
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 27 August 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.692067
This article is part of the Research Topic Challenges in Posterior Circulation Ischemic Stroke View all 17 articles
 Kosmas Macha1
Kosmas Macha1 Philip Hoelter2
Philip Hoelter2 Gabriela Siedler1
Gabriela Siedler1 Ruihao Wang1
Ruihao Wang1 Michael Knott2
Michael Knott2 Svenja Stoll1
Svenja Stoll1 Tobias Engelhorn2
Tobias Engelhorn2 Arnd Doerfler2
Arnd Doerfler2 Stefan Schwab1
Stefan Schwab1 Iris Mühlen2†
Iris Mühlen2† Bernd Kallmünzer1*†
Bernd Kallmünzer1*†Background: rt-PA for ischemic stroke in the unknown or extended time window beyond the first 4. 5 h after symptom onset is safe and effective for certain patients after selection by multimodal neuroimaging. However, the evidence for this approach comes mainly from patients with anterior circulation stroke (ACS), while the data on posterior circulation stroke (PCS) are scarce.
Methods: Ischemic stroke patients treated with IV-thrombolysis in the unknown or extended time window between January 2011 and May 2019 were identified from an institutional registry. The patients were categorized into PCS or ACS based on clinico-radiological findings. We analyzed the hemorrhagic complications, clinical and imaging efficacy outcomes, and mortality rates by comparing the PCS and ACS patient groups. Adjusted outcome analyses were performed after propensity score matching for the relevant factors.
Results: Of the 182 patients included, 38 (20.9%) had PCS and 144 (79.1%) had ACS. Symptomatic acute large vessel occlusion (LVO) was present in 123 patients on admission [27 (22.0%) PCS and 96 (78.0%) ACS]. The score on the National Institutes of Health Stroke Scale (NIHSS), the time from last seen normal, and the door-to-needle times were similar in PCS and ACS. In patients with LVO, the NIHSS score was lower [8 (5–15) vs. 14 (9–18), p = 0.005], and infarction visible on follow-up imaging was less common [70.4 vs. 87.5%; aRD, −18.9% (−39.8 to −2.2%)] in the PCS patient group. There was a trend toward a lower risk for intracranial hemorrhage (ICH) following intravenous thrombolysis in PCS vs. ACS, without reaching a statistical significance [5.3 vs. 16.9%; aRD, −10.4% (−20.4 to 4.0%)]. The incidence of symptomatic ICH [according to the ECASS III criteria: 2.6 vs. 3.5%; aRD, −2.9% (−10.3 to 9.2%)], efficacy outcomes, and mortality rates were similar in PCS and ACS patients.
Conclusions: In this real-world clinical cohort, the safety and the efficacy of rt-PA for ischemic stroke in the unknown or extended time window did not show relevant differences between PCS and ACS, with a trend toward less hemorrhagic complications in PCS. The findings reconfirm the clinician in the usage of rt-PA beyond the first 4.5 h also in selected patients with PCS.
In up to 16% of acute ischemic strokes treated with IV-thrombolysis, the territories of the posterior circulation, including the vertebral, basilar, or posterior cerebral arteries, are affected (1–4). In the subgroup of patients with severe ischemic stroke, presenting with National Institutes of Health Stroke Scale (NIHSS) scores >25, the proportion of PCS is increasing up to 36% (5). IV-thrombolysis is the standard of care for acute ischemic stroke within 4.5 h from symptom onset irrespective of the vascular territory affected (6, 7). In addition, several studies could demonstrate the safety and the efficacy of IV-thrombolysis for selected patients in the unknown or extended time window beyond 4.5 h (8–10). Multimodal CT or MRI was used for patient selection in these studies, and some of these approaches were implemented in the latest international guidelines (6–10).
The proportion of PCS was low or not reported in most randomized rt-PA trials with treatment within 4.5 h from onset and with treatment in the unknown or extended time window (8, 9, 11–14). Therefore, the transfer of the results to patients with PCS might be inappropriate.
A meta-analysis including 10,313 patients (PCS 11.9%) demonstrated a lower risk for symptomatic rt-PA-associated intracranial hemorrhage and higher rates of good functional outcome at 3 months in posterior circulation than in anterior circulation stroke patients receiving treatment in the approved time window of 4.5 h from onset (15).
