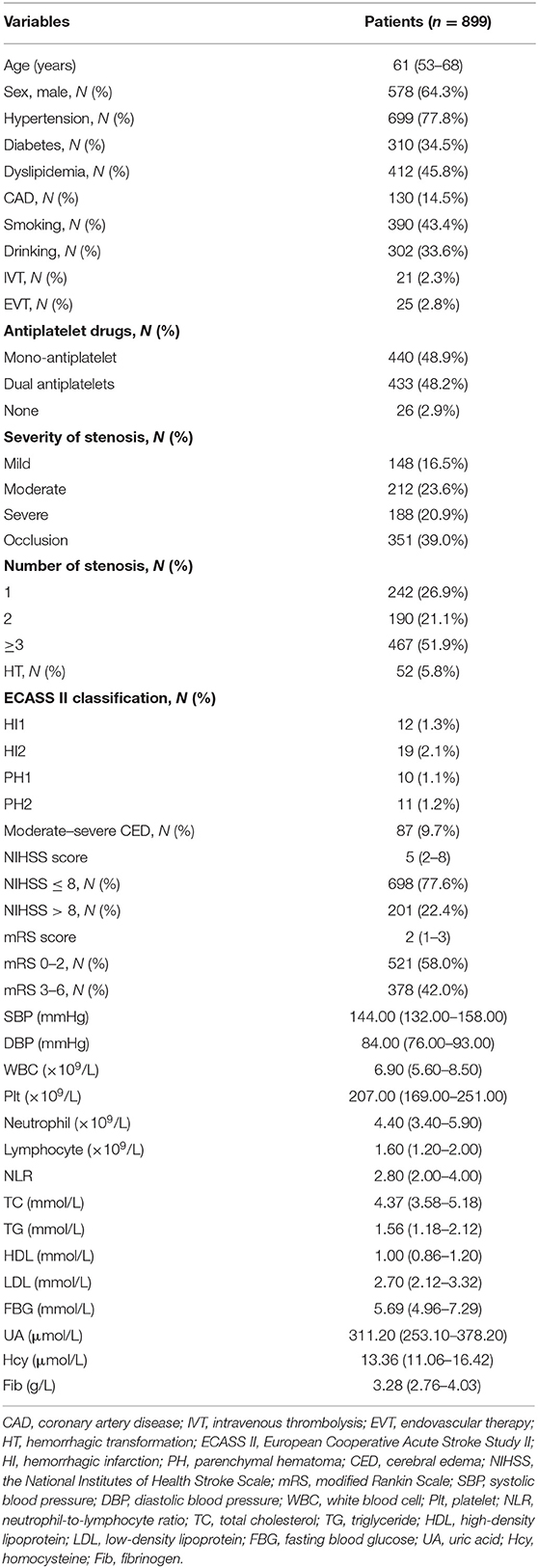
- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 2Clinical Research Center for Cerebrovascular Disease of Hunan Province, Central South University, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Background: Neutrophil-to-lymphocyte ratio (NLR) is an indicator of poor prognosis in acute ischemic stroke (AIS), but associations between NLR with stroke severity and prognosis of intracranial atherosclerotic stenosis (ICAS)-related ischemic events have not been well-elucidated; therefore, we aimed to evaluate whether admission NLR levels correlate with the early stroke severity and short-term functional prognosis in patients with symptomatic intracranial atherosclerotic stenosis (sICAS).
Methods: This retrospective study enrolled 899 consecutive patients with AIS attributed to ICAS at Xiangya Hospital stroke center between May 2016 and September 2020. The initial stroke severity was rated by the admission National Institutes of Health Stroke Scale (NIHSS) scores, and the short-term prognosis was evaluated using the 14-day modified Rankin Scale (mRS) scores after stroke onset. A severe stroke was defined as NIHSS >8; an unfavorable functional outcome was defined as mRS scores of 3–6. Admission NLR was determined based on circulating neutrophil and lymphocyte counts.
Results: The median admission NLR of all patients was 2.80 [interquartile range (IQR), 2.00–4.00]. In univariate analysis, admission NLR was significantly elevated in patients with severe stroke and poor short-term prognosis. After multivariate adjustment, admission NLR levels were significantly correlated with severe stroke [odds ratio (OR), 1.132; 95% confidence interval (95% CI), 1.038–1.234; P = 0.005] and unfavorable short-term prognosis (OR, 1.102; 95% CI, 1.017–1.195; P = 0.018) in Model 1. In Model 2, the highest NLR tertile (≥3.533) remained an independent predictor of severe stroke (OR, 2.736; 95% CI, 1.590–4.708; P < 0.001) and unfavorable functional outcome (OR, 2.165; 95% CI, 1.416–3.311; P < 0.001) compared with the lowest NLR tertile (<2.231). The receiver operating characteristic (ROC) curves showed the predictability of NLR regarding the stroke severity [area under the curve (AUC), 0.659; 95% CI, 0.615–0.703; P < 0.001] and short-term prognosis (AUC, 0.613; 95% CI, 0.575–0.650; P < 0.001). The nomograms were constructed to create the predictive models of the severity and short-term outcome of sICAS.
Conclusions: Elevated admission NLR levels were independently associated with the initial stroke severity and could be an early predictor of severity and poor short-term prognosis in AIS patients with ICAS, which might help us identify a target group timely for preventive therapies.
Introduction
Stroke is a growing public health concern and remains a leading cause of mortality and disability worldwide (1). Of all different stroke subtypes, ischemic stroke is the most common subtype, accounting for about 80% of all strokes and mainly caused by large artery atherosclerosis (LAA), especially intracranial atherosclerotic stenosis (ICAS), which has a higher prevalence in Asians, Africans, and Hispanics compared with Caucasians (1–3). Accumulating evidence suggests that ICAS was associated with increased risks of cognitive impairment, dementia, stroke recurrence, and death (4–6). However, the pathogenesis of ICAS has not been well-investigated. Previous studies have established that atherosclerosis is an inflammatory disorder mediated by activation of inflammatory markers and infiltration of various inflammatory cells, especially activated macrophages and T cells, which could release metalloproteinases, thus leading to atherosclerotic plaque instability or rupture, ischemia, and infarction (7, 8). Since ICAS is on the spectrum of atherosclerosis, inflammation might also be a pathophysiological mechanism for the development and progression of ICAS.
Neutrophil-to-lymphocyte ratio (NLR) is emerging as an objective and easily accessible indicator of systemic inflammatory status, reflecting the balance between neutrophils and lymphocytes in peripheral blood (9). An increase in NLR levels has been reported to be associated with atherosclerotic events, serving as a prognostic predictor in coronary artery disease (CAD), peripheral arterial occlusive disease (PAOD), and ischemic stroke (10–12). Specifically, it has previously been observed that high NLR levels were correlated with stroke severity, poor functional outcomes, and recurrent ischemic events in stroke patients (12, 13). Furthermore, large vessel occlusion patients with high admission NLR values had increased risks of symptomatic intracranial hemorrhage (sICH) and 3-month mortality after mechanical thrombectomy (14). Even in a healthy population, an elevated NLR has been proved to be linked to the prevalence and burdens of ICAS (15). However, few studies have examined the association between NLR and symptomatic intracranial atherosclerotic stenosis (sICAS). Although a recent study has explored the relationships between NLR with ICAS and ischemic stroke and demonstrated that the association between NLR and ischemic stroke was partially mediated by ICAS (16), there is a relative paucity of studies investigating the underlying relationships between admission NLR with the initial severity and prognosis of sICAS.
