
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 15 July 2021
Sec. Sleep Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.654158
This article is part of the Research TopicTranslation and Processing of Light by the Non-Image Forming Visual System – Context, Mechanisms and ApplicationsView all 17 articles
 Manuel Spitschan1,2,3*
Manuel Spitschan1,2,3*Light exposure profoundly affects human physiology and behavior through circadian and neuroendocrine photoreception primarily through the melanopsin-containing intrinsically photosensitive retinal ganglion cells. Recent research has explored the possibility of using temporally patterned stimuli to manipulate circadian and neuroendocrine responses to light. This mini-review, geared to chronobiologists, sleep researchers, and scientists in adjacent disciplines, has two objectives: (1) introduce basic concepts in time-varying stimuli and (2) provide a checklist-based set of recommendations for documenting time-varying light exposures based on current best practices and standards.
Light profoundly affects human circadian and neuroendocrine physiology. Signals processed by the photoreceptors in the retina encode different aspects of environmental light. There are three classes of photoreceptors: the cones (of which there are three different spectral classes, the L, M, and S cones), the rods, and the melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs). The retinal photoreceptors are sensitive to different but overlapping wavelength ranges, with melanopsin playing the primary role in mediating the circadian and neuroendocrine effects of light (1). Furthermore, the different photoreceptor classes also differ in the way they respond to light stimuli that are patterned in time, such as a train of brief flashes or sinusoidal flicker. With recent studies showing that flashes of light lead to different effects on circadian and neuroendocrine physiology than continuous light exposure (2, 3), it is worth reviewing how light stimuli changing over time can be described parametrically.
This tutorial paper is targeted to chronobiologists, sleep researchers and scientists from adjacent disciplines, such as environmental psychology, who wish to develop an understanding of specifying light exposure in time. The focus will be on how time-varying light stimuli can be described quantitatively in the time and frequency domain, learn about major classes of time-varying stimuli and their properties, and some caveats in using time-varying stimuli. This paper is providing an introduction to readers with no specific background in signal processing and analyses of time-varying signals.
An intuitive way of thinking about time-varying stimuli is by representing their variation as a function of time, i.e., as a time course (Figure 1A). This representation is called the time domain. In this representation, stimulus values vary across different time points. In most cases, this representation is discrete, i.e., there is a set of time points for which stimulus values are specified. These stimulus values could be the luminance or illuminance, or some variant of radiance or illuminance, depending on the space in which the stimuli are specified. They could also be specified in terms of the contrast relative to an explicit or implicit reference light, such as an adaptation light.
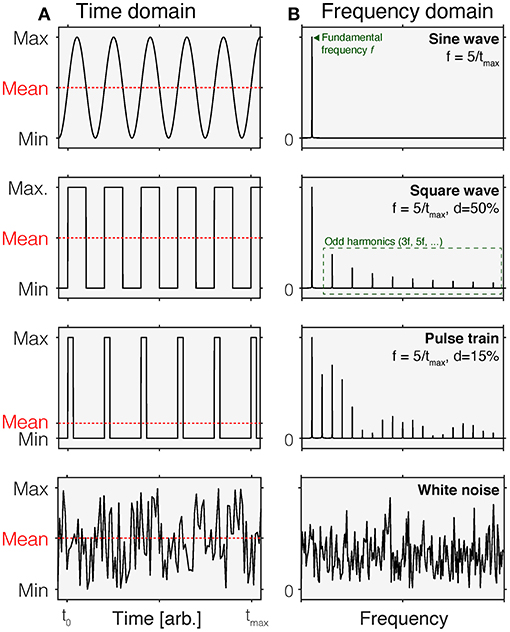
Figure 1. Time-domain (A) and normalized frequency (B) representations of common time-varying stimuli. In each panel in (A), the mean of each waveform is indicated by the dashed red line. Different waveforms in the time domain have different properties in the frequency domain. Row 1: A sine wave is a periodic waveform going between minimum and maximum values. It only contains frequency components at the fundamental frequency f. Row 2: A square wave varies in a discrete fashion between minimum and maximum values. It is composed of sine waves of different frequencies, including the fundamental frequency f and odd harmonics (3f, 5f,…). In a square wave, the duty cycle is 50%, which means that over time, the fraction of time at the maximum value is 50% within a period. Row 3: A pulse train can be thought of as a square wave with duty cycles that are <50%. This shorting of the time of that the wave is at maximum introduces additional harmonics. Row 4: White noise corresponds to a signal that is sampled from a uniform distribution. In the frequency domain, this corresponds to a flat curve, indicating that all frequencies are equally represented.