The main objective of our study was to investigate these differences in the risk of hemorrhagic complications between PCS and ACS patients in the unknown or extended time window. The secondary objectives were imaging and functional efficacy outcomes and mortality rates.
This retrospective cohort study is based on the data of consecutive acute ischemic stroke patients treated with IV-thrombolysis (rt-PA) in the unknown or extended time window >4.5 h in the period from January 2011 to May 2019 at our tertiary university stroke center. The patients were eligible for IV-thrombolysis using multimodal CT or MR imaging according to our institutional treatment algorithm for the management of ischemic stroke in the extended or unknown time window as described previously (16). Accordingly, IV-thrombolysis was performed after (1) the exclusion of intracranial hemorrhage and (2) the exclusion of major infarction on non-contrast CT or gradient echo and fluid-attenuated inversion recovery sequences using MRI and (3) evidence of salvageable tissue at risk on perfusion imaging (mismatch between hypoperfusion vs. infarcted core on perfusion CT or mismatch between perfusion-weighted and diffusion-weighted imaging using MRI; mismatch quotient >1.4 for both modalities) (Figure 1). Follow-up imaging was performed 24 h after IV-thrombolysis or earlier in the case of neurologic deterioration (NIHSS score increase of four points or more). The modality of follow-up imaging (CT or MRI) was to the discretion of the treating physician. We categorized all patients according to clinical and/or radiological findings as posterior circulation stroke or anterior circulation stroke patients (full patient cohort—clinico-radiological categorization); patients with simultaneous anterior and posterior circulation stroke were excluded. In addition, we conducted a subgroup analysis of patients with acute large vessel occlusion (LVO). Patients with occlusion of the internal carotid and middle or anterior cerebral artery were categorized as ACS patients, and patients with occlusion of the vertebral, basilar, or posterior cerebral artery were categorized as PCS patients. Patients with complete fetal posterior cerebral artery were excluded from the study.
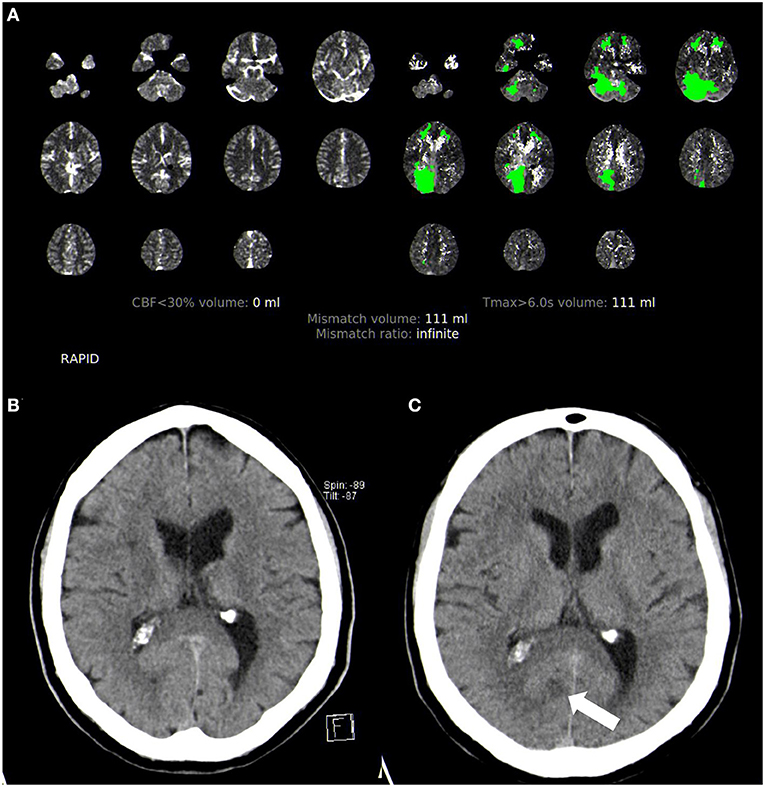
Figure 1. Acute multimodal CT and follow-up non-contrast CT imaging in posterior circulation stroke. (A) Perfusion CT analyzed with Rapid CTP. (B) Initial non-contrast CT showing no infarction. (C) Follow-up non-contrast CT with a visible small infarction in the posterior cerebral artery territory (arrow).
