
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 14 June 2021
Sec. Dementia and Neurodegenerative Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.663637
 Yuting Ren1,2
Yuting Ren1,2 Siyuan Li1
Siyuan Li1 Siyu Chen3
Siyu Chen3 Xiaosun Sun1,4
Xiaosun Sun1,4 Fei Yang1
Fei Yang1 Hongfen Wang1
Hongfen Wang1 Mao Li1
Mao Li1 Fang Cui1
Fang Cui1 Xusheng Huang1*
Xusheng Huang1*Objective: The aim of this study was to measure both plasma and cerebrospinal fluid (CSF) TAR DNA-binding protein 43 (TDP-43) and phosphorylated TDP-43 (pTDP-43) levels in sporadic amyotrophic lateral sclerosis (sALS) patients, and to compare them with that of healthy controls. The correlation between plasma or CSF TDP-43/pTDP-43 and clinical indicators of ALS patients was assessed.
Methods: Paired plasma and CSF TDP-43/pTDP-43 levels in 69 ALS patients and 59 healthy controls were measured by sandwich ELISA. Time to generalization (TTG), an indicator suggested that the time of symptoms spreading from spinal or bulbar localization to both, was evaluated in all patients screened for mutations in genes associated with ALS.
Results: Both of the plasma TDP-43 and pTDP-43 levels were significantly higher in ALS patients than HCs (P < 0.001). The pTDP-43/TDP-43 ratios in plasma were significantly higher in HCs than ALS patients (P < 0.001). The area under the curve (AUC) value was 0.924 for plasma TDP-43 level, with a 91.3% sensitivity and 91.5% specificity. Moreover, the correlation between plasma and CSF TDP-43 was observed in each ALS patient (r = 0.195, P = 0.027). A correlation between CSF pTDP-43 levels and the ALSFRS-R (r = −0.245; P = 0.042) was established. A correlation was observed between plasma TDP-43 levels and TTG in ALS patients, which indicated that high levels of plasma TDP-43 correlated with prolonged TTG (r = 0.415; P = 0.004).
Conclusion: The plasma TDP-43 and pTDP-43 levels might play an important role in diagnosis in the future study of ALS. The plasma TDP-43 might differentiate ALS and HC groups based on high sensitivity and specificity, and as an indicator of progression of disease.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by the degeneration of both upper and lower motor neurons, with a median survival of 3–5 years (1). The pathogenesis of the disease is not fully elucidated and there is still lack of effective treatments. Thus, a specific and reliable biomarker is essential for diagnosis, assessment of disease progression and prognosis in ALS patients.
Previous reports indicated that plasma TDP-43 protein levels was elevated in 46% patients with frontotemporal dementia (FTD) as compared to the control subjects as assessed by ELISA (2). Interestingly, based on the autopsy studies, the proportion of FTD patients showing increased plasma TDP-43 levels was similar to that of patients known to harbor TDP-43 pathological changes in brains. These results concluded that increased biofluids such as plasma or cerebrospinal fluid (CSF) levels of TDP-43 may indicate the presence and degree of TDP-43 pathology within the brain (2). Although a range of antibodies can be used to detect TDP-43 in biofluids, an incredibly highly variability of detection in samples from ALS and FTD patients has been reported (3). Several studies explored the role of plasma and CSF TDP-43 levels as a biomarker in patients with FTD or ALS and demonstrated variable results (2, 4–8). Foulds et al. developed an ELISA-based method to assess the levels of phosphorylated TDP-43 (pTDP-43) within plasma samples, which seemed to be more reliable than total TDP-43 in distinguishing FTD-TDP from other forms of FTD or Alzheimer's disease (5). Noto et al. showed that the CSF TDP-43 levels were elevated only in ALS patients and lower CSF TDP-43 levels may be associated with worse survival (8). Steinacker et al. reported that patients with ALS and FTD had higher TDP-43 levels than controls in CSF, which indicated that CSF TDP-43 might aid in characterizing the subgroups of patients across the ALS and FTD disease spectrum (6). Suarez-Calvet et al. used ELISA to estimate the pTDP-43 levels in plasma and CSF in FTD patients and healthy controls, thereby suggesting that plasma pTDP-43 levels may be increased in subjects with mutations in GRN and C9orf72 genes (9). Majumder et al. conducted a meta-analysis suggesting that CSF TDP-43 levels could be a promising biomarker in FTD-ALS spectrum disorders in particular ALS patients (10). Junttila et al. showed that higher CSF TDP-43 levels in ALS patients and this finding was independent of the C9orf72 expansion carrier status, and suggested that CSF TDP-43 levels might be an indicator of a more rapid progression in ALS (11). Lower TDP-43 levels in CSF have also been observed in C9orf72 repeat expansion carriers with underlying TDP-43 pathology and a shorter survival