
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 06 July 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.693524
This article is part of the Research Topic Blood-Based Biomarkers in Acute Ischemic Stroke and Hemorrhagic Stroke View all 36 articles
Background: Stroke is a leading cause of morbidity and mortality. Over the past decade, plasma D-dimer levels have emerged as a biomarker for predicting stroke outcome. However, no consensus in the literature currently exists concerning its utility for predicting post-stroke functional outcome and mortality.
Objective: To systematically review the effectiveness of plasma D-dimer levels for predicting functional outcome and mortality following stroke.
Methods: Five academic databases were screened according to PRISMA guidelines for eligible studies. With these studies, we conducted a random-effect meta-analysis to evaluate the impact of plasma D-dimer levels for predicting functional outcome and mortality post-stroke. We also conducted subgroup analyses to evaluate differences in predictive capacity for different stroke subtypes.
Results: Nineteen studies were included, containing data on 5,781 stroke patients (mean age: 65.26 ± 6.4 years). Overall methodological quality for the included studies was high. Meta-analysis showed that increased D-dimer levels were predictive of worsened functional outcomes (Hazard ratio: 2.19, 95% CI: 1.63–2.93) and elevated overall mortality (2.29, 1.35–3.88). Subgroup analysis showed that plasma D-dimer levels were more predictive of poorer functional outcomes for ischemic (2.08, 1.36–3.18) stroke as compared to intracerebral hemorrhage (2.62, 1.65–4.17). We also noted that predictive capacity was similar when it came to mortality in patients with cryptogenic ischemic stroke (2.65, 0.87–8.08) and intracerebral hemorrhage (2.63, 1.50–4.59).
Conclusion: The study provides preliminary evidence concerning the capacity of plasma D-dimer levels for predicting functional outcomes and mortality following stroke and reports that higher D-dimer levels of are associated with poorer functional outcomes and higher mortality.
Stroke is the second most common cause of death or disability worldwide (1, 2). Characterized as a cerebrovascular accident that hampers blood flow resulting in brain damage (3), stroke accounts for almost 5.5 million deaths and 116.4 million disability-adjusted life-years per year (4, 5).
Brain structural damage in stroke patients occurs due to either blood vessel occlusion or intracerebral hemorrhage (6, 7). The resultant ischemic damage then initiates a signaling cascade that triggers excitotoxic and/or inflammatory mechanisms eventually resulting in cellular apoptosis (8). Studies suggest that hemodynamic restoration is the primary mode for limiting neural injury (9, 10). However, this approach does not completely eliminate morbidity and mortality (7, 11). As such, preemptive diagnosis is imperative and is widely recommended (12–16).
D-dimers, such as circulating fibrin-degradation products, have recently been shown to be critical for predicting short- and long-term stroke-related outcomes (12, 17, 18). The presence of D-dimers can be representative of total fibrin concentrations, thereby serving as a biomarker for intravascular fibrinolysis and intravascular thrombus formation (19, 20). For stroke patients, this biomarker can detect disrupted vessels, dissolved clots, and the release of stroke-related tissue factors. D-dimers also serve as a good biomarker because of its prolonged stability, half-life, cost-effectiveness, and high sensitivity (> 97%) (21–24).
To date, only a few individual retrospective cohort studies have attempted to evaluate whether plasma D-dimer levels can predict future functional outcomes and mortality post-stroke (25–28). These studies have not established a consensus here. While some studies reported a positive correlation between mortality and plasma D-dimer levels (29–32), others have reported weaker or no correlation (27, 33, 34). Similarly, there is also no consensus concerning whether D-dimer levels are predictive for overall functional outcome. Some studies noted that plasma D-dimer levels were related to worse functional outcomes (26, 31, 33, 35), other have reported limited correlations (25, 28). To date, we have located one systematic review that attempted to evaluate the predictive capacity for plasma D-dimers (12). However, this review failed to include a meta-analysis. Moreover, since it was published in 2009, an update centered around the current evidence is strongly warranted. While a recently published meta-analysis did attempt to evaluate the prognostic impact of plasma D-dimer levels on mortality, it only contained two studies (17). We therefore, in this present systematic review and meta-analysis, attempt to evaluate the capacity for plasma D-dimer levels to predict post-stroke functional outcome and mortality.
The database search for this meta-analysis was done according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (36). Five databases (Web of Science, MEDLINE, CENTRAL, EMBASE, and Scopus) were screened for studies published prior to February 2021. The search was performed across a combination of MeSH keywords, including “D-dimer,” “stroke,” “intracerebral stroke,” “ischemic stroke,” “cryptogenic stroke,” “subarachnoid stroke,” “hemorrhage,” “cerebrovascular disease,” “cerebrovascular accident,” “functional outcome,” and “mortality.” A sample search strategy for EMBASE database has been provided in Supplementary Table 1. References cited in included studies were manually examined to identify further relevant hits. Study inclusion criteria were as follows:
a) Studies evaluating the impact of D-dimer levels in population groups following stroke.
b) Studies evaluating functional outcome and mortality outcome.
c) Studies investigating human participants.
d) Case-control studies, prospective trials, or retrospective cohort trials.
e) Studies published in peer-reviewed scientific journals.
f) Studies published in English.
Study screening and data collection was independently conducted by two reviewers. The extraction of data was done manually while using Microsoft excel. In cases of disagreements concerning eligibility of studies, discussions were held with a third independent reviewer. Moreover, in conditions where required data was not mentioned in the included studies, repeated attempts were made to contact respective corresponding authors for additional data. We extracted the following data from the included studies: author information, country of research, type of study, descriptive data of the sample, type of cerebrovascular incident, baseline D-dimer levels, functional outcomes, and mortality outcomes.
Risk of bias appraisal for included studies was performed using Cochrane's risk of bias assessment tool for non-randomized controlled trials (37). This tool evaluates study outcomes for possible selective reporting, confounding bias, measurement of outcomes, and incomplete data availability. Appraisal was carried out by two reviewers, with a third reviewer called in to arbitrate in case of disagreement. In addition, we also assessed the overall level of evidence presented in the literature by using Oxford Centre for Evidence Based Medicine tool (38).
