
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 16 June 2021
Sec. Movement Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.680254
This article is part of the Research Topic Tremor Syndromes: Current Concepts and Future Perspectives View all 12 articles
 Florian Holtbernd1,2,3*
Florian Holtbernd1,2,3* N. Jon Shah1,2,3
N. Jon Shah1,2,3Background: The pathophysiology underlying essential tremor (ET) still is poorly understood. Recent research suggests a pivotal role of the cerebellum in tremor genesis, and an ongoing controversy remains as to whether ET constitutes a neurodegenerative disorder. In addition, mounting evidence indicates that alterations in the gamma-aminobutyric acid neurotransmitter system are involved in ET pathophysiology. Here, we systematically review structural, functional, and metabolic neuroimaging studies and discuss current concepts of ET pathophysiology from an imaging perspective.
Methods: We conducted a PubMed and Scopus search from 1966 up to December 2020, entering essential tremor in combination with any of the following search terms and their corresponding abbreviations: positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and gamma-aminobutyric acid (GABA).
Results: Altered functional connectivity in the cerebellum and cerebello-thalamico-cortical circuitry is a prevalent finding in functional imaging studies. Reports from structural imaging studies are less consistent, and there is no clear evidence for cerebellar neurodegeneration. However, diffusion tensor imaging robustly points toward microstructural cerebellar changes. Radiotracer imaging suggests that the dopaminergic axis is largely preserved in ET. Similarly, measurements of nigral iron content and neuromelanin are unremarkable in most studies; this is in contrast to Parkinson's disease (PD). PET and MRS studies provide limited evidence for cerebellar and thalamic GABAergic dysfunction.
Conclusions: There is robust evidence indicating that the cerebellum plays a key role within a multiple oscillator tremor network which underlies tremor genesis. However, whether cerebellar dysfunction relies on a neurodegenerative process remains unclear. Dopaminergic and iron imaging do not suggest a substantial overlap of ET with PD pathophysiology. There is limited evidence for alterations of the GABAergic neurotransmitter system in ET. The clinical, demographical, and genetic heterogeneity of ET translates into neuroimaging and likely explains the various inconsistencies reported.
Essential tremor (ET) is among the most common movement disorders in adulthood. Its prevalence in the general population is estimated at ~0.5% (1). ET can manifest at any age, but there is a strong association with older age, as demonstrated by a much higher prevalence (4–5%) in people aged >65 years (2). ET can manifest sporadically, but 30–70% of ET cases have a positive family history, suggesting the disease has a genetic background (3). Familial cases usually show early disease manifestation in the first two to four decades (4). The clinical hallmark of ET is a symmetric action tremor of the upper limbs (5). However, tremor may spread to other regions, such as the head, tongue, torso, jaw, and legs or can manifest as voice tremor. In some patients, signs of cerebellar impairment, such as subtle oculomotor disturbances and gait ataxia are present. Cognitive impairment and psychiatric symptoms, such as depression also can occur in ET patients (4). The term “essential tremor plus” has been coined for ET cases presenting with these additional symptoms (5). Given the heterogeneity of clinical manifestation, the variable hereditary background, and wide range of age at onset, it is likely that ET does not constitute a single disease entity, but rather a disease spectrum (4).
Despite its high prevalence, the neuronal mechanisms underpinning ET are still not fully understood. Originally, the inferior olive nucleus (ION) had been considered the central oscillator of tremor genesis in ET (6); however, this hypothesis has since been disputed, and a multiple oscillator tremor network comprising the ION, brainstem, cerebellum, thalamus, and motor cortical areas has been indicated in tremor genesis (7). Moreover, a series of histopathological studies reporting a loss and morphological alterations of cerebellar Purkinje cells gave rise to the hypothesis that cerebellar neurodegeneration may be the primary cause of ET (8–10). However, this concept has been challenged by others (11, 12). In addition, there is mounting evidence that alterations in the integrity of the inhibitory gamma-aminobutyric acid (GABA) neurotransmitter system is a contributory factor in ET pathophysiology (13). Lastly, particularly in the early course of the disease, clinical differentiation of ET from Parkinson's disease (PD) can be challenging, and some authors have suggested common pathophysiological features of the two diseases (14).
In recent decades, a substantial number of imaging techniques have emerged that enable the assessment of structural, functional, and metabolic alterations of the ET brain in a non-invasive and easily accessible way, resulting in a large body of literature. Whereas some findings corroborate with current concepts of ET pathophysiology (15–17), others do not (18, 19). More recently, novel techniques have been established to assess distinct neurotransmitter systems and their role in tremor genesis in vivo. Furthermore, studies exploring the dopaminergic system and cerebral iron depositions have tried to establish a connection between ET and progressive neurodegeneration, particularly with PD, providing equivocal findings (14). Here, we systematically review the advances in structural, functional, and metabolic imaging and discuss pathophysiological concepts underlying ET based on evidence from neuroimaging.
We conducted a PubMed and Scopus search, including publications from 1966 up to December 2020, entering “essential tremor” in combination (“AND”) with any of the following terms and their corresponding abbreviations: positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), gamma-aminobutyric, and γ-aminobutyric acid (GABA). In addition, we browsed the reference lists of original and review articles retrieved in this primary search. We only considered articles that were (1) written in English, (2) included >5 ET subjects, (3) directly compared ET subjects with a healthy control (HC) cohort, (4) were performed on human subjects, and (5) provided quantitative or semiquantitative data analyses. We did not consider case reports, case series, or research papers that primarily focused on therapeutic interventions, such as thalamotomy, MRI-guided focused ultrasound, or deep brain stimulation (DBS). If ET patients were additionally compared with other disease groups (e.g., dystonic tremor), we solely considered comparisons with HC. We followed the PRISMA guidelines for systematic reviews (20).
We sought to address the following questions: (1) does evidence from neuroimaging support the hypothesis of cerebellar neurodegeneration in ET? (2) Do findings from neuroimaging corroborate with the postulated concept of a tremor network? (3) Is there support from neuroimaging for alterations of the GABAergic system in ET? (4) Is there evidence from neuroimaging for striatal dopaminergic degradation and nigral iron accumulation in ET as typically observed in PD?
Our search revealed 1,135 hits. References retrieved were imported into a reference manager (Endnote X8), and duplicates were removed. FH screened all titles and abstracts for eligibility. A total of 86 papers met our inclusion criteria. Fifteen additional abstracts were identified by browsing the reference lists of papers retrieved in the database search. The senior author (JS) cross-checked papers selected for qualitative data synthesis for eligibility. A flowchart of the selection process is presented in Figure 1. Thirty-one studies were assigned to volumetric MRI, 19 to diffusion tensor imaging (DTI), 26 to functional MRI (fMRI), six to MRS, six to imaging of brain iron, three to GABAergic imaging, 17 to dopaminergic imaging, seven to perfusion imaging (PET or SPECT), and five to metabolic radiotracer imaging. Some studies applied more than one modality and were assigned to different categories accordingly. A summary of all studies included is presented in Table 1 (MRI and GABAergic imaging) and Table 2 (radiotracer imaging).