In this study, we aimed to determine the potential predictive capacity of admission NLR levels for stroke severity and short-term prognosis of patients with acute ischemic stroke (AIS) attributed to ICAS to help identify a promising therapeutic strategy.
Materials and Methods
Patients and Population
This study retrospectively enrolled and analyzed 899 consecutive patients with AIS attributed to ICAS, who were admitted to Xiangya Hospital stroke center between May 2016 and September 2020, satisfying the following inclusion criteria: (1) age ≥18 years; (2) time interval from symptom onset to admission ≤72 h; and (3) blood sampling performed within 24 h after hospital admission. Those patients, who had cardioembolic stroke or evidence of cardioembolic propensity, severe stenosis of extracranial carotid artery, malignant tumors, severe renal or hepatic diseases, hematological diseases, inflammatory or infectious diseases, or history of immunosuppressant medications within the past 3 months, were excluded from the current study. The criteria for diagnosing AIS have been described in detail previously (17). Each participant in this study has provided the informed consent form. The study was approved by the Medical Ethics Committee of Xiangya Hospital, Central South University, China.
Stroke Severity and Outcome Assessment
The National Institutes of Health Stroke Scale (NIHSS) scores have been collected on admission for the assessment of initial stroke severity, stratifying patients into two groups: mild stroke (NIHSS score ≤8) and severe stroke (NIHSS score >8). The 14-day modified Rankin Scale (mRS) scores after stroke onset have been used to evaluate the short-term prognosis after stroke, and patients were therefore categorized into two groups: favorable functional outcome group with mRS scores of 0–2 and unfavorable functional outcome group with mRS scores of 3–6.
Clinical and Laboratory Data Collection
Baseline characteristics including demographics, cardiovascular risk factors, clinical data, and laboratory data were collected and recorded on admission. Histories of hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease (CAD), as well as cigarette smoking and alcohol intake, have been considered cardiovascular risk factors. Besides, systolic and diastolic blood pressure levels were measured on admission. Admission NIHSS scores and 14-day mRS scores were collected as previously mentioned. The treatments including intravenous thrombolysis (IVT), endovascular treatment (EVT), and antiplatelet therapy were collected during hospitalization. Blood samples were collected within 24 h after admission for the examination of laboratory parameters, including white blood cell (WBC), platelet (Plt), neutrophil, lymphocyte, NLR, fasting blood glucose (FBG), uric acid (UA), homocysteine (Hcy), fibrinogen (Fib), and a lipid profile which consists of total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). The diagnostic criteria and parameters have been described in detail in our prior work (17, 18). Admission NLR was calculated based on neutrophil and lymphocyte counts in the peripheral blood.
Radiological Assessment
Standard magnetic resonance imaging (MRI) of the brain was performed in all participants on 1.5 or 3.0 T scanners, including sequences of T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI). The lesions of stenosis or occlusion were measured using three-dimensional time-of-flight (3D TOF) magnetic resonance angiography (MRA), computerized tomography angiography (CTA), or digital subtraction angiography (DSA). The presence of ICAS was defined as stenosis or occlusion of intracranial atherosclerotic arteries rated by two experienced radiologists, and the percentage of luminal stenosis was calculated according to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) criteria, classifying the severity of stenosis into mild (<50%), moderate (50–69%), severe (70–99%), and occlusion (100%) (19). Moreover, hemorrhagic transformations (HT) and cerebral edema (CED) were defined using the computed tomography (CT) or MRI and classified according to the European Cooperative Acute Stroke Study-2 (ECASS-II) criteria by at least two experienced radiologists (20, 21). In this study, we stratified HT into hemorrhagic infarction (HI) 1, HI2, parenchymal hemorrhage (PH) 1, and PH2; and we dichotomized CED into none or mild (grades 0–1) and moderate-to-severe (grades 2–3) (20, 22).
Statistical Analysis
Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR) for data with normal and non-normal distributions, respectively, whereas categorical variables were described as number and percentage. For continuous variables, Student t-test and Mann-Whitney U-test were used for intergroup comparisons, while for categorical variables, Pearson χ2-test was used to assess the intergroup difference. Multivariate binary logistic regression analysis was conducted to evaluate associations between NLR with early severity and short-term prognosis after adjustment for age, sex, and other possible confounders, involving all variates with a P < 0.05 in initial univariate analysis, with results exhibited as odds ratios (OR) and 95% confidence interval (CI). Model 1 (the laboratory parameters as continuous variables) and Model 2 (the laboratory parameters as dichotomous or trichotomous variables) were used in multivariate binary logistic regression analysis, and NLR levels were categorized into tertiles: the first tertile, <2.231; the second tertile, 2.231–3.533; and the third tertile, ≥3.533 in Model 2. To evaluate the predictive capacity of NLR to discriminate early severity and short-term prognosis, we illustrated receiver operating characteristic (ROC) curves, measured the area under the curve (AUC), and determined the CIs. The nomograms were plotted using the independent significant variables based on the results of multivariate logistic regression analysis. All statistical analyses were conducted with Empower Stats (http://www.empowerstats.com) and R software (http://www.R-project.org/). P < 0.05 was considered statistically significant.
Results
Patient Baseline Characteristics
From May 2016 to September 2020, totally 899 consecutive AIS patients with ICAS were enrolled in the study analysis. The median age of all participants was 61 years (IQR, 53–68 years) and 64.3% (n = 578) were male (as shown in Table 1). Cardiovascular risk factors included histories of hypertension (n = 699, 77.8%), diabetes mellitus (n = 310, 34.5%), dyslipidemia (n = 412, 45.8%), CAD (n = 130, 14.5%), smoking (n = 390, 43.4%), and drinking (n = 302, 33.6%) (as shown in Table 1). During hospitalization, almost all patients were on antiplatelet therapy, in which 48.9% (n = 440) were using mono-antiplatelet drugs, and 48.2% (n = 433) were using dual antiplatelet drugs (as shown in Table 1). The rates of participants treated with IVT and EVT were 2.3% (n = 21) and 2.8% (n = 25), respectively (Table 1). Additionally, patients were stratified as having mild stenosis (n = 148, 16.5%), moderate stenosis (n = 212, 23.6%), severe stenosis (n = 188, 20.9%), and occlusion (n = 351, 39.0%) (Table 1). The median admission NLR of all included patients was 2.80 (IQR, 2.00–4.00) (Table 1). Patients with intracranial atherosclerotic artery occlusion had statistically higher admission NLR levels compared to patients with mild stenosis (P = 0.024) and moderate stenosis (P = 0.013) (as shown in Figure 1A). Regarding the number of stenosis, higher admission NLR levels were documented in patients with multiple intracranial artery stenosis (the number of stenosis ≥3) (n = 467, 51.9%) compared to patients with single stenosis (n = 242, 26.9%; P = 0.007) (as shown in Table 1 and Figure 1B). Besides, patients with HT (n = 52, 5.8%) had statistically higher admission NLR levels compared to patients without HT (P = 0.014), and patients with moderate-severe CED (n = 87, 9.7%) had statistically elevated admission NLR levels compared to patients with none-mild CED (P = 0.001) (as shown in Table 1 and Figure 2). The median admission NIHSS score of the participants was 5 (IQR, 2–8), and the initial stroke severity was stratified according to admission NIHSS scores as follows: mild stroke, NIHSS score ≤8 (n = 698, 77.6%); severe stroke, NIHSS score >8 (n = 201, 22.4%) (as shown in Table 1). The median mRS score on the 14th day after stroke onset of the participants was 2 (IQR, 1–3), and the short-term prognosis was stratified by 14-day mRS scores as follows: a favorable outcome, mRS scores ≤2 (n = 521, 58.0%); an unfavorable outcome, mRS scores >2 (n = 378, 42.0%) (as shown in Table 1). Baseline clinical characteristics, as well as the admission laboratory data of the participants, were summarized in Table 1.
