Yeates KO, Crossley L, Hearps SJ, Catroppa C. Social competence at 6 months following childhood traumatic brain injury. J Int Neuropsychol Soc. (2013) 19:539–50. doi: 10.1017/S1355617712001543
15. Anderson V, Catroppa C. Recovery of executive skills following paediatric traumatic brain injury (TBI): a 2 year follow-up. Brain Inj. (2005) 19:459–70. doi: 10.1080/02699050400004823
16. Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. (2005) 116:1374–82. doi: 10.1542/peds.2004-1728
17. Catroppa C, Anderson V, Ditchfield M, Coleman L. Using magnetic resonance imaging to predict new learning outcome at 5 years after childhood traumatic brain injury. J Child Neurol. (2008) 23:486–96. doi: 10.1177/0883073807309773
18. Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI). J Pediatr Psychol. (2008) 33:707–18. doi: 10.1093/jpepsy/jsn006
19. Ryan NP, Anderson V, Godfrey C, Beauchamp MH, Coleman L, Eren S, et al. Predictors of very-long-term sociocognitive function after pediatric traumatic brain injury: evidence for the vulnerability of the immature “social brain”. J Neurotrauma. (2014) 31:649–57. doi: 10.1089/neu.2013.3153
21. Tong KA, Ashwal S, Holshouser BA, Nickerson JP, Wall CJ, Shutter LA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. (2004) 56:36–50. doi: 10.1002/ana.20123
22. Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. (2003) 227:332–9. doi: 10.1148/radiol.2272020176
23. Meaney DF, Smith DH, Shreiber DI, Bain AC, Miller RT, Ross DT, et al. Biomechanical analysis of experimental diffuse axonal injury. J Neurotrauma. (1995) 12:689–94. doi: 10.1089/neu.1995.12.689
24. Cory CZ, Jones MD, James DS, Leadbeatter S, Nokes LD. The potential and limitations of utilising head impact injury models to assess the likelihood of significant head injury in infants after a fall. Forensic Sci Int. (2001) 123:89–106. doi: 10.1016/S0379-0738(01)00523-0
25. Hirsch C, Evans FG. Studies on some physical properties of infant compact bone. Acta Orthop Scand. (1965) 35:300–13. doi: 10.3109/17453676508989361
26. Lindsey HM, Wilde EA, Caeyenberghs K, Dennis EL. Longitudinal neuroimaging in pediatric traumatic brain injury: current state and consideration of factors that influence recovery. Front Neurol. (2019) 10:1296. doi: 10.3389/fneur.2019.01296
27. Chapman SB, McKinnon L. Discussion of developmental plasticity: factors affecting cognitive outcome after pediatric traumatic brain injury. J Commun Disord. (2000) 33:333–44. doi: 10.1016/S0021-9924(00)00029-0
28. Wilde EA, Hunter, Bigler ED. Pediatric traumatic brain injury: neuroimaging and neurorehabilitation outcome. NeuroRehabilitation. (2012) 31:245–60. doi: 10.3233/NRE-2012-0794
29. Anderson SW, Damasio H, Tranel D, Damasio AR. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol. (2000) 18:281–96. doi: 10.1207/S1532694202Anderson
30. Eslinger PJ, Biddle KR. Adolescent neuropsychological development after early right prefrontal cortex damage. Dev Neuropsychol. (2000) 18:297–329. doi: 10.1207/S1532694203Eslinger
31. De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. (2002) 22:9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002
32. Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. (2015) 18:690–7. doi: 10.1038/nn.3988
33. Moreno JA, McKerral M. Differences according to sex in sociosexuality and infidelity after traumatic brain injury. Behav Neurol. (2015) 2015:914134. doi: 10.1155/2015/914134
34. Gerring JP, Slomine B, Vasa RA, Grados M, Chen A, Rising W, et al. Clinical predictors of posttraumatic stress disorder after closed head injury in children. J Am Acad Child Adolesc Psychiatry. (2002) 41:157–65. doi: 10.1097/00004583-200202000-00009
35. Despins EH, Turkstra LS, Struchen MA, Clark AN. Sex-Based differences in perceived pragmatic communication ability of adults with traumatic brain injury. Arch Phys Med Rehabil. (2016) 97:S26–32. doi: 10.1016/j.apmr.2014.06.023
36. Scott C, McKinlay A, McLellan T, Britt E, Grace R, MacFarlane M. A comparison of adult outcomes for males compared to females following pediatric traumatic brain injury. Neuropsychology. (2015) 29:501–8. doi: 10.1037/neu0000074
37. Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J Pediatr Psychol. (2003) 28:251–63. doi: 10.1093/jpepsy/jsg013
38. Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. (2006) 30:718–29. doi: 10.1016/j.neubiorev.2006.06.001
39. Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. (1973) 63:195–204. doi: 10.1016/0006-8993(73)90088-7
40. Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. (1979) 163:195–205. doi: 10.1016/0006-8993(79)90349-4
41. Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci USA. (1991) 88:10218–21. doi: 10.1073/pnas.88.22.10218
42. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. (1999) 2:861–3. doi: 10.1038/13158
43. Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. (2000) 20:1294–300. doi: 10.1097/00004647-200009000-00003
44. Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. (2007) 5:96–105. doi: 10.1111/j.1528-1167.2007.01295.x
45. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. (2010) 468:562–6. doi: 10.1038/nature09513
46. Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, et al. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci. (2002) 70:1909–22. doi: 10.1016/S0024-3205(02)01492-3
47. Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr Protoc Toxicol Chapter. (2005) 13:Unit13 10. doi: 10.1002/0471140856.tx1310s26
48. Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore SJ. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc Cogn Affect Neurosci. (2014) 9:123–31. doi: 10.1093/scan/nss113
49. Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev. (2011) 35:1654–64. doi: 10.1016/j.neubiorev.2010.10.011
50. Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. (2008) 9:267–77. doi: 10.1038/nrn2353
51. Bondar NP, Lepeshko AA, Reshetnikov VV. Effects of early-life stress on social and anxiety-like behaviors in adult mice: sex-specific effects. Behav Neurol. (2018) 2018:1538931. doi: 10.1155/2018/1538931
52. Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. (1970) 206:419–36. doi: 10.1113/jphysiol.1970.sp009022
53. Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. (1984) 52:819–47. doi: 10.1152/jn.1984.52.5.819
54. Fine, Wade AR, Brewer AA, May MG, Goodman DF, Boynton GM, et al. Long-term deprivation affects visual perception and cortex. Nat Neurosci. (2003) 6:915–6. doi: 10.1038/nn1102
55. Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. (2005) 46:163–83. doi: 10.1002/dev.20055
56. Semple BD, Canchola SA, Noble-Haeusslein LJ. Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J Neurotrauma. (2012) 29:2672–83. doi: 10.1089/neu.2012.2595
57. Levin HS, Hanten G, Chang CC, Zhang L, Schachar R, Ewing-Cobbs L, et al. Working memory after traumatic brain injury in children. Ann Neurol. (2002) 52:82–8. doi: 10.1002/ana.10252
58. Anderson VA, Spencer-Smith MM, Coleman L, Anderson PJ, Greenham M, Jacobs R, et al. Predicting neurocognitive and behavioural outcome after early brain insult. Dev Med Child Neurol. (2014) 56:329–36. doi: 10.1111/dmcn.12387
59. Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. (2012) 129:e254–61. doi: 10.1542/peds.2011-0311
60. Karver CL, Kurowski B, Semple EA, Stancin T, Taylor HG, Yeates KO, et al. Utilization of behavioral therapy services long-term after traumatic brain injury in young children. Arch Phys Med Rehabil. (2014) 95:1556–63. doi: 10.1016/j.apmr.2014.03.030
61. Karver CL, Wade SL, Cassedy A, Taylor HG, Stancin T, Yeates KO, et al. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol. (2012) 57:256–65. doi: 10.1037/a0029522
62. Kozlowski DA, Schallert T. Relationship between dendritic pruning and behavioral recovery following sensorimotor cortex lesions. Behav Brain Res. (1998) 97:89–98. doi: 10.1016/S0166-4328(98)00030-8
63. Kolb B, Gibb R, van der Kooy D. Neonatal frontal cortical lesions in rats alter cortical structure and connectivity. Brain Res. (1994) 645:85–97. doi: 10.1016/0006-8993(94)91641-1
64. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/S0749-3797(98)00017-8
65. Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. (2009) 163:1135–43. doi: 10.1001/archpediatrics.2009.214
66. Giovanelli A, Reynolds AJ, Mondi CF, Ou SR. Adverse childhood experiences and adult well-being in a low-income, urban cohort. Pediatrics. (2016) 137:e20154016. doi: 10.1542/peds.2015-4016
67. Flaherty EG, Thompson R, Dubowitz H, Harvey EM, English DJ, Proctor LJ, et al. Adverse childhood experiences and child health in early adolescence. JAMA Pediatr. (2013) 167:622–9. doi: 10.1001/jamapediatrics.2013.22
68. Richards M, Wadsworth ME. Long term effects of early adversity on cognitive function. Arch Dis Child. (2004) 89:922–7. doi: 10.1136/adc.2003.032490
69. Kelly-Irving M, Lepage B, Dedieu D, Bartley M, Blane D, Grosclaude P, et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol. (2013) 28:721–34. doi: 10.1007/s10654-013-9832-9
70. Banyard VL. Childhood maltreatment and the mental health of low-income women. Am J Orthopsychiatry. (1999) 69:161–71. doi: 10.1037/h0080418
71. Rorty M, Yager J, Rossotto E. Childhood sexual, physical, and psychological abuse in bulimia nervosa. Am J Psychiatry. (1994) 151:1122–6. doi: 10.1176/ajp.151.8.1122
72. Cavaiola AA, Schiff M. Behavioral sequelae of physical and/or sexual abuse in adolescents. Child Abuse Negl. (1988) 12:181–8. doi: 10.1016/0145-2134(88)90026-9