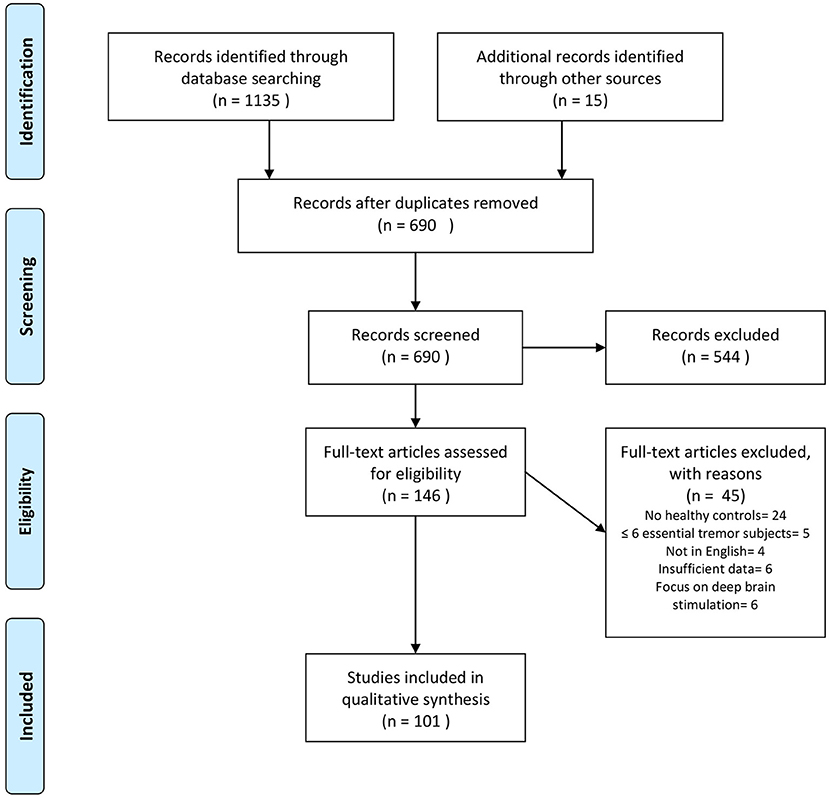
Figure 1. Flowchart depicting the study selection process. Search was performed in PubMed and Scopus up to December 2020. Modified from Moher et al. (20).
A number of imaging techniques have been applied to visualize the brain morphology of ET patients in vivo.
Voxel-based morphometry (VBM) allows for a voxel-based automated and rater-independent analysis of brain volumes between groups, either in specified regions-of-interest (ROI) or at a whole-brain level without a priori hypotheses (117). Alternatively, automated segmentation methods can be applied to quantitatively measure brain volumes, e.g., the cortical thickness, using freely available software, such as FreeSurfer (118).
Cerebellar atrophy is commonly reported in ET patients (15, 22, 26, 28–32, 36, 37, 43, 46, 48). However, an equivocal number of studies found no morphological cerebellar changes (18, 21, 25, 33–35, 38–42, 49), and even increased cerebellar gray matter volume in young ET subjects has been reported (27, 45). Findings of cerebral cortical and subcortical structural changes are even more heterogeneous. There is no consistent pattern of atrophy. Moreover, alongside volume loss, gray matter volume gain has been observed in various cortical regions, and some studies did not identify any cortical differences between ET patients and HC (15, 22, 23, 26, 33–36, 38, 41, 44, 45, 47). Of note, the clinical phenotype of ET is associated with distinct morphological brain changes. For example, ET patients presenting with additional head tremor display more pronounced or distinct patterns of cerebellar atrophy, as well as various cortical structural changes compared with classic ET (25, 26, 28, 30, 31, 46). Indeed, some of the studies reporting cerebellar atrophy found significant volume loss only in ET individuals exhibiting additional head or voice tremor (28, 30, 31, 46). Moreover, cognitive dysfunction in ET has been linked to specific cortical atrophy patterns not apparent in cognitively intact ET individuals (24). The heterogeneity of structural brain alterations reported in ET has been highlighted in a recent meta-analysis including 16 VBM studies and more than 350 ET individuals (119). The latter study did not identify any brain regions, including the cerebellum, that exhibited consistent gray matter volume loss in ET patients compared with HC (119).
DTI is utilized for the assessment of the brain's microstructural integrity and is particularly sensitive to alterations in cerebral white matter. DTI measures the random movement of water molecules, which is mainly directed along white matter fiber tracts (120). Two important measures are the mean diffusivity (MD) and the fractional anisotropy (FA). The MD depicts the average movement of water molecules in organic tissue, whereas the FA refers to the directionality of movement. FA values close to 1 reflect anisotropy, whereas values nearing 0 are isotropic and are suggestive of tissue damage. Conversely, high MD values are a surrogate for a loss of cellular integrity and indicative of neuronal damage (121).
Compared with conventional MRI, DTI studies more consistently point toward microstructural alterations of the cerebellum, particularly of the cerebellar peduncles and dentate nuclei (38, 49, 54, 55, 57, 59–61). In contrast to these 11 studies, only two studies, both employing an ROI-based approach, did not find any differences in DTI between ET patients and HC (19, 21). Beyond cerebellar changes, widespread microstructural alterations have been reported in various cerebral white matter tracts related to both motor and non-motor function and in the red nuclei (37, 38, 52, 53, 56, 59). For example, in a recent study by Revuelta et al., the authors reported decreased MD of fiber tracts connecting the ventral intermediate nucleus of the thalamus (Vim), the typical target for DBS in ET, with motor and supplementary motor cortical regions (58). Even though no alterations of FA were observed in the same tracts, both MD and FA in the Vim supplementary motor area tract correlated with tremor severity, suggesting a pathological reinforcement of this tract in ET (58). Similar to VBM studies, phenotype-specific changes of FA and MD have been reported. Specifically, ET patients presenting with additional resting tremor, but unremarkable dopamine transporter imaging, showed reduced structural connectivity in a network comprised of the globus pallidus, caudate nucleus, and supplemental motor area that was not apparent in ET patients without resting tremor (51). Moreover, distinct cortical microstructural changes, including the hippocampi, have been linked to cognitive dysfunction (41, 49, 50).
The initial model of ET tremor genesis proposed that rhythmic discharges originating in the ION propagate tremor in ET (6). However, based on current research, it seems more likely that tremor genesis is not governed by a single oscillator, but is rather driven by a number of oscillators within a tremor network comprising the ION, cerebellum, thalamus, motor cortical regions, and the brainstem (7). This hypothesis is supported by evidence from neurophysiological studies confirming abnormal oscillatory activity within the tremor network in ET (122).
fMRI measures the blood oxygen level-dependent (BOLD) contrast—generally called “the BOLD signal” (123). The BOLD signal is affected by hemodynamic, vascular, and metabolic factors, but is generally assumed to be closely related to neural activity (123, 124). The first task-based fMRI study in ET patients identified increased activity in the contralateral sensory and motor cortices, thalamus, and globus pallidus and bilateral overactivation of the cerebellar hemispheres and dentate nuclei during arm posturing. In contrast, the authors observed increased activity in the ION in only two out of 12 patients, supporting a pivotal role of the cerebellum in tremor genesis and refuting the single oscillator ION hypothesis (16). Subsequently, numerous task-based fMRI studies have confirmed that altered cerebellar and cerebello-thalamico-cortical activity is correlated to clinical tremor manifestation and task performance (36, 40, 62–64, 66, 67). One study did not report a significant difference in functional connectivity during an emotion processing and finger tapping task in ET patients compared with HC. However, in the latter study, HC were not age matched to the ET cohort (32). In line with the findings from structural imaging, cognitive function has been associated with specific activity changes outside the classical tremor network (65, 68).
More recently, neuronal activity has been assessed in the resting state. Resting-state fMRI is advantageous over task-based paradigms in that it is independent of individual variability in task performance and interference of tremor with motor function. The most consistent finding reported by these studies was altered intrinsic cerebellar and cerebello-thalamo-cortical activation/connectivity, particularly of cerebello-motor cortical projections (33–35, 37, 61, 71–75, 77). There is also evidence that complex functional alterations outside the classical tremor axis are present in ET, including visual networks (69, 70). In this vein, Archer et al. have demonstrated that activity within the tremor and visual networks during a grip motor task could be modulated by visual feedback (21).
Of note, surgical interventions to treat ET, such as Vim-DBS or thalamotomy have been shown to restore connectivity in the tremor network partially and to cause widespread remodeling of other brain networks outside the classical tremor axis [e.g., (125, 126)]. In line with observations from structural MRI, the clinical phenotype appears to be associated with distinct functional brain changes. For example, ET individuals exhibiting head tremor showed distinct cerebellar activity compared with those who did not (76), and ET patients with resting tremor showed different activation patterns of various cortical and subcortical brain regions compared with classical ET (73).