We analyzed the clinical and imaging stroke characteristics, risk factors, procedure times for IV-thrombolysis and mechanical thrombectomy (if performed), clinical course including intracranial hemorrhagic complications, evidence of cerebral infarction on follow-up imaging, mortality rates, and 90-day outcome. Demographic, clinical, and radiologic data were collected during inpatient stay. The assessment of day 90 follow-up was conducted via telephone interview, outpatient visit, or medical reports by trained raters.
The primary outcome was the incidence of hemorrhagic complication [any intracranial hemorrhage (ICH), symptomatic ICH according to different definitions—European Cooperative Acute Stroke Study (ECASS) III criteria (13), ECASS II criteria (11), and Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria (12)] on follow-up imaging at 24 h after IV-thrombolysis.
The secondary outcomes were the rate of early major neurologic improvement (defined as a reduction of the NIHSS score by at least eight points or a score of 0 or 1 after 24 h), evidence of cerebral infarction on follow-up imaging after 24 h, mortality, and favorable functional outcome at day 90 using the modified Rankin Scale (mRS). A favorable outcome was defined as either a score on the mRS 0–2 or an improvement to the level prior to stroke onset, respectively.
Data were presented as absolute/relative numbers for categorical variables and median/interquartile range (IQR) for continuous variables. The significance of differences between patient groups (PCS and ACS) was calculated using the Mann–Whitney U-test, the χ2 test, and the Fisher exact test as appropriate. Statistical significance was set a priori at p-value < 0.05.
For the investigation of primary and secondary outcomes, adjusted analyses after propensity score matching (estimation algorithm: logistic regression, matching algorithm: nearest neighbor approach, caliper 0.2, match 1:3) were performed. The PCS and ACS patients were matched for age, female sex, arterial hypertension, diabetes mellitus, atrial fibrillation, NIHSS on admission, large vessel occlusion (in clinico-radiological cohort), and mechanical thrombectomy (Supplementary Figures 1, 2). We calculated absolute differences between PCS and ACS patients in percentage (absolute risk difference, aRD) with corresponding 95% confidence interval. Negative values indicate a decrease of measurement from ACS patients as reference.
Missing data of the 90-day follow-up were excluded from the outcome analyses. Statistical analyses were performed using IBM SPSS Statistics 21 software package (www.spss.com) and R 2.12.1 (www.r-project.org).
One hundred eighty-four acute ischemic stroke patients treated with IV-thrombolysis in the unknown or extended time window were identified. Two patients were excluded due to simultaneous anterior and posterior circulation stroke, resulting in the final study cohort of 182 patients including 38 (20.9%) PCS and 144 (79.1%) ACS patients (Figure 2). Acute LVO was present in 123 patients (67.6%) and constituted the LVO patient subgroup.
Patients with PCS were less commonly female (28.9 vs. 56.9%, p = 0.002) but similar regarding comorbidities and concurrent medication (Table 1). The proportion of patients in the confirmed time window >4.5 h was higher in the PCS group (23.7 vs. 9.0%, p = 0.023), and the time from last seen normal to treatment was similar in PCS and ACS patients [450 min (362–719) vs. 558 min (354–819), p = 0.148]. Differences in NIHSS scores on admission between PCS and ACS patients [8 (5–14) vs. 11 (6–17), p = 0.068] did not reach statistical significance. Door-to-needle times were similar in PCS and ACS patients [56 min (42–91) vs. 59 min (41–76), p = 0.510]. Further clinical and imaging characteristics of stroke are shown in Table 2.