An equivalent, but to some perhaps less intuitive way of representing time-varying stimuli is the frequency-domain representation (Figure 1B). While the time-domain representation shows the change of a signal over time, the frequency-domain representation describes the extent to which a given signal happens at different frequencies. For example, a sinusoidal change in intensity with a period length of 1 s has a frequency of 1 Hz but has no power at any other frequencies. The power a given signal has at all frequencies (within a given range) is called the power spectrum. Time-domain and frequency-domain representations can be converted between each other using the Fourier Transform, which decomposes the periodic signal into its constituent, single-frequency components (4).
The previous discussion mentioned two general classes of specifying waveforms: intensity and contrast. It is worth considering what actual quantities might be used for the specification of temporal waveforms and which units these might be expressed in. Here, we will review different ways of specifying the temporal waveform, focusing on absolute (intensity) and relative (contrast) units.
The most basic representation of time-varying stimuli is a time course of spectral radiance or irradiance distributions, giving the radiance or irradiance of the stimulus at each wavelength for each point in time. At one point in time, this corresponds to a physical measurement (5) with no direct reference to the human photoreceptors. Spectra are weighted by the spectral sensitivities of the different photoreceptor classes (6), and then summed to arrive at alpha-opic radiance (for spectral radiance measurements) or alpha-opic irradiance (for spectral irradiance measurements). Box 1 provides a glossary of relevant terms. This can be accomplished using an Excel-based toolbox provided by the CIE (7, 8) and the recently published platform-independent open-access and open-source web software luox (https://luox.app/) (9).
In cases where there is a well-defined background light around which stimuli are presented, e.g., sinusoidally flickering lights against a mean background, it is useful to specify the contrast of the flickering stimuli as the relative change in (il)luminance or alpha-opic (ir)radiance relative to the background (which is the mean of the signal for sinusoidal flicker). In certain cases, time-varying contrast could be specified in a cone-opponent space (10). This is essential in cases where the photoreceptor excitation is actively controlled, such as using metameric lights changing over time, or stimuli generated using the method of silent substitution (11). In the method of silent substitution, lights are designed in such a way that they only produce differences in the activation of a selected class of photoreceptors, with no difference in the activation of the other photoreceptor classes, which are “silent” to the exchange of lights.
It is important to note that time-varying stimuli defined in one stimulus space may not be linear when represented in another stimulus space. One such example is the mapping between correlated color temperature (CCT), a common way to specify the color appearance of lights relative to reference spectra (blackbody, <5,000 K or daylight spectrum, >5,000 K). A specification of CCT varying over time does not linearly map onto cone contrast changing over time, as CCT and cone activations are not linearly correlated. Consequently, such a non-linearity may lead to undesired frequency components in another space.
Box 1. Glossary.
Radiance-derived quantities
Spectral radiance: The spectral radiance is the absolute spectrum of light of a surface (self-emitting, e.g., displays, or reflecting, light reflected from a wall) per united solid angle per unit projected area. Spectral radiance is measured in W/m2/sr/nm. From the radiance, we can calculate various quantities:
• Luminance (cd/m2): Spectrum weighted by the photopic luminosity function.
• α-opic radiances (W/m2/sr): Spectrum weighted by the five α-opic effect functions corresponding to the spectral sensitivities of the human photoreceptors and summed. As a consequence, there are α-opic radiances (the letter “α” is just a placeholder). The melanopic radiance (W/m2/sr) is the spectrum weighted by the melanopsin spectral sensitivity function and summed.
• α-opic equivalent daylight luminance (EDL) (cd/m2): Luminance of a D65 daylight spectrum that produces the same α-opic radiance is the spectrum in question. The melanopic equivalent daylight luminance (mEDL) is in reference to the melanopic radiance.
Irradiance-derived quantities
Spectral irradiance: The spectral radiance is the absolute spectrum of light of a received by a given area. Spectral irradiance is measured in W/m2/nm. From the irradiance, we can calculate various quantities:
• Illuminance (lux): Spectrum weighted by the photopic luminosity function.
• α-opic irradiances (W/m2): Spectrum weighted by the five α-opic effect functions corresponding to the spectral sensitivities of the human photoreceptors and summed. As a consequence, there are α-opic radiances (the letter “α” is just a placeholder). The melanopic irradiance (W/m2) is the spectrum weighted by the melanopsin spectral sensitivity function and summed.
• α-opic equivalent daylight luminance (EDL) (lux): Illuminance of a D65 daylight spectrum that produces the same α-opic radiance is the spectrum in question. The melanopic equivalent daylight illuminance (mEDI) is in reference to the melanopic irradiance.
Chromaticity is an (il)luminance-independent way of specifying the color of an object, surface, or spectrum.
Correlated color temperature (CCT) is the temperature of a black-body radiator that matches the chromaticity of a spectrum in question.
Contrast is the relative different of activation (e.g., melanopic irradiance) between two different spectra.
