
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 18 August 2021
Sec. Epilepsy
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.668439
Objective: Central nervous system infections (CNSIs), especially viral encephalitis and meningitis, are well-recognized causes of medically refractory epilepsy. Although surgery is an effective and durable intervention against these infections, the seizure control outcomes described in previous surgical series have been variable. Accordingly, it is not clear which variables are most valuable in predicting seizure control following surgery for CNSI. The aim of this meta-analysis was to identify the predictors of favorable surgical outcomes in CNSI-related epilepsy.
Methods: The PubMed, EMBASE, Cochrane Library, WANGFANG, VIP, CBM, and CNKI databases were searched for studies according to the inclusion criteria. Prognostic factors, surgical outcomes, and patient characteristics were extracted. Heterogeneity was detected by the I2 and Q statistics.
Results: Seventeen studies were included in our meta-analysis. Eight predictors of favorable outcomes (Engel Class I/II) were determined, including abnormal MRI findings, meningitis, temporal location only, regional ictal pattern, unilateral ictal pattern, older age at epilepsy, longer silent period, and longer time from infection, as follows: OR = 3.34 (95% CI 1.44–7.74), OR = 0.31 (95% CI 0.13–0.70), OR = 0.34 (95% CI 0.16–0.74), OR = 5.65 (95% CI 1.75–18.30), and OR = 9.53 (95% CI 2.36–38.48), respectively, and MD = 2.15 (95% CI 0.20–4.11), MD = 2.40 (95% CI 0.09–4.70), and MD = 8.49 (95% CI 1.50–15.48), respectively. A subgroup analysis found the following associations: regional and unilateral ictal patterns in viral encephalitis, a younger age at infection in parasitic encephalopathy, an older age at surgery, a longer time from onset, and a longer time from infection in unexplained meningitis. A sensitivity analysis restricted to studies that included each variable yielded robust results. Little evidence of publication bias was observed.
Conclusions: This meta-analysis suggests that abnormal MRI findings, meningitis, temporal location only, regional and unilateral ictal patterns, older age at epilepsy, longer silent period, and longer time from infection are predictive factors in patients with favorable surgical outcomes in CNSI-related epilepsy. In addition, different infective agents influenced the results in regional and unilateral ictal patterns in ictal electroencephalography, as well as the relationship between age at infection and surgery and the time from epilepsy onset and infection.
Central nervous system infections (CNSIs) are a frequent cause of acquired epilepsy worldwide (1, 2). Seven percent of CNSI survivors have a risk of developing late symptomatic epilepsy (3), especially in the first 5 years (4). Early (acute) symptomatic seizures present within 2 weeks after infection (5), whereas late unprovoked seizures occur months to years after CNSI. According to a small number of reports, infection eventually leads to epilepsy in 12–22% of affected children (6, 7) and 25% of affected adults (7). Various infectious agents can cause late symptomatic epilepsy, including viruses (38% of cases), neurocysticercosis (NCC) (34%), and tuberculosis (25%) (7). Epilepsy can also occur after CNSI involving other pathogens, such as bacteria, and other diseases, such as schistosomiasis.
Late symptomatic epilepsy is strongly related to adverse outcomes, including progressive cognitive and behavior impairment, pharmacoresistance and other epilepsy-associated morbidities, such as traumatic injury, depression, and sudden unexpected death (8–10). A multicenter French study reported that 40% of epilepsy cases that initiated after CNSI-developed drug resistance (11), although only 6% of pediatric and 8% of adult cases of intractable epilepsy were caused by “postnatal infections and other postnatal factors” (12). Despite the high rate of pharmacoresistance, surgery is a highly effective and durable intervention that can improve quality of life in patients with intractable epilepsy (13, 14). Moreover, early surgical intervention might release or reverse impaired psychosocial outcomes in patients with uncontrolled seizures during adolescence or adulthood (14–16).
The surgical outcomes of postinfectious epilepsy are encouraging in general; however, the prognostic factors that predict surgical outcomes remain unclear, although various potential risk factors have been identified. A recent case-control study showed that longer epilepsy durations and multiple lobe involvement predicted worse outcomes in NCC-related epilepsy (17), whereas another retrospective study reported that the age of occurrence of previous meningitis or a history of encephalitis contributed to outcomes after anterior temporal lobectomy. However, the predictors that have been related to surgical outcomes have varied among numerous studies. Therefore, we conducted the first meta-analysis to identify the real risk factors associated with seizure freedom and surgical outcomes in CNSI-related epilepsy.
A systematic literature review and meta-analysis was performed adhering to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix 1) (18, 19).
We performed a systematic search of the PubMed (MEDLINE), Embase, Cochrane Library, WANGFANG, VIP Database for China Science and Technology Journal (VIP), Chinese National Knowledge Infrastructure (CNKI), and Chinese Biomedical and Medical Database (CBM) databases from database inception to April 1, 2018. We applied the following terms as a search strategy: “surgery,” “central nervous system infections,” and “epilepsy” (the detailed strategy is presented in the Supplementary Material). No geography or language restrictions were imposed. When necessary, we contacted the study authors for additional information.