in ALS patients. Kasai et al. showed that increased levels of CSF TDP-43 in sALS compared with control groups. The elevated CSF TDP-43 levels may preempt the accumulation of TDP-43 pathology in the central nervous system, or parallel with TDP-43 pathology in the early stage thereby serving as a biomarker for ALS (4). The combined use of CSF neurofilament light chain (NfL) and CSF TDP-43 may be a useful biomarker for the diagnosis of ALS (12). Bourbouli et al. found that combined analysis of CSF TDP-43, total tau protein (τT), and tau protein phosphorylated at threonine 181 would be of help in the antemortem diagnosis of ALS (13). Hosokawa et al. reported that TDP-43 concentrations in CSF were significantly higher in ALS than in Guillain-Barré syndrome (GBS), which suggested that quantitative measurement of CSF TDP-43 levels is a potential laboratory testing for differentiating ALS from peripheral neuropathies (14). In a relatively large cohort of 219 sALS patients and 100 healthy controls, Verstraete et al. confirmed that TDP-43 plasma levels were significantly increased in ALS patients and found a positive correlation with age in all subjects (7). However, whether phosphorylation of TDP-43 is a useful biomarker or an early pathological event in disease, perhaps even promoting mislocalization and aggregation, or is secondary to degradation processes or aggregate formation stays to be elucidated (3). Therefore, the present study aimed to measure both plasma and CSF TDP-43 and pTDP-43 levels in ALS patients. Furthermore, the correlations between TDP-43/pTDP-43 levels and clinical indicators such as age, disease duration, amyotrophic lateral sclerosis functional rating scale revised (ALSFRS-R) score and disease progression rate were also examined. In addition, we applied an early clinical parameter of survival that previously reported, the time of symptoms spreading from spinal or bulbar localization to generalization, known simply as time to generalization (TTG) (15, 16).
The study enrolled 78 patients admitted to the Department of Neurology, Chinese PLA General Hospital, from November 2013–May 2018, from ALS patients with definite or probable ALS according to the revised El Escorial criteria (17) diagnosed by a neurologist specialized in motor neuron diseases. All the ALS patients who participated in our study were apparently sporadic cases. A family history of dementia was excluded and in the main routine a comprehensive neuropsychological evaluation including Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MOCA) and Frontal Assessment Battery (FAB) were conducted on all ALS patients, where no patient was found to have cognitive impairment. We also screened the ALS patients for mutations in TARDBP, SOD1, FIG4, FUS, CHMP2B, VCP, ELP3, SETX, HNRNPA1, SPG11, NEFH, VAPB, ANG, OPTN, UBQLN2, SQSTM1, MATR3, ATXN2, PFN1 and DCTN1 genes using multiplex PCR, variants identified in our study had been classified as pathogenic, likely pathogenic, uncertain significance, likely benign, or benign in accordance with Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology and ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar). Given the potential consequences of gene heterogeneity, nine patients with ALS carrying pathogenic variants were excluded (Supplementary Material 1). Lastly 69 ALS patients were recruited for this study. The present study was approved by the Ethics Committee of the Chinese PLA General Hospital. Informed consent was obtained from all patients and controls. The clinical indicators of the ALS patients were recorded, including age, age of onset, site of symptom onset, diagnostic category (clinically definite, clinically probable, clinically probable-laboratory-supported, or clinically possible ALS). The functional status of the ALS patients was rated using the ALSFRS-R (18), and the disease progression rate (DPR) was calculated based on the established formula: ΔFS = (48–ALSFRS-R score)/(disease duration from initial symptoms onset to evaluation in months) (19). TTG, an early clinical parameter of disease progression, was also assessed in all ALS patients (20).
The healthy control (HC) group comprised of 59 age-matched subjects who initially visited Neurology Clinic and underwent lumbar puncture for the purpose of making the exact diagnosis; however, the final diagnosis were free from neurological diseases.
CSF and blood samples were collected at the time of diagnosis in our department for analysis as described previously (21). All samples were processed within 5h following collection and stored at −80°C until further use. CSF was collected into polypropylene tubes by lumbar puncture and blood was collected into ethylenediaminetetracetic acid (EDTA) tubes, followed by centrifuged at 3,000 rpm for 15 min (4°C) to remove the cells and debris.
