
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 19 May 2021
Sec. Endovascular and Interventional Neurology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.667835
This article is part of the Research Topic Search for optimal treatment for MeVO; thrombectomy, thrombolysis, adjunctive therapy View all 7 articles
 Takeshi Yoshimoto1*
Takeshi Yoshimoto1* Kanta Tanaka2,3
Kanta Tanaka2,3 Junpei Koge3
Junpei Koge3 Masayuki Shiozawa3
Masayuki Shiozawa3 Hiroshi Yamagami4
Hiroshi Yamagami4 Manabu Inoue2,3
Manabu Inoue2,3 Naruhiko Kamogawa1,3
Naruhiko Kamogawa1,3 Tetsu Satow5
Tetsu Satow5 Hiroharu Kataoka5
Hiroharu Kataoka5 Kazunori Toyoda3
Kazunori Toyoda3 Masafumi Ihara1
Masafumi Ihara1 Masatoshi Koga3
Masatoshi Koga3Introduction: The usefulness of the blind exchange with mini-pinning (BEMP) technique has recently been reported for mechanical thrombectomy in patients with stroke owing to medium vessel occlusion (MeVO). The Tron stent retriever can be delivered and deployed through a 0.0165-inch microcatheter. This retriever has potential as an effective and safe treatment for acute ischemic stroke (AIS) due to occlusion of the M2 segment of the middle cerebral artery (MCA). Here, we report the outcomes of the BEMP technique using Tron stent retrievers for M2 occlusion thrombectomy.
Methods: Consecutive patients with AIS owing to M2 occlusion who underwent the BEMP technique using 2 × 15-mm or 4 × 20-mm Tron stent retrievers were included. The technique involves deploying a Tron stent retriever through a 0.0165-inch microcatheter, followed by microcatheter removal and blind navigation of a 3MAX or 4MAX aspiration catheter over the bare Tron delivery wire until the aspiration catheter reaches the clot. A Tron stent retriever is inserted into the aspiration catheter like a cork and subsequently pulled as a unit. We assessed procedural outcomes [first-pass expanded thrombolysis in cerebral infarction (eTICI) score 2c/3 and 2b/2c/3], safety outcomes [symptomatic intracranial hemorrhage (sICH)], and clinical outcomes (good outcome rate defined as modified Rankin Scale score 0–2 at 90 days and mortality at 90 days).
Results: Eighteen M2 vessels were treated in 15 patients (six female, median age: 80 years, and median National Institutes of Health Stroke Scale score: 18). The BEMP technique was performed successfully in all cases. Whether to use a 3MAX or 4MAX catheter was determined by considering one of the following target vessels: dominant, non-dominant, or co-dominant M2 (3MAX, n = 9; 4MAX, n = 9). The first-pass eTICI 2c/3 and 2b/2c/3 rates were 47 (7/15) and 60% (9/15), respectively; sICH was not observed. Seven patients (47%) achieved good outcomes, and one patient (7%) died within 90 days.
Conclusions: The Tron stent retriever was safely and effectively used in the BEMP technique for acute MCA M2 occlusion and can be combined with a 0.0165-inch microcatheter, which may be useful for treating MeVO, in general.
The usefulness of the blind exchange with mini-pinning (BEMP) technique has recently been reported for mechanical thrombectomy in patients with acute ischemic stroke (AIS) owing to medium vessel occlusion (MeVO), i.e., occlusions of the M2/M3 middle cerebral artery (MCA), A2/A3 anterior cerebral artery, and P2/P3 posterior cerebral artery segments (1, 2).
The BEMP technique, which is the blind catheter exchange technique and originally devised using a Trevo 3 × 20-mm retriever (Stryker, Fremont, CA, USA) delivery wire to advance the thromboaspiration catheter and the pinning technique with small devices (“mini-pinning”) for thrombectomy of intracranial distal occlusions, has been reported to be helpful and safe for optimizing procedural performance when treating MeVO (1, 2). Although original mini-pinning was required to pull the stent retriever slightly and lock the stent retriever and aspiration catheter (1), performing this operation for MCA distal M2 occlusion is challenging. The Tron stent retriever is a new stent retriever for AIS owing to not only large intracranial vessel occlusion but also MeVO (3). As the most significant feature, the Tron stent retriever can be deployed via a relatively small 0.0165-inch (0.42-mm) microcatheter, similar to the Aperio (Acandis, Pforzheim, Germany) (2 × 16 mm and 3.5 × 28 mm) (4, 5) or Catch View mini (Catch View; Balt, Montmorency, France) (3.5 × 20 mm), (6) and has a stent retriever diameter profile of 2 mm (3). The Trevo stent retriever, which was used in the first reported BEMP technique (1), has a minimum low profile of 3 mm, and a microcatheter can be used only up to 0.0175 in. (0.44 mm), whereas, the Tron stent retriever has a minimum profile of 2 mm, and a catheter can be used up to 0.0165 in. These differences provide the advantage that the Tron stent retriever can be deployed distally, and that less friction between the stent retriever and vessel wall is also expected to reduce hemorrhagic events (3). Furthermore, the Tron stent retriever is more useful for MCA M2 occlusion thrombectomy in the BEMP technique. Although the traditional combination technique using a Tron stent retriever with a 3MAX or 4MAX aspiration catheter may be effective in enhancing the first-pass effect (FPE) (7) in MCA M2 occlusion thrombectomy, we occasionally experience that a 4MAX aspiration catheter is often outsized for the target vessel, and that a 3MAX aspiration catheter for the target thrombus volume is insufficient when trying to engage these aspiration catheters proximal to the thrombus. In the BEMP technique, the aspiration catheter (3MAX or 4MAX aspiration catheter) can be determined after confirming the target vessel diameter (1, 2). Therefore, the BEMP technique using Tron stent retriever has potential as an effective and safe treatment for MeVO, including occlusion of the MCA M2. Here, we report the outcomes of the BEMP technique using Tron stent retriever for MCA M2 occlusion thrombectomy.