MRS is utilized to assess neurometabolic alterations in brain tissue in vivo. N-acetylaspartate (NAA) is an abundant amino acid derivative synthesized in neurons. A reduction of NAA, therefore, is indicative of neuronal damage. The choline (Cho) fraction comprises several soluble components mainly located in myelin and cell membranes. Conditions resulting in increased turnover or damage to cellular membranes and myelin, such as inflammation, tumor, or neurodegenerative processes, result in increased Cho concentrations. Creatine (Cr) is found in most neurons and astrocytes. The Cr peak is very robust, which is why Cr is commonly used as a denominator to offset changes of NAA and Cho (127). MRS can also be applied to measure GABA (please see below).
Few studies have exploited MRS to investigate neurometabolite changes in ET. Louis et al. were the first to report a reduced NAA/Cr ratio in the cerebellum of 16 ET patients compared with 11 HC that was inversely correlated to tremor severity (81). The same group later found that NAA/Cr changes were similar between cerebellar hemispheres, in accordance with the symmetric clinical manifestation of ET (82). Similarly, reduced NAA/Cr ratios have been reported by Pagan et al. in a small cohort of 10 ET patients (83). That said, others found normal NAA/Cr ratios in the thalami of ET patients (78–80), whereas there was an increase in the excitatory neurotransmitter glutamate/glutamine evident in one of these studies (79).
The role of GABA in ET has been a topic of ongoing discussion for many years (13), and different lines of research have vindicated the significance of the GABAergic system in ET pathophysiology.
ET patients show lower GABA levels in the cerebrospinal fluid (128), and GABA receptor density in the cerebellar dentate nucleus has been reported to be reduced (129). Alcohol has agonistic GABAergic properties and alleviates tremor in many patients with ET (130), and the majority of drugs used to treat ET act via GABAergic pathways (131). Moreover, GABA-A1 receptor knockout mice develop an ET-like disease that responds to GABAergic drugs commonly used to treat ET (132). The impact of reduced cerebellar GABAergic tone on neuronal activity in cerebello-thalamico-cortical tremor network activity has also been highlighted in a recent study applying a complex computational simulation model of ET (133).
Very few in vivo imaging studies have explored the role of GABA in ET. Using PET and 11C-flumazenil, a ligand of the benzodiazepine site of the GABA receptor, Boecker et al. observed increased cerebellar, thalamic, and premotor cortical tracer uptake in a small cohort of ET patients compared with HC, hinting at reduced GABAergic function (90). In contrast, a separate study employing MRS failed to demonstrate a significant difference in GABA concentration in the dentate nuclei between ET individuals and HC (91). Another MRS study reported a positive correlation of the cerebellar GABA/glutamate + glutamine ratio with clinical tremor scores in a small ET cohort. However, neither GABA nor glutamate/glutamine levels differed between ET patients and HC in the latter study (92). Given the limited number of available imaging studies focused on GABA, we would like to mention the work by Gironell et al. even though their study did not meet our inclusion criteria; they found a significant correlation of cerebellar 11C-flumazenil uptake and tremor severity in a cohort of 10 ET patients (134).
Different MRI techniques, such as susceptibility-weighted imaging, T2*-weighted, or its inverse R2*-weighted gradient echo imaging, can be used to measure brain iron (135). More recently, novel methods, such as neuromelanin and nigrosome-1 imaging have been developed to visualize the neuronal integrity of the substantia nigra (136). These techniques have been extensively used to detect iron depositions, which are assumed to be a surrogate of cellular damage in neurodegenerative disorders, such as PD (137).
In one study comparing 25 ET patients with 25 matched HC, no significant difference was found in the R2* relaxation times of the substantia nigra (85). Similarly, three other studies reported normal nigral neuromelanin concentrations in ET patients (86, 88, 89), and nigral nigrosome-1 integrity has been found to be comparable with that of HC (84, 86). The focus of all these studies was on the substantia nigra, and only one study applied a whole-brain voxel-based approach (87). The authors reported increased iron levels in the bilateral pallidum, substantia nigra, and the right dentate nucleus. That being said, only the pallidal iron increase survived correction for multiple comparisons and was correlated to tremor severity (87).
Alongside MRI, PET and SPECT have been applied using a variety of tracers to study the integrity of the dopaminergic axis, brain perfusion, and metabolism in ET.
Epidemiological studies suggest that ET populations have an increased risk of developing PD, and there is an ongoing controversy about a potential pathophysiological overlap between the two diseases (14).
FP-CIT SPECT (commercially known as DaTScan) is commonly used to assess the presynaptic striatal dopaminergic integrity (138). Striatal tracer uptake is significantly reduced in typical and atypical parkinsonism (138).
Most studies using FP-CIT SPECT or comparable techniques found no alterations of the dopaminergic system in ET (51, 78, 93–96, 100, 102–105, 107, 139). These findings were extended by two longitudinal studies showing constant tracer uptake over time (98, 100). Of note, a third longitudinal study not meeting our inclusion criteria did not reveal a decline of striatal dopamine transporter availability over a mean follow-up period of 28 months (140). One study reported normal DaTScan in classical ET patients, whereas tracer uptake was reduced in ET patients with additional resting tremor (101). However, resting tremor ET individuals were about 7 years older than the corresponding HC, and several subjects presented with subtle parkinsonian features, raising the question of whether they may have subsequently developed PD. Conversely, others have found normal striatal dopaminergic integrity in ET patients with resting tremor (102). That being said, some authors reported signs of slight striatal dopaminergic degradation in classical ET (97–99, 106). Of note, ET patients may show reductions of tracer uptake primarily in the caudate nucleus, contrasting the typical pattern of pronounced posterior putamenal reductions observed in PD (99, 106).
A series of small exploratory PET studies conducted in the 1990s, mostly using 15O-labeled H2O and PET, revealed increased regional cerebellar blood flow (rCBF) during both rest and posture in ET patients compared with HC (17, 109, 110). These studies showed overactivation of additional regions comprising the tremor network, whereas olivary overactivation was not present in any of these studies (17, 109, 110). Boecker et al. demonstrated that abnormally increased cerebellar rCBF decreased toward normal values after ingestion of ethanol, and this decrease was correlated to concurrent tremor alleviation (108). Furthermore, there was an increase in ION activation following ethanol ingestion, suggesting increased afferent olivary input as a consequence of normalizing synaptic cerebellar activity (108). More recently, SPECT and 99mTc-hexamethylpropylenaminoxom (HMPAO) have been used to measure rCBF in ET cohorts. Sahin et al. did not observe any difference of rCBF between 16 ET patients and matched HC, but reported an inverse correlation of frontal cortical rCBF with tremor severity (111). Employing the same method, Song et al. found no significant differences in rCBF between ET patients with and without head tremor (113). Interestingly, rCBF was reduced in various brain regions, including the cerebellum, in the overall ET cohort compared with HC in the latter and in a subsequent study conducted by the same group (112, 113).
18F-fluorodeoxyglucose (FDG) and PET can be used to quantitatively assess brain glucose consumption, which is largely equivalent to neuronal activity (141). FDG PET has been extensively used to characterize metabolic brain abnormalities in neurodegenerative disorders, such as PD, and has revealed disease-specific abnormal brain networks that correlate with disease severity and can discriminate PD from atypical parkinsonism (142).
Hallett and Dubisnky were among the first to report increased brainstem and thalamic activity in ET patients, whereas they did not observe significant changes in cerebellar metabolism (114). Similarly, two recently published studies did not find changes in cerebellar metabolism, but widespread cortical hypometabolism was reported in one of these studies (105, 115). In contrast, Song et al. found cerebellar hypometabolism accompanied by reduced tracer uptake in various cortical regions (116). In yet another study using an ROI-based approach, no metabolic differences were identified in ET patients compared with controls in the basal ganglia (95).