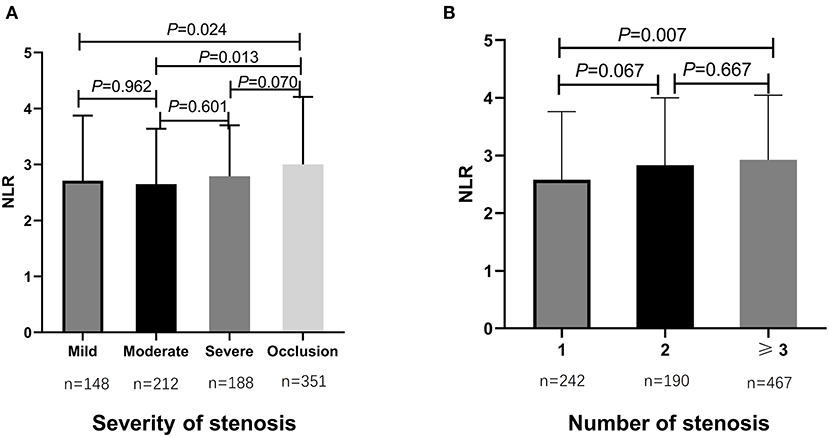
Figure 1. Comparisons of admission NLR levels according to the severity and number of intracranial atherosclerotic stenosis in sICAS patients. (A) Comparison of admission NLR levels according to the severity of intracranial atherosclerotic stenosis. (B) Comparison of admission NLR levels according to the number of intracranial atherosclerotic stenosis. P < 0.05 was considered statistically significant. NLR, neutrophil-to-lymphocyte ratio; sICAS, symptomatic intracranial atherosclerotic stenosis.
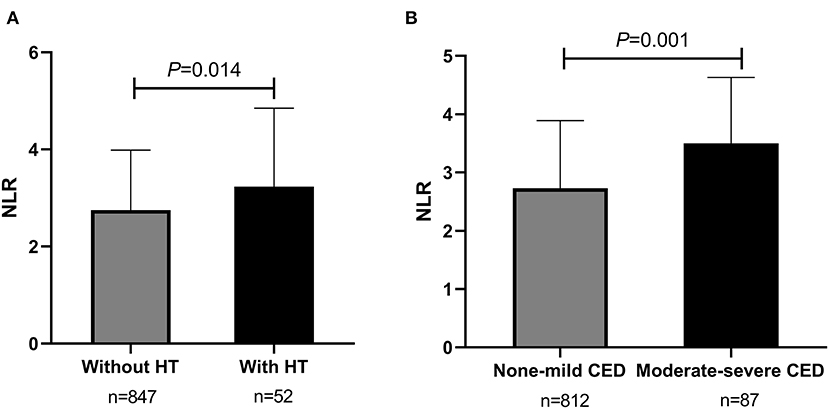
Figure 2. Comparisons of admission NLR levels according to HT and CED in sICAS patients. (A) Comparison of admission NLR levels according to HT (without HT vs. with HT). (B) Comparison of admission NLR levels according to CED (none-mild CED vs. moderate-severe CED). P < 0.05 was considered statistically significant. NLR, neutrophil-to-lymphocyte ratio; sICAS, symptomatic intracranial atherosclerotic stenosis; HT, hemorrhagic transformation; CED, cerebral edema.
Association Between NLR and Initial Stroke Severity in sICAS Patients
Of all participants, 698 (77.6%) were classified as having a mild stroke, and 201 (22.4%) had a severe stroke as mentioned before. In univariate analysis, sex, dual antiplatelet therapy, stenosis severity, moderate-severe CED, WBC, neutrophils, lymphocytes, NLR, FBG, UA, and Fib were significantly related to stroke severity at admission with a P < 0.05 (as shown in Table 2). Patients with severe stroke had statistically elevated admission NLR levels (median, 3.53; IQR, 2.43–5.25) compared to patients with mild stroke (median, 2.63; IQR, 1.93–3.64; P < 0.001) (as shown in Table 2 and Figure 3A). Moreover, the proportions of female gender, occlusion, and moderate-severe CED, and levels of WBC, neutrophil, FBG, and Fib in the severe stroke group were higher than those in the mild stroke group, whereas the proportion of dual antiplatelet therapy and the levels of lymphocyte and UA were lower in the severe stroke group (as shown in Table 2). Multivariate logistic regression analysis indicated that admission NLR levels were significantly correlated with severe stroke (OR, 1.132; 95% CI, 1.038–1.234; P = 0.005) in Model 1 and both the middle and the highest NLR tertiles were significantly correlated with severe stroke (tertile 2: OR, 1.851; 95% CI, 1.089–3.145; P = 0.023; and tertile 3: OR, 2.736; 95% CI, 1.590–4.708; P < 0.001) compared with the lowest NLR tertile in Model 2 after adjustment for age, sex, dual antiplatelet therapy, stenosis severity, moderate-severe CED, WBC, FBG, UA, and Fib (as shown in Table 3).