This study performed a within-group meta-analysis using Comprehensive Meta-analysis (CMA) software version 2.0 (39). This meta-analysis was conducted based on a random-effects model (40). Hazard ratios were calculated to determine the impact of D-dimer levels on functional outcomes and mortality following stroke. Heterogeneity among studies was assessed using I2 statistics (0–25%: negligible heterogeneity, 25–75%: moderate heterogeneity, and ≥75%: substantial heterogeneity) (41). To ensure clinical heterogeneity we also carried out subgroup analyses on the basis of stroke subtypes i.e., intracerebral hemorrhage, subarachnoid hemorrhage, central nervous system infarction (including ischemic stroke and silent infarction). Besides, we also carried out subgroup analyses for two studies reporting the outcomes of cryptogenic ischemic stroke (i.e., a subtype of ischemic stroke). In the included studies cryptogenic ischemic stroke was defined as per the TOAST criteria which defines it as a brain infarction that is not attributable to a definite cardioembolism, large artery atherosclerosis, or small artery disease despite extensive vascular, cardiac, and serologic evaluation (42). Publication bias was evaluated using Duval and Tweedy's trim and fill procedure (43), which examines publication bias by adding studies on either side of the plotted graph. The significance level for this study was determined at 5%.
Database screening yielded 950 studies, while manual screening added another 13 to this total. After applying inclusion criteria, 19 studies remained (Figure 1). Thirteen of these were retrospective cohort studies (25–28, 30–35, 44, 45), while the other six were prospective cohort studies (29, 46–50). Relevant data from each study was extracted and tabulated (Table 1).
The 19 included studies featured data from 5,781 total patients (2,821 females and 2,701 males). Four studies did not report gender distributions (30, 35, 47, 48). Average patient age was 65.26 ± 6.4 years, with one study reporting age as only a range (31) and one omitting age altogether (48).
Risk of methodological bias for the included non-randomized controlled trials was assessed with the ROBINS-I tool (Table 2). Overall risk among the included studies was low, with missing data, selection of reported results, and selection bias the most prominent aspects (Figure 2). We also found that the overall level of evidence according to the Oxford Centre for Evidence Based Medicine to be 2b.
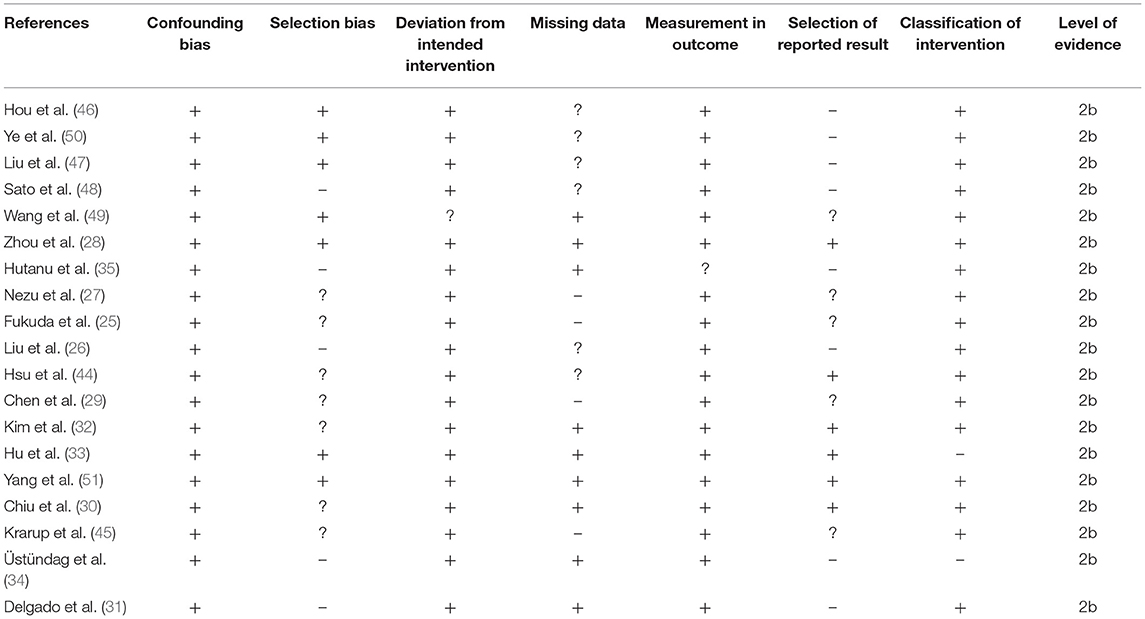
Table 2. Risk of bias according to Cochrane's risk of bias assessment tool for included non-randomized controlled trials.
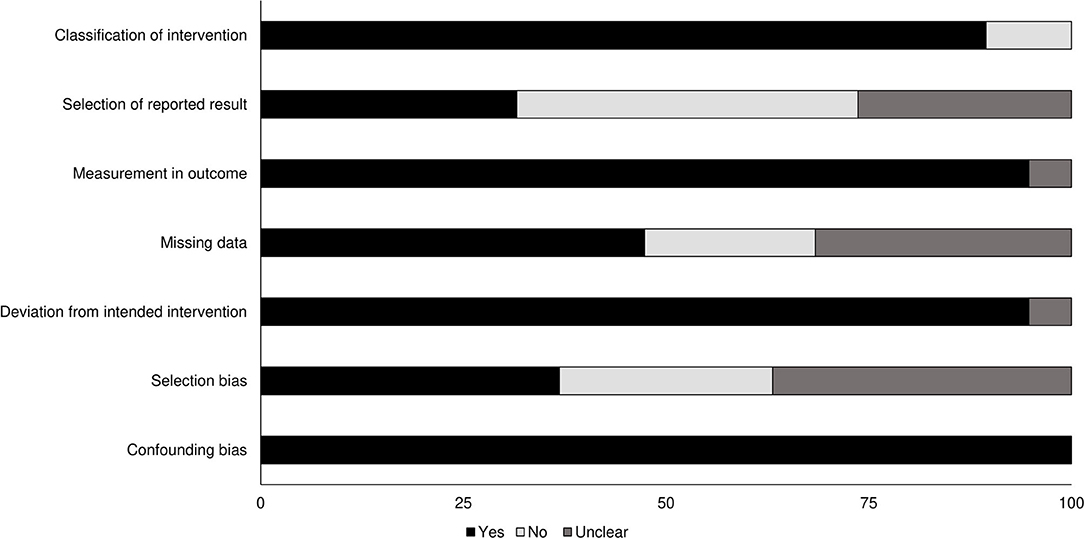
Figure 2. Risk of bias for non-randomized controlled trials according to the Cochrane risk of bias assessment.