Whereas there is relatively little support from neuroimaging for the hypothesis that the ION is the primary oscillator of abnormal neuronal activity, there is robust evidence indicating that the cerebellum plays a major role in ET pathophysiology. Findings from volumetric MRI studies are, however, heterogeneous, and VBM studies do not unequivocally corroborate with histopathological findings of cerebellar neurodegeneration. Importantly, the topography of cerebellar regions displaying atrophy is inconsistent across studies, countering arguments in favor of a uniform pattern of cerebellar cell loss. DTI studies have more consistently revealed microstructural alterations of the cerebellum, and fMRI studies have clearly demonstrated abnormal cerebellar function and altered connectivity in cerebello-thalamico-cortical circuitry. Along these lines, radiotracer studies have shown increased cerebellar rCBF in ET patients, further underpinning a pivotal role of this structure in tremor genesis. That said, in view of the widespread functional alterations reported, it is likely that the cerebellum is not the sole driver of tremor genesis but rather constitutes a major hub within a multiple oscillator tremor network, thus validating neurophysiological data (122).
Findings from FDG PET studies are ambiguous. Some studies have reported extensive cortical hypometabolism, and there is evidence for increased thalamic activity. However, other studies have reported opposing results, and in particular, there are conflicting findings with respect to cerebellar metabolic activity. Data from MRS studies are scarce and insufficient to draw firm conclusions. However, the few available studies provide some evidence for thalamic and cerebellar neuronal damage.
The dopaminergic axis appears to be largely preserved in ET. This is also illustrated by the fact that DaTScan has been certified for use in the differentiation of PD from ET by both the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA), and some studies on PD even use ET cohorts as “normal” controls [e.g., (143)]. That being said, some authors have suggested slightly reduced striatal dopaminergic integrity in ET subjects that does not, however, seem to decline over time.
With the exception of one study, there is no evidence for pathological nigral iron accumulation as typically observed in PD. However, only one study did not apply an ROI-based approach limited to the substantia nigra, and this study did observe a significant increase of iron accumulation in the bilateral globus pallidus internus. Therefore, it seems that there is no relevant nigral iron accumulation in ET, but this could well be the case for other brain regions not commonly assessed by imaging studies thus far, arguing in favor of neurodegenerative processes.
Finally, MRS and radiotracer studies lend some support to the hypothesis that dysfunction of the GABAergic system is involved in ET pathophysiology. It remains to be elucidated whether the reduced GABAergic tone is a primary contributing factor to ET pathophysiology or rather a consequence of disturbed Purkinje cell function or even cell death.
Based on the epidemiological, genetic, and clinical heterogeneity, it is likely that no single ET entity exists, but rather an ET spectrum. This is supported by the notion that clinical phenotype, e.g., the distribution of tremor manifestation, the presence of cognitive impairment, or resting tremor, is linked to specific functional and structural brain changes. A summary of the main findings reported in this review is depicted in Figure 2.
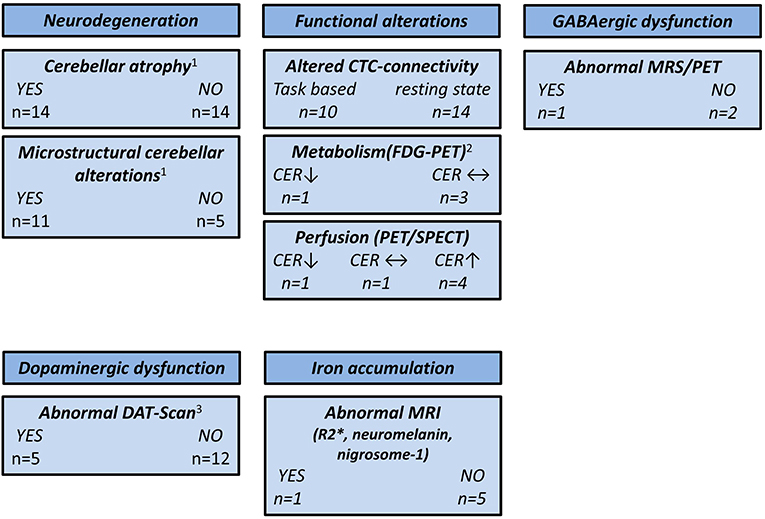
Figure 2. Overview of neuroimaging studies in essential tremor. CER, cerebellum; CTC, cerebello-thalamico-cortical; DAT, dopamine transporter; FDG, 18F-fluorodeoxyglucose; GABA, γ-aminobutyric acid; MRS, magnetic resonance spectroscopy; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography; ↑, higher compared with the healthy controls; ↓, lower compared with the healthy controls; ↔, no difference compared with the healthy controls. 1CER not assessed by three studies. 2CER not assessed by one study. 3One study used 11C-CFT PET and two studies used [99mTc]-TRODAT SPECT.
Taking genetic background (familiar vs. sporadic), age at onset, disease duration, therapeutic responsiveness, and clinical phenotype (e.g., presence of head tremor, symptoms of “ET plus”) into account is important when studying ET populations, but these factors have not been consistently implemented in study designs so far. There are additional issues likely contributing to the heterogeneous findings from neuroimaging studies, such as limited sample size (this is particularly true for PET and SPECT studies), subject age (the mean age of ET cohorts included in this review ranged from 28 to 74 years), nature of the analytical approach (e.g., ROI-based vs. whole-brain analysis, statistical threshold applied), and the different field strengths of the MRI scanners. Moreover, several groups have published multiple papers on related topics using similar cohorts or did not specify if there was an overlap of cohorts across their studies, a potential source of reporting bias.
It is desirable that future studies more rigorously focus on the demographical, genetic, and clinical heterogeneity of ET. Multimodal imaging, including the simultaneous assessments of MRI, PET, and electroencephalography, may shed further light on the complex neuronal alterations underlying ET. Furthermore, the much higher spatial resolution of ultra-high field MRI may enable researchers to solve the remaining controversy of whether cerebellar neurodegeneration is the pathological foundation of ET.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
FH: study design, data collection and analysis, and drafting of the manuscript. NS: study design, revision of the manuscript, and supervision. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. (2010) 25:534–41. doi: 10.1002/mds.22838
2. Louis ED. The roles of age and aging in essential tremor: an epidemiological perspective. Neuroepidemiology. (2019) 52:111–8. doi: 10.1159/000492831
3. Louis ED, Dogu O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology. (2007) 29:208–12. doi: 10.1159/000111584
4. Hopfner F, Deuschl G. Is essential tremor a single entity? Eur J Neurol. (2018) 25:71–82. doi: 10.1111/ene.13454
5. Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. (2018) 33:75–87. doi: 10.1002/mds.27121
6. Louis ED, Lenka A. The olivary hypothesis of essential tremor: time to lay this model to rest? Tremor Other Hyperkinet Mov (N Y). (2017) 7:473. doi: 10.7916/D8FF40RX
7. Raethjen J, Deuschl G. The oscillating central network of essential tremor. Clin Neurophysiol. (2012) 123:61–4. doi: 10.1016/j.clinph.2011.09.024
8. Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. (2008) 70:1452–5. doi: 10.1212/01.wnl.0000310425.76205.02