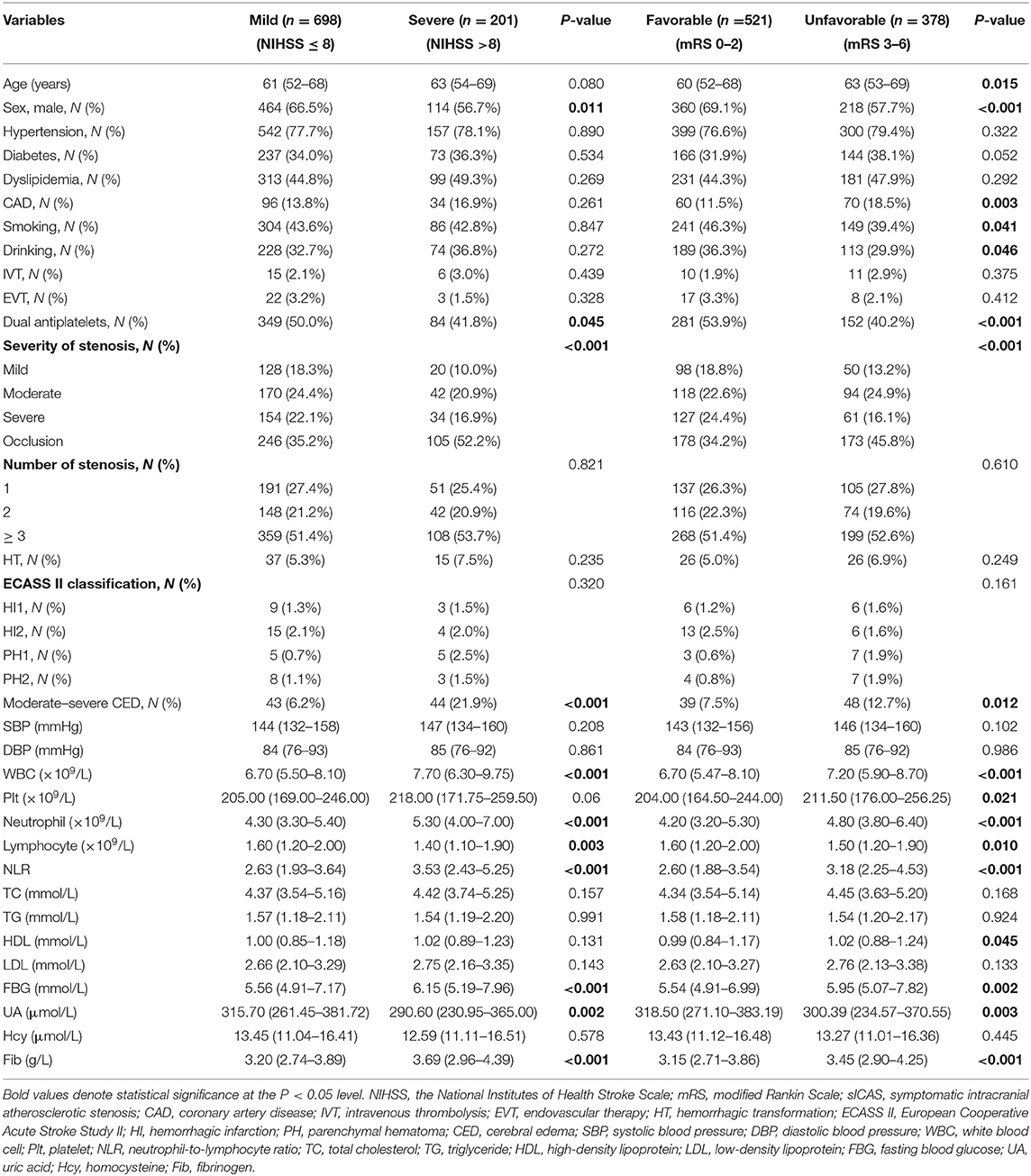
Table 2. Clinical characteristics of sICAS patients according to the initial stroke severity and short-term prognosis.
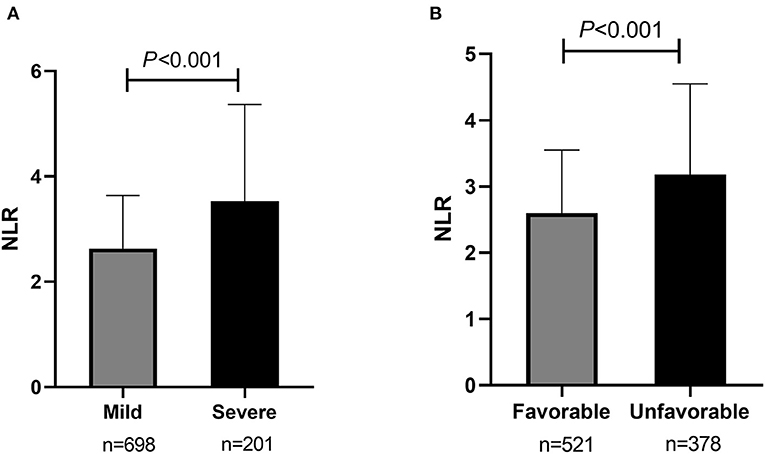
Figure 3. Comparisons of admission NLR levels according to the initial stroke severity and short-term prognosis of sICAS. (A) Comparison of admission NLR levels according to the initial stroke severity (severe stroke vs. mild controls). (B) Comparison of admission NLR levels according to the short-term prognosis (unfavorable outcome vs. favorable outcome). NLR, neutrophil-to-lymphocyte ratio; sICAS, symptomatic intracranial atherosclerotic stenosis.
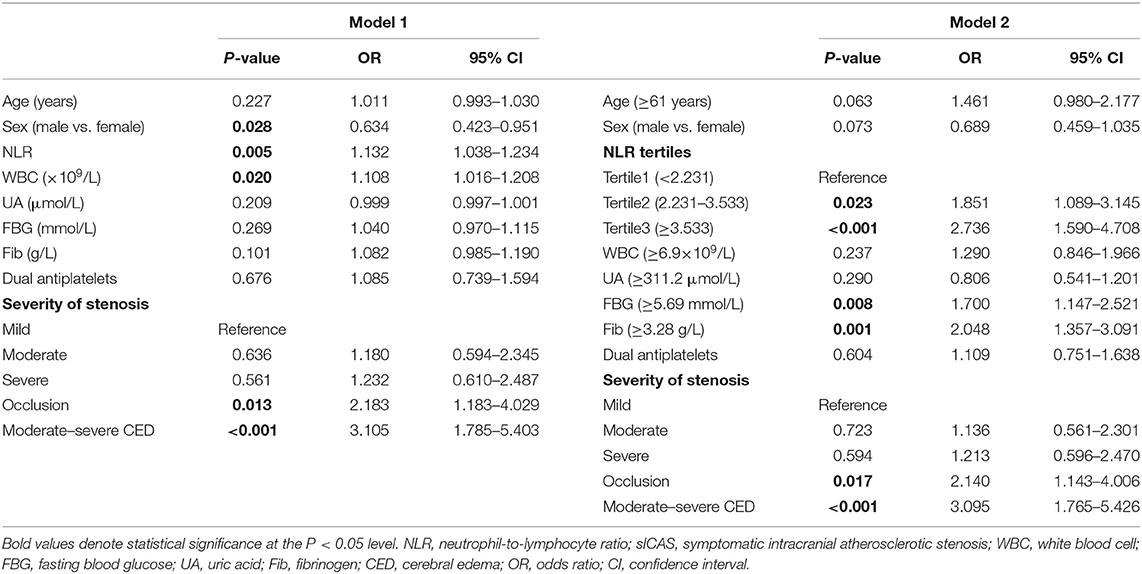
Table 3. Multivariate logistic regression analysis of the relationship between NLR and the initial stroke severity of sICAS.