There was a statistical trend for a lower incidence of any intracranial hemorrhage post IV-thrombolysis in PCS than in ACS patients (5.3 vs. 16.9%, p = 0.075). The proportion of patients with symptomatic ICH following different definitions (ECASS III criteria: 2.6 vs. 3.5%, p = 1.000) and fatal ICH (0.0 vs. 1.4%, p = 1.000) was similar in PCS and ACS patients. There was no significant difference in the rate of infarction visible on follow-up imaging (65.8 vs. 77.5%, p = 0.140), early neurologic improvement (15.8 vs. 18.1%, p = 1.000), or functional outcome at day 90 (pre-mRS or mRS score 0–2: 33.3 vs. 39.3%, p = 0.532). The mortality rates during inpatient stay and at day 90 were similar in PCS and ACS patients (13.2 vs. 11.8%, p = 0.784 and 23.5 vs. 26.8%, p = 0.827) (Figure 3). Two patients in the ACS group died prior to follow-up imaging (the suspected cause of death was lung embolism and circulatory failure, respectively).
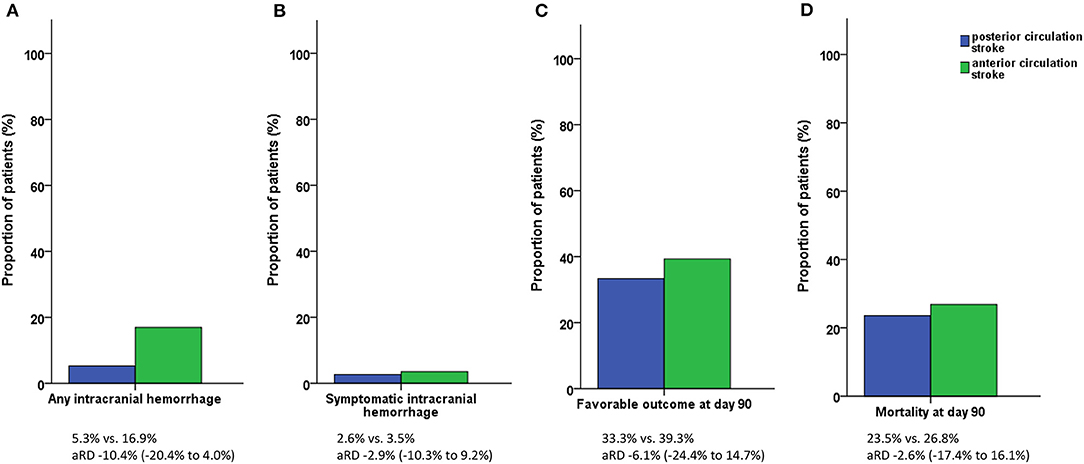
Figure 3. Study outcomes. (A) Incidence of any intracranial hemorrhage. (B) Incidence of symptomatic intracranial hemorrhage. (C) Functional outcome at day 90. (D) Mortality at day 90. Symptomatic intracranial hemorrhage according to the ECASS III criteria. Favorable outcome defined as pre-modified Rankin Scale (mRS) or mRS score 0–2.
The analyses after propensity score matching included 124 patients (PCS, n = 35; ACS, n = 89) and did not lead to statistical differences in the incidence of any ICH [aRD −10.4% (−20.4 to 4.0%)], symptomatic ICH, evidence of infarction on follow-up imaging, early neurologic improvement, functional outcome, or mortality (Table 3).