A pulse is a change in stimulus intensity or contrast that has a limited duration. For example, a brief light flash in an otherwise dim environment is a pulse. When the intensity is pulsed around a background light, the more intuitive way to specify the stimulus properties is in terms of contrast, i.e., the relative difference in stimulus intensity with respect to the background light.
Ramps are increases or decreases in intensity or contrast up or down to a specified level. An example of such a stimulus is a gradual increase of light intensity in “dawn simulation” lights (12–19). The term ramp itself is ill-defined, as ramps can be linear, exponential, logarithmic, or modulated in some other way, e.g., using a cosine window [e.g., (20)]. Different ramps have different parameters in the frequency space. Ramps are often used to remove transient signals at the onset of a light, smoothing out the abrupt transition between different spectra.
Square-wave flicker changes between two different intensity or contrast settings at a given frequency. A simple intuitive example is turning on the light switch in a room for 1 s, and turning it off again for 1 s.
Sinusoidal flicker is a light that is gradually changing intensity or contrast in accordance to the sine function. Sinusoidal flicker is parameterized by the frequency, phase, and the amplitude. In the frequency domain, sinusoidal flicker has the property that it only contains power at one frequency, the fundamental frequency. Square-wave flicker has power at odd harmonics as well.
A sequence or train of pulses can be parameterized by the ratio between the duration that the light is on and the duration that the light is off. This is called the duty cycle and expresses the percentage that a light is turned on as part of the entire period. A duty cycle of 0% is of course simply no light, and a duty cycle of 100% is a continuous light. A repeating sequence of pulses with 50% duty cycle corresponds to the special case of square wave flicker (see above).
Of course, the space of possible waveforms is infinite. However, in studies employing stimuli parametrically, the waveforms above describe most use cases. Time courses sampled from different underlying distributions are called colored noise. For example, white noise (Figure 1, bottom row) has a flat frequency spectrum.
Documenting and reporting of lighting conditions is key to ensuring that studies can be reproduced, aggregated, and placed into context. While guidelines detailing how static aspects of light should be documented have been developed recently (5, 21) and are worth consulting for details on spectral characterization of light, technical details on time-varying light exposure are currently not typically documented and reported in standard form. Recently, the International Commission on Illumination (CIE) published technical note CIE TN 011:2020 (6), describing which information should be captured in studies investigating non-visual responses to light. The document lists the following primary aspects that should be captured and documented: (1) the timeline of the experiment explained in detail, in clock time; (2) the duration of exposure in minutes or hours; (3) the sequence of exposures, including pre-experimental light and environmental exposures to the greatest degree of detail possible. The document expands this description to include (1) the overall duration of the experiment, (2) timing of light exposure (in clock time), (3) dim light exposure, (4) duration of the light exposure (in min or h), (5) duration of dim up and dim down (in min), and (6) pattern.
In an earlier document, CIE 213:2014 (22), the CIE suggested the following descriptors for dynamic light: (1) initial conditions (luminance, light source color, direction, etc.), specified as above; (2) intermediate and ending conditions, specified as above; (3) rate of change; (4) rate of cycle, if any; (5) change profile, particularly if the change is not linear; (6) movement of the observer's head and eyes relative to the light source or illuminated area. It is suggested to consult the two CIE documents already in the design stage of a research project.
In Table 1, we propose a reporting workflow based on the recommendations of CIE TN 011:2020 (6) and CIE 213:2014 (22) specifically for experiments in chronobiology and sleep research. In addition to naming specific quantities of interest and their derivatives, the proposed workflow also includes guidance on whether a specific item should be essential, optional, or recommended. The workflow is expandable and versatile. While it may be possible to derive this information from the full text of a given published study, future work should consider the development of a formalized schema which facilitates automated analyses based on stimulus descriptions as well as appropriate software tools that make it easy for investigators to provide this information. This may help make research future-proof (23) and more sustainable.
Light sources are generally controlled using an input parameter, which we will call the input settings. In a conventional 8-bit RGB monitor, for example, three primary lights can be controlled at 255 levels. However, the output radiance is not linear with the input RGB settings but instead follows a non-linear gamma function. As a consequence, for example, an RGB triplet of [127 127 127] does not represent the expected 50% of the maximum output radiance, but less. To be able to control a monitor linearly then requires a measurement of the gamma function. The same of course applies to other light sources.
Unless they are corrected, non-linearities in output radiance lead to distortions in the output signal. Consider, for example, the case of a sinusoidal modulation that is displayed using a non-linear light source. In the time domain, the resulting waveform is no longer symmetric around the mean. In the frequency domain, it becomes obvious that the non-linearities have introduced additional frequencies that were not present in the target modulation.
