A Commentary on
Enhanced Hemodynamic and Clinical Response to αCGRP in Migraine Patients—A TCD Study
by Visočnik, D., Zaletel, M., Žvan, B., and Zupan, M. (2021). Front. Neurol. 12:638903. doi: 10.3389/fneur.2021.638903
Historically, meningeal vessel nociceptive sensitization has been a milestone of the migraine pathophysiology knowledge and the starting point of implementing new therapeutic strategies. Activated trigeminovascular terminals release peptides, which modulate pain and vascular activity (1). This neurogenic influence on cerebral hemodynamics is part of a very fine orchestral action with myogenic (i.e., autoregulation), endothelial (i.e., endothelial reactivity), and metabolic responses (i.e., vasomotor reactivity) (Figure 1). The astrocyte production of prostaglandins and nitric oxide (NO) responds to the neuronal firing (i.e., neurovascular coupling), mediating smaller intraparenchymal arterioles' dilatation. The neurogenic control of medium and small size arteries, on the other hand, occurs through the activation of sympathetic, parasympathetic, and sensory neurons. The last ones act secreting calcitonin gene-related peptide (CGRP), NO, serotonin, and Pituitary Adenylate Cyclase-Activating Polypeptide [PACAP] (2). Of these, CGRP is probably the most potent vasodilatory agent released in the cerebral circulation (3). Visočnik et al. (4) investigated by transcranial doppler the effect of the infusion of αCGRP on cerebral hemodynamics in migraine patients and healthy controls. Confirming what was observed by Lassen (5) in a placebo-controlled design, they observed a reduction in mean velocity of the middle cerebral artery (MCA) in both groups reflecting vaso-dilation. The relative contribution of cerebral vessels' dilation in determining migraine pain is much debated.
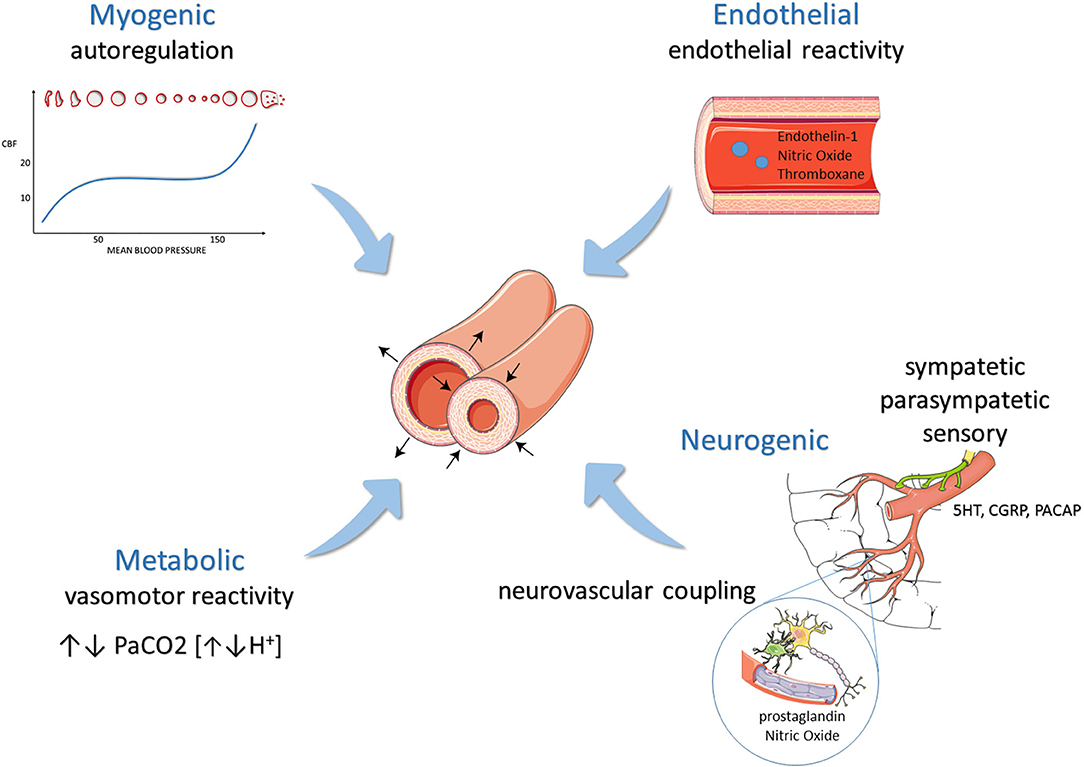
Figure 1. Modulation of cerebral hemodynamics: mechanisms balancing vasoconstriction and vasodilation.