Total TDP-43 was determined in plasma and CSF using a commercially available ELISA kit (Human TDP-43, KE00005, Proteintech, Chicago, USA) according to the manufacturer's instructions. The standard provided by the manufacturer consisted of a His-tag recombinant human full-length TDP-43 protein (Cat. # ag13119, Proteintech). All the analysis were performed on coded samples by the analyst blinded to the patient data. Results based on duplicates with CV>10% were excluded. Each experiment was repeated at least twice. Plasma and CSF samples were tested undiluted with a single batch of reagents, respectively.
pTDP-43 levels in plasma and CSF were measured in duplicate by a commercially available ELISA kit (pTDP-43 ELISA Kit, E9442h EIAab, Wuhan, China) according to the manufacturer's instructions. In this assay, a biotinylated rabbit polyclonal antibody against TDP-43 phosphorylated at Ser409 [antiphospho-TDP-43 (pSer409), Cat. # SAB4200223 Sigma-Aldrich, Saint Louis, MI, USA] was used as a detection antibody. The standard provided by the manufacturer consisted of recombinant human TDP-43 phosphorylated at Ser409. The results were expressed as relative units (plasma or CSF), generated from concentration values normalized to a standard sample loaded on all plates as previously described (9). Results based on duplicates with CV>15% were excluded. The plasma and CSF samples were tested undiluted.
Median levels and interquartile range (IQR) were used to indicate the central value and range, respectively. The χ2 test was used to compare the differences in categorical variables, and Fisher's exact test was used to analyze the contingency tables, wherein the sample sizes were <5. Non-parametric statistics (Kruskal–Wallis and Mann–Whitney post-hoc test) were used because of the non-normal distribution and heterogeneity of variance of a majority of the variables and parametric statistics were determined by Spearman's rank correlation coefficient (r). Receiver operating characteristic (ROC) curve analysis was performed for evaluation in the ALS patients; the area under the curve (AUC) was calculated and optimal cut-off values were derived by Youden index. P-values were two-sided, and ≤ 0.05 were considered statistically significant. All analyses were performed using the SPSS for Windows (Version 21.0, IBM Corp., Armonk, NY, USA).
Sixty nine ALS patients were recruited for this study which comprised 46 males and 23 females with a mean (SD) age of 51.46 (9.42) years. The onset site was classified as spinal onset in 59 patients and as bulbar onset in 10 patients. The median average time from clinical onset to diagnosis was 19.59 months in ALS. The clinical characteristics of all participants are summarized in Table 1. The mean plasma level of TDP-43 was 113.10 and 25.19 pg/ml in ALS and HC groups, respectively; whereas, that in the CSF was 35.90 and 30.81 pg/ml, respectively. The mean pTDP-43 level in the plasma was 1.34 and 1.11% for ALS and HC groups, respectively, while the mean levels in CSF were 2.44 and 2.16%, respectively. The plasma TDP-43 level was significantly higher in ALS than HC (P < 0.001) (Figure 1A). There was no significant difference in CSF TDP-43 and pTDP-43 levels between ALS and HCs (P > 0.05) (Figures 1B,D). In addition, the plasma pTDP-43 level was also significantly higher in ALS than HC (P < 0.001) (Figure 1C). Apart from that, the pTDP-43/TDP-43 ratios in plasma were significantly higher in HCs than ALS patients (P < 0.001), but no significant difference was found in CSF (P = 0.409).
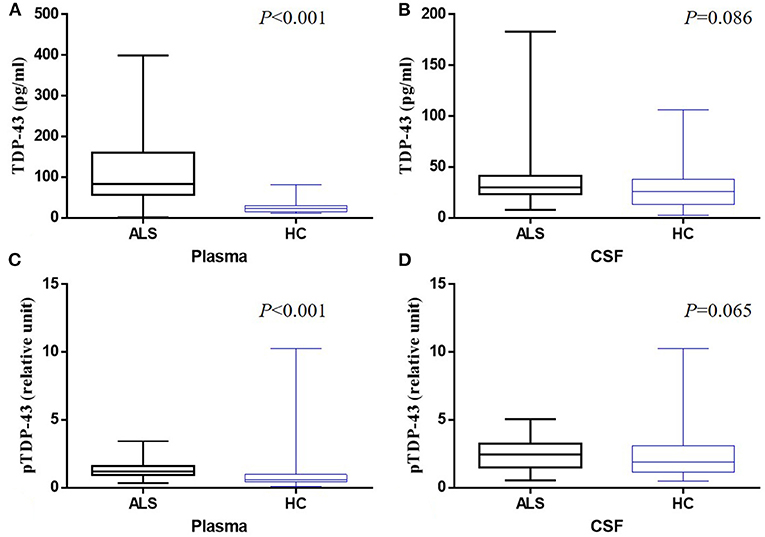
Figure 1. Box-whisker plots illustrate concentrations of TDP-43 in plasma (A) and CSF (B) and pTDP-43 in plasma (C) and CSF (D) from ALS patients and healthy controls (HC). The horizontal line in the box represents the median. Bottom and top of the box represent the 25th and 75th percentile, respectively. The whiskers (error bars) represent the range.
