
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 May 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.668360
This article is part of the Research Topic Machine Learning in Action: Stroke Diagnosis and Outcome Prediction View all 12 articles
Objective: Hypoperfusion is an important factor determining the prognosis of ischemic stroke patients. The present study aimed to investigate possible predictors of hypoperfusion on MRI of ischemic stroke patients within 7 days of stroke onset.
Methods: Ischemic stroke patients, admitted to the comprehensive Stroke Center of Shanghai Fourth People's Hospital affiliated to Tongji University within 7 days of onset between January 2016 and June 2017, were recruited to the present study. Magnetic resonance imaging (MRI), including both diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI), was performed within 7 days of the symptom onset. Time to maximum of the residue function (Tmax) maps were automatically evaluated using the RAPID software. The volume of hypoperfusion was measured outside the infarct area based on ADC < 620 × 10−6 mm2/s. The 90 d mRS score was assessed through either clinic visits or telephone calls. Multivariate step-wise analysis was used to assess the correlation between MR findings and clinical variables, including the demographic information, cardio-metabolic characteristics, and functional outcomes.
Results: Among 635 patients admitted due to acute ischemic stroke within 7 days of onset, 241 met the inclusion criteria. Hypoperfusion volume of 38 ml was the best cut-off value for predicting poor prognosis of patients with cerebral infarction (90 d-mRS score ≥ 2). The incidences of MR perfusion Tmax > 4–6 s maps with a volume of 0–38 mL or >38 mL were 51.9% (125/241) and 48.1% (116/241), respectively. Prior stroke and vascular stenosis (≥70%) were associated with MR hypoperfusion. Multivariate step-wise analysis showed that prior stroke and vascular stenosis (≥70%) were risk factors of Tmax > 4–6 s maps, and the odds ratios (OR) were 3.418 (adjusted OR 95% CI: 1.537–7.600), and 2.265 (adjusted OR, 95% CI: 1.199–4.278), respectively.
Conclusion: Our results suggest that prior stroke and vascular stenosis (≥70%) are strong predictors of hypoperfusion in patients with acute ischemic stroke within 7 days of stroke onset.
Intravenous thrombolysis and endovascular therapy are effective methods for the treatment of acute ischemic stroke (1, 2). However, due to time window restrictions, only a small number of people receive timely treatment, and over 70% of stroke patients still have disabilities (modified Rankin classification, mRS2-6) due to the presence of hypoperfused tissues (3, 4). Quantitative assessment of hemodynamic indices of acute stroke patients will facilitate the discovery of potential predictors of hypoperfusion, which will reveal new targets for early and effective intervention. Currently, a number of factors have been reported to influence functional outcomes of acute ischemic stroke patients, including blood glucose, blood pressure, history of atrial fibrillation, baseline NIHSS, volume of core infarction, blood perfusion, and vascular lesions (5–12). Previous studies have shown that abnormal brain perfusion is closely related to stroke recurrence and functional outcome, but there are few studies on risk factors impacting brain perfusion.
Tmax is a widely used parameter of magnetic resonance perfusion for patients with acute ischemic stroke and has been used in clinical trials (13, 14). Different Tmax thresholds reflect different degrees of hypoperfused volumes, with a high threshold reflecting a low degree of hypoperfusion (15). Changes of Tmax may reflect the microvascular integrity of collaterals and the perfusion status of brain tissue (16). In view of the fact that perfusion imaging is closely related to the status of collateral circulation, the cerebral perfusion parameters on MRI may be a good biomarker of collateral circulation. Therefore, it is reasonable to use Tmax to evaluate the status of tissue hypoperfusion and facilitate decision-making on the choice of treatments for patients with AIS (17–19).