Studies were included if they fulfilled the following criteria: (1) patients underwent surgery for postinfectious epilepsy; (2) surgical outcomes were documented in accordance with Engel's classification (20), the International League Against Epilepsy (ILAE) classification (21), or any other classifications match description based on the Engel (or ILAE) classification or the detailed outcomes of each patient were reported; (3) the predictive variables included in the study were associated with an Engel or ILAE classification or another acceptable classification match description similar to the Engel (or ILAE) classification for surgery for CNSI-related epilepsy; and (4) studies published as full articles, meeting abstracts with full data, theses, case series, or case reports. Studies were excluded if they failed to meet the inclusion criteria or provided incomplete or non-retrievable data. When the same population was used in more than one study, we selected the study that had the longest follow-up time.
Two independent authors screened the search results for potential inclusion according to the inclusion and exclusion criteria. All disputes were resolved by consensus.
Data were extracted systematically from the studies and compiled independently by two reviewers using standard electronic sheets and cross-checks to reach a consensus. In the case of any disagreement, a consensus was reached by discussion. Trial and patient characteristics were documented and included the name of the first author, year of publication, country, period, infective type, infective agent, gender, number of participants, mean age at surgery, disease course, standard outcome classification, and predictors. The predictors of interest were gender; age; side (dominat or non-dominant hemisphere and unilateral or bilateral), location (temporal and extratemporal locations), and number of lesions on magnetic resonance imaging (MRI)/computed tomography (CT); ictal and interictal electroencephalography (EEG); MRI structural abnormalities; type of disease (encephalitis or meningitis); choice of operation [anterior temporal lobectomy (ATL) or extratemporal cortisectomy (ETC)], and time from first seizure. A favorable outcome was defined as Engel I and II or ILAE OC1 and 2 (22). In five studies without an Engel or ILAE classification, only seizure freedom was regarded as a good prognosis for statistical purposes (23–27). If the lesion was located in both the temporal and the extratemporal locations, we considered it to have an extratemporal location. The time from first seizure/infection was defined as the time between surgery and the first seizure onset or infection. The silent period was defined as the time between acute infection and epilepsy onset.
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of observational studies (28). The quality of case-control studies was also assessed according to three factors: the selection of cases and controls (0–4 stars), comparability of cases and controls (0–2 stars), and ascertainment of exposure (0–3 stars) (28).
The main outcomes were MRI findings, the type of infection, the location, side and number of epileptic lesions, age of onset and silent period of epilepsy, and the time from infection to epilepsy.
We calculated individual and pooled odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) for each study. Heterogeneity in OR was estimated by the I2 statistic and P-values. A P < 0.05 or I2 > 50% (29) was defined as indicative of significant heterogeneity (30, 31), and in those instances, a random-effects model was used for the meta-analysis. Otherwise, we selected a fixed-effects model (32). Furthermore, we conducted a sensitivity analysis to test the robustness of the pooled results by omitting one study each in turn. A subgroup analysis was conducted to investigate the potential effects of different infective agents on outcomes. All analyses were performed using Review Manager Version 5.1.7 (provided by The Cochrane Collaboration, available at http://www.cc-ims.net/revman) and STATA Version 11.0 (StataCorp, College Station, TX). A P-value < 0.05 was considered to be significant, except where otherwise specified. Begg's correlation and Egger's regression tests were used to assess potential publication bias (33, 34). If publication bias was evident, the trim and fill method was applied to provide an adjusted summary OR that included potentially missing trials (35).
We initially retrieved 2,227 potential references from databases and bibliographies (Figure 1). Based on the inclusion criteria, 1,347 studies were excluded after title screening, and 636 trials were excluded after a review of the abstracts. A full-text review of the remaining 244 papers excluded 227 studies for the following reasons: 211 had an unrelated population or outcome, and 16 did not report outcomes of interest. Finally, 17 articles containing 390 patients who met all inclusion criteria were enrolled in the meta-analysis (17, 23–27, 36–46). All articles were subjected to intention-to-treat (ITT) analysis.