All AIS patients admitted to our institute within 7 days from symptom onset or last-known-well date were prospectively registered in the National Cerebral and Cardiovascular Center (NCVC) Stroke Registry (8–13). Data for AIS patients with MCA M2 occlusion undergoing the BEMP technique were collected retrospectively from the NCVC Stroke Registry from March 2019 to June 2020. The decision to proceed with endovascular therapy (EVT), including the BEMP technique, was made at the discretion of the investigators in a nonrandomized fashion. This study was approved by the institutional review board of the NCVC (approval number: M23-073-4). The NCVC Stroke Registry is registered at ClinicalTrials.gov (NCT02251665).
The Tron stent retriever is a self-expanding stent retriever made of a nickel–titanium alloy that consists of two types of cells with different shapes (3). The large cell captures the thrombus, and the small cell reduces stent extension at the flexion. When deploying and retracting from an elongated target vessel, the stent retriever stretches against the flexion force and passes through the lesser curvature side of an elongated target vessel. However, the unique cell structure of the Tron stent retriever allows the stent structure to stretch at the bend; therefore, this stent retriever is less likely to miss a blood clot. The details of the Tron stent retriever are illustrated in Figure 1.
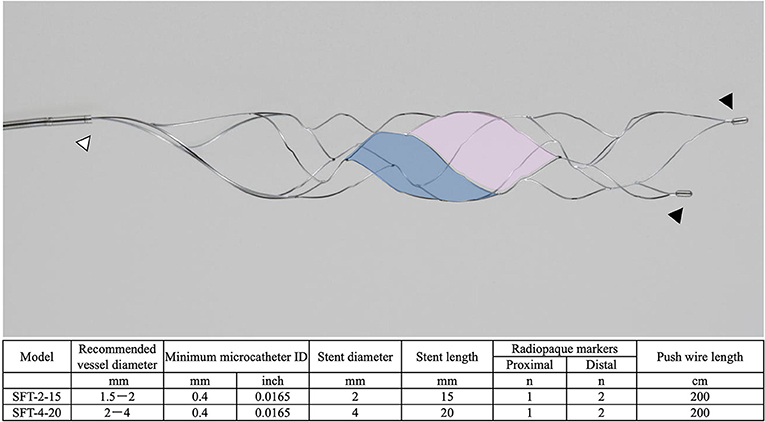
Figure 1. The details of the Tron stent retriever. Photograph of the Tron stent retriever, which is constructed of two types of cells. There are a radiopaque marker in the proximal end (white arrowhead) and two markers attached to the distal end (black arrowheads). The large cell (pink) captures the thrombus, and the small cell (blue) reduces stent extension at the flexion.
All procedures in the BEMP technique using the Tron stent retriever were performed by four experienced neurointerventionalists (TY, KTa, JK, and HY) as a front-line thrombectomy with local anesthesia or conscious sedation. The BEMP technique was performed through a right femoral artery approach, and the occluded-side cervical internal carotid artery (ICA) was catheterized using a balloon guide catheter (BGC). The proximal BGC used in all procedures was a 9-Fr Optimo BGC (Tokai Medical Products Inc., Aichi, Japan). The BEMP technique using the Tron stent retriever is performed initially by advancing a 0.0165-inch microcatheter (Excelsior SL-10 microcatheter; Target Therapeutics/Stryker, Fremont, CA, USA) distal to the occlusion site. Next, a 2 × 15-mm or 4 × 20-mm Tron stent retriever (JIMRO; Terumo, Takasaki, Gunma, Japan) is deployed via a 0.0165-inch microcatheter, followed by microcatheter removal and immediate blind navigation of a low-profile distal aspiration catheter (DAC), namely, a 3MAX or 4MAX aspiration catheter (Penumbra, Alameda, CA, USA), over the bare Tron delivery wire (“blind exchange”) until the DAC reaches the clot. The aspiration catheter is then aspirated using a commercially available aspiration pump. The Tron stent retriever is pulled into the DAC like placing a bottle cork, and the pinned clot with the stent retriever and the DAC then forms a single unit. The Tron stent retriever and DAC are subsequently retracted as a unit to retrieve the clot (1). Figure 2 shows the BEMP technique performed as the primary maneuver. Figure 3 illustrates an example of the BEMP technique using the Tron stent retriever for MCA M2 occlusion.