It is well-known that the penumbra is the area surrounding the ischemic core, which has a high risk of progressing to infarct. Tmax > 6 s can accurately define the penumbra (20). Albers et al. screened patients with salvageable penumbra for endovascular thrombectomy using Tmax > 6 s with the assistance of the RAPID software (17). Time to peak contrast concentration (TTP) or time at which the deconvolved residue function reaches its maximum value (Tmax) is generally used to evaluate hypoperfusion status. Compared with TTP, Tmax has the advantage of reducing dependence on bolus shape and cardiac output (21). Therefore, Tmax seems to be more appropriate in evaluating tissue lesions with hypoperfusion. It has been reported that Tmax > 6 s or Tmax > 4 s is more accurate than Tmax > 2 s in predicting the salvageable penumbra or stroke progression. Difference between the volumes of Tmax > 4 s and Tmax > 6 s seems to be the best biomarker in identifying severe hypoperfusion (22). Studies have shown benefit from prolonged reperfusion therapy with increased likelihood of good prognosis through evaluating the ischemic penumbra with the perfusion parameter Tmax (17, 19). However, few studies have reported risk factors of low perfusion in Chinese populations. Therefore, the present study aimed to quantitatively evaluate the hypoperfusion status of AIS patients and to explore the potential predictors of hypoperfusion on MRI.
Acute ischemic stroke patients, admitted to the comprehensive Stroke Center of Shanghai Fourth People's Hospital affiliated to Tongji University within 7 days of onset between January 2016 and June 2017, were recruited to the present study.
The inclusion criteria were: (a) Patients who were admitted within 7 days of onset and evaluated by two stroke neurologists (23, 24); (b) MR images including both DWI and PWI were available at the time of hospitalization; (c) Tmax maps were assessed using the RAPID software (iSchemaView USA, Version 4.9) (25) independently.
The exclusion criteria: patients did not have their perfusion status assessed using DWI and PWI within 7 days of stroke onset.
Demographic data, clinical variables, risk factors, neurologic deficits, time between MRI scan and stroke onset were documented for each patient. The 90 d mRS was evaluated by experienced neurologists.
MRI scans were collected using a 1.5-T Avanto scanner (Siemens, Erlangen, Germany). The imaging protocol for this study included diffusion-weighted imaging (DWI), perfusion weighted imaging (PWI), apparent diffusion coefficient (ADC), fluid-attenuated inversion recovery (FLAIR), and magnetic resonance angiography (MRA). Imaging parameters were listed below, DWI: 19 slices, 192 × 192 matrices; slice thickness = 5.5 mm; TR/TE, 3,600/102 ms; field of view = 230 mm2, b = 0 and 1,000 s/mm2; EPI factor = 192; bandwidth = 964 Hz/pixel. FLAIR: 18 slices, 256 × 190 matrices; slice thickness = 5.5 mm; TR/TE, 4,000/92 ms; field of view = 230 mm2; TI = 1,532.6 ms; bandwidth = 190 Hz/Px; flip angle = 150°. Dynamic susceptibility contrast PWI (DSC-PWI): 19 slices, 128 × 128 matrices; slice thickness = 5 mm; TR/TE, 1,590/32 ms; measurements = 50; field of view = 230 mm2; band width = 1,346 Hz/pixel; flip angle = 90°. A Gd-DTPA contrast agent (gadopentetate dimeglumine injection; Shanghai Pharmaceutical Corporation, Shanghai, China) was injected intravenously (0.2 mmol/kg body weight) at a rate of 4 mL/s after a bolus with 30 ml normal saline. Three-dimensional time-of-flight MRA of the internal carotid artery (ICA) and intracranial circulation: 241 × 256 matrices, slice thickness = 0.7 mm; TR/TE, 25/7 ms; field of view = 180 mm2; Bandwidth = 100 Hz/PX; flip angle = 25°.
Estimates of hypoperfusion on PWI were calculated using the RAPID software (iSchemaView USA, Version 4.9), which is an automated imaging post-processing system. ADC < 620 × 10−6 mm2/s was adopted to define the infarct core (26). Volumes of Tmax > 4 s and >6 s were used to determine hypoperfusion in ischemic stroke patients. The volume of hypoperfusion was measured outside the infarct area, based on ADC < 620 × 10−6 mm2/s. Measurement of vascular stenosis was performed on Magnetic Resonance Angiography by two independent radiologists using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method (27). The extent of reduction of the internal arterial diameter was then graded. If the measurements of two radiologists were inconsistent, repetition will be required before reaching a conclusion.
The primary outcome was assessed using the 90-day modified Rankin Scale (mRS). mRS ranges from 0 (asymptomatic) to 6 (death). Excellent functional outcomes are defined by a 90 d-mRS score ≤ 1, and poor functional outcomes are defined by a 90 d-mRS score ≥ 2. Three months after stroke onset, mRS scores were collected through clinic visits or telephone calls.