9. Louis ED, Lee M, Babij R, Ma K, Cortes E, Vonsattel JP, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. (2014) 137:3142–8. doi: 10.1093/brain/awu314
10. Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. (2008) 65:101–7. doi: 10.1001/archneurol.2007.8
11. Rajput AH, Robinson CA, Rajput A. Purkinje cell loss is neither pathological basis nor characteristic of essential tremor. Parkinsonism Relat Disord. (2013) 19:490–1. doi: 10.1016/j.parkreldis.2012.11.019
12. Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. (2014) 29:496–500. doi: 10.1002/mds.25845
13. Gironell A. The GABA hypothesis in essential tremor: lights and shadows. Tremor Other Hyperkinet Mov (N Y). (2014) 4:254. doi: 10.7916/d8sf2t9c
14. Tarakad A, Jankovic J. Essential tremor and Parkinson's disease: exploring the relationship. Tremor Other Hyperkinet Mov (N Y). (2018) 8:589. doi: 10.7916/D8MD0GVR
15. Benito-León J, Alvarez-Linera J, Hernández-Tamames JA, Alonso-Navarro H, Jiménez-Jiménez FJ, Louis ED. Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurol Sci. (2009) 287:138–42. doi: 10.1016/j.jns.2009.08.037
16. Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. (1997) 41:32–40. doi: 10.1002/ana.410410108
17. Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. (1994) 36:636–42. doi: 10.1002/ana.410360413
18. Daniels C, Peller M, Wolff S, Alfke K, Witt K, Gaser C, et al. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology. (2006) 67:1452–6. doi: 10.1212/01.wnl.0000240130.94408.99
19. Martinelli P, Rizzo G, Manners D, Tonon C, Pizza F, Testa C, et al. Diffusion-weighted imaging study of patients with essential tremor. Mov Disord. (2007) 22:1182–5. doi: 10.1002/mds.21287
20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Archer DB, Coombes SA, Chu WT, Chung JW, Burciu RG, Okun MS, et al. A widespread visually-sensitive functional network relates to symptoms in essential tremor. Brain. (2018) 141:472–85. doi: 10.1093/brain/awx338
22. Bagepally BS, Bhatt MD, Chandran V, Saini J, Bharath RD, Vasudev MK, et al. Decrease in cerebral and cerebellar gray matter in essential tremor: a voxel-based morphometric analysis under 3T MRI. J Neuroimaging. (2012) 22:275–8. doi: 10.1111/j.1552-6569.2011.00598.x
23. Benito-León J, Serrano JI, Louis ED, Holobar A, Romero JP, Povalej-BrŽan P, et al. Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Ann Clin Transl Neurol. (2019) 6:83–97. doi: 10.1002/acn3.681
24. Bhalsing KS, Upadhyay N, Kumar KJ, Saini J, Yadav R, Gupta AK, et al. Association between cortical volume loss and cognitive impairments in essential tremor. Eur J Neurol. (2014) 21:874–83. doi: 10.1111/ene.12399
25. Buijink AW, Broersma M, van der Stouwe AM, Sharifi S, Tijssen MA, Speelman JD, et al. Cerebellar atrophy in cortical myoclonic tremor and not in hereditary essential tremor–a voxel-based morphometry study. Cerebellum. (2016) 15:696–704. doi: 10.1007/s12311-015-0734-0
26. Cameron E, Dyke JP, Hernandez N, Louis ED, Dydak U. Cerebral gray matter volume losses in essential tremor: A case-control study using high resolution tissue probability maps. Parkinsonism Relat Disord. (2018) 51:85–90. doi: 10.1016/j.parkreldis.2018.03.008
27. Cao H, Wang R, Luo X, Li X, Hallett M, Thompson-Westra J, et al. A voxel-based magnetic resonance imaging morphometric study of cerebral and cerebellar gray matter in patients under 65 years with essential tremor. Med Sci Monit. (2018) 24:3127–35. doi: 10.12659/msm.906437
28. Cerasa A, Messina D, Nicoletti G, Novellino F, Lanza P, Condino F, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. Am J Neuroradiol. (2009) 30:1240–3. doi: 10.3174/ajnr.A1544
29. Cerasa A, Nistico R, Salsone M, Bono F, Salvino D, Morelli M, et al. Neuroanatomical correlates of dystonic tremor: a cross-sectional study. Parkinsonism Relat Disord. (2014) 20:314–7. doi: 10.1016/j.parkreldis.2013.12.007
30. Choi SM, Kim BC, Chang J, Choi KH, Nam TS, Kim JT, et al. Comparison of the brain volume in essential tremor and Parkinson's disease tremor using an automated segmentation method. Eur Neurol. (2015) 73:303–9. doi: 10.1159/000381708
31. Dyke JP, Cameron E, Hernandez N, Dydak U, Louis ED. Gray matter density loss in essential tremor: a lobule by lobule analysis of the cerebellum. Cereb Ataxias. (2017) 4:10. doi: 10.1186/s40673-017-0069-3
32. Espay AJ, Maloney T, Vannest J, Norris MM, Eliassen JC, Neefus E, et al. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin. (2018) 17:179–87. doi: 10.1016/j.nicl.2017.10.020
33. Fang W, Lv F, Luo T, Cheng O, Liao W, Sheng K, et al. Abnormal regional homogeneity in patients with essential tremor revealed by resting-state functional MRI. PLoS ONE. (2013) 8:e69199. doi: 10.1371/journal.pone.0069199
34. Fang W, Chen H, Wang H, Zhang H, Liu M, Puneet M, et al. Multiple resting-state networks are associated with tremors and cognitive features in essential tremor. Mov Disord. (2015) 30:1926–36. doi: 10.1002/mds.26375
35. Fang W, Chen H, Wang H, Zhang H, Puneet M, Liu M, et al. Essential tremor is associated with disruption of functional connectivity in the ventral intermediate nucleus–motor cortex–cerebellum circuit. Hum Brain Mapp. (2016) 37:165–78. doi: 10.1002/hbm.23024
36. Boscolo Galazzo I, Magrinelli F, Pizzini FB, Storti SF, Agosta F, Filippi M, et al. Voxel-based morphometry and task functional magnetic resonance imaging in essential tremor: evidence for a disrupted brain network. Sci Rep. (2020) 10:15061. doi: 10.1038/s41598-020-69514-w
37. Gallea C, Popa T, García-Lorenzo D, Valabregue R, Legrand AP, Marais L, et al. Intrinsic signature of essential tremor in the cerebello-frontal network. Brain. (2015) 138:2920–33. doi: 10.1093/brain/awv171
38. Klein JC, Lorenz B, Kang JS, Baudrexel S, Seifried C, van de Loo S, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp. (2011) 32:896–904. doi: 10.1002/hbm.21077
39. Lin CH, Chen CM, Lu MK, Tsai CH, Chiou JC, Liao JR, et al. VBM reveals brain volume differences between Parkinson's disease and essential tremor patients. Front Hum Neurosci. (2013) 7:247. doi: 10.3389/fnhum.2013.00247
40. Nicoletti V, Cecchi P, Frosini D, Pesaresi I, Fabbri S, Diciotti S, et al. Morphometric and functional MRI changes in essential tremor with and without resting tremor. J Neurol. (2015) 262:719–28. doi: 10.1007/s00415-014-7626-y
41. Pelzer EA, Nelles C, Pedrosa DJ, Eggers C, Burghaus L, Melzer C, et al. Structural differences in impaired verbal fluency in essential tremor patients compared to healthy controls. Brain Behav. (2017) 7:e00722. doi: 10.1002/brb3.722
42. Pietracupa S, Bologna M, Bharti K, Pasqua G, Tommasin S, Elifani F, et al. White matter rather than gray matter damage characterizes essential tremor. Eur Radiol. (2019) 29:6634–42. doi: 10.1007/s00330-019-06267-9
43. Prasad S, Pandey U, Saini J, Ingalhalikar M, Pal PK. Atrophy of cerebellar peduncles in essential tremor: a machine learning-based volumetric analysis. Eur Radiol. (2019) 29:7037–46. doi: 10.1007/s00330-019-06269-7