In addition to non-linearities in output radiance at different input settings, light sources may also shift in their spectral output. This is most notable in LEDs, which can shift up to a few nanometers, depending on how they are driven. Of course, such a spectral shift will manifest in uncertainties in the effective stimulus and may also introduce undesirable artifacts (such as the unwanted stimulation of photoreceptors). As the direction and size of these spectral shifts cannot be predicted easily, a practical solution is to measure the spectral output at different input settings. These measurements can then be used to design stimuli that account for the spectral shifts or to characterize the uncertainty in stimulus presentation post-hoc, or both.
A historical precedent cautioning to calibrate the temporal output of light generators carefully was offered by Mollon and Polden (24). Measuring the time constants of tachistoscopes, which were devices enabling very brief light exposures pre-dating the common usage of computer displays in research, they found that the time constants were too slow to present stimuli at the millisecond scale accurately. More than 40 years later, accuracy of timing is still a topic in visual psychophysics, with each generation of novel display technology bringing its own potential idiosyncratic temporal artifacts (25–29).
In the previous discussion, the near-exclusive focus has been on accurately capturing the temporal properties of the stimulus in the physical domain. In psychophysics, this is sometimes called the distal stimulus, while the pattern of light impinging on the retina is called the proximal stimulus. Notably, the distal stimulus and the proximal stimulus are related to one another through the optics of image formation and projection onto the retina, but they are not the same. Before light excites the photoreceptors, it is modified (relative to the cornea) by passing through the pupil and the ocular media.
Pupil size, being the aperture of the eye, changes the overall retinal irradiance over a factor of ~16 × , or 1.2 log units, given by the ratio of the largest possible pupil area under full dark adaptation (8 mm) to the smallest possible pupil area under bright light conditions (2 mm) (30). The pupil is not static and responds to light in a wavelength- and time-specific fashion. Under time-varying stimuli, then, the pupil size is also dynamically changing, modifying the temporal properties of the distal stimulus in a way that is not very easy to predict. In addition, the pupil responds to other factors unrelated to light, such as cognitive processing (31), and displays spontaneous fluctuations (32, 33) which have been found to be related sleepiness (34). Furthermore, pupil size varies with age, with older people having smaller pupils on average (35), and is subject to diurnal variations (36–41).
As expected, when pupil size is controlled through pharmacological dilation and the stimulus is viewed through this maximally dilated pupil, the same corneal irradiance leads to more melatonin suppression compared with the undilated pupil (42). As a consequence, dose–response curves collected under undilated conditions [e.g., (43)] represent a mixture of two effects: a pupil size effect that modifies retinal light exposure, and a melatonin-suppressive effect. Special care must be taken when dose–response curves collected under different pupil conditions are compared (1, 44).
One solution to estimating retinal illuminance may be the use of mobile eye trackers during a given experiment [e.g., (45)], which enable the determination of pupil size at all time points. In conjunction with head-referenced irradiance or radiance measurements of the corneal irradiance, capturing an individual's “spectral diet” (46), the time course of light exposure at the cornea, it is then possible to determine the actual retinal irradiance, as this is the biologically relevant quantity. Development efforts for such a system are currently underway [e.g., (47)].
Of course, observers are moving their trunk, head, and eyes during the waking day, thereby displacing the retinal image at a high frequency (48). Saccadic eye movements displace the retinal image (49), thereby repositioning different parts of the visual world into the fovea, thereby supporting various visually guided tasks and support various tasks (50, 51). Saccadic eye movements are guided by a variety of factors, including salience and higher-level factors (52). In free-viewing of pictures, saccades can make up to 20% of time (53). In addition, during periods of fixation, three types of fixational eye movements occur: tremor, also called physiological nystagmus, occurring at ~90 Hz, drift, and microsaccades, occurring at 1–2 Hz (54). The extent to which the trunk, head, and eye movements decorrelate the distal from the proximal (retinal) stimulus depends on the spatial characteristics of the scene. One can imagine two extremes. In a completely unarticulated homogenous environment such as those produced by a ganzfeld stimulus, the retinal stimulus to an observer may be nearly constant. On the other extreme, a point light source in an otherwise dark room under free-viewing conditions will stimulate different retinal locations between and—due to fixational eye movements—during fixation, thereby producing very large spatial and temporal contrast.
Box 2. Recommended further reading.
• The CIE has published two documents on documenting lighting and light exposures: CIE 213:2014 (22) and CIE TN 011:2020 (6). The articles published Spitschan et al. (5) and Knoop et al. (21) represent independent proposal for standardized ways of reporting lighting conditions in experimental situations.
• Watson (73) provides a solid introduction into linear-systems modeling of temporal sensitivity in human psychophysics.
• Kronauer et al. (56) synthesize temporal non-linearities in the circadian system's response to light and subject it to rigorous modeling.
• Schlangen & Price (74) provide an extensive introduction in measuring the lighting environment.