73. Briere J, Runtz M. Multivariate correlates of childhood psychological and physical maltreatment among university women. Child Abuse Negl. (1988) 12:331–41. doi: 10.1016/0145-2134(88)90046-4
74. Chu JA, Dill DL. Dissociative symptoms in relation to childhood physical and sexual abuse. Am J Psychiatry. (1990) 147:887–92. doi: 10.1176/ajp.147.7.887
75. Riggs S, Alario AJ, McHorney C. Health risk behaviors and attempted suicide in adolescents who report prior maltreatment. J Pediatr. (1990) 116:815–21. doi: 10.1016/S0022-3476(05)82679-4
76. Moeller TP, Bachmann GA, Moeller JR. The combined effects of physical, sexual, and emotional abuse during childhood: long-term health consequences for women. Child Abuse Negl. (1993) 17:623–40. doi: 10.1016/0145-2134(93)90084-I
77. Gould DA, Stevens NG, Ward NG, Carlin AS, Sowell HE, Gustafson B. Self-reported childhood abuse in an adult population in a primary care setting. Prevalence, correlates, and associated suicide attempts. Arch Fam Med. (1994) 3:252–6. doi: 10.1001/archfami.3.3.252
78. McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. (1997) 277:1362–8. doi: 10.1001/jama.277.17.1362
79. Bensley LS, Van Eenwyk J, Spieker SJ, Schoder J. Self-reported abuse history and adolescent problem behaviors. Antisocial I, suicidal behaviors. J Adolesc Health. (1999) 24:163–72. doi: 10.1016/S1054-139X(98)00111-6
80. Merrill LL, Newell CE, Thomsen CJ, Gold SR, Milner JS, Koss MP, et al. Childhood abuse and sexual revictimization in a female navy recruit sample. J Trauma Stress. (1999) 12:211–25. doi: 10.1023/A:1024789723779
81. Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. (2010) 13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x
82. Wilson RS, Boyle PA, Levine SR, Yu L, Anagnos SE, Buchman AS, et al. Emotional neglect in childhood and cerebral infarction in older age. Neurology. (2012) 79:1534–9. doi: 10.1212/WNL.0b013e31826e25bd
83. van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Personality traits and childhood trauma as correlates of metabolic risk factors: the Netherlands study of depression and anxiety (NESDA). Prog Neuropsychopharmacol Biol Psychiatry. (2012) 36:85–91. doi: 10.1016/j.pnpbp.2011.10.001
84. van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand. (2010) 122:30–9. doi: 10.1111/j.1600-0447.2010.01565.x
85. Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, et al. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res. (2007) 16:148–55. doi: 10.1111/j.1365-2869.2007.00581.x
86. Kalsbeek A, Yi CX, la Fleur SE, Buijs RM, Fliers E. Suprachiasmatic nucleus and autonomic nervous system influences on awakening from sleep. Int Rev Neurobiol. (2010) 93:91–107. doi: 10.1016/S0074-7742(10)93004-3
87. Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. (2008) 149:4892–900. doi: 10.1210/en.2008-0633
88. Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. (2003) 301:386–9.
89. R. Dalle Molle, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, et al. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry. (2012) 2:e195. doi: 10.1038/tp.2012.126
90. Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM. Behavioral changes after closed head injury in children. J Consult Clin Psychol. (1990) 58:93–8. doi: 10.1037/0022-006X.58.1.93
91. Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull. (2007) 133:535–56. doi: 10.1037/0033-2909.133.3.535
92. McNally KA, Bangert B, Dietrich A, Nuss K, Rusin J, Wright M, et al. Injury versus noninjury factors as predictors of postconcussive symptoms following mild traumatic brain injury in children. Neuropsychology. (2013) 27:1–12. doi: 10.1037/a0031370
93. Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, et al. Gene profiling reveals unknown enhancing suppressive actions of glucocorticoids on immune cells. FASEB J. (2002) 16:61–71. doi: 10.1096/fj.01-0245com
94. Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. (1993) 365:756–9. doi: 10.1038/365756a0
95. Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. (1995) 377:68–71. doi: 10.1038/377068a0
96. Tertil M, Skupio U, Barut J, Dubovyk V, Wawrzczak-Bargiela A, Soltys Z, et al. Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Transl Psychiatry. (2018) 8:255. doi: 10.1038/s41398-018-0300-x
97. Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. (2013) 339:332–5. doi: 10.1126/science.1226767
98. Fitzsimons CP, van Hooijdonk LW, Schouten M, Zalachoras I, Brinks V, Zheng T, et al. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry. (2013) 18:993–1005. doi: 10.1038/mp.2012.123
99. Reus GZ, Fernandes GC, de Moura AB, Silva RH, Darabas AC, de Souza TG, et al. Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. J Psychiatr Res. (2017) 95:196–207. doi: 10.1016/j.jpsychires.2017.08.020