44. Prasad S, Shah A, Bhalsing KS, Ingalhalikar M, Saini J, Pal PK. Clinical correlates of abnormal subcortical volumes in essential tremor. J Neural Transm (Vienna). (2019) 126:569–76. doi: 10.1007/s00702-019-02004-0
45. Qi S, Cao H, Wang R, Jian Z, Bian Y, Yang J. Relative increase in cerebellar gray matter in young onset essential tremor: evidence from voxel-based morphometry analysis. J Clin Neurosci. (2020) 79:251–6. doi: 10.1016/j.jocn.2020.07.003
46. Quattrone A, Cerasa A, Messina D, Nicoletti G, Hagberg GE, Lemieux L, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. Am J Neuroradiol. (2008) 29:1692–7. doi: 10.3174/ajnr.A1190
47. Serrano JI, Romero JP, Castillo MDD, Rocon E, Louis ED, Benito-Leon J. A data mining approach using cortical thickness for diagnosis and characterization of essential tremor. Sci Rep. (2017) 7:2190. doi: 10.1038/s41598-017-02122-3
48. Shin H, Lee DK, Lee JM, Huh YE, Youn J, Louis ED, et al. Atrophy of the cerebellar vermis in essential tremor: segmental volumetric MRI analysis. Cerebellum. (2016) 15:174–81. doi: 10.1007/s12311-015-0682-8
49. Novellino F, Vasta R, Saccà V, Nisticò R, Morelli M, Arabia G, et al. Hippocampal impairment in patients with essential tremor. Parkinsonism Relat Disord. (2020) 72:56–61. doi: 10.1016/j.parkreldis.2020.02.006
50. Bhalsing KS, Kumar KJ, Saini J, Yadav R, Gupta AK, Pal PK. White matter correlates of cognitive impairment in essential tremor. Am J Neuroradiol. (2015) 36:448–53. doi: 10.3174/ajnr.A4138
51. Caligiuri ME, Arabia G, Barbagallo G, Lupo A, Morelli M, Nisticò R, et al. Structural connectivity differences in essential tremor with and without resting tremor. J Neurol. (2017) 264:1865–74. doi: 10.1007/s00415-017-8553-5
52. Jia L, Jia-Lin S, Qin D, Qing L, Yan Z. A diffusion tensor imaging study in essential tremor. J Neuroimaging. (2011) 21:370–4. doi: 10.1111/j.1552-6569.2010.00535.x
53. Nestrasil I, Svatkova A, Rudser KD, Chityala R, Wakumoto A, Mueller BA, et al. White matter measures correlate with essential tremor severity–a pilot diffusion tensor imaging study. Brain Behav. (2018) 8:e01039. doi: 10.1002/brb3.1039
54. Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. (2010) 74:988–94. doi: 10.1212/WNL.0b013e3181d5a460
55. Novellino F, Nicoletti G, Cherubini A, Caligiuri ME, Nisticò R, Salsone M, et al. Cerebellar involvement in essential tremor with and without resting tremor: a diffusion tensor imaging study. Parkinsonism Relat Disord. (2016) 27:61–6. doi: 10.1016/j.parkreldis.2016.03.022
56. TantIk Pak A, SengUl Y, Otcu Temur H, Alkan A. Impaired integrity of commissural and association fibers in essential tremor patients: evidence from a diffusion tensor imaging study. Turk J Med Sci. (2020) 51:328–34. doi: 10.3906/sag-2004-305
57. Prasad S, Rastogi B, Shah A, Bhalsing KS, Ingalhalikar M, Saini J, et al. DTI in essential tremor with and without rest tremor: two sides of the same coin? Mov Disord. (2018) 33:1820–1. doi: 10.1002/mds.27459
58. Revuelta G, McGill C, Jensen JH, Bonilha L. Characterizing thalamo-cortical structural connectivity in essential tremor with diffusional kurtosis imaging tractography. Tremor Other Hyperkinet Mov (N Y). (2019) 9. doi: 10.7916/tohm.v0.690
59. Saini J, Bagepally BS, Bhatt MD, Chandran V, Bharath RD, Prasad C, et al. Diffusion tensor imaging: tract based spatial statistics study in essential tremor. Parkinsonism Relat Disord. (2012) 18:477–82. doi: 10.1016/j.parkreldis.2012.01.006
60. Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. Am J Neuroradiol. (2008) 29:151–3. doi: 10.3174/ajnr.A0744
61. Tikoo S, Pietracupa S, Tommasin S, Bologna M, Petsas N, Bharti K, et al. Functional disconnection of the dentate nucleus in essential tremor. J Neurol. (2020) 267:1358–67. doi: 10.1007/s00415-020-09711-9
62. Broersma M, van der Stouwe AMM, Buijink AWG, de Jong BM, Groot PFC, Speelman JD, et al. Bilateral cerebellar activation in unilaterally challenged essential tremor. Neuroimage Clin. (2016) 11:1–9. doi: 10.1016/j.nicl.2015.12.011
63. Buijink AW, Broersma M, van der Stouwe AM, van Wingen GA, Groot PF, Speelman JD, et al. Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor. Parkinsonism Relat Disord. (2015) 21:383–8. doi: 10.1016/j.parkreldis.2015.02.003
64. Buijink AW, van der Stouwe AM, Broersma M, Sharifi S, Groot PF, Speelman JD, et al. Motor network disruption in essential tremor: a functional and effective connectivity study. Brain. (2015) 138:2934–47. doi: 10.1093/brain/awv225
65. Cerasa A, Passamonti L, Novellino F, Salsone M, Gioia MC, Morelli M, et al. Fronto-parietal overactivation in patients with essential tremor during Stroop task. Neuroreport. (2010) 21:148–51. doi: 10.1097/WNR.0b013e328335b42c
66. Muthuraman M, Raethjen J, Koirala N, Anwar AR, Mideksa KG, Elble R, et al. Cerebello-cortical network fingerprints differ between essential, Parkinson's and mimicked tremors. Brain. (2018) 141:1770–81. doi: 10.1093/brain/awy098
67. Neely KA, Kurani AS, Shukla P, Planetta PJ, Wagle Shukla A, Goldman JG, et al. Functional brain activity relates to 0–3 and 3–8 Hz force oscillations in essential tremor. Cereb Cortex. (2015) 25:4191–202. doi: 10.1093/cercor/bhu142
68. Passamonti L, Novellino F, Cerasa A, Chiriaco C, Rocca F, Matina MS, et al. Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain. (2011) 134:2274–86. doi: 10.1093/brain/awr164
69. Benito-León J, Louis ED, Romero JP, Hernández-Tamames JA, Manzanedo E, Álvarez-Linera J, et al. Altered functional connectivity in essential tremor: a resting-state fMRI study. Medicine. (2015) 94:e1936. doi: 10.1097/md.0000000000001936
70. Benito-León J, Sanz-Morales E, Melero H, Louis ED, Romero JP, Rocon E, et al. Graph theory analysis of resting-state functional magnetic resonance imaging in essential tremor. Hum Brain Mapp. (2019) 40:4686–702. doi: 10.1002/hbm.24730
71. Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, et al. Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology. (2017) 59:157–68. doi: 10.1007/s00234-016-1771-1
72. Li JY, Lu ZJ, Suo XL, Li NN, Lei D, Wang L, et al. Patterns of intrinsic brain activity in essential tremor with resting tremor and tremor-dominant Parkinson's disease. Brain Imaging Behav. (2020) 14:2606–17. doi: 10.1007/s11682-019-00214-4
73. Li JY, Suo XL, Li NN, Lei D, Lu ZJ, Wang L, et al. Altered spontaneous brain activity in essential tremor with and without resting tremor: a resting-state fMRI study. Magma. (2020) 34:201–12. doi: 10.1007/s10334-020-00865-1
74. Mueller K, Jech R, Hoskovcová M, Ulmanová O, Urgošík D, Vymazal J, et al. General and selective brain connectivity alterations in essential tremor: a resting state fMRI study. Neuroimage Clin. (2017) 16:468–76. doi: 10.1016/j.nicl.2017.06.004
75. Nicoletti V, Cecchi P, Pesaresi I, Frosini D, Cosottini M, Ceravolo R. Cerebello-thalamo-cortical network is intrinsically altered in essential tremor: evidence from a resting state functional MRI study. Sci Rep. (2020) 10:16661. doi: 10.1038/s41598-020-73714-9