Interestingly, not all cerebral vasodilatory agents (e.g., VIP, CO2) trigger migraine. Conversely, PACAP, which can dilate meningeal vessels but not MCA, can provoke headache in most subjects (6, 7) with a more pronounced response in migraine patients. These observations suggest that cerebral vessel dilation is an epiphenomenon of attacks rather than a trigger.
On the other hand, the MCA velocity reduction induced by αCGRP infusion was higher in migraineurs than controls, suggesting that they have a vascular tree more prone to sensitization (neurogenic modulation of hemodynamics) (4, 5). Similar hyperactive responses were observed under other conditions influencing hemodynamics. Nitric oxide super-sensitivity of the cerebral circulation has been consistently demonstrated in migraineurs (endothelial and parasympathetic control) (8). Similarly, migraine patients, in particular those with migraine with aura (MA), can display a higher response to hypercapnia (i.e., vasomotor reactivity, metabolic control) (9, 10). Finally, the vascular tree of MA patients is also more reactive to sensory inputs such as visual stimulation (i.e., neurovascular coupling) (11).
In the study by Visočnik and colleagues, mean arterial pressure was not modified during α-CGRP infusion, even if it increased significantly soon after only in migraine patients. This finding suggests that CGRP has no direct effect on systemic circulation. Accordingly, erenumab does not impair brachial flow-mediated dilation under physiological conditions in migraineurs without aura (12). On the other hand, peripheral vascular dysfunction was proposed in patients with migraine (13, 14). However, the studies addressing this topic reported highly conflicting findings, with some authors hypothesizing a hyperactive NO-supersensitive peripheral circulation in migraine (15).
It begs the question: if cerebral vessel dilation is just an epiphenomenon of migraine attacks, why is it promoted by the different mechanisms of hemodynamic control? The answer to this question would shed light on migraine pathophysiology and help understand the pathological link between migraine and stroke (16).
The monoclonal antibodies targeting the CGRP pathway are the first pharmacological therapies specifically developed for migraine prevention (17). These antibodies do not pass the blood-brain barrier for their high molecular weight; thus, their rapid efficacy in migraine prevention is mainly due to their peripheral action. Accordingly, Erenumab does not impair cerebral hemodynamics under physiological conditions in migraineurs without aura (12), nor the vasoactive response in isolated cranial arteries (18).
These observations suggest that CGRP is not involved in the control of hemodynamics under physiological conditions, reassuring the use of CGRP–targeted therapy in the clinical practice.
On the other hand, CGRP receptor antagonists seem to worsen cerebral ischemia in mice after MCA occlusion (19). Similarly, the autoregulatory vasodilation in response to hypotension was found attenuated by CGRP receptor desensitization in animal models (20). Altogether, this evidence points toward the hypothesis that CGRP can be called upon the need to rescue vasodilation when other compensatory mechanisms fail in extreme conditions.
If so, why is it released during migraine attacks? What would it be the physiological meaning and evolutionary advantage in migraine? To provide an answer to these questions, one could look at the relationship between cortical spreading depression (CSD) and CGRP (21, 22). CGRP seems not to trigger CSD while it is true the opposite (23). Genetic studies also support the primary role of cortical activation (i.e., CSD) in the migraine cascade, ultimately producing vessel dilation (24).
On the other hand, CGRP can modulate CSD propagation (23). Interestingly, cerebral blood flow can contra-regulate neural activity (the so-called vascular-neural coupling) (25). In this view, the physiological advantage of CGRP release would be to support the rapid wave of depolarization, increasing blood flow to meet the amplified metabolic demands. In this line, as opposed to the historical view of migraine as a neurovascular disorder, recent evidence bring light to the predominant role of the brain cortex and its metabolic demands due to aberrant plasticity and excitability as the pathophysiological substrate of the migraine cycle (26). Thus, more amply, the evolutionary role of CGRP is likely to prepare the brain to face stressful conditions (i.e., increased metabolic demand) via multiple mechanisms (27).
Finally, CGRP could counterbalance the action of other substances released during CSD with vasoconstrictive properties, such as endothelin-1 (28). In this line, CGRP related vasodilation can be considered a vascular adaptation to the metabolic and hemodynamic consequences of CSD.
This hypothesis would also explain the apparently paradoxical observation of a progressive improvement of cerebral hemodynamics along with migraine disease history (29, 30). These reports are also clinically reflected by the relative lower risk of stroke in MA patients with longer disease history, as in subjects with early-onset, compared with those with shorter disease history (31).