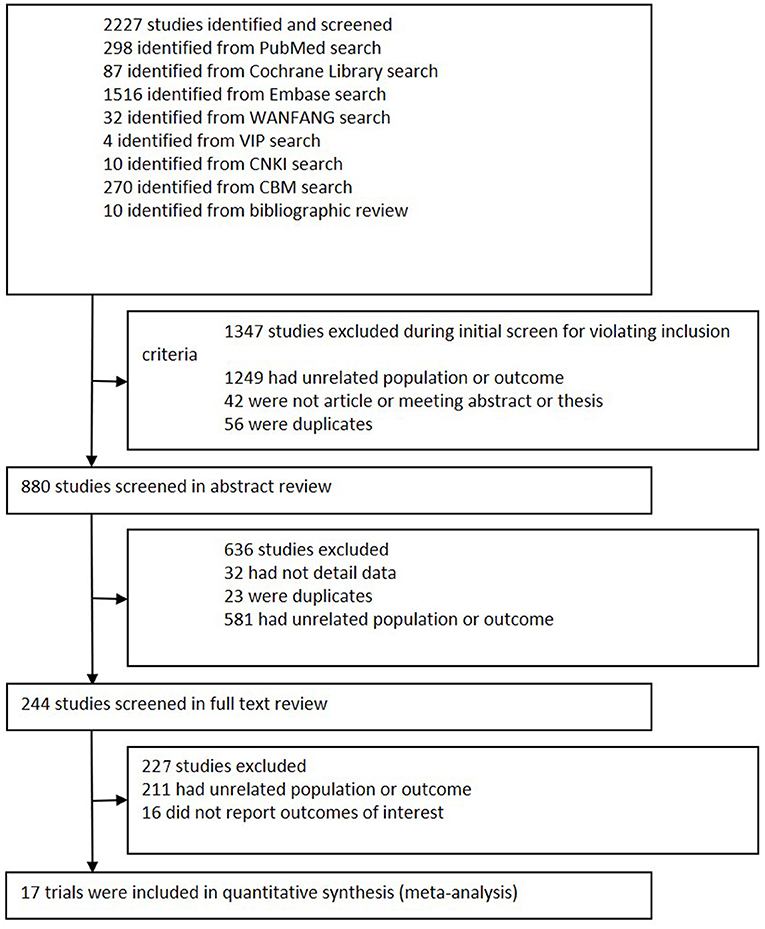
Figure 1. Flowchart of study selection. This flowchart shows the procedure used to select studies according to the inclusion criteria.
The basic characteristics of the included studies are presented in Table 1. All the studies were published between 1984 and 2016. Of the included studies, seven were conducted in North America (four in the USA and three in Canada) (23–27, 39, 44), six in Asia (two in China, two in India, one in the State of Qatar, and one in South Korea) (36, 37, 40, 41, 43, 46), two in Europe (one in Spain and one in Austria) (38, 45), one in South America (Brazil) (17), and one in Oceania (Australia) (42). Regarding the writing language, 15 articles were written in English, and two were published in Chinese. For most studies, the mean age at the time of surgery ranged from 3 to 69 years old. The quality of observational studies was low to moderate, and only five studies received more than seven stars according to the NOS (Table 2).
Regarding general patient information, such as gender, disease type (encephalitis or meningitis), age at infection, onset, and surgery, time from seizure, silent period duration, and time from infection, meningitis was more frequently associated with favorable outcomes when compared with encephalitis (OR 0.31, 95% CI 0.13–0.70, p = 0.005, Figure 2A). Additionally, older age at surgery (MD = 2.15, 95% CI 0.20–4.11), longer time from seizure onset to surgery (MD = 2.40, 95% CI 0.09–4.70), and longer time from infection to surgery (MD = 8.49, 95% CI 1.50–15.48) were associated with better outcomes (Figures 2B–D). Regarding manifestations viewed on MRI, in CNSI epilepsy, an abnormal MRI finding was associated with a higher rate of favorable outcomes achieved in patients than a normal MRI result (OR 3.34, 95% CI 1.44–7.74, p = 0.005, Figure 3). With regard to ictal EEG features (temporal or extratemporal ictal pattern, regional or non-localizable ictal pattern, and unilateral or bilateral ictal pattern), we identified that temporal ictal pattern, regional ictal pattern, and unilateral ictal pattern were protective factors in the prognosis of postinfective epilepsy (extratemporal location, OR 0.34, 95% CI 0.16–0.74, p = 0.007; regional ictal pattern, OR 5.65, 95% CI 1.75–18.30, p = 0.004; unilateral ictal pattern, OR 9.53, 95% CI 2.36–38.48, p = 0.002, Figures 4A–C).