76. Wang L, Lei D, Suo X, Li N, Lu Z, Li J, et al. Resting-state fMRI study on drug-naive patients of essential tremor with and without head tremor. Sci Rep. (2018) 8:10580. doi: 10.1038/s41598-018-28778-z
77. Yin W, Lin W, Li W, Qian S, Mou X. Resting state fMRI demonstrates a disturbance of the cerebello-cortical circuit in essential tremor. Brain Topogr. (2016) 29:412–8. doi: 10.1007/s10548-016-0474-6
78. Barbagallo G, Arabia G, Morelli M, Nistico R, Novellino F, Salsone M, et al. Thalamic neurometabolic alterations in tremulous Parkinson's disease: a preliminary proton MR spectroscopy study. Parkinsonism Relat Disord. (2017) 43:78–84. doi: 10.1016/j.parkreldis.2017.07.028
79. Barbagallo G, Arabia G, Novellino F, Nistico R, Salsone M, Morelli M, et al. Increased glutamate + glutamine levels in the thalamus of patients with essential tremor: a preliminary proton MR spectroscopic study. Parkinsonism Relat Disord. (2018) 47:57–63. doi: 10.1016/j.parkreldis.2017.11.345
80. Kendi AT, Tan FU, Kendi M, Erdal HH, Tellioglu S. Magnetic resonance spectroscopy of the thalamus in essential tremor patients. J Neuroimag. (2005) 15:362–6. doi: 10.1177/1051228405279039
81. Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. (2002) 333:17–20. doi: 10.1016/s0304-3940(02)00966-7
82. Louis ED, Shungu DC, Mao X, Chan S, Jurewicz EC. Cerebellar metabolic symmetry in essential tremor studied with 1H magnetic resonance spectroscopic imaging: implications for disease pathology. Mov Disord. (2004) 19:672–7. doi: 10.1002/mds.20019
83. Pagan FL, Butman JA, Dambrosia JM, Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. (2003) 60:1344–7. doi: 10.1212/01.wnl.0000065885.15875.0d
84. Cheng Z, He N, Huang P, Li Y, Tang R, Sethi SK, et al. Imaging the Nigrosome 1 in the substantia nigra using susceptibility weighted imaging and quantitative susceptibility mapping: an application to Parkinson's disease. Neuroimage Clin. (2020) 25:102103. doi: 10.1016/j.nicl.2019.102103
85. Homayoon N, Pirpamer L, Franthal S, Katschnig-Winter P, Kogl M, Seiler S, et al. Nigral iron deposition in common tremor disorders. Mov Disord. (2019) 34:129–32. doi: 10.1002/mds.27549
86. Jin L, Wang J, Wang C, Lian D, Zhou Y, Zhang Y, et al. Combined visualization of nigrosome-1 and neuromelanin in the substantia nigra using 3T MRI for the differential diagnosis of essential tremor and de novo Parkinson's disease. Front Neurol. (2019) 10:100. doi: 10.3389/fneur.2019.00100
87. Novellino F, Cherubini A, Chiriaco C, Morelli M, Salsone M, Arabia G, et al. Brain iron deposition in essential tremor: a quantitative 3-Tesla magnetic resonance imaging study. Mov Disord. (2013) 28:196–200. doi: 10.1002/mds.25263
88. Reimão S, Pita Lobo P, Neutel D, Guedes LC, Coelho M, Rosa MM, et al. Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson's disease. Mov Disord. (2015) 30:953–9. doi: 10.1002/mds.26182
89. Wang J, Huang Z, Li Y, Ye F, Wang C, Zhang Y, et al. Neuromelanin-sensitive MRI of the substantia nigra: an imaging biomarker to differentiate essential tremor from tremor-dominant Parkinson's disease. Parkinsonism Relat Disord. (2019) 58:3–8. doi: 10.1016/j.parkreldis.2018.07.007
90. Boecker H, Weindl A, Brooks DJ, Ceballos-Baumann AO, Liedtke C, Miederer M, et al. GABAergic dysfunction in essential tremor: an 11C-flumazenil PET study. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. (2010) 51:1030–5. doi: 10.2967/jnumed.109.074120
91. Louis ED, Hernandez N, Dyke JP, Ma RE, Dydak U. In vivo dentate nucleus gamma-aminobutyric acid concentration in essential tremor vs. controls. Cerebellum. (2018) 17:165–72. doi: 10.1007/s12311-017-0891-4
92. Tapper S, Göransson N, Lundberg P, Tisell A, Zsigmond P. A pilot study of essential tremor: cerebellar GABA+/Glx ratio is correlated with tremor severity. Cereb Ataxias. (2020) 7:8. doi: 10.1186/s40673-020-00116-y
93. Asenbaum S, Pirker W, Angelberger P, Bencsits G, Pruckmayer M, Brucke T. [123I]beta-CIT and SPECT in essential tremor and Parkinson's disease. J Neural Transm (Vienna). (1998) 105:1213–28. doi: 10.1007/s007020050124
94. Benamer HTS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [(123) I]-FP-CIT SPECT imaging: The [(123) I]-FP-CIT study group. Mov Disord. (2000) 15:503–10. doi: 10.1002/1531-8257(200005)15:3<503::AID-MDS1013>3.0.CO;2-V
95. Breit S, Reimold M, Reischl G, Klockgether T, Wüllner U. [(11)C]d-threo-methylphenidate PET in patients with Parkinson's disease and essential tremor. J Neural Transm (Vienna). (2006) 113:187–93. doi: 10.1007/s00702-005-0311-7
96. Di Giuda D, Camardese G, Bentivoglio AR, Cocciolillo F, Guidubaldi A, Pucci L, et al. Dopaminergic dysfunction and psychiatric symptoms in movement disorders: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging. (2012) 39:1937–48. doi: 10.1007/s00259-012-2232-7
97. Fang YH, Chiu SC, Lu CS, Yen TC, Weng YH. Fully automated quantification of the striatal uptake ratio of [(99m)Tc]-TRODAT with SPECT imaging: evaluation of the diagnostic performance in Parkinson's disease and the temporal regression of striatal tracer uptake. Biomed Res Int. (2015) 2015:461625. doi: 10.1155/2015/461625
98. Gerasimou G, Costa DC, Papanastasiou E, Bostanjiopoulou S, Arnaoutoglou M, Moralidis E, et al. SPECT study with I-123-Ioflupane (DaTSCAN) in patients with essential tremor. Is there any correlation with Parkinson's disease? Ann Nucl Med. (2012) 26:337–44. doi: 10.1007/s12149-012-0577-4
99. Isaias IU, Canesi M, Benti R, Gerundini P, Cilia R, Pezzoli G, et al. Striatal dopamine transporter abnormalities in patients with essential tremor. Nucl Med Commun. (2008) 29:349–53. doi: 10.1097/MNM.0b013e3282f4d307
100. Isaias IU, Marotta G, Hirano S, Canesi M, Benti R, Righini A, et al. Imaging essential tremor. Mov Disord. (2010) 25:679–86. doi: 10.1002/mds.22870
101. Lee MS, Kim YD, Im JH, Kim HJ, Rinne JO, Bhatia KP. 123I-IPT brain SPECT study in essential tremor and Parkinson's disease. Neurology. (1999) 52:1422–6. doi: 10.1212/wnl.52.7.1422
102. Nistico R, Pirritano D, Novellino F, Salsone M, Morelli M, Valentino P, et al. Blink reflex recovery cycle in patients with essential tremor associated with resting tremor. Neurology. (2012) 79:1490–5. doi: 10.1212/WNL.0b013e31826d5f83
103. Nisticò R, Salsone M, Vescio B, Morelli M, Trotta M, Barbagallo G, et al. Blink reflex recovery cycle distinguishes essential tremor with resting tremor from de novo Parkinson's disease: an exploratory study. Parkinsonism Relat Disord. (2014) 20:153–6. doi: 10.1016/j.parkreldis.2013.10.006
104. Novellino F, Arabia G, Bagnato A, Cascini GL, Salsone M, Nicoletti G, et al. Combined use of DAT-SPECT and cardiac MIBG scintigraphy in mixed tremors. Mov Disord. (2009) 24:2242–8. doi: 10.1002/mds.22771
105. Sun X, Liu F, Liu Q, Gai Y, Ruan W, Wimalarathne DN, et al. Quantitative research of (11)C-CFT and (18)F-FDG PET in Parkinson's disease: a pilot study with NeuroQ software. Front Neurosci. (2019) 13:299. doi: 10.3389/fnins.2019.00299