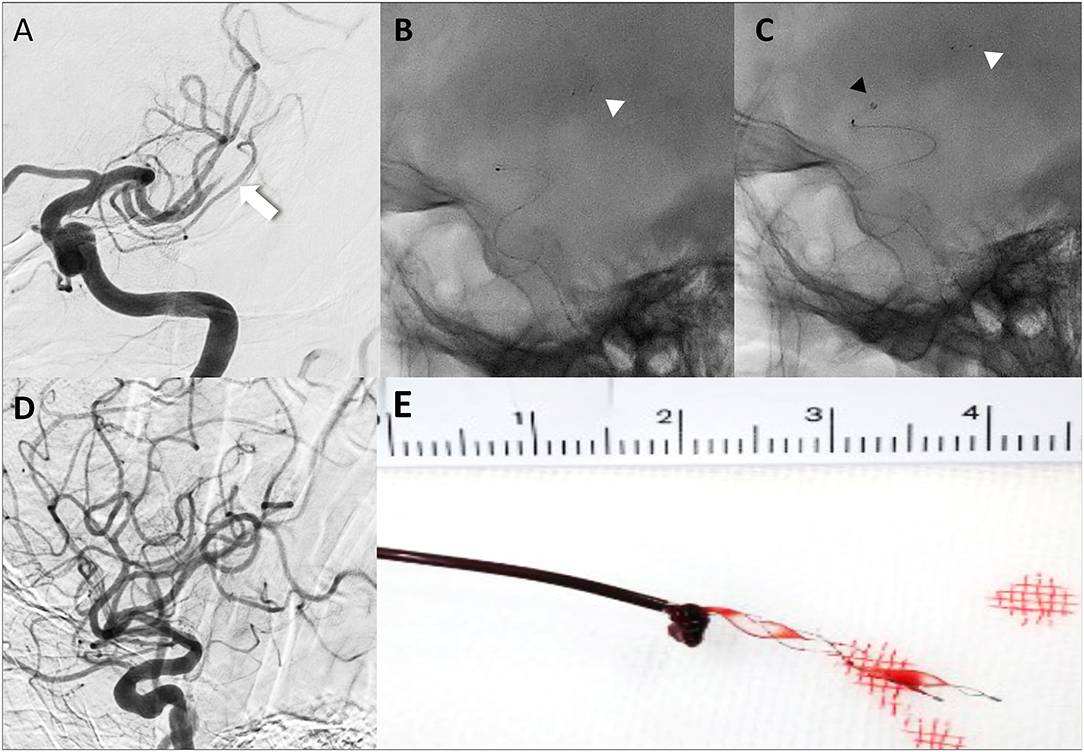
Figure 2. Illustrative cases of the blind exchange and mini-pinning technique using a Tron stent retriever to remove a clot. Illustration of blind exchange and mini-pinning using the Tron stent retriever in a dominant and proximal middle cerebral artery M2 occlusion. (white arrow: proximal end of the clot) (A) Lateral angiogram showing an M2 occlusion. (B) A 0.0165-inch microcatheter in place after deployment (white arrowhead: distal end of the retriever). (C) The 3MAX aspiration catheter is advanced into the face of the clot (black arrowhead) over the bare retriever delivery wire. (D) Final angiogram showing successful reperfusion. (E) Mini-pinning (2-mm Tron stent retriever and 3MAX aspiration catheter) with “corking” of the thrombus.
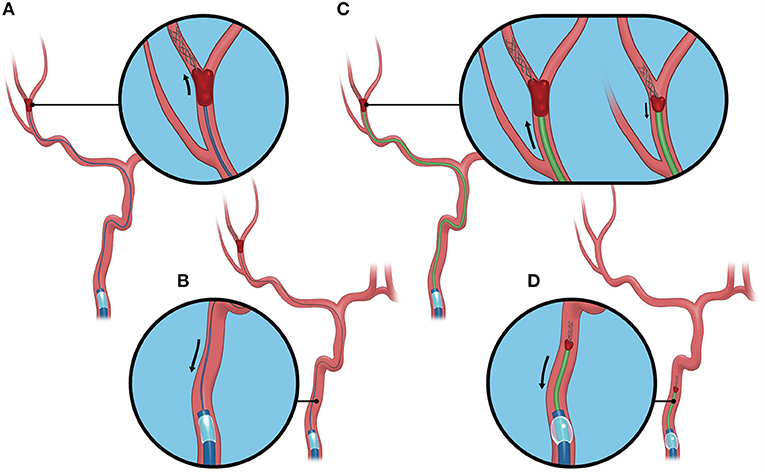
Figure 3. Blind exchange and mini-pinning using a Tron stent retriever to retrieve a clot. Illustrative example of the blind exchange and mini-pinning technique using a Tron stent retriever to treat a middle cerebral artery M2 occlusion. We routinely place a large-bore balloon guide catheter in the cervical internal carotid artery. (A) The biaxial system, including the microcatheter and microguidewire, is advanced as a unit, with the microcatheter and guidewire traversing the occluded segment. The Tron stent retriever is deployed via a 0.0165-inch microcatheter distal to the clot. (B) In the next step, the microcatheter is removed from the body. (C) A 3MAX or 4MAX aspiration catheter, as a distal aspiration catheter, is blindly navigated over the bare Tron delivery wire (“blind exchange”) until the aspiration catheter reaches the clot. (C, left) The Tron stent retriever is pulled into the distal aspiration catheter like placing a bottle cork, and the pinned clot with the stent retriever and the distal aspiration catheters then forms a single unit. (C, right). (D) The Tron stent retriever and aspiration catheter are subsequently retracted as a unit to retrieve the clot.