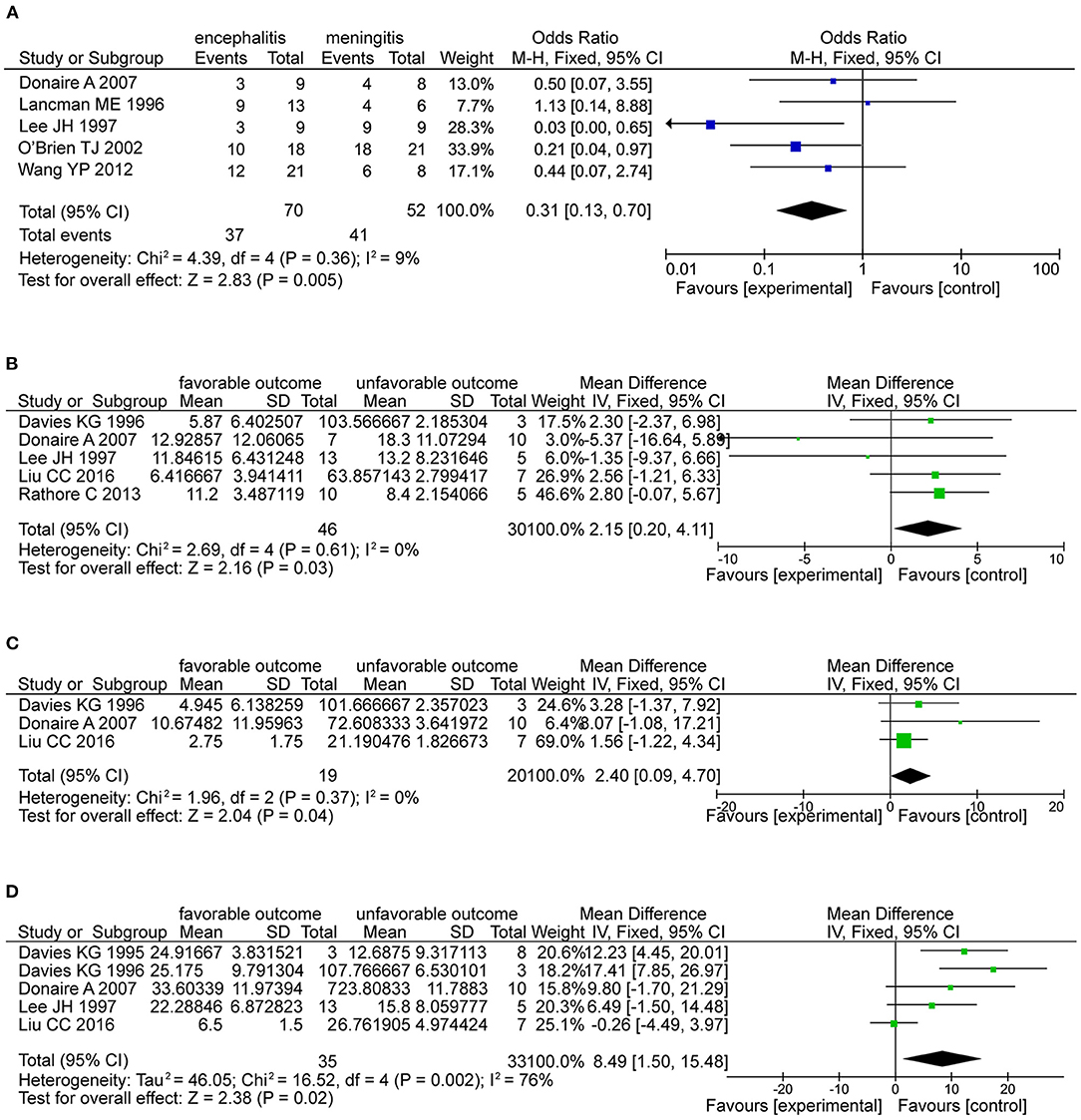
Figure 2. The factors associated with favorable outcomes. (A) The forest plot showed that meningitis was associated with favorable outcomes than encephalitis (OR 0.31, 95% CI 0.13–0.70, P = 0.005). (B) The forest plot showed older age at epilepsy (OR 2.15, 95% CI 0.20-4.11, P = 0.03) is associated with favorable outcomes. (C) Forest plot showed longer silent period (OR 2.40, 95% CI 0.09-4.70, P = 0.04) is associated with favorable outcomes. (D) Forest plot showed the time from infection (OR 8.49, 95% CI 1.50-15.48, P = 0.02) is associated with favorable outcomes.
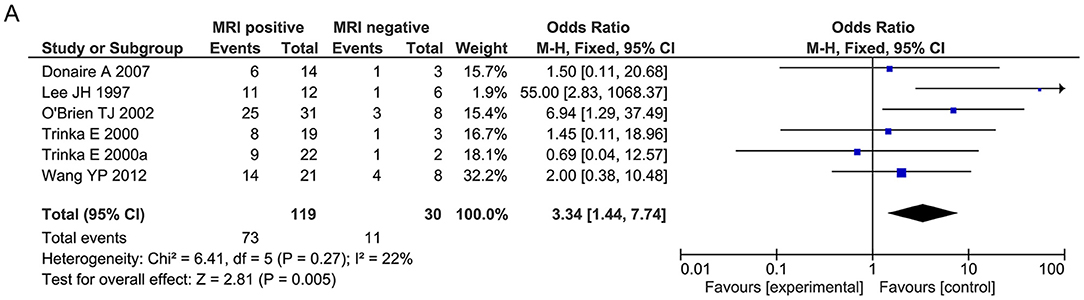
Figure 3. Meta-analysis for MRI findings. (A) Forest plot showed favorable outcomes were achieved in patients with positive MRI findings.