100. Hartmann J, Dedic N, Pohlmann ML, Hausl A, Karst H, Engelhardt C, et al. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry. (2017) 22:466–75. doi: 10.1038/mp.2016.87
101. Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation conditioning. Proc Natl Acad Sci USA. (2008) 105:12004–9. doi: 10.1073/pnas.0803216105
102. Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: a role for the NLRP3 inflammasome. Brain Behav Immun. (2016) 55:215–224. doi: 10.1016/j.bbi.2015.10.009
103. Kochanek PM, Wallisch JS, Bayir H, Clark RSB. Pre-clinical models in pediatric traumatic brain injury-challenges and lessons learned. Childs Nerv Syst. (2017) 33:1693–701. doi: 10.1007/s00381-017-3474-2
104. Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. (2005) 22:42–75. doi: 10.1089/neu.2005.22.42
105. Chen CY, Noble-Haeusslein LJ, Ferriero D, Semple BD. Traumatic injury to the immature frontal lobe: a new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits. Dev Neurosci. (2013) 35:474–90. doi: 10.1159/000355874
106. Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. (2007) 130:2508–19. doi: 10.1093/brain/awm216
107. Osier ND, Dixon CE. The controlled cortical impact model: applications, considerations for researchers, future directions. Front Neurol. (2016) 7:134. doi: 10.3389/fneur.2016.00134
108. Fox GB, LeVasseur RA, Faden AI. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J Neurotrauma. (1999) 16:377–89. doi: 10.1089/neu.1999.16.377
109. Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. (1998) 15:599–614. doi: 10.1089/neu.1998.15.599
110. Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, et al. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. (1999) 16:109–22. doi: 10.1089/neu.1999.16.109
111. Chauhan NB, Gatto R. Restoration of cognitive deficits after statin feeding in TBI. Restor Neurol Neurosci. (2011) 29:23–34. doi: 10.3233/RNN-2011-0573
112. Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. (1992) 9:11–20. doi: 10.1089/neu.1992.9.11
113. Zhao S, Yu Z, Liu Y, Bai Y, Jiang Y, van Leyen K, et al. CD47 deficiency improves neurological outcomes of traumatic brain injury in mice. Neurosci Lett. (2017) 643:125–30. doi: 10.1016/j.neulet.2016.12.006
114. Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP. The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J Neurotrauma. (2012) 29:2283–96. doi: 10.1089/neu.2012.2456
115. Byrnes KR, Loane DJ, Stoica BA, Zhang J, Faden AI. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J Neuroinflammation. (2012) 9:43. doi: 10.1186/1742-2094-9-43
116. Kamper JE, Pop V, Fukuda AM, Ajao DO, Hartman RE, Badaut J. Juvenile traumatic brain injury evolves into a chronic brain disorder: behavioral and histological changes over 6months. Exp Neurol. (2013) 250:8–19. doi: 10.1016/j.expneurol.2013.09.016
117. Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res. (1996) 95:272–82. doi: 10.1016/0165-3806(96)00098-3
118. Levine S. Infantile experience and resistance to physiological stress. Science. (1957) 126:405. doi: 10.1126/science.126.3270.405
119. Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. (1962) 135:795–6. doi: 10.1126/science.135.3506.795-a
120. Ader R. The effects of early experience on the adrenocortical response to different magnitudes of stimulation. Physiol Behav. (1970) 5:837–9. doi: 10.1016/0031-9384(70)90168-X
121. Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. (1991) 128:1385–95. doi: 10.1210/endo-128-3-1385
122. Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol. (1991) 24:547–58. doi: 10.1002/dev.420240803
123. Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. (1993) 18:195–200. doi: 10.1016/0169-328X(93)90189-V
124. Spencer-Booth Y, Hinde RA. The effects of 13 days maternal separation on infant rhesus monkeys compared with those of shorter and repeated separations. Anim Behav. (1971) 19:595–605. doi: 10.1016/S0003-3472(71)80117-3
125. Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol. (1993) 26:433–46. doi: 10.1002/dev.420260802
126. Schmidt MV, Oitzl MS, Levine S, de Kloet ER. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. (2002) 139:39–49. doi: 10.1016/S0165-3806(02)00519-9
127. Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. (1996) 15:114–9. doi: 10.1016/0887-8994(96)00153-1
128. Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. (2001) 13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x
129. Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. (2005) 25:9328–38. doi: 10.1523/JNEUROSCI.2281-05.2005
130. Moussaoui N, Larauche M, Biraud M, Molet J, Million M, Mayer E, et al. Limited nesting stress alters maternal behavior and in vivo intestinal permeability in male wistar pup rats. PLoS ONE. (2016) 11:e0155037. doi: 10.1371/journal.pone.0155037