Duval and Tweedy's trim and fill method was used to determine if studies were missing from either side of the mean effect. The method observed that six studies were missing on the left side of the mean effect. The overall random effects model determined point estimates and 95% confidence intervals for all studies combined as 2.13 (95% CI: 1.69–2.67). Imputed point estimate using the trim and fill method was 1.74 (95% CI: 1.41–2.15) (Figure 3).
Thirteen studies examined the impact of D-dimer levels on post-stroke functional outcome (25, 26, 28, 31, 33, 35, 44, 49, 51). Hazard ratio was 2.19 (95% CI: 1.63–2.93, p < 0.001) with no heterogeneity (I2: 0%) (Figure 4).
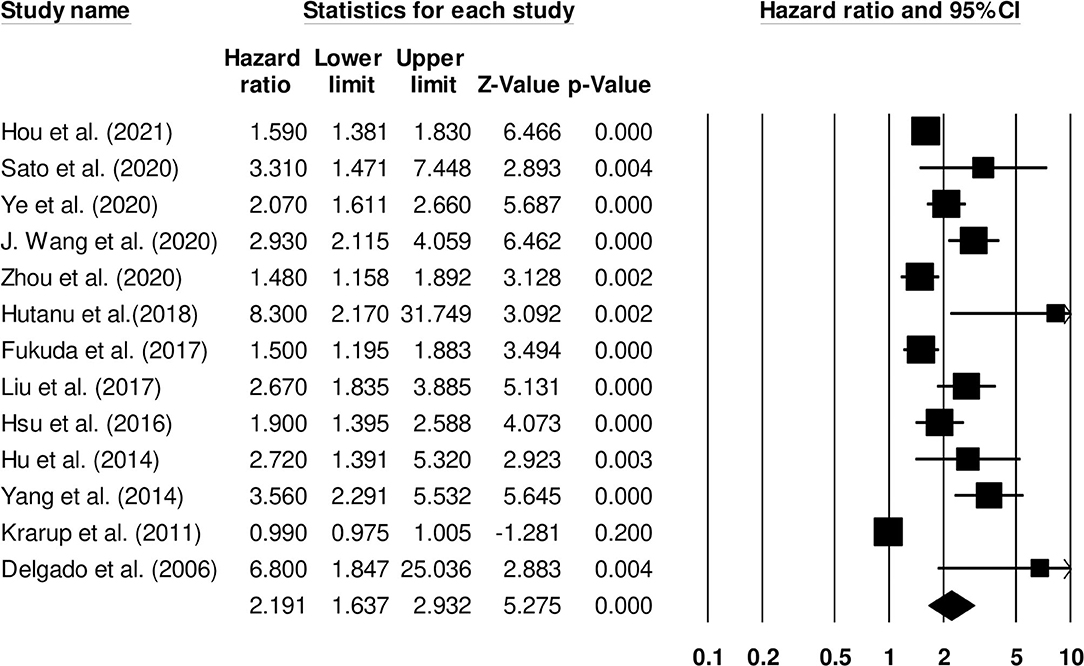
Figure 4. Forest plot for studies evaluating the impact of D-dimer level on post-stroke functional outcomes. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
Further subgroup analysis for functional outcome post-stroke was carried out to examine the effect of stroke type. Six studies reported functional outcomes for patients with ischemic stroke (Hazard ratio: 2.08, 95% CI: 1.36–3.18, p = 0.001; I2: 0%; Figure 5) while three included studies evaluated outcomes for intracerebral hemorrhage patients with negligible heterogeneity (Hazard ratio: 2.62, 95% CI: 1.65–4.17, p = 0.001; I2: 23.52%; Figure 6).