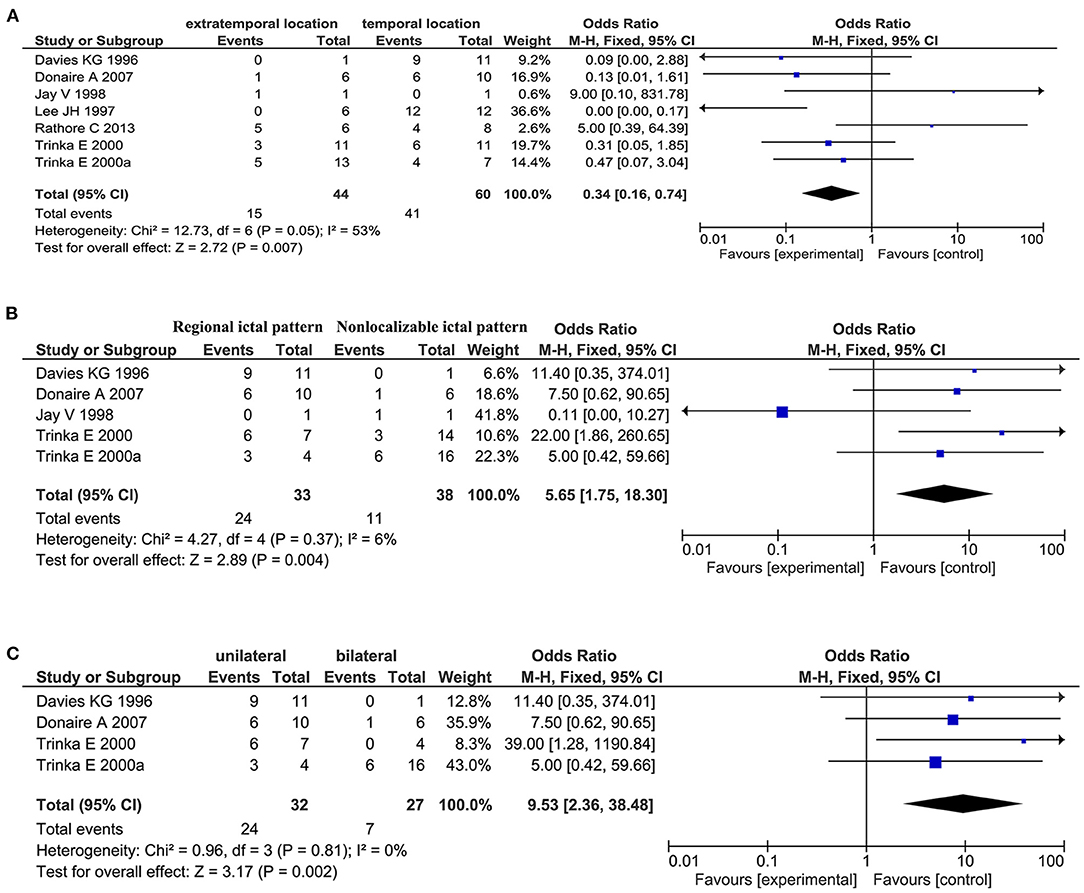
Figure 4. Meta-analysis of ictal EEG findings. (A) Forest plot showed a temporal location of only lesion was associated with favorable outcomes in patients. (B) Forest plot showed a regional ictal pattern of EEG findings was associated with favorable outcomes in patients. (C) Forest plot showed favorable outcomes were achieved in patients with unilateral ictal EEG findings.
According to disease type, all the studies were classified into the following groups: viral encephalitis, parasitic encephalopathy, bacterial meningitis, and unexplained meningitis (the etiological agent of meningitis was not clearly identified). Among all variables of interest, single location of ictal EEG patterns or unilateral location of ictal EEG patterns are markers of a good prognosis in patients with history of viral encephalitis. This finding was consistent with the pooled results obtained by combining all groups, although no significant association was found in any of the other individual groups. The remaining primary outcomes failed to show any significant results among the four groups (Table 3).
Sensitivity analyses were conducted by sequentially omitting individual studies. The combined ORs of the primary results were not excessively altered by the omission of any individual study, confirming the robustness of the results.
No heterogeneity was observed. Several outcomes with a high I2 (>50%) and non-significant P-values for the Q statistic (>0.05) were still regarded as having low heterogeneity and were analyzed with a fixed-effects model (extratemporal or temporal location in ictal EEG patterns: p = 0.05, I2 = 53%; surgical strategy: p = 0.05, I2 = 53%). A random-effects model was used for age at infection (p = 0.02, I2 = 67%) and surgery (p = 0.006, I2 = 70%), time from seizure (p = 0.004, I2 = 74%) and infection (p = 0.002, I2 = 76%), and unilateral or bilateral ictal pattern on interictal EEG (p = 0.06, I2 = 71%).
There was no significant potential publication bias among all the combined results according to a funnel plot and Begg's and Egger's tests. Because the number of articles was limited, publication bias was not evaluated in the outcomes for interictal EEG.
In recent decades, the risk factors associated with surgical outcomes in postinfective epilepsy have varied among different infectious agents and different studies. The present meta-analysis identified the following five predictors, which were associated with favorable outcomes: abnormal MRI finding, meningitis, temporal location, and regional and unilateral ictal patterns. In addition, regional and unilateral ictal patterns were related to a good prognosis for viral encephalitis-related epilepsy surgery; however, we failed to identify any significant risk factors for other types of CNSI-related epilepsy.
There was no heterogeneity in most of the pooled results. Different outcome classification standards may influence the heterogeneity of combined results. Two studies conducted by Davies (23, 24) regarded seizure freedom as predictive of a favorable prognosis; however, this was not defined as the Engel classification (I/II). There was significant heterogeneity in age at the time of surgery and the time between seizure onset and infection.