The baseline clinical characteristics for the following variables were collected: sex, age, prestroke modified Rankin Scale (mRS) score, medical history (hypertension, dyslipidemia, diabetes mellitus, current smoking, ischemic stroke, and atrial fibrillation), systolic blood pressure at admission, baseline National Institutes of Health Stroke Scale (NIHSS) score, baseline Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) on diffusion-weighted imaging (DWI) or computed tomography (CT), baseline ischemic core volume, time logistics (onset-to-groin puncture time and groin puncture-to-revascularization time), treatment profile (intravenous thrombolysis), type of presentation by vessel occlusion (isolated M2 occlusion, tandem occlusion, or multi-vessel M2 occlusion), M2 occlusion site (proximal, distal), M2 type (dominant, co-dominant, or non-dominant), M2 division (superior, inferior), and stroke causative mechanism. Baseline ischemic core volume was calculated according to an apparent diffusion coefficient of <620 × 10−6 mm2/s (14) on DWI or a relative reduction in cerebral blood flow of <30% (15) on CT perfusion using an automated image postprocessing system (RAPID, version 4.9.2.2; iSchemaView, Menlo Park, CA, USA). Hypoperfused tissue, which represents the penumbral tissue, was estimated in accordance with previously validated thresholds as time-to-maximum (Tmax) >6 s (16, 17). Occlusion sites were determined using digital subtraction angiography. The stroke causative mechanism was determined according to the Trial of ORG 10172 in Acute Stroke Treatment criteria by board-certified stroke neurologists.
The MCA M2 segment was defined as the vessels from the main MCA bifurcation/trifurcation to the circular sulcus of the insula (18, 19). M2 occlusions in which the occluded MCA bifurcated after the horizontal segment were considered proximal occlusions. Proximal M2 was defined as the horizontal M2 segment from the main MCA bifurcation/trifurcation within 1 cm from the MCA bifurcation and distal M2 as the Sylvian M2 segment, starting 1 cm from the bifurcation/trifurcation to the circular sulcus of the insula (19–21). M2 caliber dominance was considered present if the M2 branch had a larger diameter than the other branches on digital subtraction angiography or if the perfusion defect associated with the occluded M2 branch was larger than 50% of the MCA territory. Only when the diameters of both the inferior and superior branches were equal and the associated perfusion defect was <50% of the MCA territory, were the branches considered co-dominant (22). M2 caliber dominance can be complicated to determine with initial angiograms, such as when the vessel is small but secondary to occlusion. Therefore, we also referred to hypoperfused tissue volume (Tmax: >6 s) to determine M2 caliber dominance. Angiograms were evaluated for the presence of M2 occlusion, and occlusions were classified according to their clinical scenario: isolated M2 occlusion, tandem occlusion, or multi-vessel M2 occlusion. Tandem occlusion was defined as an M2 occlusion with a concomitant larger vessel/proximal occlusion. Multi-vessel M2 occlusion was defined as ipsilateral occlusions of the M2 superior trunk of the MCA and M2 inferior trunk of the MCA.
The procedural outcomes were defined as a first-pass expanded thrombolysis in cerebral infarction (eTICI) score of 2c/3 and 2b/2c/3 for the targeted M2 (23). Safety outcomes were defined as the presence of any parenchymal hematoma (PH) [hemorrhagic infarction according to the European Cooperative Acute Stroke Study (ECASS); PH type 1 or 2], any intracranial hemorrhage (ICH), symptomatic ICH (sICH), which was defined according to the ECASS II criteria (any ICH with a ≥4-point increase in the NIHSS score from baseline) (24), subarachnoid hemorrhage (SAH) (diffuse or focal within the territory of the artery treated for distal occlusion), arterial spasm (defined as any degree of spasm in treated vessels), emboli to the same or different territory after thrombectomy of a proximal occlusion, active extravasation, and mortality at 90 days. Efficacy outcome was defined as the rate of good outcome (mRS score of 0–2 at 90 days).
Continuous variables are reported as median [interquartile range (IQR)], and categorical variables are reported as proportions. Analyses were performed using Stata 15.1 (StataCorp, College Station, TX, USA).
The study flowchart is provided in Figure 4. A total of 774 patients had a diagnosis of AIS within 24 h of onset between March 2019 and June 2020 in the NCVC Stroke Registry, and of these, 62 patients had MCA M2 occlusion, including 25 patients who underwent EVT. Of the 25 M2 occlusion patients, 18 MCA M2 occlusion patients were treated with the BEMP technique. Of the seven patients who did not undergo the BEMP technique, two patients were enrolled in another device clinical trial, and in one patient, we were unable to advance the BGC into the ICA; this patient underwent a simple stent retrieval technique. The remaining four patients underwent another EVT technique (Aspiration-Retriever Technique for Stroke technique (25) using the EmboTrap II device [Neuravi/Cerenovus, Miami, FL, USA] or a Trevo stent retriever and a large-bore aspiration catheter) at the investigator's discretion. The BEMP technique using a Trevo stent retriever was performed in three patients before the use of the Tron stent retriever was approved.

Figure 4. Study flowchart. ACA, anterior cerebral artery; BA, basilar artery; BEMP, blind exchange with mini-pinning; BGC, balloon guide catheter; CCA, common carotid artery; EVT, endovascular therapy; ICA, internal carotid artery; MCA, middle cerebral artery; NCVC, National Cerebral and Cardiovascular Center; PCA, posterior cerebral artery; VA, vertebral artery.