ROC curve analysis was used to differentiate ALS from HC, and the AUC values were 0.924 and 0.588 for plasma and CSF TDP-43 levels, respectively. A cut-off level of 37.58 pg/ml for TDP-43 in plasma generated a sensitivity of 91.30% and specificity of 91.50% (Figure 2A). The optimal cut-off value for TDP-43 levels in CSF (15.08 pg/ml) generated a sensitivity of 94.20% and specificity of 30.50% (Figure 2B). The AUC values were 0.765 and 0.595 for plasma pTDP-43 levels and CSF pTDP-43 levels, respectively. A cut-off level of 0.86% for pTDP-43 in plasma provided a 82.60% sensitivity and 67.80% specificity (Figure 2C). The optimal cut-off value for pTDP-43 levels in CSF (2.31%) provided a 56.50% sensitivity and 66.10% specificity (Figure 2D).
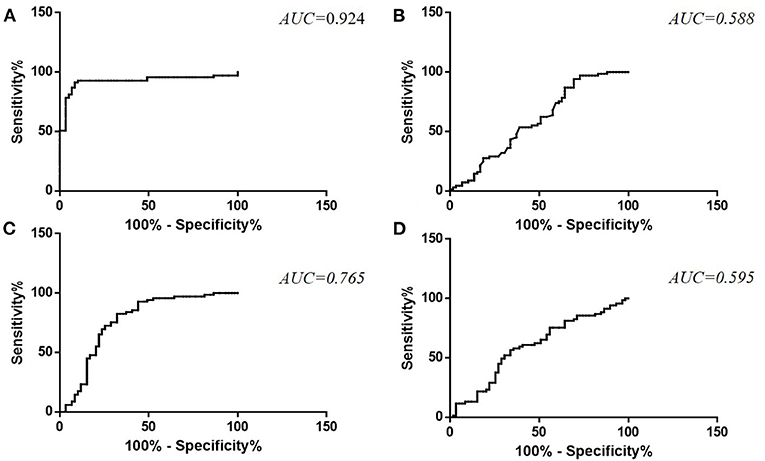
Figure 2. ROC curve for TDP-43 levels in plasma (A) and CSF (B) and pTDP-43 levels in plasma (C) and CSF (D) in ALS patients as compared to healthy controls.
In ALS patients, no significant difference was observed between males and females in TDP-43 (plasma: P = 0.410, CSF: P = 0.462) and pTDP-43 (plasma: P = 0.223, CSF: P = 0.800) levels. Moreover, no significant difference was noted between bulbar onset and spinal onset in ALS patients in TDP-43 (plasma: P = 0.179, CSF: P = 0.357) and pTDP-43 (plasma: P = 0.373, CSF: P = 0.159) levels (Table 2). Moreover, a correlation was observed between CSF pTDP-43 levels and the ALSFRS-R (r = −0.245, P = 0.042) (Figure 3B); Moreover, the correlation between plasma and CSF TDP-43 was observed in each ALS patient (r = 0.195, P = 0.027), however, no correlation was found between plasma or CSF pTDP-43 levels and other clinical indicators (Supplementary Material 2).
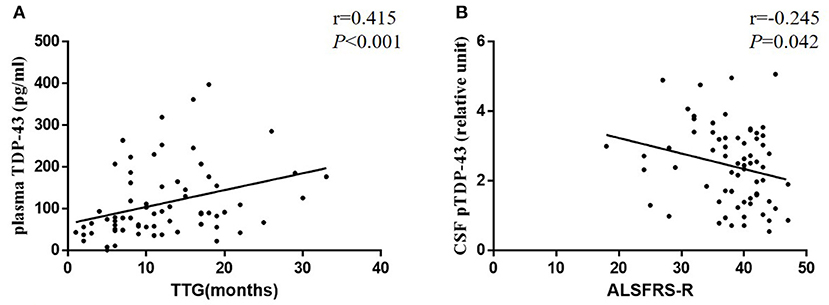
Figure 3. Scatter plots comparing plasma TDP-43 levels with TTG (A) and CSF pTDP-43 levels with age of onset (B) in each ALS patient.
All ALS patients, who participated in our study, were followed up for the outcome of the disease. The mean TTG was 12.03 (range 1–33) months. No significant difference was observed between the bulbar onset (mean, 8.60 months; range 1–22 months) and spinal onset (mean, 12.61 months; range, 2–33 months) in TTG (P = 0.100). Moreover, a correlation was established between plasma TDP-43 levels and TTG (r = 0.415, P < 0.001), which indicated that high levels of plasma TDP-43 correlated with a long TTG (Figure 3A).