Ethical approval for this study was obtained from the Human Research Ethics Committee of Shanghai Fourth People's Hospital Affiliated to Tongji University School of Medicine. Written informed consent was obtained from all subjects.
Data analysis was performed using IBM SPSS (version 22.0) for Windows (SPSS Inc., Chicago, IL, USA). Continuous parameters were presented as mean ± standard deviation (SD) or median with interquartile range (IQR); categorical variables were summarized as independent proportions. Baseline information of patients with or without MRI perfusion abnormalities was compared using either t-test or Mann-Whitney U-test for continuous variables and χ2 or Fisher's exact test for categorical variables. In this study, the cutoff value was 38 ml for hypoperfusion. Logistic regression analysis was used to identify independent predictors of Tmax> 4–6 s maps. Multivariate step-wise regression modeling was used to correlate Tmax > 4–6 s maps with potential risk factors with their P-values < 0.01. All correlation data were presented as odds ratios (OR) with their corresponding 95% confidence intervals (CI) and P-values. Statistical significance was considered when P < 0.05.
In the present study, 635 patients with acute ischemic stroke were admitted within 7 days of onset, but only 241 (73 women, 168 men; median age: 67 years) had technically adequate DWI and PWI scans. Among those 394 patients who were excluded, 383 of them did not have MRI, 3 could not provide the information of hematological data, 10 had their MRI more than 7 days after stroke onset, 1 had poor MRI image quality. Among these 241 patients, 107 patients had excellent functional outcomes and 134 poor functional outcomes. Among these patients, 125 had Tmax > 4–6 s volume in the range of 0–38 ml and the other 116 had a volume > 38 ml (Figure 1, Table 1).

Table 1. Comparison of demographic and clinical characteristics among ischemic stroke patients within 7 days of onset based on Tmax >4 s −6 s volume 38 ml.
Baseline characteristics of patients included in the present study were shown in Tables 1, 2. The median (IQR) age of these patients was 67 (61–79) years and their median Admission NIHSS score was 3 (IQR: 1–9). Perfusion status was evaluated after a median (IQR) delay of 3 (1–6) days from the symptom onset. The median ADC volume was 0 (IQR: 0–7.5) ml. The median fasting blood glucose (FBG) was 6.0 (IQR: 5.2–8.2) mmol/L. A history of hypertension was present in 68.5% (165/241) of patients, diabetes mellitus in 34.4% (83/241), atrial fibrillation in 14.5% (35/241), prior stroke in17% (41/241) and smoking in 33.2% (80/241). The patients were divided into four groups: intravenous thrombolysis (36/241), Bridge endovascular thrombectomy (2/241), Direct endovascular thrombectomy (12/241), and standard medical therapy alone (191/241) (Table 1).
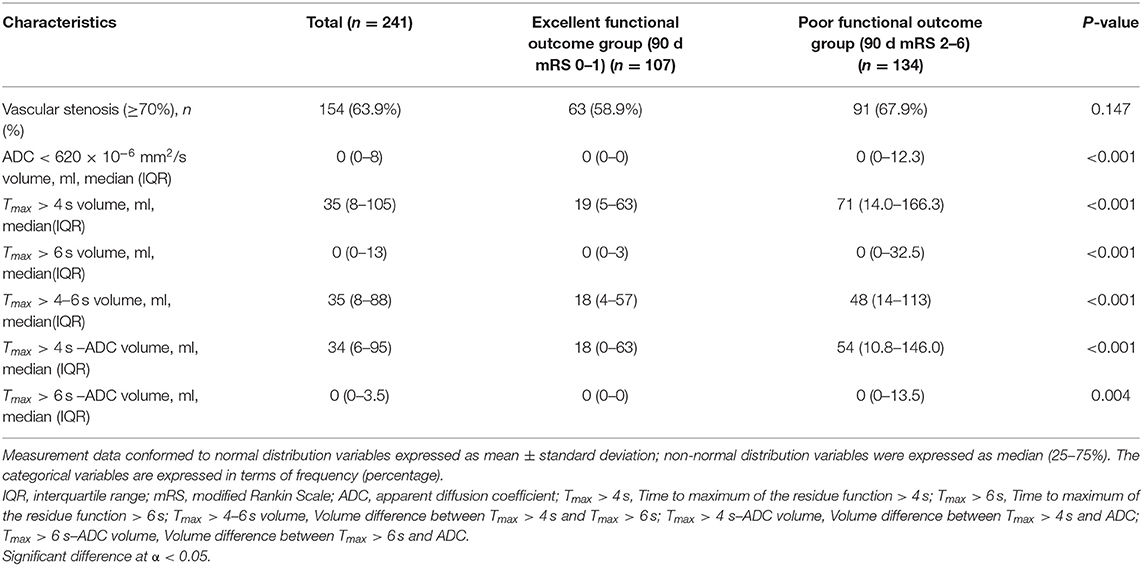
Table 2. Imaging characteristics of ischemic stroke patients with excellent and poor functional outcomes.