Association Between NLR and Short-Term Prognosis in sICAS Patients
Among all participants, 521 (58.0%) had a favorable short-term functional outcome, and 378 (42.0%) had an unfavorable short-term functional outcome as mentioned before. In univariate analysis, age, sex, histories of CAD, smoking, and drinking, dual antiplatelet therapy, stenosis severity, moderate-severe CED, and levels of WBC, Plt, neutrophils, lymphocytes, NLR, HDL, FBG, UA, and Fib were significantly correlated with short-term prognosis in sICAS patients (as shown in Table 2). NLR levels were statistically elevated in patients with unfavorable outcomes (median, 3.18; IQR, 2.25–4.53) compared with those with favorable outcomes (median, 2.60; IQR, 1.88–3.54; P < 0.001) (as shown in Table 2 and Figure 3B). Moreover, age, the proportions of female gender, occlusion, and moderate-severe CED, the prevalence of CAD, and levels of WBC, Plt, neutrophil, HDL, FBG, and Fib in the unfavorable outcome group were higher than those in the favorable outcome group, whereas the proportion of dual antiplatelet therapy, the frequencies of smoking and drinking, and the levels of lymphocyte and UA were lower in the unfavorable outcome group (as shown in Table 2). Multivariate logistic regression analysis showed that admission NLR levels were significantly correlated with unfavorable short-term prognosis (OR, 1.102; 95% CI, 1.017–1.195; P = 0.018) in Model 1 and the highest NLR tertile was positively associated with unfavorable short-term prognosis (OR, 2.165; 95% CI, 1.416–3.311; P < 0.001) compared with the lowest NLR tertile in Model 2 after adjusting for age, sex, CAD, smoking, drinking, dual antiplatelet therapy, severity of stenosis, moderate-severe CED, WBC, Plt, HDL, FBG, UA, and Fib (as shown in Table 4).
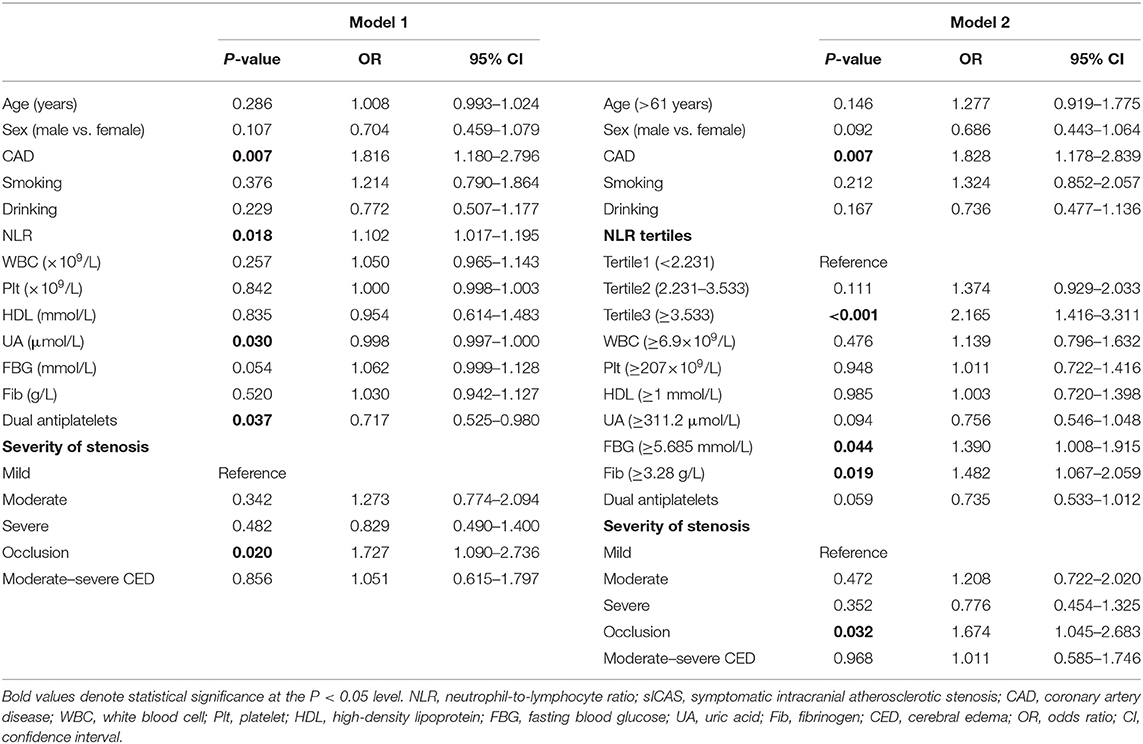
Table 4. Multivariate logistic regression analysis of the relationship between NLR and the short-term prognosis of sICAS.
Predictive Values of NLR for the Severity and Short-Term Prognosis of sICAS
Receiver operating characteristic curve analysis was conducted to determine the predictability of NLR with the results illustrated in Figure 4. Area under the curve values for discriminating stroke severity and short-term prognosis were 0.659 (95% CI, 0.615–0.703; P < 0.001) and 0.613 (95% CI, 0.575–0.650; P < 0.001), respectively, and the optimal cut-off values of NLR were 3.33 (specificity, 69.49%; sensitivity, 54.87%) for stroke severity and 3.31 (specificity, 70.45%; sensitivity, 47.43%) for short-term prognosis (as shown in Figure 4).
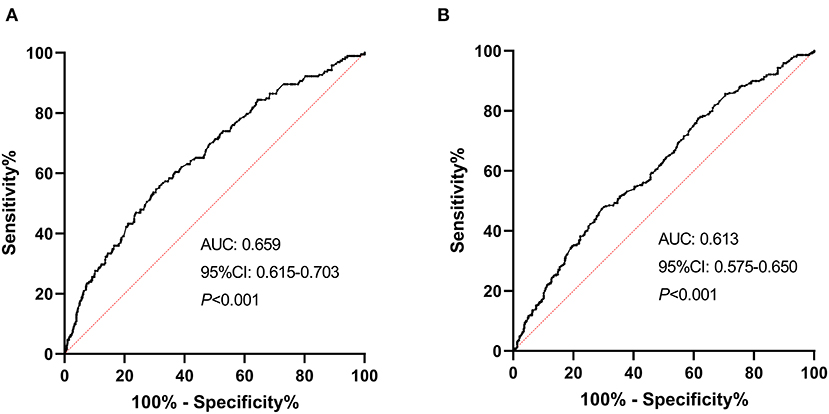
Figure 4. ROC curves for the initial stroke severity and short-term prognosis of sICAS with NLR. (A) ROC curve for the initial stroke severity of sICAS with NLR (severe stroke vs. mild controls). (B) ROC curve for the short-term prognosis of sICAS with NLR (unfavorable outcome vs. favorable outcome). ROC, receiver operating characteristic; sICAS, symptomatic intracranial atherosclerotic stenosis; AUC, area under the curve; NLR, neutrophil-to-lymphocyte ratio; CI, confidence interval.
Predictive Models of the Severity and Short-Term Outcome of sICAS
To establish a more direct correlation of NLR with sICAS, we constructed nomograms using the variables identified by multivariate logistic regression analysis. We chose the independent risk factors identified in Model 1. The selected factors were shown by lines in the nomograms. Higher nomogram total scores indicated a higher risk of severe neurological deficit or unfavorable short-term outcome of sICAS patients. Figures 5A, 6A showed the nomograms for predicting the severity and short-term prognosis of sICAS patients. Figures 5B, 6B showed the performance of the nomograms. Supplementary Figures 1A–D showed the nomograms using the variables identified by multivariate logistic regression in Model 2 as well as their performances.