Six patients (40%) were female; the median (IQR) age was 80 (75–83) years; the median NIHSS score was 18 (11–25); baseline ASPECTS on DWI or CT: 7 (6–10); onset-to-groin puncture time: 141 (101–170) min. The median groin puncture-to-revascularization time was 55 (45–75) min, of which the median groin puncture-to-revascularization time in isolated M2 occlusion was 35 (27–41) min, and that for tandem occlusion or multi-vessel M2 occlusion was 82 (58–112) min. Intravenous alteplase was used in 9 (60%) patients, and a BGC was used in 15 (100%) patients; eight patients had isolated M2 occlusions. Tandem occlusion included the M2 plus the intracranial ICA (n = 3) or MCA M1 (n = 1). Three patients had multi-vessel M2 occlusion. At the M2 occlusion site, 11/18 (61%) vessels had proximal M2 occlusion, and 7/18 vessels had distal M2 occlusion. Dominance of either superior or inferior division was observed in 12/15 (80%) patients, and co-dominance of M2 branches was observed in 3 (20%) patients with M2 occlusion. Dominance of a branch was more commonly seen in the inferior division (7/10). A first-pass eTICI score of 2c/3 was achieved in 7/15 (47%) vessels, and a first-pass eTICI score of 2b/2c/3 was achieved in 9/15 (60%) vessels. A final eTICI score of 2c/3 was obtained in nine patients (60%), and a final eTICI score of 2b/2c/3 was obtained in 14/15 patients (93%). The median (IQR) number of passes was 1 (1–3).
Concerning the safety and efficacy outcomes, PH, SAH, arterial spasm, and emboli to the same territory after thrombectomy of a proximal occlusion were seen in one patient each, respectively. ICH was seen in 6 patients (40%); no sICH, active extravasation, or emboli to the different territory after thrombectomy of a proximal occlusion were observed. The rate of good outcome was 47% (7/15), and 1 patient died with a history of severe heart failure. The patients' baseline characteristics and outcomes are summarized in Table 1, and the cohort clinical outcomes and the outcomes in the M2 occlusion patients undergoing BEMP using the Tron stent retriever and other stent retrievers (Trevo stent retriever) are summarized in Table 2.
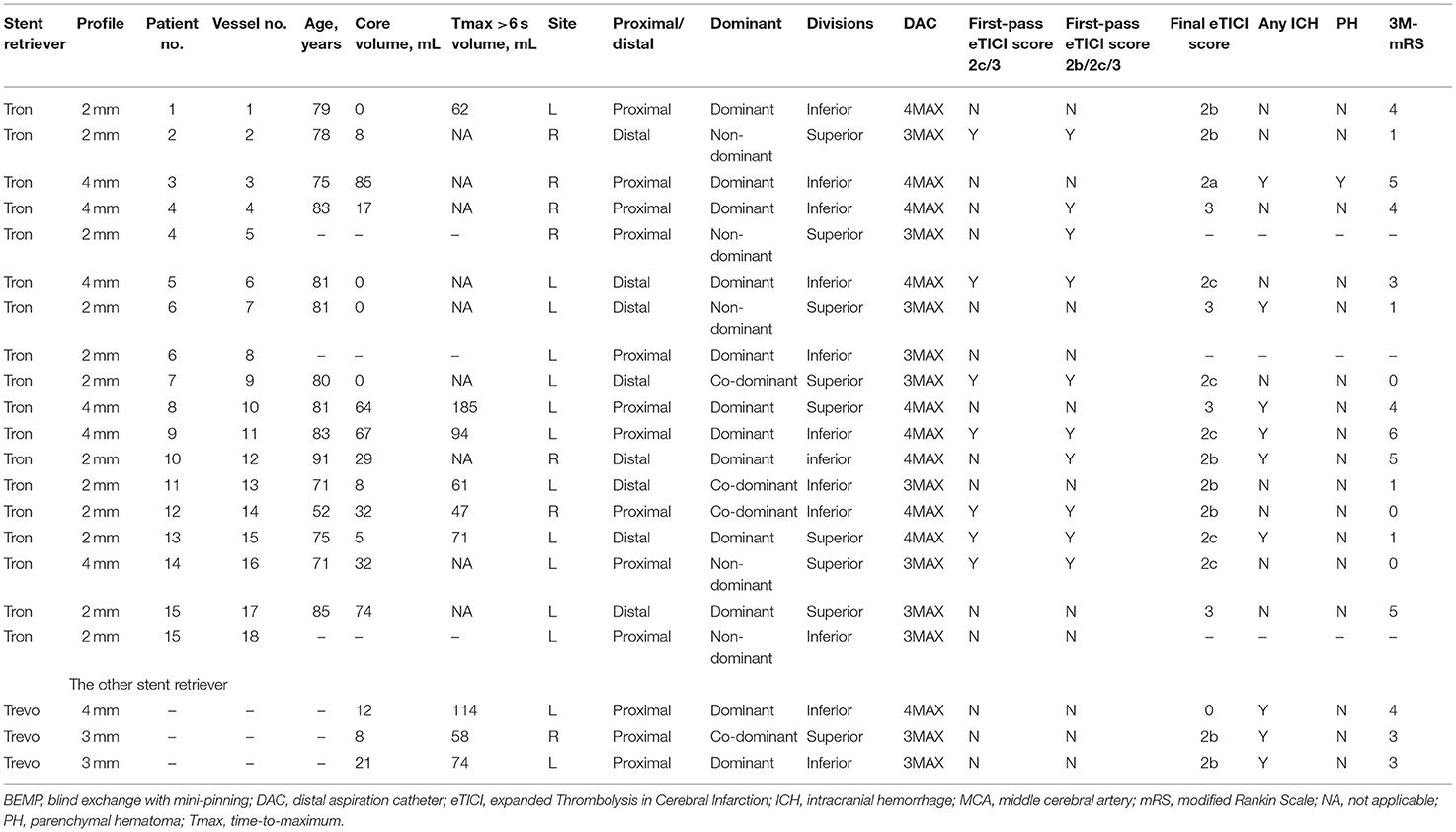
Table 2. The clinical characteristics and outcomes in MCA M2 occlusion patients undergoing the BEMP technique using the Tron vs. other stent retrievers.