Among a total of 123 patients with acute large vessel occlusion, 27 (22.0%) were PCS patients and 96 (78.0%) were ACS patients. The distribution of occlusion sites in PCS and ACS patients is given in Table 4. The PCS patients were less commonly female (29.6 vs. 59.4%, p = 0.008). There were no significant differences in comorbidities and concurrent medication (Table 5). The proportion of patients in the confirmed extended time window beyond 4.5 h was higher in PCS patients (22.2 vs. 6.3%, p = 0.023), and the time from symptom recognition to admission was shorter in ACS patients [68 min (50–123) vs. 89 min (67–153), p = 0.049]. The NIHSS scores on admission were higher in ACS patients [14 (9–18) vs. 8 (5–15), p = 0.005]. The time from last seen normal to the start of IV-thrombolysis did not differ significantly between the PCS and ACS patients [457 min (368–745) vs. 613 (385–843), p = 0.141]; mechanical thrombectomy was equally performed in the PCS and ACS patient groups (48.1 vs. 51.0%, p = 0.790). The door-to-needle times [55 min (42–91) vs. 61 min (42–78), p = 0.886] and the door-to-groin times [97 min (78–121) vs. 94 min (77–111), p = 0.621] were similar in PCS and ACS patients. Further clinical and imaging characteristics of stroke are shown in Table 6. The incidence of any ICH (7.4 vs. 18.9%, p = 0.239), symptomatic ICH (ECASS III criteria: 3.7 vs. 2.1%, p = 0.531), and fatal ICH (0.0 vs. 1.0%, p = 1.000) was similar in PCS and ACS patients. Infarction on follow-up imaging was significantly more often evident in ACS patients than in PCS patients (87.5 vs. 70.4%, p = 0.034). Early neurologic improvement (18.5 vs. 16.7%, p = 0.779), functional outcome at day 90 (pre-mRS or mRS score 0–2: 21.7 vs. 28.9%, p = 0.603), and mortality rates (inpatient stay: 18.5 vs. 14.6%, p = 0.563; day 90: 29.2 vs. 30.7%, p = 1.000) were similar in PCS and ACS patients. One ACS patient died prior to follow-up imaging (the suspected cause of death was lung embolism).
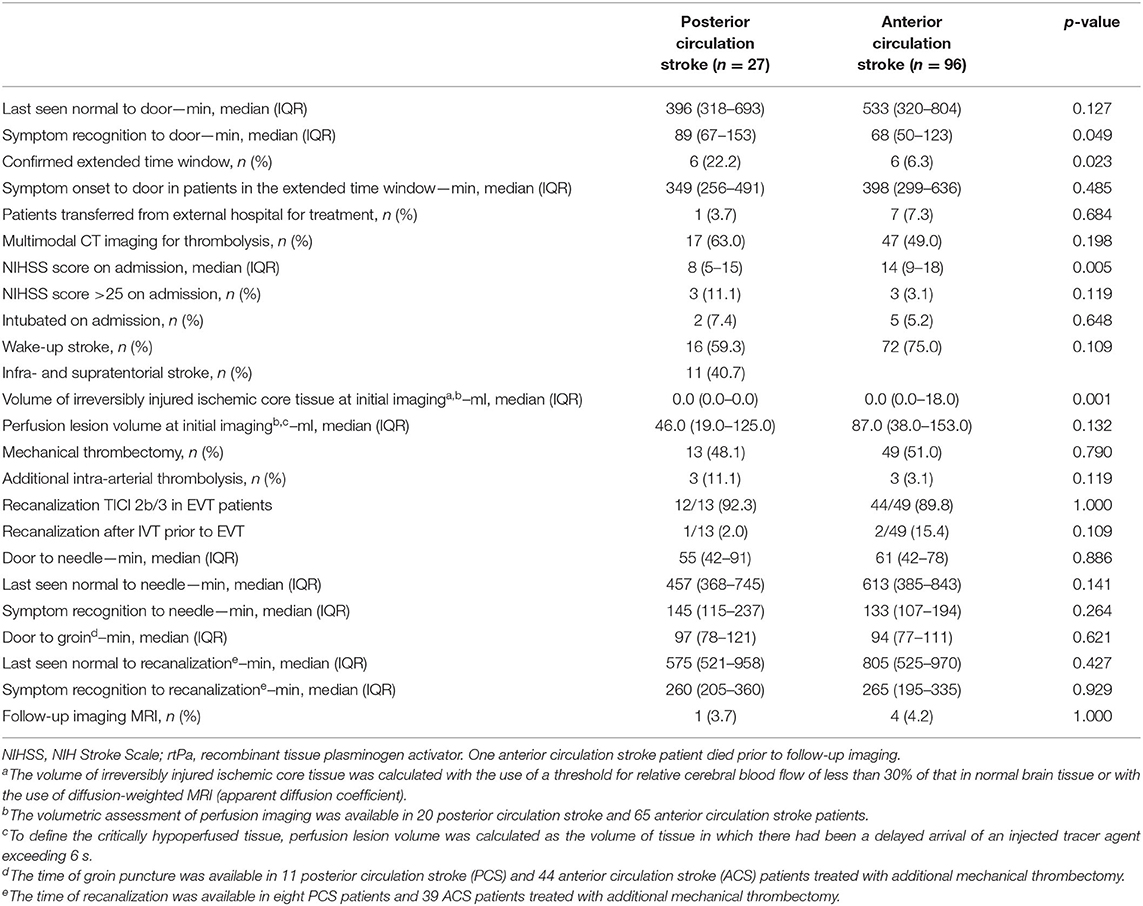
Table 6. Clinical and imaging characteristics of stroke in the large vessel occlusion patient cohort.