Demographic characteristics, possible risk factors associated with the Tmax > 4–6 s map, and comparisons of these variables between groups with volume = 0–38 ml and >38 ml were presented in Table 1. Hypoperfusion volume of 38 ml was the best cut-off value for predicting poor prognosis of patients with cerebral infarction (90 d-mRS score ≥ 2) as shown by the ROC (AUC: 0.67, 95% CI: 0.603–0.738, sensitivity: 0.612, specificity 0.673, p < 0.001) (Figures 2, 3). In univariate analyses, factors associated with hypoperfusion were: age [66 (59–75) vs. 70 (62–80), p = 0.009], atrial fibrillation (7.2 vs. 22.4%, p = 0.001), previous ischemic stroke (9.6 vs. 25.0%, p = 0.001), homocysteine [12.4 (10.9–15.85) vs. 14.25 (12.0–17.0), p = 0.011], admission NIHSS score [3 (1–6) vs. 6 (2–10), p < 0.001], symptom onset to the MR perfusion [4 (2–6) vs. 3 (1–6), p = 0.006], vascular stenosis (≥70%) (49.6 vs. 79.3%, p < 0.001), ADC volume [0 (0–0) vs. 0 (0–24.5), p < 0.001]. Other variables were not statistically different (Table 1).
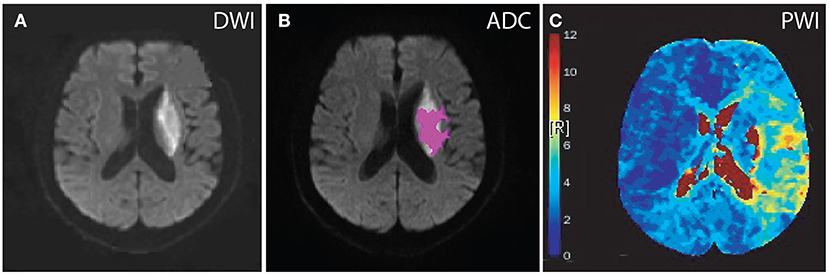
Figure 2. Diffusion and perfusion abnormalities of a patient treated with intravenous tPA. A 63-year-old male who presented with dysarthria and weakness in the right arm, hypertension for more than 3 years, vascular stenosis (≥70%), no previous ischemic stroke, MRI scan was completed 6 days after stroke onset. DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging; ADC, apparent diffusion coefficient; Tmax, Time to maximum of the residue function. Tmax color scale: 4 s < Tmax ≤ 6 s (blue); 6 s < Tmax ≤ 8 s (green); 8 s < Tmax ≤ 10 s (yellow); 10 s < Tmax (red). (A) DWI, lesion volume was 22 ml. (B) ADC, lesion volume was 9 ml. (C) PWI, lesion volumes according to Tmax delay were as follows: Tmax > 4 s, 132 ml; Tmax > 4 s–ADC volume, 123 ml; Tmax > 6 s, 22 ml; Tmax > 6 s–ADC volume, 13 ml; Tmax > 8 s, 4 ml; and Tmax > 10 s, 3 ml.
Excellent functional outcomes (mRS 0–1) were present in 44.4% (107/241) of patients, among whom the median (IQR) Tmax > 4–6 s volume, Tmax > 6 s volume, and Tmax > 4 s volume were 18 (4–57) ml, 0 (0–3) ml, and 19 (5–63) ml, respectively. MRA scans with adequate quality of both the carotid and intracranial vessels were available in the present study. MRA showed that vascular stenosis (>70%) was detected in 154 (63.9%) patients (Table 2).