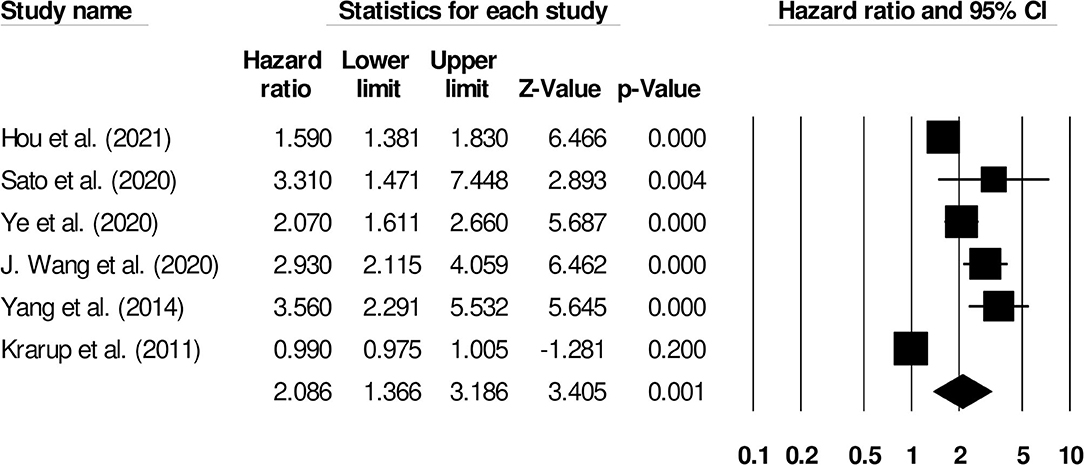
Figure 5. Forest plot for studies evaluating the impact of D-dimer level on post-ischemic stroke functional outcomes. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
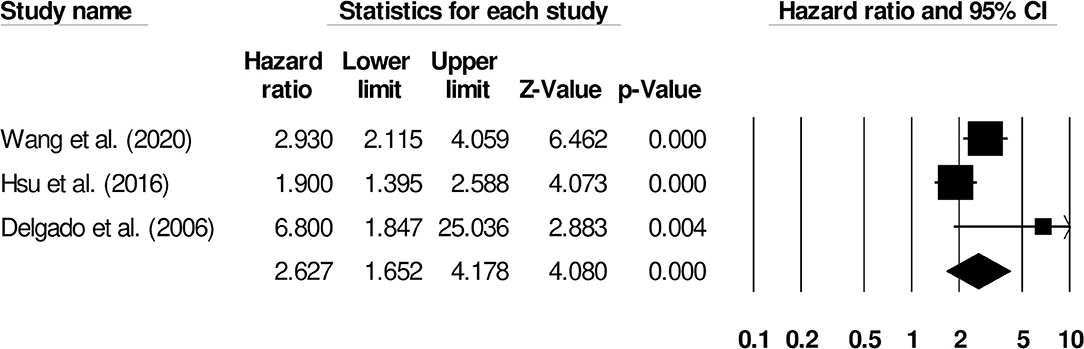
Figure 6. Forest plot for studies evaluating the impact of D-dimer level on post-intracerebral hemorrhage functional outcomes. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
We also conducted two subgroup analyses based on different follow-up periods and assessment methods. Firstly, we identified only six studies that had reported a uniform follow-up of 3 months and they had used modified rankin scale for assessing functional outcome. We observed increased mortality outcomes for patients with moderate heterogeneity (Hazard ratio: 2.08, 95% CI: 1.53–2.84, p < 0.001; Figure 7; I2:31.1%). Secondly, we identified two studies that had reported a uniform follow-up of 2 months and they had also used modified rankin scale for assessing functional outcome. We observed increased mortality outcomes for patients with no heterogeneity (Hazard ratio: 3.28, 95% CI: 2.27–4.74, p < 0.001; Figure 8; I2:0%).
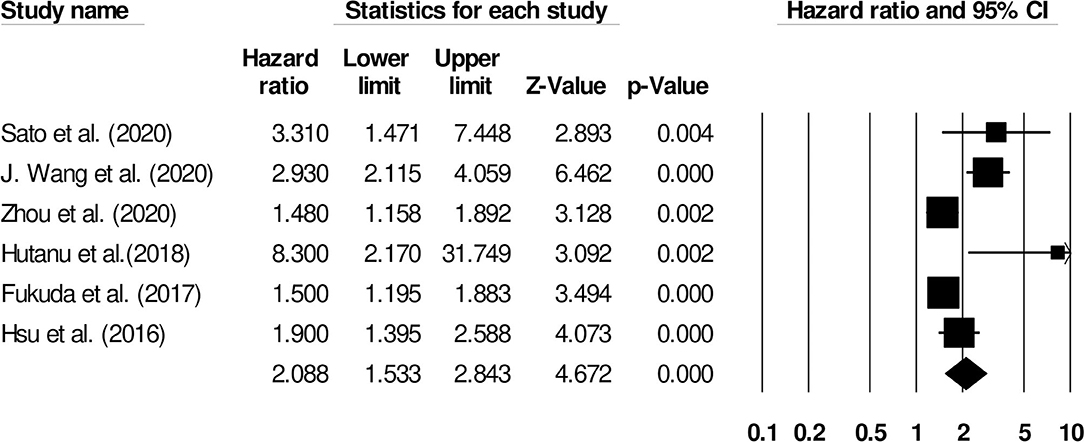
Figure 7. Forest plot for studies evaluating the impact of D-dimer level on post-stroke functional outcomes for 3 months follow up. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
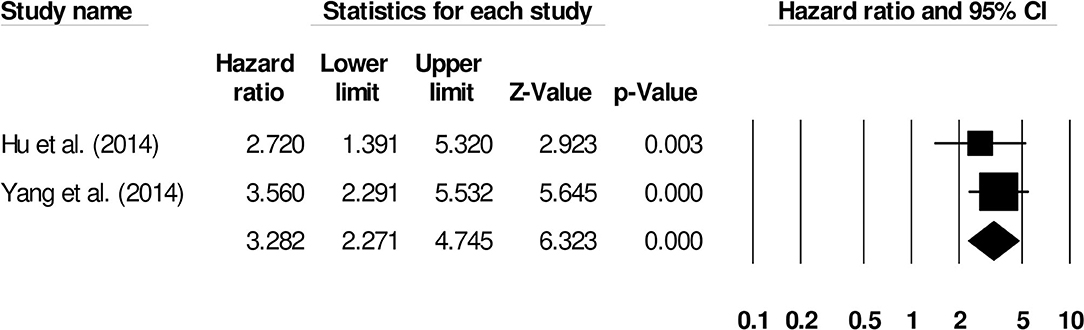
Figure 8. Forest plot for studies evaluating the impact of D-dimer level on post-intracerebral hemorrhage functional outcomes for 2 months follow up. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
Nine studies evaluated the impact of D-dimer levels on post-stroke mortality (26–34). A hazard ratio of 2.29 (95% CI: 1.35–3.88, p = 0.002, Figure 9) was observed, with moderate heterogeneity (I2: 39.03%).