• Münch et al. (75) and Knoop et al. (76) provide recent syntheses on the status of daylight for humans, which may serve as a reference when thinking about naturalistic light exposure and its temporal properties.
Ultimately, the use of temporally patterned stimuli biases processing in the retina to a specific set of photoreceptors. While previous work has examined how different photoreceptors drive the pupillary light reflex in different temporal regimes (55), there are at present no parametric measurements for the effects of photoreceptor-selective stimuli varying in their frequency properties on neuroendocrine and circadian physiology. A few studies (2, 3, 56) address the question of temporal integration, though a lot remains unknown in humans. The growing body of literature in animal models [rodents: (57–60), Drosophila: (61, 62)] can serve as a useful starting point.
It may be desirable to derive a summary metric for time-varying stimuli. For pulse stimuli, one intuitive way to summarize illuminance or irradiance and duration is the product of the two. This yields a summary quantity with units in e.g., “lux minutes.” This approach has been used in literature to describe total light doses [e.g., (12–14, 63–65)], in line with the notion that the circadian system acts as a photon counter (66). The extent to which this is a useful metric, however, is not necessarily clear. It relies on reciprocity, which refers to the notion that irradiance and duration can be traded off without any difference in effect. Further data mapping out how intensity and duration can be traded off (67) are required before the adoption of such a summary metric.
In field experiments where both light exposure and an outcome metric (such as sleep timing and duration, or other circadian, neuroendocrine, behavioral, and cognitive outputs) are measured conjointly, summarizing the pattern of light exposure is often a necessary step to relate input to output. A data-driven approach to summarizing light exposures in these contexts is to use time above threshold (TAT), as a well as distributional characteristics of the light exposure above the threshold, such as the mean and standard deviation (68).
As the temporal properties of the proximal (retinal) stimulus cannot easily be determined, understanding the properties of the distal stimulus is a key first step. One strategy is to quantitatively characterize the propagation of light in specific study context using simulation software (69). This can be achieved using specialized architectural lighting design tools. An integrated workflow to go from physically realistic simulations of illuminated spaces to physiologically plausible simulations of retinal illumination [such as those available in the iSETBIO toolbox (70–72)] is a promising and exciting path forward. For readers wishing to learn more about the fundamentals touched upon in this review, Box 2 provides advanced reading for readers with a background in signal processing and analyses of time-varying signals but no specific background in chronobiology and sleep research.
MS: conceptualization, methodology, software, resources, writing—original draft preparation, writing—review and editing, and visualization.
This work was supported by Wellcome Trust 204686/Z/16/Z and 204686/Z/16/C.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Brown TM. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J Pineal Res. (2020) 69:e12655. doi: 10.1111/jpi.12655
2. Najjar RP, Zeitzer JM. Temporal integration of light flashes by the human circadian system. J Clin Invest. (2016) 126:938–47. doi: 10.1172/JCI82306
3. Rahman SA, St Hilaire MA, Chang AM, Santhi N, Duffy JF, Kronauer RE, et al. Circadian phase resetting by a single short-duration light exposure. JCI Insight. (2017) 2:e89494. doi: 10.1172/jci.insight.89494
4. Smith SW. The Scientist and Engineer's Guide to Digital Signal Processing 1st ed. San Diego, CA: California Technical Pub. (1997).
5. Spitschan M, Stefani O, Blattner P, Gronfier C, Lockley SW, Lucas RJ. How to report light exposure in human chronobiology and sleep research experiments. Clocks Sleep. (2019) 1:280–9. doi: 10.3390/clockssleep1030024
6. CIE. CIE TN 011:2020: What to Document and Report in Studies of ipRGC-Influenced Responses to Light. Vienna: CIE Central Bureau (2020). doi: 10.25039/TN.011.2020
7. CIE. CIE S 026 Toolbox (v1.49a - November 2020) [Computer Program]. Vienna: CIE (2020). doi: 10.25039/S026.2018.TB
8. CIE. User Guide to the α-opic Toolbox for Implementing CIE S 026 [Computer Program]. Vienna: CIE (2020). doi: 10.25039/S026.2018.UG
9. Spitschan M, Mead J, Roos C, Lowis C, Griffiths B, Mucur P, et al. luox: novel open-access and open-source web platform for calculating and sharing physiologically relevant quantities for light and lighting. Wellcome Open Res. (2021) 6:1. doi: 10.12688/wellcomeopenres.16595.1
10. Brainard DH. Cone contrast and opponent modulation color spaces. In: Kaiser B, Boynton R, editors. Human Color Vision. 2nd ed. Washington, DC: Optical Society of America (1996).