In seven patients with MCA M2 occlusion who did not undergo the BEMP technique, the rate of a first-pass eTICI score of 2c/3 was 29% (2/7), and the rate of a first-pass eTICI score of 2b/2c/3 was 43% (3/7). ICH was seen in four patients (57%); no sICH or active extravasation was observed. The rate of good outcome was 43% (3/7).
We describe the BEMP technique using the Tron stent retriever, which appears to be helpful and safe in optimizing the procedural performance in the treatment of MCA M2 occlusion. In the traditional combination technique, we sometimes experience that a 4MAX aspiration catheter (1.42-mm distal outer diameter) is outsized for the target vessel, and a 3MAX aspiration catheter (1.27-mm distal outer diameter) is insufficient for the target thrombus volumes. Therefore, before navigating these catheters in a coaxial manner, it is necessary to thoroughly judge what types of aspiration catheters are required in advance concerning the diameter and sites of the occluded MCA M2 vessels. The blind exchange technique was derived from the need to circumvent this limitation. Furthermore, the stiffness of the retriever delivery wire, distal anchoring by the deployed Tron stent retriever, which has less friction on M2 vessels, and coverage of the wire by the BGC eliminate the risk of pushing/losing the delivery wire in the patient. The subsequent straightening of the vessels that can be achieved by gently pulling the retriever wire greatly facilitates overcoming the barriers to M2 navigation of DACs. Pérez-García et al. reported that the BEMP technique using 3 × 15-mm, 3 × 20-mm, or 3.5 × 28-mm stent retrievers led to higher the first-pass eTICI 2c/3 recanalization rates (57.1%) and a lower incidence of sICH (1.9%) than that using mini-stent retrievers alone in thrombectomy for intracranial MeVOs (2). In our BEMP technique using 2 × 15-mm or 4 × 20-mm Tron stent retrievers, the first-pass eTICI 2c/3 recanalization was observed in 47% of the M2 vessels, and no sICH was seen for MCA M2 occlusion. These results may indicate clinical benefit in the BEMP technique using Tron stent retrievers for MCA M2 occlusion. Additionally, the minimum sizes of low-profile stent retrievers reported in the BEMP technique were in the 3-mm range (Aperio: 3.5 mm and Catch Mini: 3 mm). Few reports of the BEMP technique used tiny, 2-mm stent retrievers. By combining a stent retriever with a 2-mm profile and the effective BEMP technique, it may be possible to more effectively treat MeVO, such as M2 distal and M3 occlusions, which have been somewhat challenging.
The concept of the BEMP technique using the Tron stent retriever for MCA M2 occlusion is to deploy the retriever and advance the DAC until it reaches the clot. Relatively similar techniques for proximal occlusions have been described, mostly consisting of single-arm studies (25–31) and some comparative studies (26, 30–32) showing that the combined use of contact aspiration and the stent retriever technique may be superior to either retriever or aspiration thrombectomy alone. However, there are few reports of techniques specifically analyzing MCA M2 occlusions. The critical aim of the BEMP technique using the Tron stent retriever is to push the DAC slightly past the proximal end of the clot in order to “cork” (partially insert) the thrombus into the catheter and to pull the Tron stent retriever a little into the DAC to “plug” the DAC with the thrombus. This increases the retrieval force by combining the retriever's traction with aspiration, which may explain the relatively higher rates of the first-pass eTICI 2b/2c/3 and the trend toward increased the first-pass eTICI 2c/3 in our results. Moreover, the friction for retrieval is attenuated because less retriever is exposed, consequently decreasing stretching and torsion in tortuous and small distal vessels (32).
It has been reported that the outer diameters of MCA M2 and M3 segments are 1.4–2.3 and 0.8–1.5 mm, respectively (33). The diameters of previous reported mini-stent retrievers are 3–3.5 mm (1, 2, 6), which is considered slightly large for MCA M2 or M3 segments (34). Since the Tron stent retriever has a 2-mm profile, it can be safely deployed even in non-dominant distal M2 occlusions with a small diameter. Finally, by adding the distal support in the clot's vicinity, the force vector is optimized as it approaches the angle of movement. The frequency of hemorrhagic complications was relatively small, in our study, and only one PH, which was caused by recanalization, could be directly attributed to the BEMP technique for MCA M2 occlusion. Although the number was as small as three, any ICH frequency was almost the same as that of the BEMP technique using the Tron stent retriever compared with other stent retrievers. Additionally, although the effect of clot composition on the performance of the different techniques is not well-defined, this appears to play an integral role in procedural performance (31, 32).
One of the present technique's strengths is that an optimal DAC (4MAX or 3MAX aspiration catheter) can be determined after deploying a stent retriever, confirming intermediate flow restoration, and determining the target vessel diameter if the combined use of contact aspiration and the stent retriever technique is considered effective (1, 26). By capturing part of the Tron stent retriever by the low-profile DAC (mini-pinning), there is less contact surface between the stent retriever and the arterial wall, reducing the radial and tractional force exerted by the stent retriever on medium-sized branches and reducing the risk of vessel laceration in MCA M2 thrombectomy. However, the following should be noted when performing the BEMP technique with the Tron stent retriever: if the inside of the aspiration catheter is not coated, such as with the SOFIA (MicroVention, Tustin, CA, USA), the inside of the aspiration catheter may be damaged when blindly guiding the aspiration catheter to the proximal thrombus. Moreover, several pitfalls with the BEMP technique should be noted. When the ICA bifurcation was strongly angled or when the BGC was placed in the common carotid artery owing to difficulty in access, the bare Tron delivery wire flexed more strongly, and the DAC entered the external carotid artery. The key to addressing this pitfall is to follow the bare Tron delivery wire by advancing the DAC while removing the deflection by pulling the bare Tron delivery wire slightly, without forcing the DAC to advance. In our study, the BEMP technique with the Tron stent retriever caused this pitfall in 3/15 patients, but the above method solved this problem in all three patients.