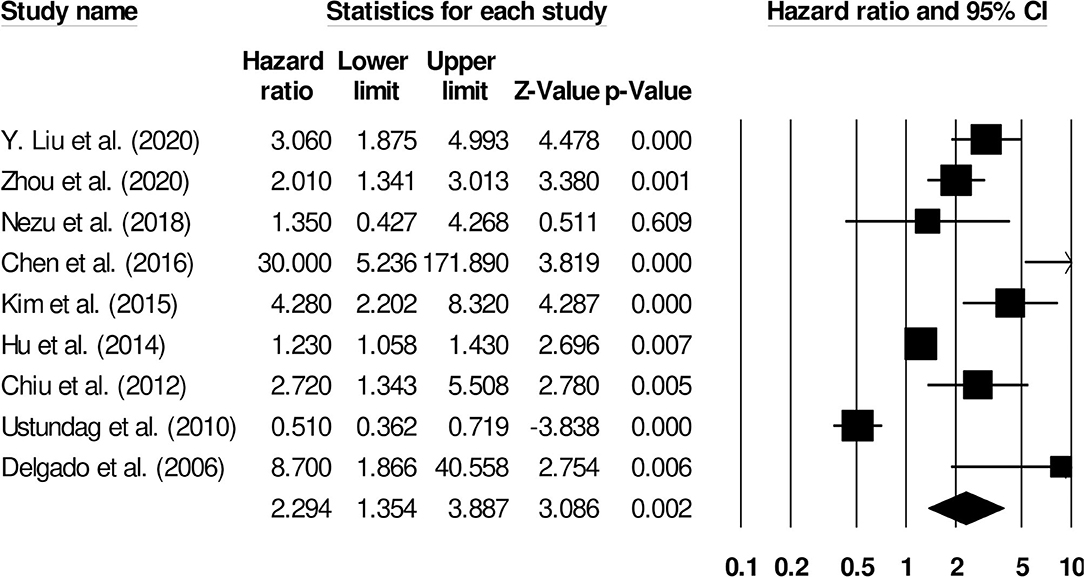
Figure 9. Forest plot for studies evaluating the impact of D-dimer level on post-stroke mortality outcomes. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
Further subgroup analysis for overall mortality was carried out examining the impact of stroke type. Two studies reported mortality outcomes for patients with cryptogenic ischemic stroke (Hazard ratio: 2.65, 95% CI: 0.87–8.08, p = 0.08; Figure 10; I2: 0%) while three included studies evaluated mortality outcomes for intracerebral hemorrhage patients with negligible heterogeneity (Hazard ratio: 2.63, 95% CI: 1.50–4.59, p = 0.001; Figure 11; I2: 18.8%).
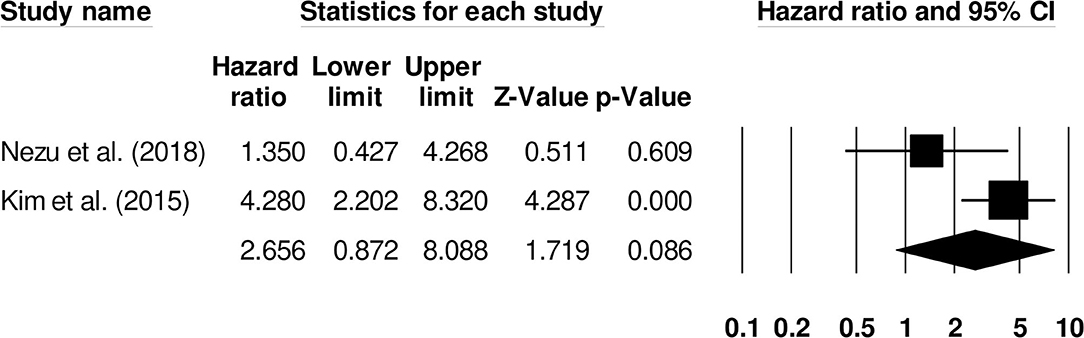
Figure 10. Forest plot for studies evaluating the impact of D-dimer level on post-cryptogenic ischemic stroke mortality outcomes. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.

Figure 11. Forest plot for studies evaluating the impact of D-dimer level on post-intracerebral hemorrhage mortality outcomes. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
We also conducted subgroup analyses based on different follow-up periods. Here, we identified only two studies that had reported a uniform follow-up of 3 months. We observed increased mortality outcomes for patients (Hazard ratio: 3.43, 95% CI: 0.86–13.71, p = 0.08; Figure 12; I2: 0%).
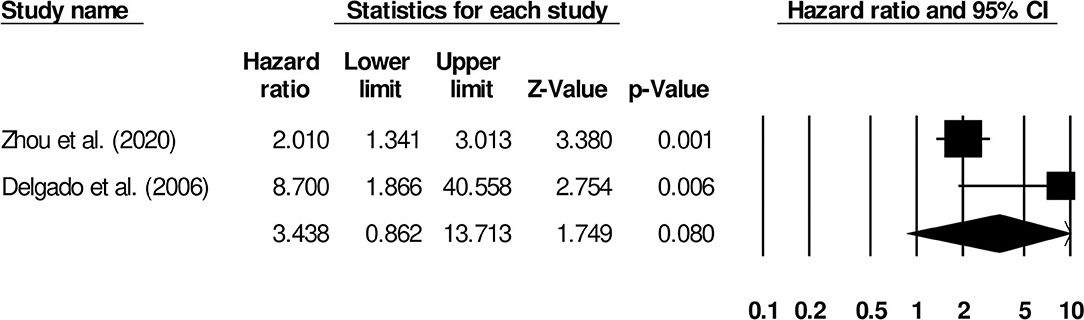
Figure 12. Forest plot for studies evaluating the impact of D-dimer level on post-stroke mortality outcomes at 3 months follow up. Hazard ratios are presented as black boxes while 95% confidence intervals are presented as whiskers. A small hazard ratio represents a lower influence of D-dimer levels on stroke patient functional outcome while a higher hazard ratio represents a higher influence.
This systematic review and meta-analysis suggest that poorer functional outcome and increased mortality incidence following stroke is associated with increased plasma D-dimer levels. We also noted that the association between plasma D-dimer levels and functional outcomes was stronger for ischemic stroke than intracerebral hemorrhage. However, plasma D-dimer predictive capacity for mortality between patients with cryptogenic ischemic stroke and intracerebral hemorrhage was similar.