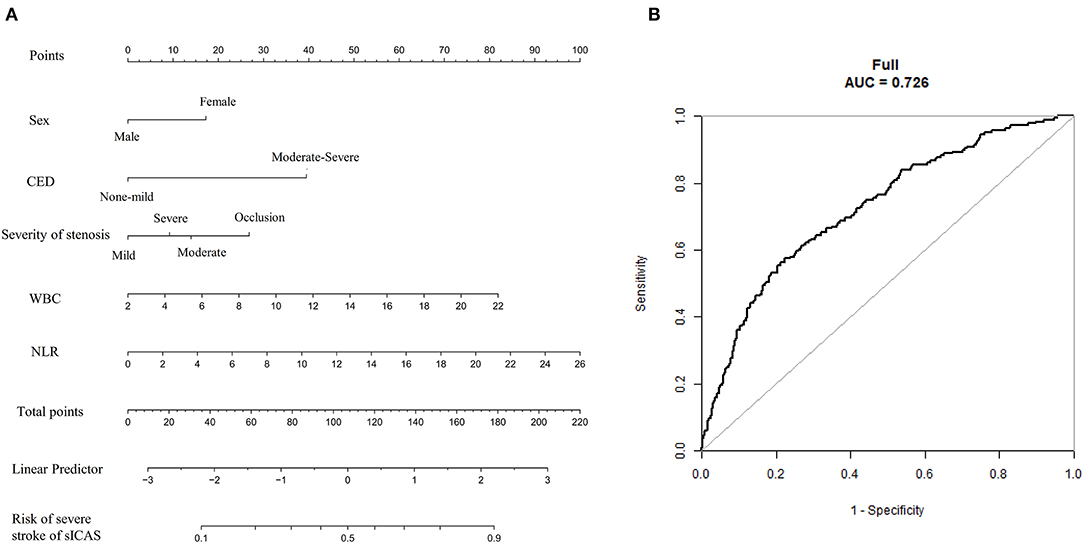
Figure 5. The nomogram for predicting the severity of sICAS patients. (A) The nomogram for predicting the risk of severe neurological deficit of sICAS patients. (B) The ROC curve of the nomogram. The predictors were chosen based on the results of multivariate logistic regression in Model 1. Each selected factor was shown by a line in the nomograms. sICAS, symptomatic intracranial atherosclerotic stenosis; CED, cerebral edema; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; ROC, receiver operating characteristic; AUC, area under the curve.
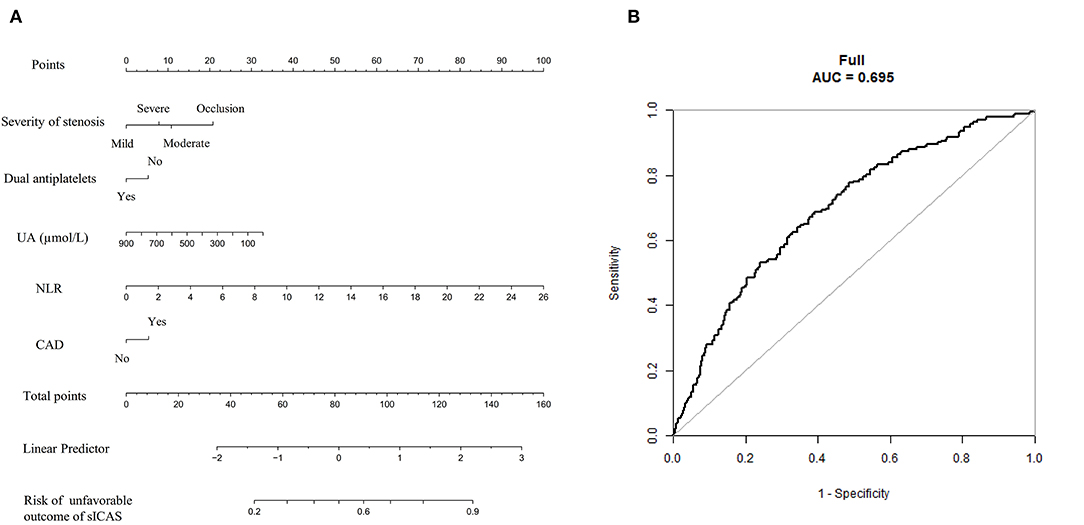
Figure 6. The nomogram for predicting the short-term prognosis of sICAS patients. (A) The nomogram for predicting the risk of unfavorable short-term outcome of sICAS patients. (B) The ROC curve of the nomogram. The predictors were chosen based on the results of multivariate logistic regression in Model 1. Each selected factor was shown by a line in the nomograms. sICAS, symptomatic intracranial atherosclerotic stenosis; NLR, neutrophil-to-lymphocyte ratio; UA, uric acid; CAD, coronary artery disease; ROC, receiver operating characteristic; AUC, area under the curve.
Discussion
In the present study, we demonstrated that elevated admission NLR was significantly correlated with initial stroke severity and poor short-term prognosis in sICAS patients. The statistical significance remained after adjustment for clinical and laboratory variables when NLR was 3.533 or more. Moreover, we demonstrated that NLR had good predictive power for the early severity and short-term prognosis of sICAS.
Our results are in accordance with prior studies relating to NLR and ischemic stroke. Researchers have reported that NLR was independently associated with stroke severity, hemorrhagic complications, poor short-term and long-term functional outcomes, high mortality rates, and stroke-associated pneumonia in acute cerebral ischemia (23–26). In patients with AIS attributable to LAA, high NLR was associated with increased risks for HT after LAA (27). In our study, we also observed that NLR values were higher in sICAS patients with HT, but HT was not a risk factor for the severity and prognosis of sICAS. More recently, a study in Portugal found that increased NLR was linked to the greater severity of CED, early neurological deterioration (END), and worse long-term prognosis in AIS patients undergoing reperfusion therapy (21). However, the predictive role of NLR in the prognosis of ischemic stroke due to ICAS has not been defined before. Our study revealed a similar role of NLR; that is, NLR was an early predictor of the early stroke severity and poor short-term outcome in patients with AIS attributed to ICAS, independent of other risk factors including CED, whereas the underlying mechanisms remain unclear.