The present study shows that a temporal location and abnormal MRI findings are predictors of a favorable outcome, which is consistent with the results obtained in other studies of focal cortical dysplasia (47, 48). There are many large adequate studies demonstrating that temporal lobectomies with structural abnormalities are the best patients for favorable outcome not only in patients with cortical dysplasia but with tumors especially developmental and mesial temporal sclerosis (MTS) (49). Meningitis is another protective factor that lowers the risk of epilepsy by 69% relative to the risk associated with encephalitis. Meningitis is a diffuse inflammatory change in the meninges. Compared with viral encephalitis, meningitis has less of an effect on neurons or connections between neurons, which may explain the above phenomenon. The differences between bacterial meningitis and viral encephalitis indicate an increased likelihood of a more extensive (less localized) impact of the latter, with a potential impact on the worst outcome. Moreover, patients with an older age at onset, a longer silent period, and a longer time from infection to seizure have more favorable surgical outcomes. In line with the results of previous studies of viral encephalitis-related epilepsy (38, 45, 50, 51), we found that a longer silent period between acute infection and the onset of epilepsy is a predictor of favorable postsurgical outcomes, although different types of infection did not produce significant effects in the subgroup analysis. Older age at onset may also reflect a longer silent period, which is a significant predictor of postsurgical outcomes. However, the time between surgery and encephalitis or meningitis is a novel factor that has not been reported in previous studies. A longer time from infection to one of these conditions suggests that the patient is a good candidate for epilepsy surgery, particularly in unexplained meningitis. Interestingly, better outcomes associated with patients with a history of meningitis, longer silent period, and temporal location are consistent with the diagnosis of secondary mesial temporal sclerosis, with well-known high odds of a good surgical outcome. Additionally, patients presenting with regional and unilateral ictal patterns have more favorable surgical outcomes than those with non-localizable and bilateral ictal patterns, which is consistent with a previous systematic review of focal cortical dysplasia (48). Non-localizable and bilateral ictal patterns may imply incomplete resection of the epileptogenic focus, which is likely to result in seizure recurrence.
The mechanisms by which different infectious agents produce acute seizures and then later on unprovoked seizures have not been fully addressed. The occurrence and development of epilepsy after brain infection vary with the type of infection, and it is often multifactorial. In meningitis and encephalitis, triggering of the inflammatory cascade seems to be a common potential factor that contributes to epileptogenesis. In patients with CNS infections, structural destruction and damage, such as infarction in meningitis, cortical necrosis with herpes simplex virus, hypoxic–ischemic injury in cerebral malaria, space-occupying effect of neurocysticercosis, and gliosis around calcified neurocysticercosis, might all form epileptogenic zones (3). Unfortunately, there are no published articles on the intergroup comparison of epilepsy susceptibility to bacterial meningitis, viral encephalitis, parasitic encephalopathy, and unexplained meningitis.
Potential limitations to this research should be acknowledged. First, all the studies used a retrospective design, and the results are, therefore, subject to selection and recall bias. Second, some of the studies we selected from the databases mainly focused on patients who achieved a seizure-free status instead of a favorable outcome according to the Engel classification. To include more potential studies in the present meta-analysis, we also accepted seizure freedom. Finally, unmeasured factors may have confounded the true association for unadjusted data used in the original studies.
Our meta-analysis also has some implications for future research. Few recent systematic reviews have focused on outcome data in postinfective epilepsy surgery, and different clinical studies of diverse infective agents have provided various predictors of prognoses (2). Therefore, the predictive factors identified here, including abnormal MRI findings, meningitis, temporal location only, regional and unilateral ictal patterns, older age at epilepsy, longer silent period, and longer time from infection, will be useful in the perioperative period. Based on a clinical diagnosis and identified predictors, doctors will be able to choose effective surgical procedures, determine nursing methods, and design dosage regimens in patients with a potentially high recurrence rate.
In conclusion, this meta-analysis identified eight factors, including abnormal MRI findings, meningitis, temporal location only, regional and unilateral ictal patterns, older age at epilepsy onset, longer silent period, and longer time from infection, that act as favorable predictors of surgical outcomes in CNSI-related epilepsy. In viral encephalitis-related epilepsy, regional and unilateral ictal patterns predicted favorable surgical outcomes. Additionally, a longer time from onset and a longer time from infection for unexplained meningitis were predictors of favorable surgical outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
ZH: designing the study, searching and screening the literature, extracting and analyzing the data, and drafting the manuscript. NA, HY, and S-YL: collating the data. C-QZ: designing study and guiding manuscript writing. Q-TD: revising the manuscript. Y-YK: processing the data sets and manuscript revision. All authors read and approved the final manuscript.