In the analyses after propensity score matching (the total included 80 patients: PCS, n = 23; ACS, n = 57), the lower rate of infarction on follow-up imaging in PCS patients remained statistically significant [aRD −18.9% (−39.5 to −2.2%)]. No significant differences were detected regarding any ICH [aRD −7.4% (−20.7 to 12.2%)], symptomatic ICH, fatal ICH, and early or late neurologic/functional outcome or mortality rates (Table 7).
The major findings of our real-world clinical cohort study investigating the treatment of acute ischemic stroke with IV-thrombolysis in the unknown or extended time window are as follows: (1) the rates of symptomatic ICH were low, without a significant difference between PCS and ACS patients, (2) there was a trend toward more hemorrhages among ACS patients than in PCS patients, and (3) the functional outcome and mortality rates at day 90 did not differ.
The rates of symptomatic ICH in our study were similar to the ones reported in the literature, without a difference between PCS and ACS patients (2, 3). Controversially, a large meta-analysis including 10,313 patients (1,224 PCS) reported the risk for sICH in PCS as half of that in ACS (15). However, while the rate of symptomatic hemorrhages did not differ in our cohort, the incidence of any ICH (including asymptomatic ones) seemed slightly higher in ACS, without reaching statistical significance. The higher volumes of ischemic core on initial multimodal imaging may have contributed to the higher risk for hemorrhagic complications in ACS patients, as infarct size is an established risk factor for hemorrhagic transformation (17, 18). These differences in ischemic core on initial imaging might be responsible for the higher rates of cerebral infarction visible on follow-up imaging in ACS patients of our LVO cohort. In addition, cerebral infarction in the posterior circulation including the brainstem might be more difficult to detect compared to anterior circulation stroke infarction on follow-up imaging (19). The rates of MRI for follow-up imaging, improving the detection of infarction especially in the posterior cranial fossa, were low and did not differ between PCS and ACS patients.
The differences in infarction on follow-up imaging did not translate into a functional outcome in PCS and ACS patients in our LVO patient cohort. The absence of correlation between the volume of infarcted tissue and the functional outcome was reported earlier for LVO patients receiving recanalization therapy (20).
Several studies demonstrated similar functional outcomes in PCS and ACS patients (1–3, 15, 21). Consistently, we did not find differences in outcome between patient groups in the analysis of the full patient cohort.
The mortality rates in PCS and ACS are reported inconsistently in the literature (2, 3, 15). This heterogeneity may be attributed to differences in the proportion of severe stroke patients between study cohorts in ACS and PCS patients. In our study, the proportion of severe stroke patients presenting with NIHSS score >25 on admission seemed higher in PCS patients, without reaching statistical significance. Nevertheless, we found no difference in mortality rates at day 90 between PCS and ACS patients in our study.
The proportion of patients with PCS in our study was slightly higher than the one reported in previous cohorts, including patients within 4.5 h from symptom onset (1–4, 15). Considering the poor prognosis of severe posterior circulation stroke without recanalizing therapies, the higher proportion of PCS in our cohort might refer to the aggressive treatment also beyond the first 4.5 h after onset (5, 22). Furthermore, a higher proportion of patients treated in the confirmed extended time window >4.5 h in PCS might have contributed to this finding and leads to the higher proportion of PCS patients in our cohort.
The higher proportion of female sex in ACS patients in our study was consistent to previous findings, demonstrating female sex as a known risk factor of ACS (15, 23). The PCS patients presented with lower median NIHSS scores on admission statistically significant in LVO patients confirming a known underrepresentation of PCS symptoms in the NIHSS (15, 21, 24).