Altogether, these observations draw the picture of CRGP as “the goodfella” of cerebral hemodynamics, even if it is the ultimate guilty for migraine pain. Nevertheless, we are far from a full understanding of the relative role of CGRP in the physiology of cerebral hemodynamics and the physiopathology of migraine. It is particularly elusive the precise pathway conducting from neuro-excitability (32) to CGRP release (33). In the perspective of the development of new CGRP-targeted therapies for migraine, further research addressing this complex interrelationship is necessary, possibly combining neurophysiological and neurosonological investigations.
Author Contributions
CA performed literature revision and drafted the manuscript. FV reviewed the manuscript content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Dodick DW. A phase-by-phase review of migraine pathophysiology. Headache. (2018) 58:4–16. doi: 10.1111/head.13300
2. Frederiksen SD, Haanes KA, Warfvinge K, Edvinsson L. Perivascular neurotransmitters: regulation of cerebral blood flow and role in primary headaches. J Cereb Blood Flow Metab. (2019) 39:610–32. doi: 10.1177/0271678X17747188
3. Goadsby PJ, Duckworth JW. Effect of stimulation of trigeminal ganglion on regional cerebral blood flow in cats. Am J Physiol Regul Integr Comp Physiol. (1987) 253: R270–4. doi: 10.1152/ajpregu.1987.253.2.r270
4. Visočnik D, Zaletel M, Zvan B, Zupan M. Enhanced hemodynamic and clinical response to αCGRP in migraine patients -A TCD study. Front Neurol. (2021) 12:638903. doi: 10.3389/FNEUR.2021.638903
5. Lassen LH, Jacobsen VB, Haderslev PA, Sperling B, Iversen HK, Olesen J, et al. Involvement of calcitonin gene-related peptide in migraine: regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain. (2008) 9:151–7. doi: 10.1007/s10194-008-0036-8
6. Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. (2009) 132:16–25. doi: 10.1093/brain/awn307
7. Hansen JM, Schankin CJ. Cerebral hemodynamics in the different phases of migraine and cluster headache. J Cereb Blood Flow Metab. (2019) 39:595–609. doi: 10.1177/0271678X17729783
8. Olesen J, Thomsen LL, Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol Sci. (1994) 15:149–53. doi: 10.1016/0165-6147(94)90075-2
9. Vernieri F, Tibuzzi F, Pasqualetti P, Altamura C, Palazzo P, Rossini PM, et al. Increased cerebral vasomotor reactivity in migraine with aura: an autoregulation disorder? A transcranial Doppler and near-infrared spectroscopy study. Cephalalgia. (2008) 28:689–95. doi: 10.1111/j.1468-2982.2008.01579.x
10. Altamura C, Paolucci M, Brunelli N, Rizzo AC, Cecchi G, Assenza F, et al. Right-to-left shunts and hormonal therapy influence cerebral vasomotor reactivity in patients with migraine with aura. PLoS ONE. (2019) 14:e0220637. doi: 10.1371/journal.pone.0220637
11. Wolf M, Jäger T, Bäzner H, Hennerici M. Changes in functional vasomotor reactivity in migraine with aura. Cephalalgia. (2009) 29:1156–64. doi: 10.1111/j.1468-2982.2009.01843.x
12. Altamura C, Viticchi G, Fallacara A, Costa CM, Brunelli N, Fiori C, et al. Erenumab does not alter cerebral hemodynamics and endothelial function in migraine without aura. Cephalalgia. (2021) 41:90–8. doi: 10.1177/0333102420956692
13. Tietjen G. Migraine as a systemic vasculopathy. Cephalalgia. (2009) 29:989–96. doi: 10.1111/j.1468-2982.2009.01937.x
14. Sacco S, Ripa P, Grassi D, Pistoia F, Ornello R, Carolei A, et al. Peripheral vascular dysfunction in migraine: a review. J Headache Pain. (2013) 14:80. doi: 10.1186/1129-2377-14-80
15. Vernieri F, Moro L, Altamura C, Palazzo P, Antonelli Incalzi R, Rossini PM, et al. Patients with migraine with aura have increased flow mediated dilation. BMC Neurol. (2010) 10:18. doi: 10.1186/1471-2377-10-18
16. Ornello R, Frattale I, Caponnetto V, Pistoia F, Sacco S. Cerebral vascular reactivity and the migraine-stroke relationship: a narrative review. J Neurol Sci. (2020) 414:116887. doi: 10.1016/j.jns.2020.116887
17. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - Successful translation from bench to clinic. Nat Rev Neurol. (2018) 14:338–50. doi: 10.1038/s41582-018-0003-1
18. Ohlsson L, Haanes KA, Kronvall E, Xu C, Snellman J, Edvinsson L. Erenumab (AMG 334), a monoclonal antagonist antibody against the canonical CGRP receptor, does not impair vasodilatory or contractile responses to other vasoactive agents in human isolated cranial arteries. Cephalalgia. (2019) 39:1745–52. doi: 10.1177/0333102419867282
19. Mulder IA, Li M, de Vries T, Qin T, Yanagisawa T, Sugimoto K, et al. Anti-migraine calcitonin gene–related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. (2020) 88:771–84. doi: 10.1002/ana.25831
20. Shin HK, Hong KW. Importance of calcitonin gene-related peptide, adenosine and reactive oxygen species in cerebral autoregulation under normal and diseased conditions. Clin Exp Pharmacol Physiol. (2004) 31:1–7. doi: 10.1111/j.1440-1681.2004.03943.x
21. Close LN, Eftekhari S, Wang M, Charles AC, Russo AF. Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia. (2019) 39:428–34. doi: 10.1177/0333102418774299
22. Dussor G. New discoveries in migraine mechanisms and therapeutic targets. Curr Opin Physiol. (2019) 11:116–24. doi: 10.1016/j.cophys.2019.10.013
23. Melo-Carrillo A, Schain AJ, Stratton J, Strassman AM, Burstein R. Fremanezumab and its isotype slow propagation rate and shorten cortical recovery period but do not prevent occurrence of cortical spreading depression in rats with compromised blood-brain barrier. Pain. (2020) 161:1037–43. doi: 10.1097/j.pain.0000000000001791
24. Di Stefano V, Rispoli MG, Pellegrino N, Graziosi A, Rotondo E, Napoli C, et al. Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. (2020) 91:764–71. doi: 10.1136/jnnp-2020-322850
25. Kim KJ, Diaz JR, Iddings JA, Filosa JA. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci. (2016) 36:12624–39. doi: 10.1523/JNEUROSCI.1300-16.2016
26. Barbanti P, Brighina F, Egeo G, Di Stefano V, Silvestro M, Russo A. Migraine as a cortical brain disorder. Headache. (2020) 60:2103–14. doi: 10.1111/head.13935
27. Borkum JM. CGRP and brain functioning: cautions for migraine treatment. Headache. (2019) 59:1339–57. doi: 10.1111/head.13591
28. Iljazi A, Ayata C, Ashina M, Hougaard A. The role of endothelin in the pathophysiology of migraine—a systematic review. Curr Pain Headache Rep. (2018) 22:27. doi: 10.1007/s11916-018-0682-8
29. Gollion C, Nasr N, Fabre N, Barège M, Kermorgant M, Marquine L, et al. Cerebral autoregulation in migraine with aura: a case control study. Cephalalgia. (2019) 39:635–40. doi: 10.1177/0333102418806861
30. Lee MJ, Cho S, Woo SY, Chung CS. Paradoxical association between age and cerebrovascular reactivity in migraine: a cross-sectional study. J Neurol Sci. (2019) 398:204–9. doi: 10.1016/j.jns.2019.01.039
31. Androulakis XM, Sen S, Kodumuri N, Zhang T, Grego J, Rosamond W, et al. Migraine age of onset and association with ischemic stroke in late life: 20 years follow-up in ARIC. Headache J Head Face Pain. (2019) 59:556–66. doi: 10.1111/head.13468
32. Brighina F, Bolognini N, Cosentino G, MacCora S, Paladino P, Baschi R, et al. Visual cortex hyperexcitability in migraine in response to sound-induced flash illusions. Neurology. (2015) 84:2057–61. doi: 10.1212/WNL.0000000000001584
Keywords: migraine, cerebral hemodynamics, calcitonin gene-related peptide, cortical spreading depolarization, ultrasound
Citation: Altamura C and Vernieri F (2021) Commentary: Enhanced Hemodynamic and Clinical Response to αCGRP in Migraine Patients—A TCD Study. Front. Neurol. 12:663818. doi: 10.3389/fneur.2021.663818
Received: 03 February 2021; Accepted: 22 February 2021;
Published: 18 March 2021.
Edited by:
Simona Sacco, University of L'Aquila, ItalyReviewed by:
Vincenzo Di Stefano, University of Palermo, ItalyRaffaele Ornello, University of L'Aquila, Italy
Copyright © 2021 Altamura and Vernieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Altamura, Yy5hbHRhbXVyYUB1bmljYW1wdXMuaXQ=
 Claudia Altamura
Claudia Altamura Fabrizio Vernieri
Fabrizio Vernieri