This work was supported by the grants from Training project of clinical medical researcher of Army Medical University (No. 2018XLC3046) and Joint Research Foundation from Chongqing Science and Technology Bureau and Municipal Health Commission (2019MSXM099) and the National Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0369).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.668439/full#supplementary-material
1. Bhalla D, Godet B, Druet-Cabanac M, Preux PM. Etiologies of epilepsy: a comprehensive review. Expert Rev Neurother. (2011) 11:861–76. doi: 10.1586/ern.11.51
2. Ramantani G, Holthausen H. Epilepsy after cerebral infection: review of the literature and the potential for surgery. Epileptic Disord. (2017) 19:117–36. doi: 10.1684/epd.2017.0916
3. Vezzani A, Fujinami RS, White HS, Preux PM, Blumcke I, Sander JW, et al. Infections, inflammation and epilepsy. Acta Neuropathol. (2016) 131:211–34. doi: 10.1007/s00401-015-1481-5
4. Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. (1988) 38:1407–10. doi: 10.1212/WNL.38.9.1407
5. Pillai SC, Mohammad SS, Hacohen Y, Tantsis E, Prelog K, Barnes EH, et al. Postencephalitic epilepsy and drug-resistant epilepsy after infectious and antibody-associated encephalitis in childhood: clinical and etiologic risk factors. Epilepsia. (2016) 57:e7–11. doi: 10.1111/epi.13253
6. Ruiz-Garcia M, Sosa-de-Martinez C, Gonzalez-Astiazaran A, Rueda-Franco F. Clinical-etiological and therapeutic profile of 719 Mexican epileptic children. Childs Nerv Syst. (2002) 18:593–8. doi: 10.1007/s00381-002-0656-2
7. Si Y, Liu L, Hu J, Mu J, Fang JJ, An DM, et al. Etiologic features of newly diagnosed epilepsy: hospital-based study of 892 consecutive patients in West China. Seizure. (2012) 21:40–4. doi: 10.1016/j.seizure.2011.09.008
8. Cardarelli WJ, Smith BJ. The burden of epilepsy to patients and payers. Am J Manag Care. (2010) 16:S331–6.
9. Nieto-Barrera M. Clinical, neuro-radiological and prognostic aspects of post-encephalitic catastrophic epilepsies. Rev Neurol. (2002) 35(Suppl. 1):S30–8.
10. van Andel J, Zijlmans M, Fischer K, Leijten FS. Quality of life of caregivers of patients with intractable epilepsy. Epilepsia. (2009) 50:1294–6. doi: 10.1111/j.1528-1167.2009.02032.x
11. Chipaux M, Szurhaj W, Vercueil L, Milh M, Villeneuve N, Cances C, et al. Epilepsy diagnostic and treatment needs identified with a collaborative database involving tertiary centers in France. Epilepsia. (2016) 57:757–69. doi: 10.1111/epi.13368
12. Alexandre V Jr., Capovilla G, Fattore C, Franco V, Gambardella A, Guerrini R, et al. Characteristics of a large population of patients with refractory epilepsy attending tertiary referral centers in Italy. Epilepsia. (2010) 51:921–5. doi: 10.1111/j.1528-1167.2009.02512.x
13. Engel J Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. (2003) 60:538–47. doi: 10.1212/01.WNL.0000055086.35806.2D
14. Kuzniecky R, Devinsky O. Surgery Insight: surgical management of epilepsy. Nat Clin Pract Neurol. (2007) 3:673–81. doi: 10.1038/ncpneuro0663
15. Chin PS, Berg AT, Spencer SS, Sperling MR, Haut SR, Langfitt JT, et al. Employment outcomes following resective epilepsy surgery. Epilepsia. (2007) 48:2253–7. doi: 10.1111/j.1528-1167.2007.01208.x
16. Widjaja E, Li B, Schinkel CD, Ritchie LP, Weaver J, Snead OC, et al. Cost-effectiveness of pediatric epilepsy surgery compared to medical treatment in children with intractable epilepsy. Epilepsy Res. (2011) 94:61–8. doi: 10.1016/j.eplepsyres.2011.01.005
17. Meguins LC, Adry RA, Silva Junior SC, Pereira CU, Oliveira JG, Morais DF, et al. Longer epilepsy duration and multiple lobe involvement predict worse seizure outcomes for patients with refractory temporal lobe epilepsy associated with neurocysticercosis. Arq Neuropsiquiatr. (2015) 73:1014–8. doi: 10.1590/0004-282X20150175
18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
19. Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. (2013) 8:e83138. doi: 10.1371/journal.pone.0083138
21. Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. (2001) 42:282–6. doi: 10.1046/j.1528-1157.2001.4220282.x
22. de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. (2011) 378:1388–95. doi: 10.1016/S0140-6736(11)60890-8
23. Davies KG, Hermann BP Jr, DF. Wyler AR. Intractable epilepsy due to meningitis: results of surgery and pathological findings. Br J Neurosurg. (1996) 10:567. doi: 10.1080/02688699646871
24. Davies KG, Hermann BP, Wyler AR. Surgery for intractable epilepsy secondary to viral encephalitis. Br J Neurosurg. (1995) 9:759–62. doi: 10.1080/02688699550040729
25. Lancman ME, Morris HH III. Epilepsy after central nervous system infection: clinical characteristics and outcome after epilepsy surgery. Epilepsy Res. (1996) 25:285–90. doi: 10.1016/S0920-1211(96)00086-1