This study has several limitations, and the first limitation was the small sample size. Second, the BEMP technique using the Tron stent retriever cannot be compared with the BEMP technique using other devices or another technique, including a simple stent retrieval technique, contact aspiration, or the combined use of contact aspiration the stent retriever technique. Third, because no formal protocol for device selection was followed, unmeasured variables could have introduced bias. Finally, the costs related to the use of an additional device must be carefully considered. However, our results are encouraging, and further studies are justified.
In conclusion, the Tron stent retriever was safely and effectively used in the BEMP technique for acute MCA M2 occlusion, which was originally devised using the Trevo 3 × 20-mm retriever (1). The features of the Tron stent retriever allow it to be combined with 0.0165-inch microcatheters, and this device may be useful for treating MeVO. Our results might indicate an extended indication and emerging thrombectomy technique in AIS to M2 and M3 MeVO using the Tron stent retriever.
The original contributions generated for this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by this study was approved by the institutional review board of the NCVC (approval number: M23-073-4). The NCVC Stroke Registry is registered at ClinicalTrials.gov (NCT02251665). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TY, KTa, JK, and HY: study conception. TY, KTa, and JK: acquisition of data. TY: analysis and interpretation of the data and drafting the manuscript. TY, KTa, JK, TS, KTo, and MK: editing the manuscript for intellectual content. All authors revising the manuscript critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Jane Charbonneau, DVM, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
1. Haussen DC, Al-Bayati AR, Eby B, Ravindran K, Rodrigues GM, Frankel MR, et al. Blind exchange with mini-pinning technique for distal occlusion thrombectomy. J Neurointervent Surg. (2019) 12:392–5. doi: 10.1136/neurintsurg-2019-015205
2. Pérez-García C, Moreu M, Rosati S, Simal P, Egido JA, Gomez-Escalonilla C, et al. Mechanical thrombectomy in medium vessel occlusions: blind exchange with mini-pinning technique versus mini stent retriever alone. Stroke. (2020) 51:3224–31. doi: 10.1161/STROKEAHA.120.030815
3. Imamura H, Sakai N, Yamagami H, Satow T, Matsumoto Y, Imai K, et al. Clinical trial of the new stent retriever Tron FX for both proximal and distal intracranial large vessel occlusions. J Stroke Cerebrovasc Dis. (2021) 30:105585. doi: 10.1016/j.jstrokecerebrovasdis.2020.105585
4. Kaschner M, Lichtenstein T, Weiss D, Turowski B, Goertz L, Kluner C, et al. The new fully radiopaque Aperio hybrid stent retriever: efficient and safe? An early multicenter experience. World Neurosurg. (2020) 141:e278–88. doi: 10.1016/j.wneu.2020.05.104
5. Kallenberg K, Solymosi L, Taschner CA, Berkefeld J, Schlamann M, Jansen O, et al. Endovascular stroke therapy with the Aperio thrombectomy device. J Neurointerv Surg. (2016) 8:834–9. doi: 10.1136/neurintsurg-2015-011678
6. Onal Y, Velioglu M, Demir U, Celikoglu E, Karakas HM. Feasibility of distal mechanical thrombectomy in M3, A3 and P3 segments via a 0.013-inch delivery system: preliminary experience. Turk Neurosurg. (2020) 30:614–20. doi: 10.5137/1019-5149.JTN.30083-20.2
7. Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al. First pass effect. A new measure for stroke thrombectomy devices. Stroke. (2018) 49:660–6. doi: 10.1161/STROKEAHA.117.020315
8. Sato S, Uehara T, Ohara T, Suzuki R, Toyoda K, Minematsu, et al. Factors associated with unfavorable outcome in minor ischemic stroke. Neurology. (2014) 8:174–81. doi: 10.1212/WNL.0000000000000572
9. Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, et al. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke. (2017) 47:3035–7. doi: 10.1161/STROKEAHA.116.015228
10. Takasugi J, Yamagami H, Noguchi T, Morita Y, Tanaka T, Okuno Y, et al. Detection of left ventricular thrombus by cardiac magnetic resonance in embolic stroke of undetermined source. Stroke. (2017) 48:2434–40. doi: 10.1161/STROKEAHA.117.018263
11. Toyoda K, Koga M, Yamagami H, Yokota C, Sato S, Inoue M, et al. Seasonal variations in neurological severity and outcomes of ischemic stroke: 5-year single-center observational study. Circ J. (2018) 82:1443–50. doi: 10.1253/circj.CJ-17-1310
12. Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, et al. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke. (2019) 50:1751–7. doi: 10.1161/STROKEAHA.119.025142
13. Yoshimoto T, Inoue M, Tanaka K, Kanemaru K, Koge J, Shiozawa M, et al. Identifying large ischemic core volume ranges in acute stroke that can benefit from mechanical thrombectomy. J NeuroInterv Surg. (2020). doi: 10.1136/neurintsurg-2020-016934. [Epub ahead of print].