In the present study, we divided patients with hypoperfusion into two groups based on the volume of Tmax > 4–6 s: Tmax > 4–6 s = 0–38 ml and Tmax > 4–6 s > 38 ml. The incidences of MR perfusion Tmax > 4–6 s = 0–38 ml and Tmax > 4–6 s > 38 ml were 51.9% (125/241) and 48.1% (116/241), respectively (Table 1).
In the univariate binary logistic regression analysis, age (P = 0.010, OR = 1.031, 95% CI: 1.007–1.056), atrial fibrillation (P = 0.001, OR = 3.723, 95% CI: 1.662–8.340), previous ischemic stroke (P = 0.002, OR = 3.139, 95% CI: 1.515–6.504), admission NIHSS score (P < 0.001, OR = 1.091, 95% CI: 1.040–1.145), symptom onset to MR perfusion (P = 0.018, OR = 0.872, 95% CI: 0.779–0.977), vascular stenosis (≥70%) (P < 0.001, OR = 3.895, 95% CI: 2.203–6.887), ADC volume (P < 0.001, OR = 1.044, 95% CI: 1.019–1.070) were independently associated with MR perfusion abnormality in ischemic stroke patients within 7 days of onset (Table 3).
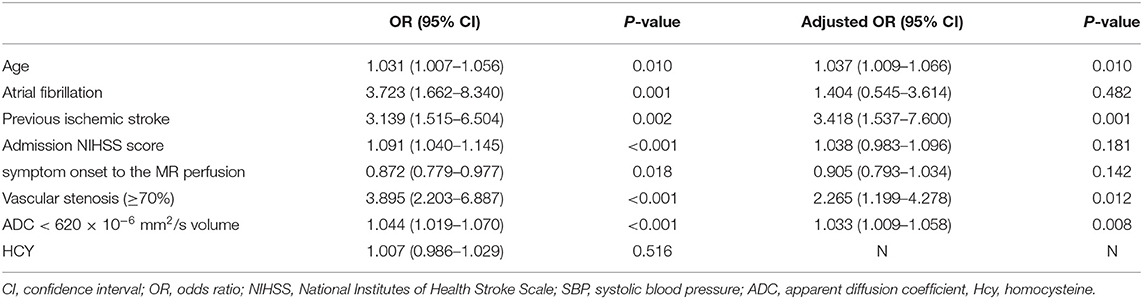
Table 3. Factors independently associated with Tmax > 4–6 s > 38 mL in ischemic stroke patients within 7 days of onset.
Multivariate step-wise regression modeling was performed for predictors with P-values < 0.01 and the multivariate logistic regression analysis performed to show the correlation between previous ischemic stroke, stenosis (≥70%), and MR perfusion abnormalities. Patients with previous ischemic stroke and stenosis were likely to develop hypoperfusion on PWI maps. The adjusted odds ratios were 3.418 (95% CI: 1.537–7.600, P = 0.001) and 2.265 (95% CI: 1.199–4.278, P = 0.012), respectively. Other variables were not significantly associated with hypoperfusion (Table 3).
In the present study, risk factors of hypoperfusion on MRI were analyzed for AIS patients admitted within 7 days of onset. It was found that the perfusion parameter Tmax > 4–6 s volume was related to clinical prognosis. Patients with previous stroke and vascular stenosis (≥70%) were more likely to have hypoperfusion, and these two were independent risk factors of low perfusion as shown by Tmax > 4–6 s map > 38 ml.
It is well-known that DWI and ADC maps were closely related to the final infarct volume and were important predictors of clinical prognosis (9, 28). The present study used non-invasive multimode magnetic resonance imaging to quantitatively evaluate Tmax > 4–6 s map. It was found that the tissue perfusion status of Tmax > 4–6 s was closely related to 90 d mRS. The greater the Tmax > 4–6 s map was, the worse the 90 d mRS was. Previous studies have shown that perfusion imaging was closely related to the status of collateral circulation, and the cerebral perfusion parameter Tmax was a good biomarker of collateral volume (29). Therefore, it is reasonable to use the Tmax > 4–6 s map to evaluate tissue hypoperfusion.