26. Leblanc R, Knowles KF, Melanson D, Maclean JD, Rouleau G, Farmer JP. Neurocysticercosis: surgical and medical management with praziquantel. Neurosurgery. (1986) 18:419. doi: 10.1097/00006123-198604000-00005
27. Mitchell WG, Snodgrass SR. Intraparenchymal cerebral cysticercosis in children: a benign prognosis. Pediatr Neurol. (1985) 1:151–6. doi: 10.1016/0887-8994(85)90054-2
28. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
29. Higgins JPT, Thompson SG. Quantifying heterogeneity in meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
30. Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. (2008) 37:1158–60. doi: 10.1093/ije/dyn204
31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
33. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
34. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
35. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
36. Bashir EFM, Taha ZM. Challenges in the management of intracranial subdural empyema. Neurosurg Quart. (2012) 13:198–206. doi: 10.1097/00013414-200309000-00005
37. Chandra PS, Bal C, Garg A, Gaikwad S, Prasad K, Sharma BS, et al. Surgery for medically intractable epilepsy due to postinfectious etiologies. Epilepsia. (2010) 51:1097–100. doi: 10.1111/j.1528-1167.2010.02538.x
38. Donaire A, Carreno M, Agudo R, Delgado P, Bargallo N, Setoain X, et al. Presurgical evaluation in refractory epilepsy secondary to meningitis or encephalitis: bilateral memory deficits often preclude surgery. Epileptic Disord. (2007) 9:127–33. doi: 10.1684/epd.2007.0098
39. Jay V, Hwang P, Hoffman HJ, Becker LE, Zielenska M. Intractable seizure disorder associated with chronic herpes infection. HSV1 detection in tissue by the polymerase chain reaction. Childs Nerv Syst. (1998) 14:15–20. doi: 10.1007/s003810050167
40. Lee JH, Lee BI, Park SC, Kim WJ, Kim JY, Park SA, et al. Experiences of epilepsy surgery in intractable seizures with past history of CNS infection. Yonsei Med J. (1997) 38:73–8. doi: 10.3349/ymj.1997.38.2.73
41. Liu CC, Chen SY, Piao YS, Lu DH. Clinicopathologic study of intractable epilepsy-related encephalitis. Zhonghua Bing Li Xue Za Zhi. (2016) 45:318–23.
42. O'Brien TJ, Moses H, Cambier D, Cascino GD. Age of meningitis or encephalitis is independently predictive of outcome from anterior temporal lobectomy. Neurology. (2002) 58:104–9. doi: 10.1212/WNL.58.1.104
43. Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia. (2013) 54:1815–22. doi: 10.1111/epi.12349
44. Trinka E, Dubeau F, Andermann F, Bastos A, Hui A, Li LM, et al. Clinical findings, imaging characteristics and outcome in catastrophic post-encephalitic epilepsy. Epileptic Disord. (2000) 2:153–62.
45. Trinka E, Dubeau F, Andermann F, Hui A, Bastos A, Li LM, et al. Successful epilepsy surgery in catastrophic postencephalitic epilepsy. Neurology. (2000) 54:2170–3. doi: 10.1212/WNL.54.11.2170
46. Wang YP, Zhang GJ, Cai LX, Tao YU. Prognostic analysis of surgical treatment on intractable epilepsy after central nervous system infections. Neurology. (2000) 54:2170–3. doi: 10.1212/wnl.54.11.2170
47. Englot DJ, Han SJ, Rolston JD, Ivan ME, Kuperman RA, Chang EF, et al. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. (2014) 14:386–95. doi: 10.3171/2014.7.PEDS13658
48. Rowland NC, Englot DJ, Cage TA, Sughrue ME, Barbaro NM, Chang EF. A meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg. (2012) 116:1035–41. doi: 10.3171/2012.1.JNS111105
49. Lamberink HJ, Otte WM, Blümcke I, Braun KPJ, European Epilepsy Brain Bank writing group, study groupet al. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. (2020) 19:748–57. doi: 10.1016/S1474-4422(20)30220-9
50. Sellner J, Trinka E. Seizures and epilepsy in herpes simplex virus encephalitis: current concepts and future directions of pathogenesis and management. J Neurol. (2012) 259:2019–30. doi: 10.1007/s00415-012-6494-6
Keywords: central nervous system infection, epilepsy, surgery, seizure freedom, meta-analysis
Citation: Hou Z, Duan Q-T, Ke Y-Y, An N, Yang H, Liu S-Y and Zhang C-Q (2021) Predictors of Seizure Freedom in Patients Undergoing Surgery for Central Nervous System Infection-Related Epilepsy: A Systematic Review and Meta-Analysis. Front. Neurol. 12:668439. doi: 10.3389/fneur.2021.668439
Received: 16 February 2021; Accepted: 03 June 2021;
Published: 18 August 2021.
Edited by:
Stephan Schuele, Northwestern University, United StatesReviewed by:
Çigdem Özkara, Istanbul University-Cerrahpasa, TurkeyCopyright © 2021 Hou, Duan, Ke, An, Yang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Qing Zhang, Y3F6aGFuZ0B0bW11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.