11. Spitschan M, Woelders T. The method of silent substitution for examining melanopsin contributions to pupil control. Front Neurol. (2018) 9:941. doi: 10.3389/fneur.2018.00941
12. Terman M, Terman JS. Controlled trial of naturalistic dawn simulation and negative air ionization for seasonal affective disorder. Am J Psychiatry. (2006) 163:2126. doi: 10.1176/ajp.2006.163.12.2126
13. Palmer CR, Kripke DF, Savage HC, Cindrich LA, Loving RT, Elliott JA. Efficacy of enhanced evening light for advanced sleep phase syndrome. Behav Sleep Med. (2003) 1:213–26. doi: 10.1207/S15402010BSM0104_4
14. Danilenko KV, Wirz-Justice A, Krauchi K, Cajochen C, Weber JM, Fairhurst S, et al. Phase advance after one or three simulated dawns in humans. Chronobiol Int. (2000) 17:659–68. doi: 10.1081/CBI-100101072
15. Gabel V, Maire M, Reichert CF, Chellappa SL, Schmidt C, Hommes V, et al. Dawn simulation light impacts on different cognitive domains under sleep restriction. Behav Brain Res. (2015) 281:258–66. doi: 10.1016/j.bbr.2014.12.043
16. Thompson A, Jones H, Gregson W, Atkinson G. Effects of dawn simulation on markers of sleep inertia and post-waking performance in humans. Eur J Appl Physiol. (2014) 114:1049–56. doi: 10.1007/s00421-014-2831-z
17. Bromundt V, Wirz-Justice A, Boutellier M, Winter S, Haberstroh M, Terman M, et al. Effects of a dawn-dusk simulation on circadian rest-activity cycles, sleep, mood and well-being in dementia patients. Exp Gerontol. (2019) 124:110641. doi: 10.1016/j.exger.2019.110641
18. Van De Werken M, Giménez MC, De Vries B, Beersma DG, Van Someren EJ, Gordijn MC. Effects of artificial dawn on sleep inertia, skin temperature, and the awakening cortisol response. J Sleep Res. (2010) 19:425–35. doi: 10.1111/j.1365-2869.2010.00828.x
19. Kavanau JL. Twilight transitions and biological rhythmicity. Nature. (1962) 194:1293–5. doi: 10.1038/1941293a0
20. Spitschan M, Bock AS, Ryan J, Frazzetta G, Brainard DH, Aguirre GK. The human visual cortex response to melanopsin-directed stimulation is accompanied by a distinct perceptual experience. Proc Natl Acad Sci USA. (2017) 114:12291–6. doi: 10.1073/pnas.1711522114
21. Knoop M, Broszio K, Diakite A, Liedtke C, Niedling M, Rothert I, et al. Methods to describe and measure lighting conditions in experiments on non-image-forming aspects. Leukos. (2019) 15:163–79. doi: 10.1080/15502724.2018.1518716
22. CIE. CIE 213:2014: Guide to Protocols for Describing Lighting. Vienna: CIE Central Bureau (2014).
23. Spitschan M. Opinion: future-proofing circadian research. Light Res Technol. (2019) 51:818–9. doi: 10.1177/1477153519873223
24. Mollon JD, Polden PG. On the time constants of tachistoscopes. Quart J Exp Psychol. (2018) 30:555–68. doi: 10.1080/00335557843000133
25. MacInnes WJ, Taylor TL. Millisecond timing on PCs and Macs. Behav Res Methods Instrum Comput. (2001) 33:174–8. doi: 10.3758/BF03195363
26. Plant RR, Quinlan PT. Could millisecond timing errors in commonly used equipment be a cause of replication failure in some neuroscience studies? Cogn Affect Behav Neurosci. (2013) 13:598–614. doi: 10.3758/s13415-013-0166-6
27. Rohr M, Wagner A. How monitor characteristics affect human perception in visual computer experiments: CRT vs. LCD monitors in millisecond precise timing research. Sci Rep. (2020) 10:6962. doi: 10.1038/s41598-020-63853-4
28. Elze T. Misspecifications of stimulus presentation durations in experimental psychology: a systematic review of the psychophysics literature. PLoS ONE. (2010) 5:12792. doi: 10.1371/journal.pone.0012792
29. Elze T. Achieving precise display timing in visual neuroscience experiments. J Neurosci Methods. (2010) 191:171–9. doi: 10.1016/j.jneumeth.2010.06.018
30. Watson AB, Yellott JI. A unified formula for light-adapted pupil size. J Vis. (2012) 12:12. doi: 10.1167/12.10.12
31. Mathôt S. Pupillometry: psychology, physiology, and function. J Cogn. (2018) 1:16. doi: 10.5334/joc.18
32. Yoss RE, Moyer NJ, Hollenhorst RW. Hippus and other spontaneous rhythmic pupillary waves. Am J Ophthalmol. (1970) 70:935–41. doi: 10.1016/0002-9394(70)92470-0
33. Bouma H, Baghuis LC. Hippus of the pupil: periods of slow oscillations of unknown origin. Vision Res. (1971) 11:1345–51. doi: 10.1016/0042-6989(71)90016-2
34. Wilhelm B, Wilhelm H, Lüdtke H, Streicher P, Adler M. Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep. (1998) 21:258–65.
35. Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci. (1994) 35:1132–7.
36. Fosnaugh JS, Bunker EB, Pickworth WB. Daily variation and effects of ambient light and circadian factors on the human light reflex. Methods Find Exp Clin Pharmacol. (1992) 14:545–53.
37. Loving RT, Kripke DF, Glazner LK. Circadian rhythms in the human pupil and eyelid. Am J Physiol. (1996) 271:R320–4. doi: 10.1152/ajpregu.1996.271.2.R320
38. Ranzijn R, Lack L. The pupillary light reflex cannot be used to measure sleepiness. Psychophysiology. (1997) 34:17–22. doi: 10.1111/j.1469-8986.1997.tb02411.x
39. Wilhelm B, Giedke H, Lüdtke H, Bittner E, Hofmann A, Wilhelm H. Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. J Sleep Res. (2001) 10:1–7. doi: 10.1046/j.1365-2869.2001.00239.x
40. Yu M, Kautz MA, Thomas ML, Johnson D, Hotchkiss ER, Russo MB. Operational implications of varying ambient light levels and time-of-day effects on saccadic velocity and pupillary light reflex. Ophthalmic Physiol Opt. (2007) 27:130–41. doi: 10.1111/j.1475-1313.2006.00450.x
41. Van Egroo M, Gaggioni G, Cespedes-Ortiz C, Ly JQM, Vandewalle G. Steady-state pupil size varies with circadian phase and sleep homeostasis in healthy young men. Clocks Sleep. (2019) 1:240–58. doi: 10.3390/clockssleep1020021
42. Gaddy JR, Rollag MD, Brainard GC. Pupil size regulation of threshold of light-induced melatonin suppression. J Clin Endocrinol Metab. (1993) 77:1398–401. doi: 10.1210/jc.77.5.1398
43. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. (2000) 526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x
44. Bracke P, Van de Putte E, Ryckaert WR. Comment concerning the effects of light intensity on melatonin suppression in the review “light modulation of human clocks, wake, and sleep” by A. Prayag et al. Clocks Sleep. (2021) 3:181–8. doi: 10.3390/clockssleep3010011
45. Kassner M, Patera W, Bulling A. Pupil: an open source platform for pervasive eye tracking and mobile gaze-based interaction. UbiComp '14 Adjunct. (2014) 2014:1151–60. doi: 10.1145/2638728.2641695
46. Webler FS, Spitschan M, Foster RG, Andersen M, Peirson SN. What is the 'spectral diet' of humans? Curr Opin Behav Sci. (2019) 30:80–6. doi: 10.1016/j.cobeha.2019.06.006
47. Lazar R, Spitschan M. Measurement of light-mediated changes in pupil size under real-world conditions. OSF Home [Preprint]. (2020). doi: 10.17605/OSF.IO/RNP2Z
48. Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. (1997) 12:537–46. doi: 10.1177/074873049701200608
49. Rayner K. Eye movements in reading and information processing. Psychol Bullet. (1978) 85:618–60. doi: 10.1037/0033-2909.85.3.618
50. Land M, Mennie N, Rusted J. The roles of vision and eye movements in the control of activities of daily living. Perception. (1999) 28:1311–28. doi: 10.1068/p2935
51. Land MF, Hayhoe M. In what ways do eye movements contribute to everyday activities? Vis Res. (2001) 41:3559–65. doi: 10.1016/S0042-6989(01)00102-X
52. Schutz AC, Braun DI, Gegenfurtner KR. Eye movements and perception: a selective review. J Vis. (2011) 11:9. doi: 10.1167/11.5.9
53. Martinez-Conde S. Fixational eye movements in normal and pathological vision. Prog Brain Res. (2006) 154:151–76. doi: 10.1016/S0079-6123(06)54008-7
54. Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nat Rev Neurosci. (2004) 5:229–40. doi: 10.1038/nrn1348
55. Spitschan M, Jain S, Brainard DH, Aguirre GK. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc Natl Acad Sci USA. (2014) 111:15568–72. doi: 10.1073/pnas.1400942111
56. Kronauer RE, St Hilaire MA, Rahman SA, Czeisler CA, Klerman EB. An exploration of the temporal dynamics of circadian resetting responses to short- and long-duration light exposures: cross-species consistencies and differences. J Biol Rhythms. (2019) 34:497–514. doi: 10.1177/0748730419862702
57. Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol. (1991) 439:115–45. doi: 10.1113/jphysiol.1991.sp018660
58. Vidal L, Morin LP. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J Neurosci. (2007) 27:3375–82. doi: 10.1523/JNEUROSCI.5496-06.2007