Collateral status could be used to predict the prognosis of patients with acute ischemic stroke as the key determinant (30, 31). Perfusion status changes temporally and spatially. Findings from perfusion imaging reflect collateral status or response to treatment for those without large vessel occlusion within 6 h or for those with large vessel occlusion within 24 h. These are closely correlated with clinical prognosis. In this study, the cutoff value 38 ml of Tmax > 4–6 s map was used to define hypoperfusion on MRI with an aim to find risk factors that were related to hypoperfusion, and to provide a reasonable direction for accurate control of these risk factors. This would improve clinical prognosis and reduce the occurrence of recurrent ischemic events.
This study found that hypoperfusion was associated with recurrent stroke and persistent deterioration of neurological functions. It might be related to a certain proportion of vascular stenosis and distal hypoperfusion in patients with previous strokes, which are likely to recur. This is consistent with the result of a previous studies (32). Previous studies have shown that stroke patients have a recurrence rate of 17% within 1 year. In symptomatic intracranial atherosclerotic stenosis (ICAS) patients, the more severe the baseline hypoperfusion was, the higher the risk of stroke recurrence was (33–35). In this study, 41 patients had a history of stroke, among whom 29 had their Tmax > 4–6 s volume > 38 ml, accounting for 25% of this type of patients. Among these 41 patients, 30 had vascular stenosis ≥ 70%, accounting for 73.2% of these patients. Previous strokes are a risk factor of hypoperfusion. The possible reason is that the majority of patients with previous strokes have vascular stenosis, which leads to distal hypoperfusion, and consequently recurrence of ischemic stroke.
Spencer and Reid first proposed the relationship between cerebral artery stenosis and cerebral blood flow, which predicted a decrease in blood flow when stenosis was >70% (36, 37). Cerebral artery stenosis or occlusion can trigger serious hemodynamic disorders. However, it has been shown that the severity of vascular stenosis does not necessarily affect the status of distal blood flow (38). In this study, it was found that vascular stenosis was related to the Tmax > 4–6 s map. 63.9% of the patients had evidence of ipsilateral proximal artery stenosis or occlusion on MRA, among whom the proportion of tissue hypoperfusion was 60.4% (93/153). Therefore, tissue hypoperfusion may be more likely to occur in patients with ipsilateral proximal artery stenosis or occlusion on MRA, which is consistent with previous studies (39, 40). This might be due to changes in the cerebral vascular structure and function resulted from intracranial atherosclerosis. Vascular stenosis affects the hemodynamic status, mainly through the decrease of cerebrovascular reserve. In the presence of insufficient collaterals, the decrease in pressure may lead to hypoperfusion.
The volume of hypoperfusion at different thresholds of Tmax non-invasively reflected the status of collaterals, which is consistent with findings of previous studies. Therefore, it is likely that perfusion parameters on MRI will be a good biomarker for volumes of collateral blood flow (29). The benefit of the present study is to non-invasively assess hypoperfusion volumes at the early stage of ischemic stroke, which reflects the collateral status. It will provide evidence for early intervention to halt stroke progression, prevent stroke recurrence, and to improve clinical prognosis.
The present study has a number of limitations. Firstly, it is a retrospective study in which all subjects were recruited from a local hospital, which may result in selection bias. In addition, it has a relatively small sample size and the conclusion from this study may not be extrapolated to all ischemic stroke patients. Therefore, prospective studies with a large sample size are required to confirm our findings. Secondly, it is a cross-sectional study and can not pinpoint the direct causality between hypoperfusion and the risk factors of ischemic stroke patients within 7 days of onset. A longitudinal design can help to investigate the direct causal relationship between risk factors and MR hypoperfusion in future studies. Thirdly, Tmax > 4–6 s and volume of hypoperfusion > 38 ml were used to determine hypoperfusion,which is based on the cutoff value of 38 ml in our analysis, whether this method has better accuracy and applicability needs to be verified by future prospective, large-scaled studies. Fourthly, The present study did not repeat MR scan between the therapeutic window and 90 days to evaluate the perfusion status, which might result in treatment bias.