Stroke management is challenging because of its atypical pathophysiology, poor prognosis, and heterogeneous manifestation (52, 53). In this light, preemptive prediction through biomarker detection has been widely recommended (54–56). Plasma D-dimer levels has been identified as a biomarker that was sensitive and specific for predicting short- and long-term functional outcomes, recurrence, and mortality post-stroke (12, 57). Johnson et al. (19) reported that D-dimer levels are indirectly indicative of hemostasis and thrombosis incidence. Furthermore, plasma D-dimers levels can be used to categorize increased risk for thromboembolic disorders (57, 58). Elevated plasma D-dimers could potentially boost interleukin-1 and 6 production (17, 59) precipitating worsened prognostic outcome following stroke (60). Nonetheless, despite pertaining several positive aspects, the routine use of plasma D-dimer in the current medical setting is complicated by its non-specificity. For instance, the plasma D-dimer levels are also susceptible to different inflammatory states, presence of infection, cancer, and venous thromboembolism (58, 61, 62). Therefore, the presence of a high plasma D-dimer at times could serve as a false positive with respect to stroke. Moreover, the clinical utility of plasma D-dimer is also limited perhaps because of limited clinical awareness this biomarker has in a stroke setting (i.e., plasma D-dimer evaluation not routinely demanded) (63).
This systematic review observed that plasma D-dimer levels could predict post-stroke functional outcome. These findings are aligned with other studies. Zhou et al. (28) showed that elevated plasma D-dimer levels measured 1-h post-hospital admission could predict poor 3-month functional outcomes for stroke patients with high precision and developed a scoring system for clinical practice. Furthermore, Hutanu et al. (35) found that plasma D-dimers could independently predict poor functional outcome in ischemic stroke patient outcomes whereas plasma c-reactive protein, neutrophil gelatinase associated lipocalin, the soluble receptor of tumor necrosis factor alfa, and neuron specific enolase could not.
We also examined the ability of plasma D-dimer levels to predict post-stroke mortality. The majority of included studies noted that plasma D-dimer levels were predictive for mortality. Hu et al. (33), for instance, noted that plasma D-dimer levels reliably predicted 7-day mortality with almost 88% sensitivity and 68% specificity—albeit the authors did note that plasma D-dimers were not as efficient as the standard Glasgow Coma scale. Similarly, Nezu et al. (27) reported that plasma D-dimer levels recorded at admission not only correlated with the National Institute of Health Stroke Scale but also with mortality. It is possible that high plasma D-dimer levels may be predictive of post-stroke mortality because it can also capture conditions such as venous thrombus, malignancy, or atrial fibrillation (64). In a novel study, Chen et al. (29) found that cerebrospinal fluid D-dimer levels were highly sensitive (88%) and specific (81%) for predicting 30-day mortality in stroke patients. The authors suggest that cerebrospinal D-dimer levels could be used reliably in patients with intracerebral or intraventricular hemorrhage. Besides, in the subgroup analyses of mortality, we observed that the risks of mortality were higher for patients with cryptogenic ischemic stroke (i.e., 2.65) when compared with the overall analyses (i.e., 2.19). In our opinion, this difference could perhaps be attributed to the small number of studies included in the subgroup analysis of cryptogenic ischemic stroke (i.e., two studies).
This study is hampered by a few limitations. This study is not pre-registered in a systematic review repository such as PROSPERO York or the Joanna Briggs Institute (65). This was because the current COVID-19 pandemic crisis has extended registration queues to over 1 year. Besides, this review does not provide a list of studies that were excluded with reasoning. This was a major flaw on our behalf, and we request future studies to address this limitation. Additionally, because of data paucity, we were unable to carry out sub-group analyses for two important parameters: the relationship between functional outcome and stroke type and the relationship between plasma D-dimer levels and short- and long-term functional outcomes. Similarly, there was a huge discrepancy in the sample sizes between the studies we included (i.e., 10,518 participants in Hou et al., and 43 participants in Chen et al.). Additionally, although we conducted subgroup analyses based on the specific follow-up periods and assessment methodologies (i.e., for functional outcomes), we were only able to include studies that reported follow-up at 3 and 2 months. Other studies for instance had reported a varied range of follow-up (i.e., at 12 months, 1 month, 48 h, 72 h) and because these were only singular studies, we could not conduct subgroup analyses for them. We presume that this could be an important source of heterogeneity in the analyses we conducted and could possibly incur bias in our results. We therefore recommend future studies to focus on these areas where there is a knowledge gap.
In conclusion, we provide preliminary 2b level of evidence concerning the capacity of plasma D-dimer levels for predicting stroke patient functional outcome and mortality. We show that increased plasma D-dimer levels are predictive of poorer functional outcomes and increased mortality. The findings from the present study may have wider implications in developing best practice guidelines for predicting post-stroke prognostic outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
PZ designed the project. CW and JW were involved in data collection and data analysis. SZ prepared the manuscript. JW edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.693524/full#supplementary-material
1. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. (2017) 120:439–48. doi: 10.1161/CIRCRESAHA.116.308413
2. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. (2019) 18:417–8. doi: 10.1016/S1474-4422(19)30030-4
3. Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. (2016) 94:634. doi: 10.2471/BLT.16.181636
4. Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. (2019) 17:191. doi: 10.1186/s12916-019-1397-3
5. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond Engl. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
6. Grotta JC, Albers GW, Broderick JP, Kasner SE, Lo EH, Mendelow AD, et al. Stroke: Pathophysiology, Diagnosis, and Management. Elsevier Inc. (2015). Available online at: https://miami.pure.elsevier.com/en/publications/stroke-pathophysiology-diagnosis-and-management (accessed April 4, 2021).
7. Woodruff TM, Thundyil J, Tang S-C, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. (2011) 6:11. doi: 10.1186/1750-1326-6-11
8. Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. (2004) 19:151–67. doi: 10.1023/B:MEBR.0000043966.46964.e6
9. Kidd PM. Integrated brain restoration after ischemic stroke–medical management, risk factors, nutrients, and other interventions for managing inflammation and enhancing brain plasticity. Altern Med Rev J Clin Ther. (2009) 14:14–35.
10. Neff KW, Horn P, Dinter D, Vajkoczy P, Schmiedek P, Düber C. Extracranial-intracranial arterial bypass surgery improves total brain blood supply in selected symptomatic patients with unilateral internal carotid artery occlusion and insufficient collateralization. Neuroradiology. (2004) 46:730–7. doi: 10.1007/s00234-004-1252-9
11. Fisher M. Stroke and TIA: epidemiology, risk factors, and the need for early intervention. Am J Manag Care. (2008) 14:S204–11.