14. Purushotham A, Campbell BCV, Straka M, Mlynash M, Olivot JM, Bammer R, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. (2015) 10:348–53. doi: 10.1111/ijs.12068
15. Campbell BCV, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. (2011) 42:3435–40. doi: 10.1161/STROKEAHA.111.618355
16. Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. (2009) 40:469–75. doi: 10.1161/STROKEAHA.108.526954
17. Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynashet M, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Stroke. (2011) 42:1608–14. doi: 10.1161/STROKEAHA.110.609008
18. Fischer E. Die Lageabweichungen der vorderen Hirnarterieim Gefassbild. Zentralblatt Fur Neurochirurgie. (1938) 3:300–12.
19. Goyal M, Ospel JM, Menon BK, Hill M.D. MeVO: the next frontier? J Neurointerv Surg. (2020) 12:545–7. doi: 10.1136/neurintsurg-2020-015807
20. Ospel JM, Menon BK, Demchuk AM, Almekhlafi MA, Kashani N, Mayank A, et al. Clinical course of acute ischemic stroke due to medium vessel occlusion with and without intravenous alteplase treatment. Stroke. (2020) 51:3232–40. doi: 10.1161/STROKEAHA.120.030227
21. Menon BK, Hill MD, Davalos A, Roos YBWEM, Campbell BCV, Dippel DWJ, et al. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: meta-analysis of data from the HERMES Collaboration. J Neurointerv Surg. (2019) 11:1065–9. doi: 10.1136/heartjnl-2014-307109.258
22. Compagne KCJ, Van Der Sluijs PM, Van Den Wijngaard IR, Roozenbeek B, Mulder MJHL, van Zwam HM, et al. Endovascular treatment: the role of dominant caliber M2 segment occlusion in ischemic stroke. Stroke. (2019) 50:419–27. doi: 10.1161/STR.0000000000000185
23. Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. (2019) 11:433–8. doi: 10.1136/neurintsurg-2018-014127
24. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
25. Massari F, Henninger N, Lozano JD, Patel A, Kuhn AL, Howk M, et al. ARTS (Aspiration-Retriever Technique for Stroke): initial clinical experience. Interv Neuroradiol. (2016) 22:325–32. doi: 10.1177/1591019916632369
26. Haussen DC, Lima A, Nogueira R. The Trevo XP 320 mm retriever ('Baby Trevo') for the treatment of distal intracranial occlusions. J Neurointerv Surg. (2016) 8:295–9. doi: 10.1136/neurintsurg-2014-011613
27. Jadhav AP, Aghaebrahim A, Horev A, Giurgiutiu DV, Ducruet AF, Jankowitz B, et al. Stent retriever-mediated manual aspiration thrombectomy for acute ischemic stroke. Interv Neurol. (2017) 6:16–24. doi: 10.1159/000449321
28. Maus V, Behme D, Kabbasch C, Borggrefe J, Tsogkas I, Nikoubashmanet O, et al. Maximizing first-pass complete reperfusion with SAVE. Clin Neuroradiol. (2018) 28:327–38. doi: 10.1007/s00062-017-0566-z
29. Hesse AC, Behme D, Kemmling A, Zapf A, Hokamp NG, Frischmuthet I, et al. Comparing different thrombectomy techniques in five large-volume centers: a 'real world' observational study. J Neurointerv Surg. (2018) 10:525–9. doi: 10.1136/neurintsurg-2017-013394
30. Delgado Almandoz JE, Kayan Y, Young ML, Fease JL, Scholz JM, Milner AM, et al. Comparison of clinical outcomes in patients with acute ischemic strokes treated with mechanical thrombectomy using either Solumbra or ADAPT techniques. J Neurointerv Surg. (2016) 8:1123–8. doi: 10.1136/neurintsurg-2015-012122
31. Bourcier R, Mazighi M, Labreuche J, Fahed R, Blanc R, Gory B, et al. Susceptibility vessel sign in the ASTER trial: higher recanalization rate and more favourable clinical outcome after first line stent retriever compared to contact aspiration. J Stroke. (2018) 20:268–76. doi: 10.5853/jos.2018.00192
32. Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA, et al. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. (2018) 10:34–8. doi: 10.1136/neurintsurg-2016-012721
33. Umansky F, Juarez SM, Dujovny M, Ausman JI, Diaz FG, Gomes F, et al. Microsurgical anatomy of the proximal segment of the middle cerebral artery. J Neurosurg. (1984) 61:458–67. doi: 10.3171/jns.1984.61.3.0458
Keywords: BEMP technique, M2 occlusion, Tron stent retriever, thrombectomy, acute ischemic stroke
Citation: Yoshimoto T, Tanaka K, Koge J, Shiozawa M, Yamagami H, Inoue M, Kamogawa N, Satow T, Kataoka H, Toyoda K, Ihara M and Koga M (2021) Blind Exchange With Mini-Pinning Technique Using the Tron Stent Retriever for Middle Cerebral Artery M2 Occlusion Thrombectomy in Acute Ischemic Stroke. Front. Neurol. 12:667835. doi: 10.3389/fneur.2021.667835
Received: 19 February 2021; Accepted: 07 April 2021;
Published: 19 May 2021.
Edited by:
Diogo C. Haussen, Emory University, United StatesReviewed by:
Carlos Pérez García, San Carlos University Clinical Hospital, SpainCopyright © 2021 Yoshimoto, Tanaka, Koge, Shiozawa, Yamagami, Inoue, Kamogawa, Satow, Kataoka, Toyoda, Ihara and Koga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takeshi Yoshimoto, eW9zaGltb3RvdGFrZXNoaTE5ODJAbmN2Yy5nby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.