In conclusion, hypoperfusion could be found in ischemic stroke patients within 7 days of onset when PWI was examined, which is related to clinical prognosis. Patients with previous ischemic strokes and vascular stenosis are more likely to have severe hypoperfusion and poor functional outcomes. Accurate control of risk factors may effectively improve functional outcomes. However, larger prospective studies are needed to confirm these findings.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of Shanghai Fourth People's Hospital Affiliated to Tongji University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
YB conceived and designed this study. JX and HL collected data and performed statistical analysis and drafted the manuscript. YueW helped with data collection and data analysis. YiW and YB revised the manuscript. All authors contributed to the article and approved the submitted version.
The present study was supported by a grant from Shanghai Municipal Commission of Health and Family Planning awarded to YB (No. 201840244), a grant from Commission of Health and Family Planning, Hongkou District, awarded to JX (1803-04), the Talent Promotion Project of Shanghai Fourth People's Hospital in 2019 (SY-XKZT-2019-3003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Yu Luo and his team in helping acquire MR images.
1. The National Institute of Neurological Disorders, and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
3. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. (2010) 41:2254–8. doi: 10.1161/STROKEAHA.110.592535
4. Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. (2007) 38:948–54. doi: 10.1161/01.STR.0000257304.21967.ba
5. Huang J, Zhang X, Li J, Tang L, Jiao X, Lv X. Impact of glucose fluctuation on acute cerebral infarction in type 2 diabetes. Can J Neurol Sci. (2014) 41:486–92. doi: 10.1017/S0317167100018539
6. Stead LG, Gilmore RM, Vedula KC, Weaver AL, Decker WW, Brown RD Jr. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. (2006) 66:1878–81. doi: 10.1212/01.wnl.0000219628.78513.b5
7. Tu HT, Campbell BC, Christensen S, Desmond PM, De Silva DA, Parsons MW, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth and hemorrhagic transformation. Int J Stroke. (2015) 10:534–40. doi: 10.1111/ijs.12007
8. Ali SF, Siddiqui K, Ay H, Singhal A, Viswanathan A, Rost N, et al. Baseline predictors of poor outcome in patients too good to treat with intravenous thrombolysis. Stroke. (2016) 47:2986–92. doi: 10.1161/STROKEAHA.116.014871
9. Wheeler HM, Mlynash M, Inoue M, Tipirneni A, Liggins J, Zaharchuk G, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. (2013) 44:681–5. doi: 10.1161/STROKEAHA.111.000135
10. Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol. (2007) 28:382–4.
11. Pan JW, Yu XR, Zhou SY, Wang JH, Zhang J, Geng DY, et al. Computed tomography perfusion and computed tomography angiography for prediction of clinical outcomes in ischemic stroke patients after thrombolysis. Neural Regen Res. (2017) 12:103–8. doi: 10.4103/1673-5374.198994
12. Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. (2010) 41:e34–40. doi: 10.1161/STROKEAHA.109.568766
13. Inoue M, Mlynash M, Straka M, Kemp S, Jovin TG, Tipirneni A, et al. Clinical outcomes strongly associated with the degree of reperfusion achieved in target mismatch patients: pooled data from the diffusion and perfusion imaging evaluation for understanding stroke evolution studies. Stroke. (2013) 44:1885–90. doi: 10.1161/STROKEAHA.111.000371
14. De Silva DA, Brekenfeld C, Ebinger M, Christensen S, Barber PA, Butcher KS, et al. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke. (2010) 41:295–9. doi: 10.1161/STROKEAHA.109.562827
15. Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. RAPID automated patient selectionfor reperfusion therapy a pooled analysis of the echoplanar imaging thrombolytic evaluation trial (EPITHET) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Stroke. (2011) 42:1608–14. doi: 10.1161/STROKEAHA.110.609008
16. Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke. (2010) 41:1169–74. doi: 10.1161/STROKEAHA.110.580670
17. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
18. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
19. Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. (2019) 380:1795–803. doi: 10.1056/NEJMoa1813046
20. Olivot JM, Mlynash M, Thijs VN, Purushotham A, Kemp S, Lansberg MG, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. (2009) 40:1692–7. doi: 10.1161/STROKEAHA.108.538082
21. Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Magnetic resonance imaging profile and response to endovascular reperfusion: results of the DEFUSE 2 prospective cohort study. Lancet Neurol. (2012) 11:860–7. doi: 10.1016/S1474-4422(12)70203-X
22. Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. (2009) 40:469–75. doi: 10.1161/STROKEAHA.108.526954
23. Powers WJ, Zivin J. Magnetic resonance imaging in acute stroke not ready for prime time. Neurology. (1998) 50:842–3. doi: 10.1212/WNL.50.4.842
24. Tourdias T, Renou P, Sibon I, Asselineau J, Bracoud L, Dumoulin M, et al. Final cerebral infarct volume is predictable by MR imaging at 1 week. AJNR Am J Neuroradiol. (2011) 32:352–8. doi: 10.3174/ajnr.A2271
25. Straka M, Albers GW, Bammer R. Real-time diffusionperfusion mismatch analysis in acute stroke. J Magn Reson Imaging. (2010) 32:1024–37. doi: 10.1002/jmri.22338
26. Purushotham A, Campbell BC, Straka M, Mlynash M, Olivot JM, Bammer R, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. (2015) 10:348–53. doi: 10.1111/ijs.12068
27. North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–3. doi: 10.1056/NEJM199108153250701
28. Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. (1995) 37:231–41. doi: 10.1002/ana.410370214
29. Cortijo E, Calleja AI, Garciá-Bermejo P, Mulero P, Pérez-Fernández S, Reyes J, et al. Relative cerebral blood volume as a marker of durable tissue-at-risk viability in hyperacute ischemic stroke. Stroke. (2014) 45:113–8. doi: 10.1161/STROKEAHA.113.003340
30. Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, et al. Collateral clock is more important than time clock for tissue fate. Stroke. (2018) 49:2102–7. doi: 10.1161/STROKEAHA.118.021484
31. Arenillas JF, Cortijo E, García-Bermejo P, Levy EI, Jahan R, Liebeskind D, et al. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab. (2018) 38:1839–47. doi: 10.1177/0271678X17740293
32. De Havenon A, Khatri P, Prabhakaran S, Yeatts SD, Peterson C, Sacchetti D, et al. Hypoperfusion distal to anterior circulation intracranial atherosclerosis is associated with recurrent stroke. J Neuroimaging. (2020) 30:468–70. doi: 10.1111/jon.12710
33. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
34. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterialstenosis. Circulation. (2006) 113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229
35. Lyu J, Ma N, Tian C, Xu F, Shao H, Zhou X, et al. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke Vasc Neurol. (2019) 4:129–34. doi: 10.1136/svn-2018-000228
36. Spencer MP, Reid JM. Quantitation of carotid stenosis with continuouswave (C-W) Doppler ultrasound. Stroke. (1979) 10:326–30. doi: 10.1161/01.STR.10.3.326
37. Carrera E, Lee LK, Giannopoulos S, Marshall RS. Cerebrovascular reactivityand cerebral autoregulation in normal subjects. J Neurol Sci. (2009) 285:191–4. doi: 10.1016/j.jns.2009.06.041
38. Powers WJ, Press GA, Grubb RL Jr, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med. (1987) 106:27–35. doi: 10.7326/0003-4819-106-1-27
39. Ge X, Zhao H, Zhou Z, Li X, Sun B, Wu H, et al. Association of fractional flow on 3D-TOF-MRA with cerebral perfusion in patients with MCA stenosis. AJNR Am J Neuroradiol. (2019) 40:1124–31. doi: 10.3174/ajnr.A6095
Keywords: ischemic stroke, magnetic resonance imaging, time to maximum of the residue function, risk factors, correlation analysis
Citation: Xiao J, Liang H, Wang Y, Wang S, Wang Y and Bi Y (2021) Risk Factors of Hypoperfusion on MRI of Ischemic Stroke Patients Within 7 Days of Onset. Front. Neurol. 12:668360. doi: 10.3389/fneur.2021.668360
Received: 16 February 2021; Accepted: 13 April 2021;
Published: 07 May 2021.
Edited by:
Ramin Zand, Geisinger Health System, United StatesReviewed by:
Seyedmehdi Payabvash, Yale University, United StatesCopyright © 2021 Xiao, Liang, Wang, Wang, Wang and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Bi, ZHJiaXlvbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.