131. Arp JM, Ter Horst JP, Loi M, den Blaauwen J, Bangert E, Fernandez G, et al. Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol Learn Mem. (2016) 133:30–8. doi: 10.1016/j.nlm.2016.05.009
132. Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. (2016) 82:64–71. doi: 10.1016/j.yhbeh.2016.04.010
133. Sanchez CM, Titus DJ, Wilson NM, Freund JE, Atkins CM. Early life stress exacerbates outcome after traumatic brain injury. J Neurotrauma. (2020) 38:555–65. doi: 10.1089/neu.2020.7267
134. Diaz-Chavez, Lajud N, Roque A, Cheng JP, Melendez-Herrera E, Valdez-Alarcon JJ, et al. Early life stress increases vulnerability to the sequelae of pediatric mild traumatic brain injury. Exp Neurol. (2020) 329:113318. doi: 10.1016/j.expneurol.2020.113318
135. Grota LJ, Ader R. Continuous recording of maternal behavior in Rattus norvegicus. Anim. Behav. (1969) 17:723–729. doi: 10.1016/S0003-3472(69)80019-9
136. Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. J Neuroendocrinol. (1995) 7:97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x
137. Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J Neurosci. (1993) 13:1097–105. doi: 10.1523/JNEUROSCI.13-03-01097.1993
138. Weinberg J. Effects of early experience on responsiveness to ethanol: a preliminary report. Physiol Behav. (1987) 40:401–6. doi: 10.1016/0031-9384(87)90068-0
139. Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol Biochem Behav. (1994) 49:961–7. doi: 10.1016/0091-3057(94)90250-X
140. Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. (1999) 32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4
141. Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci. (2005) 25:4453–62. doi: 10.1523/JNEUROSCI.4807-04.2005
142. Mehta M, Schmauss C. Strain-specific cognitive deficits in adult mice exposed to early life stress. Behav Neurosci. (2011) 125:29–36. doi: 10.1037/a0021952
143. Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. (2003) 43:561–7. doi: 10.1016/S0018-506X(03)00063-1
144. Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. (2007) 32:437–50. doi: 10.1016/j.psyneuen.2007.02.008
145. Tsuda MC, Ogawa S. Long-lasting consequences of neonatal maternal separation on social behaviors in ovariectomized female mice. PLoS ONE. (2012) 7:e33028. doi: 10.1371/journal.pone.0033028
146. de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. (2005) 29:271–81. doi: 10.1016/j.neubiorev.2004.10.008
147. Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. (2000) 405:149–60. doi: 10.1016/S0014-2999(00)00548-3
148. Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. (2003) 27:57–71. doi: 10.1016/S0149-7634(03)00009-5
149. Schmidt MV. Molecular mechanisms of early life stress–lessons from mouse models. Neurosci Biobehav Rev. (2010) 34:845–52. doi: 10.1016/j.neubiorev.2009.05.002
150. Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci. (1998) 16:187–97. doi: 10.1016/S0736-5748(98)00019-7
151. Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. (2008) 154:1132–42. doi: 10.1016/j.neuroscience.2008.04.019
152. Gallo M, Shleifer DG, Godoy LD, Ofray D, Olaniyan A, Campbell T, et al. Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front Behav Neurosci. (2019) 13:167. doi: 10.3389/fnbeh.2019.00167
153. Wang L, Jiao J, Dulawa SC. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology. (2011) 216:207–18. doi: 10.1007/s00213-011-2209-4
154. Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, et al. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci. (2013) 16:706–13. doi: 10.1038/nn.3395
155. Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. (2015) 25:309–28. doi: 10.1002/hipo.22374
156. Krugers HJ, Arp JM, Xiong H, Kanatsou S, Lesuis SL, Korosi A, et al. Early life adversity: lasting consequences for emotional learning. Neurobiol Stress. (2017) 6:14–21. doi: 10.1016/j.ynstr.2016.11.005
157. Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. (2012) 32:7758–65. doi: 10.1523/JNEUROSCI.5843-11.2012
158. Raineki C, Sarro E, Rincon-Cortes M, Perry R, Boggs J, Holman CJ, et al. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. (2015) 40:906–14. doi: 10.1038/npp.2014.266
159. Rincon-Cortes M, Sullivan RM. Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Transl Psychiatry. (2016) 6:e930. doi: 10.1038/tp.2016.205
160. Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, et al. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci. (2013) 33:19534–54. doi: 10.1523/JNEUROSCI.1337-13.2013
161. McLaughlin RJ, Verlezza S, Gray JM, Hill MN, Walker CD. Inhibition of anandamide hydrolysis dampens the neuroendocrine response to stress in neonatal rats subjected to suboptimal rearing conditions. Stress. (2016) 19:114–24. doi: 10.3109/10253890.2015.1117448
162. Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. (2016) 6:e702. doi: 10.1038/tp.2015.200
163. Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. (2013) 36:621–31. doi: 10.1016/j.tins.2013.08.002
164. Dickens AM, Tovar RLB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. (2017) 10:eaa7696. doi: 10.1126/scisignal.aai7696
165. Makinde HM, Cuda CM, Just TB, Perlman HR, Schwulst SJ. Nonclassical monocytes mediate secondary injury, neurocognitive outcome, and neutrophil infiltration after traumatic brain injury. J Immunol. (2017) 199:3583–91. doi: 10.4049/jimmunol.1700896
166. Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. (2014) 505:223–8. doi: 10.1038/nature12808
167. Russo MV, Latour LL, McGavern DB. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol. (2018) 19:442–52. doi: 10.1038/s41590-018-0086-2
168. Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. (2005) 25:8000–9. doi: 10.1523/JNEUROSCI.1748-05.2005
169. Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. (2011) 31:15511–21. doi: 10.1523/JNEUROSCI.3688-11.2011
170. Somera-Molina KC, Nair S, Van Eldik LJ, Watterson DM, Wainwright MS. Enhanced microglial activation and proinflammatory cytokine upregulation are linked to increased susceptibility to seizures and neurologic injury in a 'two-hit' seizure model. Brain Res. (2009) 1282:162–72. doi: 10.1016/j.brainres.2009.05.073
171. Chastain LG, Franklin T, Gangisetty O, Cabrera MA, Mukherjee S, Shrivastava P, et al. Early life alcohol exposure primes hypothalamic microglia to later-life hypersensitivity to immune stress: possible epigenetic mechanism. Neuropsychopharmacology. (2019) 44:1579–88. doi: 10.1038/s41386-019-0326-7
172. Vanbesien-Mailliot CC, Wolowczuk I, Mairesse J, Viltart O, Delacre M, Khalife J, et al. Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrinology. (2007) 32:114–24. doi: 10.1016/j.psyneuen.2006.11.005
173. Wang D, Levine JLS, Avila-Quintero V, Bloch M, Kaffman A. Systematic review and meta-analysis: effects of maternal separation on anxiety-like behavior in rodents. Transl Psychiatry. (2020) 10:174. doi: 10.1038/s41398-020-0856-0
174. Saavedra LM, Hernandez-Velazquez MG, Madrigal S, Ochoa-Zarzosa A, Torner L. Long-term activation of hippocampal glial cells and altered emotional behavior in male and female adult rats after different neonatal stressors. Psychoneuroendocrinology. (2021) 126:105164. doi: 10.1016/j.psyneuen.2021.105164
175. Sagae SC, Zanardini B, Ribeiro-Paz ED, Amaral AC, Bronczek GA, Lubaczeuski C, et al. Metabolic dysfunction in a rat model of early-life scarcity-adversity: modulatory role of cafeteria diet. Exp Physiol. (2018) 103:1481–93. doi: 10.1113/EP087171
176. Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. (2007) 21:47–59. doi: 10.1016/j.bbi.2006.03.005
177. De Miguel C, Obi IE, Ho DH, Loria AS, Pollock JS. Early life stress induces immune priming in kidneys of adult male rats. Am J Physiol Renal Physiol. (2018) 314:F343–55. doi: 10.1152/ajprenal.00590.2016
178. Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. (2010) 94:57–64. doi: 10.1016/j.nlm.2010.04.001
179. Mattei D, Ivanov A, Ferrai C, Jordan P, Guneykaya D, Buonfiglioli A, et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl Psychiatry. (2017) 7:e1120. doi: 10.1038/tp.2017.80
180. Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. (1995) 154:4719–25.
181. Liu Y, Cousin JM, Hughes J, Van Damme J, Seckl JR, Haslett C, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. (1999) 162:3639–46.
182. Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. (1999) 162:6473–81.
183. van der Goes A, Hoekstra K, van den Berg TK, Dijkstra CD. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J Leukoc Biol. (2000) 67:801–7. doi: 10.1002/jlb.67.6.801
184. Frank MG, Fonken LK, Watkins LR, Maier SF. Acute stress induces chronic neuroinflammatory, microglial and behavioral priming: A role for potentiated NLRP3 inflammasome activation. Brain Behav Immun. (2020) 89:32–42. doi: 10.1016/j.bbi.2020.05.063
185. Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. (2011) 286:38703–13. doi: 10.1074/jbc.M111.275370
186. Niu L, Luo SS, Xu Y, Wang Z, Luo D, Yang H, et al. The critical role of the hippocampal NLRP3 inflammasome in social isolation-induced cognitive impairment in male mice. Neurobiol Learn Mem. (2020) 175:107301. doi: 10.1016/j.nlm.2020.107301
187. Trojan E, Chamera K, Bryniarska N, Kotarska K, Leskiewicz M, Regulska M, et al. Role of chronic administration of antidepressant drugs in the prenatal stress-evoked inflammatory response in the brain of adult offspring rats: involvement of the nlrp3 inflammasome-related pathway. Mol Neurobiol. (2019) 56:5365–80. doi: 10.1007/s12035-018-1458-1