As the symptoms of PCS can be clinically less noticeable, this may have led to longer times from symptom recognition to door in LVO patients of our study. Less noticeable symptoms may have led to longer door-to-needle times reported for PCS patients compared to ACS previously (25). In our study, the procedure times did not differ significantly between PCS and ACS patients despite the lower NIHSS scores on admission in PCS patients. Similar procedure times in ACS and PCS patients might be another evidence for an aggressive treatment of PCS patients in our cohort. Overall, the times from last seen normal to treatment were similar in PCS and ACS patients in our study.
Our study has relevant limitations, mainly its small patient number and monocentric design. The limited sample size might be causal for missing statistical significance in the difference of any ICH in our study. Furthermore, the classification as posterior or anterior circulation stroke based on clinical symptoms can be challenging in some patients presenting without LVO and with no infarction visible on follow-up imaging. This may have led to wrongly classifying some patients in the clinico-radiological cohort. However, no clear contradictory results between our clinico-radiological and LVO patient cohorts regarding primary and secondary outcomes were detected.
To conclude the main result of our study, the rates of hemorrhagic complications were low in PCS patients treated with IV-thrombolysis in the unknown or extended time window. This will reaffirm the clinician in the use of rt-PA for selected PCS patients beyond the established 4.5-h time window.
Anonymized data will be shared on request to any qualified investigator.
The studies involving human participants were reviewed and approved by the Ethics Board, Medical Faculty, University of Erlangen-Nuremberg. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KM contributed to the design and conceptualization of the study, acquisition and analysis of data, and drafting of the manuscript for intellectual content. PH, GS, RW, MK, and SS contributed to the acquisition of data and revision of the manuscript for intellectual content. TE, AD, and SS contributed to the design and conceptualization of the study and revision of the manuscript for intellectual content. IM contributed to the design and conceptualization of the study, analysis of data, and revision of the manuscript for intellectual content. BK contributed to the design and conceptualization of the study, analysis of data, and drafting of the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.692067/full#supplementary-material
Supplemental Figure 1. Standardized differences in variables included in the propensity score before and after matching in the clinico-radiologic patient cohort.
Supplemental Figure 2. Standardized differences in variables included in the propensity score before and after matching in the large vessel occlusion patient cohort.
1. Sarikaya H, Arnold M, Engelter ST, Lyrer PA, Mattle HP, Georgiadis D, et al. Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke. (2011) 42:2498–502. doi: 10.1161/STROKEAHA.110.607614
2. Breuer L, Huttner HB, Jentsch K, Blinzler C, Winder K, Engelhorn T, et al. Intravenous thrombolysis in posterior cerebral artery infarctions. Cerebrovasc Dis. (2011) 31:448–54. doi: 10.1159/000323253
3. Förster A, Gass A, Kern R, Griebe M, Hennerici MG, Szabo K. Thrombolysis in posterior circulation stroke: stroke subtypes and patterns, complications and outcome. Cerebrovasc Dis. (2011) 32:349–53. doi: 10.1159/000330346
4. Sung S-F, Chen C-H, Chen Y-W, Tseng M-C, Shen H-C, Lin H-J. Predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis: stroke territory as a potential pitfall. J Neurol Sci. (2013) 335:96–100. doi: 10.1016/j.jns.2013.08.036
5. Mazya MV, Lees KR, Collas D, Rand V-M, Mikulik R, Toni D, et al. IV thrombolysis in very severe and severe ischemic stroke: results from the SITS-ISTR registry. Neurology. (2015) 85:2098–106. doi: 10.1212/WNL.0000000000002199
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute Ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STROKEAHA.119.026917
7. Berge E, Whiteley W, Audebert H, Marchis GMD, Fonseca AC, Padiglioni C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021:2396987321989865. doi: 10.1177/2396987321989865
8. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. (2018) 379:611–22. doi: 10.1056/NEJMoa1804355
9. Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. (2019) 380:1795–803. doi: 10.1056/NEJMoa1813046
10. Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. Extending thrombolysis to 4.5–9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. (2019) 394:139–47. doi: 10.1016/S0140-6736(19)31053-0
11. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
12. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
13. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with Alteplase 3 to 4. 5 Hours after Acute Ischemic Stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
14. IST-3 collaborative group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. (2012) 379:2352–63. doi: 10.1016/S0140-6736(12)60768-5
15. Keselman B, Gdovinová Z, Jatuzis D, Melo TPE, Vilionskis A, Cavallo R, et al. Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: results from the safe implementation of treatments in stroke registry and meta-analysis. Stroke. (2020) 51:876–82. doi: 10.1161/STROKEAHA.119.027071
16. Macha K, Hoelter P, Siedler G, Knott M, Schwab S, Doerfler A, et al. Multimodal CT or MRI for IV thrombolysis in ischemic stroke with unknown time of onset. Neurology. (2020) 95:e2954–64. doi: 10.1212/WNL.0000000000011059
17. Kerenyi L, Kardos L, Szász J, Szatmári S, Bereczki D, Hegedüs K, et al. Factors influencing hemorrhagic transformation in ischemic stroke: a clinicopathological comparison. Eur J Neurol. (2006) 13:1251–5. doi: 10.1111/j.1468-1331.2006.01489.x
18. Tan S, Wang D, Liu M, Zhang S, Wu B, Liu B. Frequency and predictors of spontaneous hemorrhagic transformation in ischemic stroke and its association with prognosis. J Neurol. (2014) 261:905–12. doi: 10.1007/s00415-014-7297-8
19. Hwang DY, Silva GS, Furie KL, Greer DM. Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med. (2012) 42:559–65. doi: 10.1016/j.jemermed.2011.05.101
20. Ganesh A, Menon BK, Assis ZA, Demchuk AM, Al-Ajlan FS, Al-Mekhlafi MA, et al. Discrepancy between post-treatment infarct volume and 90-day outcome in the ESCAPE randomized controlled trial. Int J Stroke. (2020) 16:593–601. doi: 10.1177/1747493020929943
21. Zürcher E, Richoz B, Faouzi M, Michel P. Differences in Ischemic anterior and posterior circulation strokes: a clinico-radiological and outcome analysis. J Stroke Cerebrovas Dis. (2019) 28:710–8. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.016
22. Sommer P, Posekany A, Serles W, Marko M, Scharer S, Fertl E, et al. Is functional outcome different in posterior and anterior circulation stroke? Stroke. (2018) 49:2728–32. doi: 10.1161/STROKEAHA.118.021785
23. Subramanian G, Silva J, Silver FL, Fang J, Kapral MK, Oczkowski W, et al. Risk factors for posterior compared to anterior ischemic stroke: an observational study of the registry of the Canadian stroke network. Neuroepidemiology. (2009) 33:12–6. doi: 10.1159/000209282
24. Linfante I, Llinas RH, Schlaug G, Chaves C, Warach S, Caplan LR. Diffusion-weighted imaging and national institutes of health stroke scale in the acute phase of posterior-circulation stroke. Arch Neurol. (2001) 58:621–8. doi: 10.1001/archneur.58.4.621
Keywords: wake-up stroke, extended time window, IV-thrombolysis, posterior circulation stroke, anterior circulation stroke
Citation: Macha K, Hoelter P, Siedler G, Wang R, Knott M, Stoll S, Engelhorn T, Doerfler A, Schwab S, Mühlen I and Kallmünzer B (2021) IV-Thrombolysis in Ischemic Stroke With Unknown Time of Onset—Safety and Outcomes in Posterior vs. Anterior Circulation Stroke. Front. Neurol. 12:692067. doi: 10.3389/fneur.2021.692067
Received: 07 April 2021; Accepted: 09 July 2021;
Published: 27 August 2021.
Edited by:
Volker Puetz, Dresden University of Technology, GermanyReviewed by:
Anita Ante Arsovska, Saints Cyril and Methodius University of Skopje, North MacedoniaCopyright © 2021 Macha, Hoelter, Siedler, Wang, Knott, Stoll, Engelhorn, Doerfler, Schwab, Mühlen and Kallmünzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernd Kallmünzer, YmVybmQua2FsbG11ZW56ZXJAdWstZXJsYW5nZW4uZGU=
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.