The presence of cytoplasmic inclusions positive for ubiquitin is a characteristic feature of degenerating motor neurons in ALS. Neumann et al. reported that TAR DNA-binding protein 43 (TDP-43), the RNA processing protein, was the major component of cytoplasmic inclusions (22). However, only little is known about the mechanism that leads to the aggregation of TDP-43 and neurodegeneration. Mutations in TARDBP, the TDP-43 gene, have been identified in a small number of ALS patients and account for ~7% of familial and ~2% of sporadic ALS cases (23, 24). Approximately, 15% of ALS cases develop FTD, that is, pure motor deficits may coexist with cognitive deficits compatible with FTD, which is well-recognized as the frontotemporal spectrum disorder of ALS (ALS-FTSD) (25–27). TDP-43-positive protein inclusions have been detected in majority cases of tau-negative FTD, providing the pathological basis for the clinical overlap between ALS and FTD (28).
In this study, we observed a significant increase in the levels of TDP-43 and pTDP-43 in plasma of the ALS group. Moreover, the pTDP-43/TDP-43 ratios in plasma were significantly higher in HCs than ALS, which revealed that plasma TDP-43/pTDP-43 levels and the pTDP-43/TDP-43 ratios in plasma appear to distinguish individuals with ALS from controls. In present study, it is of note that 10 out of 69 ALS patients with apparent decreased levels of CSF pTDP-43 had bulbar signs, and the variability may be due in part to differences between the bulbar-onset and spinal-onset clinical and pathological characteristics of ALS with neurodegeneration (29, 30), or the differences in the sequence to repair motor neuron damage. However, our results could not confirm this finding and showed no statistical significance between bulbar-onset and spinal-onset subgroups in CSF pTDP-43 in ALS (P = 0.159). Given the limited number of cases, further studies on a larger scale are required to better understand the pathology of ALS subtypes.
Previous studies stated that TDP-43 lesions in subpial/subependymal or perivascular localizations had been focused on the TDP-43-linked neurodegeneration, which might account for increased blood or CSF TDP-43 levels through mechanisms remains unclarified (31). Furthermore, the rapid neurodegeneration in ALS may release intracellular TDP-43 from inclusions, which might lead to increased TDP-43 levels in biofluids. According to the views of Feneberg et al. the quantification of TDP-43 in CSF appears limited by very low concentrations or low binding affinity of the antibodies in the presence of high abundant immunoglobulines and albumin (3). However, the question regarding the higher levels of CSF pTDP-43 in ALS patients remains unanswered. We speculate that the discrepancy could be explained by one or more of the following hypotheses: (1) higher levels of CSF pTDP-43 might be related to the more widespread pTDP-43 pathology in brain and spinal cord, which suggests that extracellular pTDP-43 could be more abundant in CSF than plasma. (2) CSF pTDP-43 levels might depend on the stage of disease or severity and release into the extracellular space by an unconventional secretory pathway which was different with TDP-43. Further longitudinal studies based on repeat sampling from the same ALS patient in the different disease stages could then be compared. (3) The permeation rate and the level of permeability of TDP-43/pTDP-43 through the damaged BBB/BSCB could potentially be different. (4) As TDP-43 is ubiquitously expressed (32) and is found in blood plasma (2), may combine with a higher level of insoluble TDP-43 in CSF, which could partly explain the relatively higher CSF pTDP-43. Previous studies showed that blood–brain barrier (BBB) and blood–spinal cord barrier (BSCB) integrity could be compromised in ALS patients. Meanwhile, evidence suggested that structural and functional damages in BBB/BSCB in the early stage of animal models (33–37). These changes might accelerate the permeation of pTDP-43 from CSF to blood, or accompanied by a extravasation of pTDP-43 due to the leaking BBB/BSCB. Further studies involving the biological or functional of pTDP-43 will address this matter.