106. Waln O, Wu Y, Perlman R, Wendt J, Van AK, Jankovic J. Dopamine transporter imaging in essential tremor with and without parkinsonian features. J Neural Transm (Vienna). (2015) 122:1515–21. doi: 10.1007/s00702-015-1419-z
107. Wang J, Jiang YP, Liu XD, Chen ZP, Yang LQ, Liu CJ, et al. 99mTc-TRODAT-1 SPECT study in early Parkinson's disease and essential tremor. Acta Neurol Scand. (2005) 112:380–5. doi: 10.1111/j.1600-0404.2005.00517.x
108. Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol. (1996) 39:650–8. doi: 10.1002/ana.410390515
109. Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowiak RS, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. (1993) 34:82–90. doi: 10.1002/ana.410340115
110. Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol. (1995) 52:299–305. doi: 10.1001/archneur.1995.00540270095025
111. Sahin HA, Terzi M, Uçak S, Yapici O, Basoglu T, Onar M. Frontal functions in young patients with essential tremor: a case comparison study. J Neuropsychiatry Clin Neurosci. (2006) 18:64–72. doi: 10.1176/jnp.18.1.64
112. Song IU, Park JW, Chung SW, Chung YA. Brain SPECT can differentiate between essential tremor and early-stage tremor-dominant Parkinson's disease. J Clin Neurosci. (2014) 21:1533–7. doi: 10.1016/j.jocn.2013.11.035
113. Song IU, Park JW, Chung SW, Chung YA. Differences in cerebral perfusion according to phenotypes of essential tremor: brain perfusion SPECT study using SPM analysis. Neurol Sci. (2014) 35:767–72. doi: 10.1007/s10072-013-1600-9
114. Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. (1993) 114:45–8. doi: 10.1016/0022-510x(93)90047-3
115. Ha SW, Yang YS, Song IU, Chung YA, Oh JK, Chung SW. Changes in regional brain glucose metabolism measured with F-18-FDG-PET in essential tremor. Acta Radiol. (2015) 56:482–6. doi: 10.1177/0284185114531414
116. Song IU, Ha SW, Yang YS, Chung YA. Differences in regional glucose metabolism of the brain measured with F-18-FDG-PET in patients with essential tremor according to their response to beta-blockers. Korean J Radiol. (2015) 16:967–72. doi: 10.3348/kjr.2015.16.5.967
117. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. (2000) 11:805–21. doi: 10.1006/nimg.2000.0582
118. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. (2000) 97:11050–5. doi: 10.1073/pnas.200033797
119. Luo R, Pan P, Xu Y, Chen L. No reliable gray matter changes in essential tremor. Neurol Sci. (2019) 40:2051–63. doi: 10.1007/s10072-019-03933-0
120. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. (1996) 111:209–19. doi: 10.1006/jmrb.1996.0086
121. Atkinson-Clement C, Pinto S, Eusebio A, Coulon O. Diffusion tensor imaging in Parkinson's disease: review and meta-analysis. Neuroimage Clin. (2017) 16:98–110. doi: 10.1016/j.nicl.2017.07.011
122. Schnitzler A, Munks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord. (2009) 24:1629–35. doi: 10.1002/mds.22633
123. Buchbinder BR. Functional magnetic resonance imaging. Handb Clin Neurol. (2016) 135:61–92. doi: 10.1016/B978-0-444-53485-9.00004-0
124. Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. (2004) 66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845
125. Gibson WS, Jo HJ, Testini P, Cho S, Felmlee JP, Welker KM, et al. Functional correlates of the therapeutic and adverse effects evoked by thalamic stimulation for essential tremor. Brain. (2016) 139:2198–210. doi: 10.1093/brain/aww145
126. Jang C, Park HJ, Chang WS, Pae C, Chang JW. Immediate and longitudinal alterations of functional networks after thalamotomy in essential tremor. Front Neurol. (2016) 7:184. doi: 10.3389/fneur.2016.00184
127. Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. (2005) 2:197–214. doi: 10.1602/neurorx.2.2.197
128. Málly J, Baranyi M, Vizi ES. Change in the concentrations of amino acids in CSF and serum of patients with essential tremor. J Neural Transm (Vienna). (1996) 103:555–60. doi: 10.1007/bf01273153
129. Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. (2012) 135:105–16. doi: 10.1093/brain/awr301
130. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. (2010) 25:2274–84. doi: 10.1002/mds.23240
131. Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. (2011) 10:148–61. doi: 10.1016/S1474-4422(10)70322-7
132. Kralic JE, Criswell HE, Osterman JL, O'Buckley TK, Wilkie ME, Matthews DB, et al. Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice. J Clin Invest. (2005) 115:774–9. doi: 10.1172/jci23625
133. Zhang X, Santaniello S. Role of cerebellar GABAergic dysfunctions in the origins of essential tremor. Proc Natl Acad Sci U S A. (2019) 116:13592–601. doi: 10.1073/pnas.1817689116
134. Gironell A, Figueiras FP, Pagonabarraga J, Herance JR, Pascual-Sedano B, Trampal C, et al. Gaba and serotonin molecular neuroimaging in essential tremor: a clinical correlation study. Parkinsonism Relat Disord. (2012) 18:876–80. doi: 10.1016/j.parkreldis.2012.04.024
135. Ghassaban K, Liu S, Jiang C, Haacke EM. Quantifying iron content in magnetic resonance imaging. Neuroimage. (2019) 187:77–92. doi: 10.1016/j.neuroimage.2018.04.047
136. Pavese N, Tai YF. Nigrosome imaging and neuromelanin sensitive MRI in diagnostic evaluation of Parkinsonism. Mov Disord Clin Pract. (2018) 5:131–40. doi: 10.1002/mdc3.12590
137. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. (2014) 13:1045–60. doi: 10.1016/S1474-4422(14)70117-6
138. Ba F, Martin WR. Dopamine transporter imaging as a diagnostic tool for Parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord. (2015) 21:87–94. doi: 10.1016/j.parkreldis.2014.11.007
139. Brooks DJ, Playford ED, Ibanez V, Sawle GV, Thompson PD, Findley LJ, et al. Isolated tremor and disruption of the nigrostriatal dopaminergic system: an 18F-dopa PET study. Neurology. (1992) 42:1554–60. doi: 10.1212/wnl.42.8.1554
140. Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffmann M, et al. Progression of dopaminergic degeneration in Parkinson's disease and atypical Parkinsonism: a longitudinal β-CIT SPECT study. Mov Disord. (2002) 17:45–53. doi: 10.1002/mds.1265
141. Holtbernd F, Gagnon JF, Postuma RB, Ma Y, Tang CC, Feigin A, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. (2014) 82:620–7. doi: 10.1212/WNL.0000000000000130
142. Holtbernd F, Eidelberg D. Functional brain networks in movement disorders: recent advances. Curr Opin Neurol. (2012) 25:392–401. doi: 10.1097/WCO.0b013e328355aa94
Keywords: essential tremor, pathophysiology, magnetic resonance imaging (MRI), tremor network, PET, SPECT, gamma-aminobutyric acid
Citation: Holtbernd F and Shah NJ (2021) Imaging the Pathophysiology of Essential Tremor—A Systematic Review. Front. Neurol. 12:680254. doi: 10.3389/fneur.2021.680254
Received: 13 March 2021; Accepted: 04 May 2021;
Published: 16 June 2021.
Edited by:
Julián Benito León, University Hospital October 12, SpainReviewed by:
Alexandre Gironell, Hospital de la Santa Creu i Sant Pau, SpainCopyright © 2021 Holtbernd and Shah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florian Holtbernd, ZmhvbHRiZXJuZEB1a2FhY2hlbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.