59. Van Den Pol AN, Cao V, Heller HC. Circadian system of mice integrates brief light stimuli. Am J Physiol. (1998) 275:R654–7. doi: 10.1152/ajpregu.1998.275.2.R654
60. Arvanitogiannis A, Amir S. Resetting the rat circadian clock by ultra-short light flashes. Neurosci Lett. (1999) 261:159–62. doi: 10.1016/S0304-3940(99)00021-X
61. Negelspach DC, Kaladchibachi S, Fernandez F. The circadian activity rhythm is reset by nanowatt pulses of ultraviolet light. Proc Biol Sci. (2018) 285:1288. doi: 10.1098/rspb.2018.1288
62. Kaladchibachi S, Negelspach DC, Zeitzer JM, Fernandez F. Optimization of circadian responses with shorter and shorter millisecond flashes. Biol Lett. (2019) 15:20190371. doi: 10.1098/rsbl.2019.0371
63. Friedman L, Zeitzer JM, Kushida C, Zhdanova I, Noda A, Lee T, et al. Scheduled bright light for treatment of insomnia in older adults. J Am Geriatr Soc. (2009) 57:441–52. doi: 10.1111/j.1532-5415.2008.02164.x
64. Wee R, Van Gelder RN. Sleep disturbances in young subjects with visual dysfunction. Ophthalmology. (2004) 111:297–302. doi: 10.1016/j.ophtha.2003.05.014
65. Terman M, Jiuan Su. Circadian rhythm phase advance with dawn simulation treatment for winter depression. J Biol Rhythms. (2010) 25:297–301. doi: 10.1177/0748730410374000
66. Brown TM. Using light to tell the time of day: sensory coding in the mammalian circadian visual network. J Exp Biol. (2016) 219:1779–92. doi: 10.1242/jeb.132167
67. Joyce DS, Spitschan M, Zeitzer JM. Integration of brief light flashes varying in intensity and duration by the human circadian system. bioRxiv [Preprint]. (2021). doi: 10.1101/759134
68. Reid KJ, Santostasi G, Baron KG, Wilson J, Kang J, Zee PC. Timing and intensity of light correlate with body weight in adults. PLoS ONE. (2014) 9:e92251. doi: 10.1371/journal.pone.0092251
69. Dawson D, Campbell SS. Bright light treatment: are we keeping our subjects in the dark? Sleep. (1990) 13:267–71.
70. Cottaris NP, Jiang H, Ding X, Wandell BA, Brainard DH. A computational-observer model of spatial contrast sensitivity: effects of wave-front-based optics, cone-mosaic structure, and inference engine. J Vis. (2019) 19:8. doi: 10.1167/19.4.8
71. Cottaris NP, Wandell BA, Rieke F, Brainard DH. A computational observer model of spatial contrast sensitivity: effects of photocurrent encoding, fixational eye movements, and inference engine. J Vis. (2020) 20:17. doi: 10.1167/jov.20.7.17
72. Farrell JE, Jiang H, Winawer J, Brainard DH, Wandell BA. 27.2: distinguished paper: modeling visible differences: the computational observer model. SID Symp Digest Technical Pap. (2014) 45:352–6. doi: 10.1002/j.2168-0159.2014.tb00095.x
73. Watson AB. Temporal sensitivity. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of Perception and Human Performance. New York, NY: Wiley (1986).
74. Schlangen LJM, Price LLA. The lighting environment, its metrology, and non-visual responses. Front Neurol. (2021) 12:624861. doi: 10.3389/fneur.2021.624861
75. Münch M, Wirz-Justice A, Brown SA, Kantermann T, Martiny K, Stefani O, et al. The role of daylight for humans: gaps in current knowledge. Clocks Sleep. (2020) 2:61–85. doi: 10.3390/clockssleep2010008
Keywords: time-varying light exposure, circadian photoreception, non-visual effects of light, temporal stimuli, ipRGCs, melanopsin, reporting
Citation: Spitschan M (2021) Time-Varying Light Exposure in Chronobiology and Sleep Research Experiments. Front. Neurol. 12:654158. doi: 10.3389/fneur.2021.654158
Received: 15 January 2021; Accepted: 06 May 2021;
Published: 15 July 2021.
Edited by:
Narong Simakajornboon, Cincinnati Children's Hospital Medical Center, United StatesReviewed by:
Thomas Dye, Cincinnati Children's Hospital Medical Center, United StatesCopyright © 2021 Spitschan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel Spitschan, bWFudWVsLnNwaXRzY2hhbkBwc3kub3guYWMudWs= orcid.org/0000-0002-8572-9268
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.