Several cohorts investigated the TDP-43 in biofluids were rather large and included various degenerative disease groups such as ALS and FTD, and the TDP-43 levels in CSF and plasma varied among the studies, which might be attributed to the difference in experimental methods, sample collections, and storage methods. However, information about the genetic background was absent in most of the studies, since the report of pathogenic missense mutations in the TARDBP gene in ALS cases, which demonstrated that defects in this gene were sufficient to cause familial ALS and sporadic ALS partially (23). Hasegawa et al. developed a ALS mouse model with a mutant TDP-43 knock in (KI) that heterozygously expressed the mutant human TARDBP gene (A382T or G348C). They found that the TDP-43 mRNA levels in white blood cells (WBCs) of A382T mutant mice were significantly higher than that of the G348C mutant. The mRNA levels of both apoptosis-related factors (Smn1 and Naip5) correlated with the TDP-43 levels in WBCs and also differed between A382T and G348C, which suggested that each mutation in TARDBP induces distinct RNA metabolism and abnormal RNA metabolism is one of the causes of neuronal cell death in ALS. The study also indicate that different mutation in TARDBP produces varying levels of TDP-43 in WBCs (38). Therefore, the concentrations of TDP-43 in plasma and CSF could be varied in ALS patients, especially in those harboring the mutation in TARDBP. A previous study described the CSF TDP-43 levels in ALS patients with hexanucleotide repeat expansion (HRE) in the C9orf72 gene, however, no difference in the CSF TDP-43 levels was observed between the C9orf72 expansion carriers and noncarriers in the cohort (11). Given the potential consequences of gene heterogeneity, nine patients with ALS carrying pathogenic variants were excluded in our study, hitherto, there has been no relevant research concerning the impact of the TARDBP mutation on the levels of TDP-43 in CSF and plasma in ALS patients. Hence, large-scale and well-controlled studies, especially including subjects with gene-confirmed ALS patients are imperative.
In the current study, the AUC value was 0.924 for plasma TDP-43 levels, which generated a high sensitivity and specificity. However, a low AUC value in CSF vs. plasma in present study. We speculate three possibilities from the difference. In present study, CSF was collected into polypropylene tubes and stored in screw-cap Eppendorf tubes, and blood was collected into EDTA tubes and stored in screw caps polypropylene tubes. EDTA is a chelating agent that inhibits protease activity by binding with mental ions such as calcium ions, magnesium ions and iron ions. Recent studies showed some evidence of physiological electrolytes induced reversible aggregation of YFP-tagged full-length TDP-43 (yTDP-43) in vitro model system. The order of aggregation induction potency was K+ < Na+ < Mg2+ < Ca2+(39). The EDTA-plasma tubes cause less aggregation of TDP-43 than polypropylene-CSF tubes, which is consistent with the results of higher levels of TDP-43 in the plasma but little in the CSF. These differences may explain the low AUC value in CSF. A second possibility is that TDP-43 could permeate the damaged BBB/BSCB freely. However, if protease could not permeate the BBB/BSCB, or only a small minority could, then the TDP-43 levels in CSF and plasma could be different. Under the assumption of degradation and metabolism of TDP-43 in CSF is influenced by the protease, a possible hypothesis is that compared with the protease-rich CSF, TDP-43 in the plasma may be more robust and less susceptible to proteases. In addition, Feneberg et al. found that the TDP-43 CSF to blood concentration ratio is about 1:200 and TDP-43 in CSF originating mainly from blood (40), and on this basis the quantification of TDP-43 appears confined to extremely low binding affinity or concentrations in CSF (3), which presenting another possibility. In the current cohort, we used the previously published pTDP-43 ELISA method (9) to estimate its sensitivity and applied this method to evaluate the levels of pTDP-43 in CSF and plasma from ALS patients and HCs. Moreover, TTG, as an early progression parameter of ALS, was evaluated and suggested a significant correlation with plasma TDP-43 levels, which indicated that plasma TDP-43 might be optimal for the assessment of disease progression. ALSFRS-R is commonly used to reflect disease severity in ALS and a negative correlation between CSF pTDP-43 and ALSFRS-R score was observed. However, these numerical associations may simply be fortuitous, or may reflect that the data we have presented here, although intriguing, is still very much preliminary, and a large-scale prospective study would be essential to substantiate the current findings.
This is the first study comprising of an Chinese population with screened genetic background of the patient and simultaneous evaluation of the plasma and CSF TDP-43/pTDP-43 levels in ALS patients. The study showed plasma TDP-43 may play an important role in disease diagnosis and as a progression predictor of ALS. However, several caveats should be kept in mind when evaluating our findings. First, the GGGGCC copy number and expansion within the C9orf72 gene was not evaluated in ALS patients allowing for an extremely low incidence of mutation among Chinese populations in our earlier limited understanding (41). Second, the study did not set up the disease control groups, particularly given the expected differential deposition of TDP-43/pTDP-43 in other TDP-43 proteinopathy (42), which was a disadvantage during differential diagnosis. Thirdly, the pathological assessment and confirmation of correlation between biofluid levels and protein deposition in the cerebrum or spinal cord were lacking.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Chinese PLA General Hospital. The patients/participants provided their written informed consent to participate in this study.