12. Haapaniemi E, Tatlisumak T. Is D-dimer helpful in evaluating stroke patients? A systematic review. Acta Neurol Scand. (2009) 119:141–50. doi: 10.1111/j.1600-0404.2008.01081.x
13. Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. (2015) 46:915–20. doi: 10.1161/STROKEAHA.114.005604
14. Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC, BRAIN Study Group. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke. (2009) 40:77–85. doi: 10.1161/STROKEAHA.108.516377
15. Schiff L, Hadker N, Weiser S, Rausch C. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol Diagn Ther. (2012) 16:79–92. doi: 10.1007/BF03256432
16. Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. (2012) 43:1323–30. doi: 10.1161/STROKEAHA.111.639401
17. Zhang J, Liu L, Tao J, Song Y, Fan Y, Gou M, et al. Prognostic role of early D-dimer level in patients with acute ischemic stroke. PLoS ONE. (2019) 14:e0211458. doi: 10.1371/journal.pone.0211458
18. Zi W-J, Shuai J. Plasma D-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS ONE. (2014) 9:e86465. doi: 10.1371/journal.pone.0086465
19. Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. (2019) 94:833–9. doi: 10.1002/ajh.25482
20. Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. (2015) 69:1–46. doi: 10.1016/bs.acc.2014.12.001
21. Kearon C, Ginsberg JS, Douketis J, Crowther M, Brill-Edwards P, Weitz JI, et al. Management of suspected deep venous thrombosis in outpatients by using clinical assessment and D-dimer testing. Ann Intern Med. (2001) 135:108–11. doi: 10.7326/0003-4819-135-2-200107170-00011
22. Penaloza A, Roy P-M, Kline J, Verschuren F, LE Gal G, Quentin-Georget S, et al. Performance of age-adjusted D-dimer cut-off to rule out pulmonary embolism. J Thromb Haemost. (2012) 10:1291–6. doi: 10.1111/j.1538-7836.2012.04769.x
23. Rathbun SW, Whitsett TL, Raskob GE. Negative D-dimer result to exclude recurrent deep venous thrombosis: a management trial. Ann Intern Med. (2004) 141:839–45. doi: 10.7326/0003-4819-141-11-200412070-00007
24. Sié P. The value of laboratory tests in the diagnosis of venous thromboembolism. Haematologica. (1995) 80:57–60.
25. Fukuda H, Lo B, Yamamoto Y, Handa A, Yamamoto Y, Kurosaki Y, et al. Plasma D-dimer may predict poor functional outcomes through systemic complications after aneurysmal subarachnoid hemorrhage. J Neurosurg. (2017) 127:284–90. doi: 10.3171/2016.5.JNS16767
26. Liu J-H, Li X-K, Chen Z-B, Cai Q, Wang L, Ye Y-H, et al. D-dimer may predict poor outcomes in patients with aneurysmal subarachnoid hemorrhage: a retrospective study. Neural Regen Res. (2017) 12:2014–20. doi: 10.4103/1673-5374.221158
27. Nezu T, Kitano T, Kubo S, Uemura J, Yamashita S, Iwanaga T, et al. Impact of D-dimer levels for short-term or long-term outcomes in cryptogenic stroke patients. J Neurol. (2018) 265:628–36. doi: 10.1007/s00415-018-8742-x
28. Zhou Q, Zhang D, Chen X, Yang Z, Liu Z, Wei B, et al. Plasma D-dimer predicts poor outcome and mortality after spontaneous intracerebral hemorrhage. Brain Behav. (2021) 11:462–8. doi: 10.1002/brb3.1946
29. Chen C-W, Wu E-H, Huang J, Chang W-T, Ao K-H, Cheng T-J, et al. Dynamic evolution of D-dimer level in cerebrospinal fluid predicts poor outcome in patients with spontaneous intracerebral hemorrhage combined with intraventricular hemorrhage. J Clin Neurosci. (2016) 29:149–54. doi: 10.1016/j.jocn.2015.10.036
30. Chiu C-C, Li Y-N, Lin L-J, Hsiao C-T, Hsiao K-Y, Chen I-C. Serum D-dimer as a predictor of mortality in patients with acute spontaneous intracerebral hemorrhage. J Clin Neurosci. (2012) 19:810–3. doi: 10.1016/j.jocn.2011.08.032
31. Delgado P, Alvarez-Sabín J, Abilleira S, Santamarina E, Purroy F, Arenillas JF, et al. Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. (2006) 67:94–8. doi: 10.1212/01.wnl.0000223349.97278.e0
32. Kim YD, Song D, Nam HS, Lee K, Yoo J, Hong G-R, et al. D-dimer for prediction of long-term outcome in cryptogenic stroke patients with patent foramen ovale. Thromb Haemost. (2015) 114:614–22. doi: 10.1160/TH14-12-1040
33. Hu X, Fang Y, Ye F, Lin S, Li H, You C, et al. Effects of plasma D-dimer levels on early mortality and long-term functional outcome after spontaneous intracerebral hemorrhage. J Clin Neurosci. (2014) 21:1364–7. doi: 10.1016/j.jocn.2013.11.030
34. Üstündag M, Orak M, Güloglu C, Tamam Y, Sayhan MB. Plasma D-Dimer levels in acute ischemic stroke: association with mortality, stroke type and prognosis. Nobel Med. (2010) 6:37–42.
35. Hutanu A, Iancu M, Bălaşa R, Maier S, Dobreanu M. Predicting functional outcome of ischemic stroke patients in Romania based on plasma CRP, sTNFR-1, D-Dimers, NGAL and NSE measured using a biochip array. Acta Pharmacol Sin. (2018) 39:1228–36. doi: 10.1038/aps.2018.26
36. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
37. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
38. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A. OCEBM Levels of Evidence — Centre for Evidence-Based Medicine (CEBM). University of Oxford. Available at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed April 9, 2021).