Intracranial atherosclerotic stenosis is one of the most predominant causes of ischemic stroke, with a higher prevalence in Asians (3). Prior studies have demonstrated that traditional cardiovascular risk factors, metabolic syndrome, and unhealthy lifestyles are crucial risk factors associated with ICAS, accounting for endothelial dysfunction and increased permeability, thereby initiating a series of inflammatory reactions, which have been considered to be implicated in the pathogenesis of atherosclerosis and might play a critical role in the development of ICAS and plaque destabilization (8, 28). Although there are various risk factors and predictive models for ICAS, more convenient and easily accessible biomarkers are required to identify high-risk patients. Neutrophil-to-lymphocyte ratio, a biomarker indicating systemic inflammatory status, has been shown to be related to intracranial atherosclerosis. A study by Chung et al. (29) revealed that high NLR was correlated with intracranial atherosclerosis (OR= 1.87; 95% CI, 1.15–3.06; P = 0.01) in healthy individuals, consistent with another study by Nam et al. (15) indicating that NLR could be a predictor for the prevalence and burdens of ICAS. However, few studies revealed the independent predictive values of NLR in the prognosis of sICAS. Our prior study has determined the association between leukocyte levels and the prognosis of sICAS, whereas NLR might be a more accurate biomarker reflecting the balance of neutrophils and lymphocytes. Therefore, we examined whether NLR levels correlate with the early stroke severity and prognosis of sICAS and indicated that NLR could be employed as a good predictor with respect to the severity and short-term prognosis of sICAS.
Since NLR represents the ratio of neutrophils to lymphocytes, both neutrophilia and lymphopenia could be associated with increased NLR levels, providing clues to the possible underlying mechanisms. A growing body of evidence suggests that elevated circulating neutrophils were related to stroke severity, infarction volume, hemorrhagic complications, and poor outcomes in ischemic stroke (24, 30, 31). When stroke events occur, blood neutrophils could be rapidly recruited to areas of ischemic brain, release matrix metalloproteinases-9 (MMP-9), which could degrade tight junction proteins and basal lamina type IV collagen, increasing the blood-brain barrier (BBB) permeability, thereby resulting in BBB disruption and tissue damage, which is accompanied by edema and hemorrhage (32–35). Besides, an experimental study demonstrated that accumulated neutrophils could impair vascular remodeling during stroke recovery via producing neutrophil extracellular traps (NETs) (36). The expanded neutrophils after ischemic stroke might result from hematopoietic stem cell activation and a hematopoiesis bias mediated by increased sympathetic tone (37). Additionally, neutrophils are implicated in the development and progression of atherosclerosis. Neutrophils could be recruited and activated on the vascular wall, secreting granule proteins and limiting the use of nitric oxide, thus leading to endothelial dysfunction and subsequent atherosclerosis (38). Also, neutrophils could induce macrophages to release cytokines and activate T helper 17 (TH17) cells via releasing NETs, further amplifying pro-inflammatory responses and causing atherosclerotic plaque destabilization and rupture (39, 40).
However, the exact role of lymphocytes in atherosclerosis and ischemic stroke has not been fully elucidated. A study by Kim et al. (41) demonstrated the associations between reduced lymphocyte counts with END and unfavorable long-term prognosis in cerebral ischemia, implying that lymphocytes might be a protector for brain injury. It could be supported by another study, which reported that a lower admission lymphocyte-to-monocyte ratio (LMR) was an independent predictor for greater stroke severity and worse 3-month functional outcomes of acute cerebral infarction (42). Since the initial severity of stroke paralleled a more robust stress response, lymphopenia has been suspected to be mechanistically mediated by stress-induced cortisol production and sympathetic tone after stroke, which could further induce redistribution of lymphocytes and lymphocyte apoptosis (43, 44). Another possible mechanism lies in the protective role of regulatory lymphocytes in stroke via secreting interleukin-10 (IL-10), a critical anti-inflammatory and neuroprotective cytokine modulating post-stroke neuroinflammation (45, 46). Collectively, both inflammation and immune responses have considerable influence on the pathogenesis of ischemic stroke.
Early risk assessment is essential in determining the appropriate treatment and improving long-term life quality in patients with sICAS. Hence, it is critical to seek early predictors of sICAS to identify patients at high risk of severe stroke and unfavorable outcomes for early clinical intervention. Admission NLR, as an easy and straightforward biomarker, has the potential to help clinicians make decisions early and accurately. Our findings suggested that high NLR levels were correlated with severe stroke and poor prognosis in AIS patients due to ICAS, highlighting a promising potential therapeutic strategy for sICAS by reducing the ratio, which requires further investigations.
Limitations
There are several limitations in this study that should be noted. First, a selection bias might exist since it was a retrospective study with participants recruited from a single stroke center. Second, our data are descriptive, so associations do not necessarily imply causality. Further studies remain to be conducted to elucidate the causality between NLR with early severity and outcomes in AIS patients with ICAS. Third, NLR was only examined on admission, and longitudinal data were lacking. Otherwise, more precise and consistent information could be provided. Moreover, the functional outcomes were only assessed on the 14th day after stroke onset, limiting the predictive values of NLR for long-term prognosis in ICAS-related stroke. Forthcoming studies should be undertaken to examine whether NLR levels could estimate stroke recurrence or dementia after sICAS.
Conclusions
This study has demonstrated that elevated admission NLR was independently correlated with severe stroke and poor functional outcome of sICAS and served as a useful parameter for early prediction of severity and short-term prognosis of sICAS. Therefore, NLR might be an essential and practical prognostic marker in patients with sICAS, helping us identify a target group for preventive therapies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Xiangya Hospital, Central South University, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JX and FY conceptualized and designed the study. YY drafted the manuscript and interpreted the data. FY analyzed the data and revised the manuscript. YL, XF, DL, MW, XL, QH, ZL, LZ, TZ, and RT collected the data. All authors contributed to the article and approved the final version to be published.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81671166) and the Fundamental Research Funds for the Central Universities of Central South University (grant number 2019zzts902).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all patients for their participation in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.705949/full#supplementary-material
References
1. Virani S, Alonso A, Benjamin E, Bittencourt M, Callaway C, Carson A, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
2. Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke. (2018) 49:814–9. doi: 10.1161/STROKEAHA.117.020031
3. Wong L. Global burden of intracranial atherosclerosis. Int J Stroke Offic J Int Stroke Soc. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
4. Wong K, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke. (2003) 34:2361–6. doi: 10.1161/01.STR.0000089017.90037.7A
5. Dearborn J, Zhang Y, Qiao Y, Suri M, Liu L, Gottesman R, et al. Intracranial atherosclerosis and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. (2017) 88:1556–63. doi: 10.1212/WNL.0000000000003837
6. Suri M, Zhou J, Qiao Y, Chu H, Qureshi A, Mosley T, et al. Cognitive impairment and intracranial atherosclerotic stenosis in general population. Neurology. (2018) 90:e1240–e7. doi: 10.1212/WNL.0000000000005250
7. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. (1999) 340:115–26. doi: 10.1056/NEJM199901143400207
8. Hansson G. Atherosclerosis–an immune disease: the Anitschkov Lecture 2007. Atherosclerosis. (2009) 202:2–10. doi: 10.1016/j.atherosclerosis.2008.08.039
9. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. (2001) 102:5–14.
10. Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. (2008) 395:27–31. doi: 10.1016/j.cca.2008.04.019
11. Erturk M, Cakmak HA, Surgit O, Celik O, Aksu HU, Akgul O, et al. Predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol. (2014) 64:371–6. doi: 10.1016/j.jjcc.2014.02.019
12. Wang L, Song Q, Wang C, Wu S, Deng L, Li Y, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. (2019) 406:116445. doi: 10.1016/j.jns.2019.116445
13. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
14. Goyal N, Tsivgoulis G, Chang J, Malhotra K, Pandhi A, Ishfaq M, et al. Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. (2018) 49:1985–7. doi: 10.1161/STROKEAHA.118.021477
15. Nam K, Kwon H, Jeong H, Park J, Kim S, Jeong S. High neutrophil to lymphocyte ratios predict intracranial atherosclerosis in a healthy population. Atherosclerosis. (2018) 269:117–21. doi: 10.1016/j.atherosclerosis.2017.12.035
16. Huang L, Sun F, Yin J, Ma Y, Li H, Zhong X, et al. Associations of the neutrophil to lymphocyte ratio with intracranial artery stenosis and ischemic stroke. BMC Neurol. (2021) 21:56. doi: 10.1186/s12883-021-02073-3
17. Yu F, Lu J, Li Z, Zhou X, Zeng S, Zhan Q, et al. Correlation of plasma vascular endothelial growth factor and endostatin levels with symptomatic intra- and extracranial atherosclerotic stenosis in a Chinese Han population. J Stroke Cerebrovasc Dis. (2017) 26:1061–70. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.021
18. Feng X, Zhou X, Yu F, Liu Z, Wang J, Li Z, et al. Low-normal free triiodothyronine and high leukocyte levels in relation to stroke severity and poor outcome in acute ischemic stroke with intracranial atherosclerotic stenosis. Int J Neurosci. (2019) 129:635–41. doi: 10.1080/00207454.2018.1503179
19. Samuels O, Joseph G, Lynn M, Smith H, Chimowitz M. A standardized method for measuring intracranial arterial stenosis. Am J Neuroradiol. (2000) 21:643–6.
20. Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. (2001) 32:438–41. doi: 10.1161/01.STR.32.2.438
21. Ferro D, Matias M, Neto J, Dias R, Moreira G, Petersen N, et al. Neutrophil-to-lymphocyte ratio predicts cerebral edema and clinical worsening early after reperfusion therapy in stroke. Stroke. (2021) 2021:STROKEAHA120032130. doi: 10.1161/STROKEAHA.120.032130
22. Strbian D, Meretoja A, Putaala J, Kaste M, Tatlisumak T. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke. (2013) 8:529–34. doi: 10.1111/j.1747-4949.2012.00781.x
23. Maestrini I, Tagzirt M, Gautier S, Dupont A, Mendyk A, Susen S, et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: Cohort study. Neurology. (2020) 95:e97–e108. doi: 10.1212/WNL.0000000000009179
24. Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. (2015) 85:1408–16. doi: 10.1212/WNL.0000000000002029
25. Yu S, Arima H, Bertmar C, Clarke S, Herkes G, Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. (2018) 387:115–8. doi: 10.1016/j.jns.2018.02.002
26. Nam K, Kim T, Lee J, Kwon H, Lee Y, Ko S, et al. High Neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. (2018) 49:1886–92. doi: 10.1161/STROKEAHA.118.021228
27. Zhang WB, Zeng YY, Wang F, Cheng L, Tang WJ, Wang XQ, et al. high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. (2020) 12:2428–39. doi: 10.18632/aging.102752
28. Wang Y, Meng R, Liu G, Cao C, Chen F, Jin K, et al. Intracranial atherosclerotic disease. Neurobiol Dis. (2019) 124:118–32. doi: 10.1016/j.nbd.2018.11.008
29. Chung D, Lee K, Choi J, Kim N, Kim O, Kim S, et al. Blood neutrophil/lymphocyte ratio is associated with cerebral large-artery atherosclerosis but not with cerebral small-vessel disease. Front Neurol. (2020) 11:1022. doi: 10.3389/fneur.2020.01022
30. Buck B, Liebeskind D, Saver J, Bang O, Yun S, Starkman S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
31. Semerano A, Laredo C, Zhao Y, Rudilosso S, Renú A, Llull L, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. (2019) 50:3456–64. doi: 10.1161/STROKEAHA.119.026743
32. Price C, Menon D, Peters A, Ballinger J, Barber R, Balan K, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. (2004) 35:1659–64. doi: 10.1161/01.STR.0000130592.71028.92
33. Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. (2015) 129:239–57. doi: 10.1007/s00401-014-1381-0
34. Gidday J, Gasche Y, Copin J, Shah A, Perez R, Shapiro S, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. (2005) 289:H558–68. doi: 10.1152/ajpheart.01275.2004
35. Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo E, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. (2008) 39:1121–6. doi: 10.1161/STROKEAHA.107.500868
36. Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. (2020) 11:2488. doi: 10.1038/s41467-020-16191-y
37. Courties G, Herisson F, Sager H, Heidt T, Ye Y, Wei Y, et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res. (2015) 116:407–17. doi: 10.1161/CIRCRESAHA.116.305207
38. Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. (2012) 110:875–88. doi: 10.1161/CIRCRESAHA.111.257535
39. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science (New York, NY). (2015) 349:316–20. doi: 10.1126/science.aaa8064
40. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. (2017) 120:736–43. doi: 10.1161/CIRCRESAHA.116.309692
41. Kim J, Song T, Park J, Lee H, Nam C, Nam H, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. (2012) 222:464–7. doi: 10.1016/j.atherosclerosis.2012.02.042
42. Ren H, Liu X, Wang L, Gao Y. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:2595–602. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.019
43. Urra X, Cervera A, Villamor N, Planas A, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. (2009) 158:1174–83. doi: 10.1016/j.neuroscience.2008.06.014
44. Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
45. Liesz A, Hu X, Kleinschnitz C, Offner H. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke. (2015) 46:1422–30. doi: 10.1161/STROKEAHA.114.008608
Keywords: ischemic stroke, intracranial atherosclerotic stenosis, neutrophil-to-lymphocyte ratio, stroke severity, short-term prognosis
Citation: Ying Y, Yu F, Luo Y, Feng X, Liao D, Wei M, Li X, Huang Q, Liu Z, Zhang L, Zhao T, Tu R and Xia J (2021) Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for Stroke Severity and Short-Term Prognosis in Acute Ischemic Stroke With Intracranial Atherosclerotic Stenosis. Front. Neurol. 12:705949. doi: 10.3389/fneur.2021.705949
Received: 06 May 2021; Accepted: 05 July 2021;
Published: 29 July 2021.
Edited by:
QinJian Sun, Shandong University, ChinaReviewed by:
P. N. Sylaja, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), IndiaLung Chan, Taipei Medical University, Taiwan
Copyright © 2021 Ying, Yu, Luo, Feng, Liao, Wei, Li, Huang, Liu, Zhang, Zhao, Tu and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xia, xjian1216@csu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Yuanlin Ying
Yuanlin Ying Fang Yu
Fang Yu Yunfang Luo
Yunfang Luo Xianjing Feng
Xianjing Feng Di Liao
Di Liao Minping Wei
Minping Wei Xi Li
Xi Li Qin Huang
Qin Huang Zeyu Liu
Zeyu Liu Lin Zhang
Lin Zhang Tingting Zhao
Tingting Zhao Ruxin Tu
Ruxin Tu Jian Xia
Jian Xia