YR: concept, data analysis, experimental studies, and manuscript preparation. XH: design, manuscript editing, and manuscript review. FC: literature search and manuscript editing. ML and HW: data acquisition. FY: data analysis. XS: data analysis and statistical analysis. SC: data acquisition and data analysis. SL: experimental studies and literature search. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.663637/full#supplementary-material
1. Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. (2011) 7:639–49. doi: 10.1038/nrneurol.2011.153
2. Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer's disease and frontotemporal lobar degeneration. Acta Neuropathol. (2008) 116:141–6. doi: 10.1007/s00401-008-0389-8
3. Feneberg E, Gray E, Ansorge O, Talbot K, Turner MR. Towards a TDP-43-based biomarker for ALS and FTLD. Mol Neurobiol. (2018) 55:7789–01. doi: 10.1007/s12035-018-0947-6
4. Kasai T, Tokuda T, Ishigami N, Sasayama H, Foulds P, Mitchell DJ, et al. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol. (2009) 117:55–62. doi: 10.1007/s00401-008-0456-1
5. Foulds PG, Davidson Y, Mishra M, Hobson DJ, Humphreys KM, Taylor M, et al. Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta Neuropathol. (2009) 118:647–58. doi: 10.1007/s00401-009-0594-0
6. Steinacker P, Hendrich C, Sperfeld AD, Jesse S, von Arnim CA, Lehnert S, et al. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol. (2008) 65:1481–7. doi: 10.1001/archneur.65.11.1481
7. Verstraete E, Kuiperij HB, van Blitterswijk MM, Veldink JH, Schelhaas HJ, van den Berg LH, et al. TDP-43 plasma levels are higher in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. (2012) 13:446–51. doi: 10.3109/17482968.2012.703208
8. Noto Y, Shibuya K, Sato Y, Kanai K, Misawa S, Sawai S, et al. Elevated CSF TDP-43 levels in amyotrophic lateral sclerosis: specificity, sensitivity, and a possible prognostic value. Amyotroph Lateral Scler. (2011) 12:140–3. doi: 10.3109/17482968.2010.541263
9. Suarez-Calvet M, Dols-Icardo O, Llado A, Sanchez-Valle R, Hernandez I, Amer G, et al. Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J Neurol Neurosurg Psychiatry. (2014) 85:684–91. doi: 10.1136/jnnp-2013-305972
10. Majumder V, Gregory JM, Barria MA, Green A, Pal S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. BMC Neurol. (2018) 18:90. doi: 10.1186/s12883-018-1091-7
11. Junttila A, Kuvaja M, Hartikainen P, Siloaho M, Helisalmi S, Moilanen V, et al. Cerebrospinal fluid TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis patients with and without the C9ORF72 hexanucleotide expansion. Dement Geriatr Cogn Dis Extra. (2016) 6:142–9. doi: 10.1159/000444788
12. Kasai T, Kojima Y, Ohmichi T, Tatebe H, Tsuji Y, Noto YI, et al. Combined use of CSF NfL and CSF TDP-43 improves diagnostic performance in ALS. Ann Clin Transl Neurol. (2019) 6:2489–502. doi: 10.1002/acn3.50943
13. Bourbouli M, Rentzos M, Bougea A, Zouvelou V, Constantinides VC, Zaganas I, et al. Cerebrospinal fluid TAR DNA-binding protein 43 combined with tau proteins as a candidate biomarker for amyotrophic lateral sclerosis and frontotemporal dementia spectrum disorders. Dement Geriatr Cogn Disord. (2017) 44:144–52. doi: 10.1159/000478979
14. Hosokawa M, Arai T, Yamashita M, Tsuji H, Nonaka T, Masuda-Suzukake M, et al. Differential diagnosis of amyotrophic lateral sclerosis from Guillain-Barre syndrome by quantitative determination of TDP-43 in cerebrospinal fluid. Int J Neurosci. (2014) 124:344–9. doi: 10.3109/00207454.2013.848440
15. Tortelli R, Copetti M, Panza F, Cortese R, Capozzo R, D'Errico E, et al. Time to generalisation as a predictor of prognosis in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. (2016) 87:678–9. doi: 10.1136/jnnp-2014-308478
16. Tortelli R, Copetti M, Panza F, Fontana A, Cortese R, Capozzo R, et al. Time to generalization and prediction of survival in patients with amyotrophic lateral sclerosis: a retrospective observational study. Eur J Neurol. (2016) 23:1117–25. doi: 10.1111/ene.12994
17. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) 1:293–9. doi: 10.1080/146608200300079536
18. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurolo Sci. (1999) 169:13–21. doi: 10.1016/S0022-510X(99)00210-5
19. Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. (2006) 66:265–7. doi: 10.1212/01.wnl.0000194316.91908.8a