188. Tapp ZM, Godbout JP, Kokiko-Cochran ON. A tilted axis: maladaptive inflammation and HPA axis dysfunction contribute to consequences of TBI. Front Neurol. (2019) 10:345. doi: 10.3389/fneur.2019.00345
189. Rowe RK, Rumney BM, May HG, Permana P, Adelson PD, Harman SM, et al. Diffuse traumatic brain injury affects chronic corticosterone function in the rat. Endocr Connect. (2016) 5:152–66. doi: 10.1530/EC-16-0031
190. Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma. (2008) 25:311–23. doi: 10.1089/neu.2007.0486
191. Taylor AN, Rahman SU, Tio DL, Sanders MJ, Bando JK, Truong AH, et al. Lasting neuroendocrine-immune effects of traumatic brain injury in rats. J Neurotrauma. (2006) 23:1802–13. doi: 10.1089/neu.2006.23.1802
192. Grundy PL, Harbuz MS, Jessop DS, Lightman SL, Sharples PM. The hypothalamo-pituitary-adrenal axis response to experimental traumatic brain injury. J Neurotrauma. (2001) 18:1373–81. doi: 10.1089/08977150152725669
193. Roe SY, McGowan EM, Rothwell NJ. Evidence for the involvement of corticotrophin-releasing hormone in the pathogenesis of traumatic brain injury. Eur J Neurosci. (1998) 10:553–9. doi: 10.1046/j.1460-9568.1998.00064.x
194. Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. (2003) 33:455–67. doi: 10.1017/S0033291702007018
195. Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, et al., Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. (2017) 22:527–36. doi: 10.1038/mp.2016.120
196. Amini-Khoei H, Haghani-Samani E, Beigi M, Soltani A, Mobini GR, Balali-Dehkordi S, et al. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int Immunopharmacol. (2019) 66:242–50. doi: 10.1016/j.intimp.2018.11.037
197. Lorigooini Z, Boroujeni SN, Sayyadi-Shahraki M, Rahimi-Madiseh M, Bijad E, Amini-Khoei H. Limonene through attenuation of neuroinflammation and nitrite level exerts antidepressant-like effect on mouse model of maternal separation stress. Behav Neurol. (2021) 2021:8817309. doi: 10.1155/2021/8817309
198. Nouri A, Hashemzadeh F, Soltani A, Saghaei E, Amini-Khoei H. Progesterone exerts antidepressant-like effect in a mouse model of maternal separation stress through mitigation of neuroinflammatory response and oxidative stress. Pharm Biol. (2020) 58:64–71. doi: 10.1080/13880209.2019.1702704
199. Hoeijmakers L, Ruigrok SR, Amelianchik A, Ivan D, van Dam AM, Lucassen PJ, et al. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an alzheimer's disease mouse model. Brain Behav Immun. (2017) 63:160–75. doi: 10.1016/j.bbi.2016.12.023
200. Delpech JC, Wei L, Hao J, Yu X, Madore C, Butovsky O, et al. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav Immun. (2016) 57:79–93. doi: 10.1016/j.bbi.2016.06.006
201. Ehrlich KB, Ross KM, Chen E, Miller GE. Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Dev Psychopathol. (2016) 28:1273–83. doi: 10.1017/S0954579416000845
202. Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. (2006) 163:1630–3. doi: 10.1176/ajp.2006.163.9.1630
203. Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. (2010) 35:2617–23. doi: 10.1038/npp.2010.159
204. Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. (2007) 104:1319–24. doi: 10.1073/pnas.0610362104
205. Milaniak, Jaffee SR. Childhood socioeconomic status and inflammation: a systematic review and meta-analysis. Brain Behav Immun. (2019) 78:161–76. doi: 10.1016/j.bbi.2019.01.018
206. Craft TK, Zhang N, Glasper ER, Hurn PD, Devries AC. Neonatal factors influence adult stroke outcome. Psychoneuroendocrinology. (2006) 31:601–13. doi: 10.1016/j.psyneuen.2006.01.002
207. McPherson RJ, Mascher-Denen M, Juul SE. Postnatal stress produces hyperglycemia in adult rats exposed to hypoxia-ischemia. Pediatr Res. (2009) 66:278–82. doi: 10.1203/PDR.0b013e3181b1bd1b
208. Tata DA, Markostamou I, Ioannidis A, Gkioka M, Simeonidou C, Anogianakis G, et al. Effects of maternal separation on behavior and brain damage in adult rats exposed to neonatal hypoxia-ischemia. Behav Brain Res. (2015) 280:51–61. doi: 10.1016/j.bbr.2014.11.033
209. Markostamou I, Ioannidis A, Dandi E, Mandyla MA, Nousiopoulou E, Simeonidou C, et al. Maternal separation prior to neonatal hypoxia-ischemia: impact on emotional aspects of behavior and markers of synaptic plasticity in hippocampus. Int J Dev Neurosci. (2016) 52:1–12. doi: 10.1016/j.ijdevneu.2016.04.002
210. Lajud N, Roque A, Cheng JP, Bondi CO, Kline AE. Early life stress preceding mild pediatric traumatic brain injury increases neuroinflammation but does not exacerbate impairment of cognitive flexibility during adolescence. J Neurotrauma. (2020) 38:411–21. doi: 10.1089/neu.2020.7354
211. Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in hospitals US, 1989-2009. NCHS Data Brief. (2012) 1–8.