39. Bax L, Yu L-M, Ikeda N, Moons KGM. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. (2007) 7:40. doi: 10.1186/1471-2288-7-40
40. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
41. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
42. Saver JL. Clinical practice. Cryptogenic Stroke. N Engl J Med. (2016) 374:2065–74. doi: 10.1056/NEJMcp1503946
43. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
44. Hsu P-J, Chen C-H, Yeh S-J, Tsai L-K, Tang S-C, Jeng J-S. High plasma D-dimer indicates unfavorable outcome of acute ischemic stroke patients receiving intravenous thrombolysis. Cerebrovasc Dis Basel Switz. (2016) 42:117–21. doi: 10.1159/000445037
45. Krarup L-H, Sandset EC, Sandset PM, Berge E. D-dimer levels and stroke progression in patients with acute ischemic stroke and atrial fibrillation. Acta Neurol Scand. (2011) 124:40–4. doi: 10.1111/j.1600-0404.2010.01409.x
46. Hou H, Xiang X, Pan Y, Li H, Meng X, Wang Y. Association of level and increase in D-dimer with all-cause death and poor functional outcome after ischemic stroke or transient ischemic attack. J Am Heart Assoc. (2021) 10:e018600. doi: 10.1161/JAHA.120.018600
47. Liu Y, Li F, Sun H, Sun Y, Sun H, Zhai Y, et al. Combined prognostic significance of D-dimer level and platelet count in acute ischemic stroke. Thromb Res. (2020) 194:142–9. doi: 10.1016/j.thromres.2020.05.021
48. Sato T, Sato S, Yamagami H, Komatsu T, Mizoguchi T, Yoshimoto T, et al. D-dimer level and outcome of minor ischemic stroke with large vessel occlusion. J Neurol Sci. (2020) 413:116814. doi: 10.1016/j.jns.2020.116814
49. Wang J, Feng A, Xu J, Liu Y, Li F, Sun Y, et al. D-dimer and its combination with blood lipid on prognosis of patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2020) 29:105394. doi: 10.1016/j.jstrokecerebrovasdis.2020.105394
50. Ye N, Liu Z, Wang X, Xu X, Wu W. Evaluation of analytic and clinical performance of thrombin-antithrombin complex and D-dimer assay in prognosis of acute ischemic stroke. Blood Coagul Fibrinolysis Int J Haemost Thromb. (2020) 31:303–9. doi: 10.1097/MBC.0000000000000915
51. Yang X-Y, Gao S, Ding J, Chen Y, Zhou X-S, Wang J-E. Plasma D-dimer predicts short-term poor outcome after acute ischemic stroke. PLoS ONE. (2014) 9:e89756. doi: 10.1371/journal.pone.0089756
52. Wang Y, Li Z, Zhao X, Wang D, Li H, Xian Y, et al. Stroke care quality in China: substantial improvement, and a huge challenge and opportunity. Int J Stroke. (2017) 12:229–35. doi: 10.1177/1747493017694392
53. Yaghi S, Elkind MSV. Cryptogenic stroke: a diagnostic challenge. Neurol Clin Pract. (2014) 4:386–93. doi: 10.1212/CPJ.0000000000000086
54. Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. (2017) 12:480–93. doi: 10.1177/1747493017714176
55. Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurother J Am Soc Exp Neurother. (2011) 8:349–60. doi: 10.1007/s13311-011-0050-4
56. Sidorov E, Sanghera DK, Vanamala JKP. Biomarker for ischemic stroke using metabolome: a clinician perspective. J Stroke. (2019) 21:31–41. doi: 10.5853/jos.2018.03454
57. Ageno W, Finazzi S, Steidl L, Biotti MG, Mera V, Melzi D'Eril G, et al. Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch Intern Med. (2002) 162:2589–93. doi: 10.1001/archinte.162.22.2589
58. Bounameaux H, de Moerloose P, Perrier A, Reber G. Plasma measurement of D-dimer as diagnostic aid in suspected venous thromboembolism: an overview. Thromb Haemost. (1994) 71:1–6. doi: 10.1055/s-0038-1642375
59. Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. (1994) 86:322–6. doi: 10.1111/j.1365-2141.1994.tb04733.x
60. Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. (2009) 6:e1000145. doi: 10.1371/journal.pmed.1000145
61. Li R, Shao H-Y, Hao L-B, Yu B-Z, Qu P-F, Zhou Y-X, et al. Plasma fibrinogen exhibits better performance than plasma D-dimer in the diagnosis of periprosthetic joint infection: a multicenter retrospective study. J Bone Joint Surg Am. (2019) 101:613–9. doi: 10.2106/JBJS.18.00624
62. Altiay G, Ciftci A, Demir M, Kocak Z, Sut N, Tabakoglu E, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol R Coll Radiol G B. (2007) 19:494–8. doi: 10.1016/j.clon.2007.04.002
63. Harvey RL, Roth EJ, Yarnold PR, Durham JR, Green D. Deep vein thrombosis in stroke. The use of plasma D-dimer level as a screening test in the rehabilitation setting. Stroke. (1996) 27:1516–20. doi: 10.1161/01.STR.27.9.1516
64. Shin Y-W, Lee S-T, Jung K-H, Kim D-Y, Park C-K, Kim TM, et al. Predictors of survival for patients with cancer after cryptogenic stroke. J Neurooncol. (2016) 128:277–84. doi: 10.1007/s11060-016-2106-0
Keywords: D-dimer, cerebrovascular accident, prognosis, morbidity, mortality
Citation: Zhang P, Wang C, Wu J and Zhang S (2021) A Systematic Review of the Predictive Value of Plasma D-Dimer Levels for Predicting Stroke Outcome. Front. Neurol. 12:693524. doi: 10.3389/fneur.2021.693524
Received: 11 April 2021; Accepted: 15 June 2021;
Published: 06 July 2021.
Edited by:
Steffen Tiedt, LMU Munich University Hospital, GermanyCopyright © 2021 Zhang, Wang, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiliang Zhang, c2R6aGFuZ3NsQHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.