20. Tortelli R, Copetti M, Ruggieri M, Cortese R, Capozzo R, Leo A, et al. Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur J Neurol. (2015) 22:215–8. doi: 10.1111/ene.12421
21. Li S, Ren Y, Zhu W, Yang F, Zhang X, Huang X. Phosphorylated neurofilament heavy chain levels in paired plasma and CSF of amyotrophic lateral sclerosis. J Neurol Sci. (2016) 367:269–74. doi: 10.1016/j.jns.2016.05.062
22. Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. (2006) 314:130–3. doi: 10.1126/science.1134108
23. Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. (2008) 40:572–4. doi: 10.1038/ng.132
24. Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. (2008) 319:1668–72. doi: 10.1126/science.1154584
25. Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. (2005) 65:586–90. doi: 10.1212/01.wnl.0000172911.39167.b6
26. Strong MJ, Abrahams S, Goldstein LH, Woolley S, McLaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Other Motor Neuron Disord. (2017) 18:153–74. doi: 10.1080/21678421.2016.1267768
27. Agarwal S, Highton-Williamson E, Caga J, Howells J, Dharmadasa T, Matamala JM, et al. Motor cortical excitability predicts cognitive phenotypes in amyotrophic lateral sclerosis. Sci Rep. (2021) 11:2172. doi: 10.1038/s41598-021-81612-x
28. Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. (2010) 9:995–1007. doi: 10.1016/S1474-4422(10)70195-2
29. Schuster C, Elamin M, Hardiman O, Bede P. The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur J Neurol. (2016) 23:1361–71. doi: 10.1111/ene.13038
30. Shellikeri S, Karthikeyan V, Martino R, Black SE, Zinman L, Keith J, et al. The neuropathological signature of bulbar-onset ALS: a systematic review. Neurosci Biobehav Rev. (2017) 75:378–92. doi: 10.1016/j.neubiorev.2017.01.045
31. Geser F, Prvulovic D, O'Dwyer L, Hardiman O, Bede P, Bokde AL, et al. On the development of markers for pathological TDP-43 in amyotrophic lateral sclerosis with and without dementia. Prog Neurobiol. (2011) 95:649–62. doi: 10.1016/j.pneurobio.2011.08.011
32. Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. (2001) 20:1774–84. doi: 10.1093/emboj/20.7.1774
33. Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. (2007) 1157:126–37. doi: 10.1016/j.brainres.2007.04.044
34. Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, et al. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE. (2007) 2:e1205. doi: 10.1371/journal.pone.0001205
35. Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. (2008) 11:420–2. doi: 10.1038/nn2073
36. Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, et al. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. (2009) 1301:152–62. doi: 10.1016/j.brainres.2009.09.018
37. Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, et al. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. (2011) 89:718–28. doi: 10.1002/jnr.22594
38. Hasegawa M, Hara-Miyauchi C, Ohta H, Sakimura K, Okano H, Okano HJ. Analysis of RNA metabolism in peripheral WBCs of TDP-43 KI mice identifies novel biomarkers of ALS. Neurosci Res. (2016) 106:12–22. doi: 10.1016/j.neures.2015.11.009
39. Sun Y, Medina Cruz A, Hadley KC, Galant NJ, Law R, Vernon RM, et al. Physiologically important electrolytes as regulators of TDP-43 aggregation and droplet-phase behavior. Biochemistry. (2019) 58:590–607. doi: 10.1021/acs.biochem.8b00842
40. Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, et al. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener. (2014) 15:351–6. doi: 10.3109/21678421.2014.905606
41. He J, Tang L, Benyamin B, Shah S, Hemani G, Liu R, et al. C9orf72 hexanucleotide repeat expansions in Chinese sporadic amyotrophic lateral sclerosis. Neurobiol Aging. (2015) 36:2660. e1–8. doi: 10.1016/j.neurobiolaging.2015.06.002
Keywords: amyotrophic lateral sclerosis, biomarker, TDP-43, pTDP-43, CSF, plasma
Citation: Ren Y, Li S, Chen S, Sun X, Yang F, Wang H, Li M, Cui F and Huang X (2021) TDP-43 and Phosphorylated TDP-43 Levels in Paired Plasma and CSF Samples in Amyotrophic Lateral Sclerosis. Front. Neurol. 12:663637. doi: 10.3389/fneur.2021.663637
Received: 03 February 2021; Accepted: 19 May 2021;
Published: 14 June 2021.
Edited by:
Emanuele Buratti, International Centre for Genetic Engineering and Biotechnology, ItalyReviewed by:
Federico Verde, Istituto Auxologico Italiano (IRCCS), ItalyCopyright © 2021 Ren, Li, Chen, Sun, Yang, Wang, Li, Cui and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xusheng Huang, bGV3aXNoMzAxQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.