212. Magno LAV, Collodetti M, Tenza-Ferrer H, Romano-Silva MA. Cylinder test to assess sensory-motor function in a mouse model of parkinson's disease. Bio Protoc. (2019) 9:e3337. doi: 10.21769/BioProtoc.3337
213. Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. (2000) 39:777–87. doi: 10.1016/S0028-3908(00)00005-8
214. Yamazaki A, Ohtsuki Y, Yoshihara T, Honma S, Honma K. Maternal deprivation in neonatal rats of different conditions affects growth rate, circadian clock, and stress responsiveness differentially. Physiol Behav. (2005) 86:136–44. doi: 10.1016/j.physbeh.2005.07.013
215. Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. (1999) 66:293–302. doi: 10.1016/S0031-9384(98)00300-X
216. McIntosh J, Anisman H, Merali Z. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Brain Res Dev Brain Res. (1999) 113:97–106. doi: 10.1016/S0165-3806(99)00005-X
217. Penke Z, Felszeghy K, Fernette B, Sage D, Nyakas C, Burlet A. Postnatal maternal deprivation produces long-lasting modifications of the stress response, feeding and stress-related behaviour in the rat. Eur J Neurosci. (2001) 14:747–55. doi: 10.1046/j.0953-816x.2001.01691.x
218. Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. (2000) 141:1593–8. doi: 10.1210/endo.141.5.7455
219. Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in long-evans rats. Pharmacol Biochem Behav. (2002) 73:131–40. doi: 10.1016/S0091-3057(02)00781-5
220. Suarez MM, Rivarola MA, Molina SM, Perassi NI, Levin GM, Cabrera R. Periodic maternal deprivation and lesion of anterodorsal thalami nuclei induce alteration on hypophyso adrenal system activity in adult rats. Life Sci. (2001) 69:803–13. doi: 10.1016/S0024-3205(01)01173-0
221. Bodnar CN, Roberts KN, Higgins EK, Bachstetter AD. A systematic review of closed head injury models of mild traumatic brain injury in mice and rats. J Neurotrauma. (2019) 36:1683–706. doi: 10.1089/neu.2018.6127
222. Hagberg H, David Edwards A, Groenendaal F. Perinatal brain damage: the term infant. Neurobiol Dis. (2016) 92:102–12. doi: 10.1016/j.nbd.2015.09.011
223. Johnson FK, Kaffman A. Early life stress perturbs the function of microglia in the developing rodent brain: new insights and future challenges. Brain Behav Immun. (2018) 69:18–27. doi: 10.1016/j.bbi.2017.06.008
224. Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. (2000) 20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000
225. Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:913–23. doi: 10.1016/j.pnpbp.2010.04.016
226. Fogelman N, Canli T. Early life stress, physiology, and genetics: a review. Front Psychol. (2019) 10:1668. doi: 10.3389/fpsyg.2019.01668
227. Gupte R, Brooks W, Vukas R, Pierce J, Harris J. Sex differences in traumatic brain injury: what we know and what we should know. J Neurotrauma. (2019) 36:3063–91. doi: 10.1089/neu.2018.6171
228. Bennett ER, Reuter-Rice K, Laskowitz DT. Genetic influences in traumatic brain injury. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton, FL (2016).
229. Cortes D, Pera MF. The genetic basis of inter-individual variation in recovery from traumatic brain injury. NPJ Regen Med. (2021) 6:5. doi: 10.1038/s41536-020-00114-y
230. Treble-Barna A, Patronick J, Uchani S, Marousis NC, Zigler CK, Fink EL, et al. Epigenetic effects on pediatric traumatic brain injury recovery (EETR): an observational, prospective, longitudinal concurrent cohort study protocol. Front Neurol. (2020) 11:460. doi: 10.3389/fneur.2020.00460
231. Kurowski BG, Treble-Barna A, Pilipenko V, Wade SL, Yeates KO, Taylor HG, et al. Genetic influences on behavioral outcomes after childhood TBI: a novel systems biology-informed approach. Front Genet. (2019) 10:481. doi: 10.3389/fgene.2019.00481
232. Neher JJ, Cunningham C. Priming microglia for innate immune memory in the brain. Trends Immunol. (2019) 40:358–374. doi: 10.1016/j.it.2019.02.001
233. Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. (2013) 27:8–12. doi: 10.1016/j.bbi.2012.06.014
234. von Leden RE, Parker KN, Bates AA, Noble-Haeusslein LJ, Donovan MH. The emerging role of neutrophils as modifiers of recovery after traumatic injury to the developing brain. Exp Neurol. (2019) 317:144–154. doi: 10.1016/j.expneurol.2019.03.004
Keywords: early life stress, traumatic brain injury, developing brain, inflammation, immune priming, stress
Citation: Parker KN, Donovan MH, Smith K and Noble-Haeusslein LJ (2021) Traumatic Injury to the Developing Brain: Emerging Relationship to Early Life Stress. Front. Neurol. 12:708800. doi: 10.3389/fneur.2021.708800
Received: 12 May 2021; Accepted: 22 July 2021;
Published: 18 August 2021.
Edited by:
Bridgette D. Semple, Monash University, AustraliaReviewed by:
Richelle Mychasiuk, Monash University, AustraliaZachary M. Weil, West Virginia University, United States
Copyright © 2021 Parker, Donovan, Smith and Noble-Haeusslein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda J. Noble-Haeusslein, bGluZGEubm9ibGVAYXVzdGluLnV0ZXhhcy5lZHU=
 Kaila N. Parker
Kaila N. Parker Michael H. Donovan
Michael H. Donovan Kylee Smith
Kylee Smith Linda J. Noble-Haeusslein
Linda J. Noble-Haeusslein